Abstract
Background:
CDC recently published Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-resistant Organisms (MDROs). We assessed the impact of implementing the strategy in a U.S. state using a mathematical model.
Methods:
We used a deterministic compartmental model, parametrized via a novel analysis of Carbapenem-resistant Enterobacteriaceae (CRE) data reported to the National Healthcare Safety Network and patient transfer data from the Center for Medicare & Medicaid Services. The simulations assumed that after the importation of the MDRO and its initial detection by clinical culture at an index hospital, fortnightly prevalence surveys for colonization and additional infection control interventions were implemented at the index facility; similar surveys were then also implemented at those facilities known to be connected most strongly to it as measured by patient transfer data; and, prevalence surveys were discontinued after two consecutive negative surveys.
Results:
If additional infection control interventions are assumed to lead to a 20% reduction in transmissibility in intervention facilities, prevalent case count in the state 3 years after importation would be reduced by 76% (IQR: 73%−77%). During the third year, these additional infection control measures would be applied in facilities accounting for 42% (37%−46%) of inpatient days.
Conclusions:
CDC guidance for containing MDROs, when used in combination with information on transfer of patients among hospitals, is predicted to be effective, enabling targeted and efficient use of prevention resources during an outbreak response. Even modestly effective infection control measures may lead to a substantial reduction in transmission events.
Keywords: multidrug-resistant organism, healthcare epidemiology, mathematical model
Summary:
Assessing the impact of implementing CDC’s Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-resistant Organisms using a mathematical model, we find substantial reduction in transmission even with modestly effective infection control measures.
Introduction
The emergence of novel pathogens and resistance mechanisms poses a serious threat to human health, and healthcare settings often act as important amplifiers for transmission of emerging pathogens [1–3]. Accordingly, prevention efforts frequently include interrupting transmission in healthcare facilities to reduce infection-associated morbidity and mortality. Since first being reported in the United States in 2001, carbapenemase-producing (CP) Klebsiella pneumoniae and other carbapenem-resistant Enterobacteriaceae (CRE) have become important causative agents of healthcare-associated infections with relatively few treatment options [4]. Patients with CP-CRE infections have been identified in all US states [5]. In addition to CP-CRE, other emerging MDROs (e.g., Candida auris) have demonstrated the ability to amplify in healthcare settings necessitating an aggressive public health response in these settings to prevent infections among patients at risk and to slow its regional spread.
CDC has issued interim guidance for slowing the spread of novel or targeted multidrug-resistant organisms (henceforth referred to as CDC guidance for containing MDROs [6]. The strategy is based upon five pillars: 1) rapid detection of targeted pathogens and their resistance mechanisms, 2) on-site infection control assessments by trained experts to identify gaps in infection prevention, 3) screening of exposed contacts to identify asymptomatic colonization for targeted pathogens, 4) coordination of the response among facilities, and 5) continuing these interventions until transmission is controlled [7]. This study aims to predict the regional effects of implementing an approach that targets a subset of facilities using a transmission model that includes patient transfer patterns in a region.
Materials and methods
Analytical background
The regional transmission model is a multifacility SIS (susceptible – infected/infectious – susceptible) model that assumes N constant occupancy facilities (hospitals, nursing homes, communities, etc.) linked through patient transfer. The dynamics of the prevalence νa at facility α are governed by
| #(1) |
Here βa is the transmissibility at facility α (the number infected per unit time by an infectious person introduced into facility α when all others at the facility are susceptible), γ is the carriage clearance rate, τa is the average length of stay at facility α, and nba⁄na represents the fraction of admissions na at facility α that are transfers nba from facility b. The first term on the right-hand side represents incident cases at the facility; the second, loss of cases through clearance and discharge; and the third, introduction of cases at admission through transfers from other facilities in the network. Further analytical details are in the online supplement.
Patient flow network
The patient flow network is characterized by the facility-to-facility transfer tallies nba, the number of admissions (and discharges) nba aggregated over a given time interval, and the average lengths of stay τa. The principal source for these quantities are the Center for Medicare & Medicaid Services (CMS) patient-level fee-for-service claims data for CMS beneficiaries. The dataset allows every claim to be tracked by an anonymized unique patient identifier, a facility identifier, and admission and discharge dates, to weave together the patient flow network, either across the United States, or by state or group of states. In practice, the tallies were aggregated over a single calendar year. For transfers among facilities, tallies were aggregated by the date of the second admission and by the time from the first discharge to the second admission (a day or less for direct transfers, longer for indirect). Discharge and admission dates were used to estimate average lengths of stay, by facility.
Tracking the flow of those with any healthcare encounter in the CMS dataset, we find that, on average, most of their time is spent in the community and not within healthcare facilities. Thus, the communities are important reservoirs in the network. In order to capture the clustering reflected in the communities, the Dartmouth Atlas of Health Care Hospital Referral Regions (HRRs) [8] were used as the community components of the patient flow network: a patient who was not transferred to another facility was ascribed to the HRR associated with the facility or its ZIP code.
The patient flow network is built from CMS fee-for-service claims data that track CMS beneficiaries only, and a census of persons 65 years and older in the HRRs. The occupancy and transfer numbers used are expected to underestimate their true values. However, in our model (equation 1), it is the relative values of these numbers, rather than their absolute values, that determine the dynamics.
Disease characteristics
The characteristics of the infectious disease that are relevant to our model are the clearance rate γ and the setting specific transmissibility, β. Since the model implicitly assumes a constant population where births and deaths balance, the death of an infectious person and clearance – both resulting in the replacement of an infectious person by a susceptible person – are quantitatively indistinguishable. Thus, γ captures both mortality and clearance rate For CRE, the clearance rate is an input parameter in our model, estimated from a review of the literature [9].
The estimation of transmissibility, which is disease and setting specific and may vary due to changes in infection control practices, etc., is particularly challenging. To estimate the transmissibility for CRE, we used data on positive laboratory tests for CRE reported to the National Healthcare Safety Network (NHSN) in 2015 from 3 states with mandatory reporting of CRE, tallied by the hospital where the specimen was collected. In addition, we made several assumptions: First, based on an examination of the annual number of cases of CRE reported to NHSN, that the situation in 2015 was approximately a steady, or endemic, state for CRE. Second, that the proportion of laboratory positive cases among all infected persons (who are assumed to be infectious) is a constant. Third, that the admission prevalence at all hospitals within each HRR were identical. And, finally, that the transmissibility depends only on the type of hospital (short-stay versus long-stay). Denoting the number of positive laboratory tests for CRE from facility α by la (and the number of admissions by na), and the proportion of laboratory positives among all infected as p, we may write
| #(2) |
For short-stay hospitals, the appropriate regression model is
| #(3) |
which yields, for βS, the transmissibility at short-stay hospitals,
| #(4) |
where cτ is the coefficient of τ in (3). For long-stay hospitals, the appropriate regression model is
| #(5) |
If c0 and c1 are the intercept and coefficient of 1⁄τ, respectively, in the model (5), then we obtain, for βL, the transmissibility at long-stay hospitals,
| #(6) |
Further, p, the fraction of all infected cases that are laboratory positive for CRE, is estimated as:
| #(7) |
Containment of multidrug-resistant organisms
The CDC guidance for containing MDROs [6] was translated for the simulation as follows. Consistent with guidance for the control of organisms with resistance mechanisms novel to the U.S. (Tier 1 organisms), the infection control intervention (i.e., timely implementation of enhanced infection control measures, and contact investigation), are assumed to lead to a specified fractional reduction in the transmissibility at the facility where it is implemented. We varied the specified fractional reduction in transmission within intervention facilities to assess the regional effects for interventions of varying effectiveness. Implementation is facility-wide, and is triggered when the cumulative number of admitted and incident infectious persons at a facility exceeds 1⁄p, corresponding to one expected lab positive patient. Enhanced infection control measures decreasing the transmissibility, β, starts 30 days after the trigger event (allowing time for testing of the index patient and to institute improvements in infection control measures) at the targeted facilities, which include the triggering facility, the upstream facility most likely to supply infectious persons, and the downstream facility most likely to receive infectious persons (representing healthcare investigation). Point prevalence surveys are conducted at the targeted facilities starting two weeks after initial implementation and every two weeks thereafter; if the number of infected persons (carriers and clinical cases) detected is less than one at two successive point prevalence surveys at a facility, enhanced infection control measures are stopped. Enhanced infection control may resume at a facility if an additional trigger event is detected there or at a connected facility.
Simulations
Simulations were run on a network of short-stay and long-stay acute care hospitals within a US state (State A). (Nursing home transmissibility could not be estimated using the methods discussed here since NHSN does not cover nursing homes, which were excluded from the simulation network.) Outbreaks were simulated on the network by seeding a selected facility with an index case and then solving (1) for the hospital and community prevalence values as functions of time using a numerical differential equation solver (package deSolve on R). For each run, the value of the transmissibility β at each hospital was selected randomly from a probability distribution parametrized by the statistically fitted values from (3) and (5). The containment strategy was evaluated by comparing outbreaks that were identically initiated and parametrized, run with and without the infection control measures triggered by the containment strategy (Table 1).
Table 1.
Summary of parameter values and sources
| Parameter | Value | Source |
|---|---|---|
| Disease parameters | ||
| Carriage clearance rate, γ | 1/387 per day | Ref [9] |
| Transmissibility (short stay), βS | 0.104 (95% CI 0.071–0.125) per day | Estimated (NHSN, CMS Claims) |
| Transmissibility (long stay), βL | 0.042 (95% CI 0.036–0.048) per day | Estimated (NHSN, CMS Claims) |
| Intervention parameters | ||
| Intervention start delay | 30 days | Informed guess (CDC) |
| Interval between PPSs | 14 days | Informed guess (CDC) |
| Negative PPSs to stop intervention | 2 | Informed guess (CDC) |
Results
Patient flow network
The regional model (Figure 1), constructed using CMS claims data tracking the flow of CMS beneficiaries in State A, included 160 hospitals [median Medicare beneficiary patient census52 [interquartile range (IQR) 19–92]; total 11,184 patients; median facility-level mean length of stay 7.0 days (IQR 6.0–8.0 days)]; 10 HRRs (population range 52,240–623,900; total population 2,410,291); 27,580 direct hospital to hospital transfers and 502,270 discharges from hospitals to the HRRs, annually.
Figure 1.
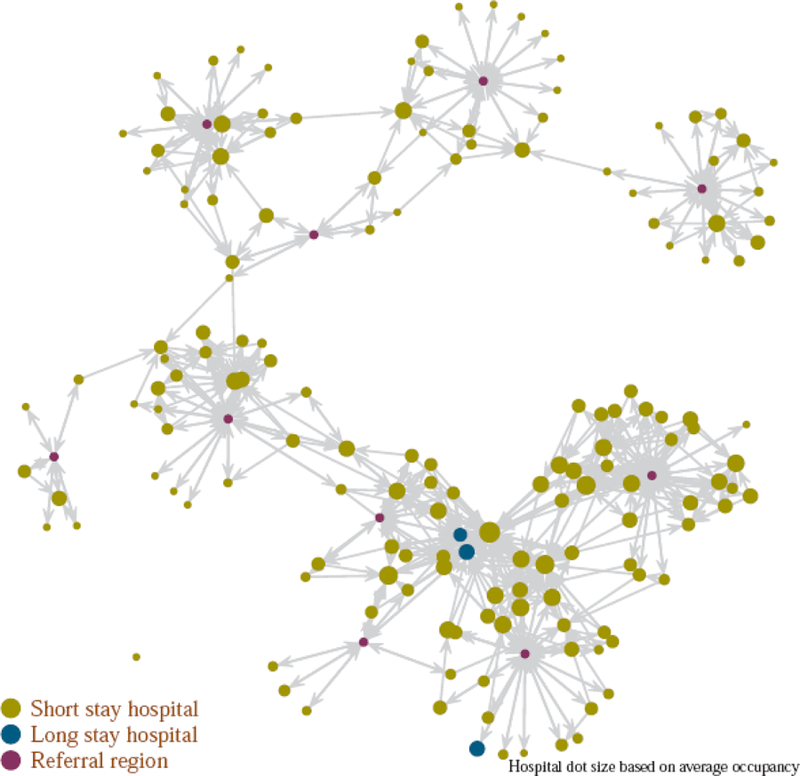
Patient transfer network in State A, based on the movement of Medicare fee-for-service beneficiaries. The location of hospitals do not correspond to geographical coordinates. Arrows correspond to at least 10 transfers per year.
Disease characteristics
Estimates of transmissibility β at hospitals were based on 3,085 positive CRE laboratory test results reported to NHSN from samples collected at 196 hospitals across 30 HRRs in the U.S. in 2015. The average length of stay τ, and the number of positive results l divided by the number of admissions in 2015 at each hospital (plotted in Figure 2) were fitted using regression models (3) and (5), using the break in the average length of stay data at around 15 days to separate short and long stay hospitals. Finally, using (8), βS= 0.104 (95% CI 0.071–0.125), and using (11), βL = 0.042 (0.036–0.048) per day, or approximately one transmission for every 9.6 or 23.8 days of stay of an index infected or colonized person at a short or long stay hospital, respectively (Table 1). In addition, equation (7) yields p = 0.137 or approximately 1 in 7.3 [the proportion of infected or colonized persons who have a positive clinical (i.e., non-surveillance) culture].
Figure 2.
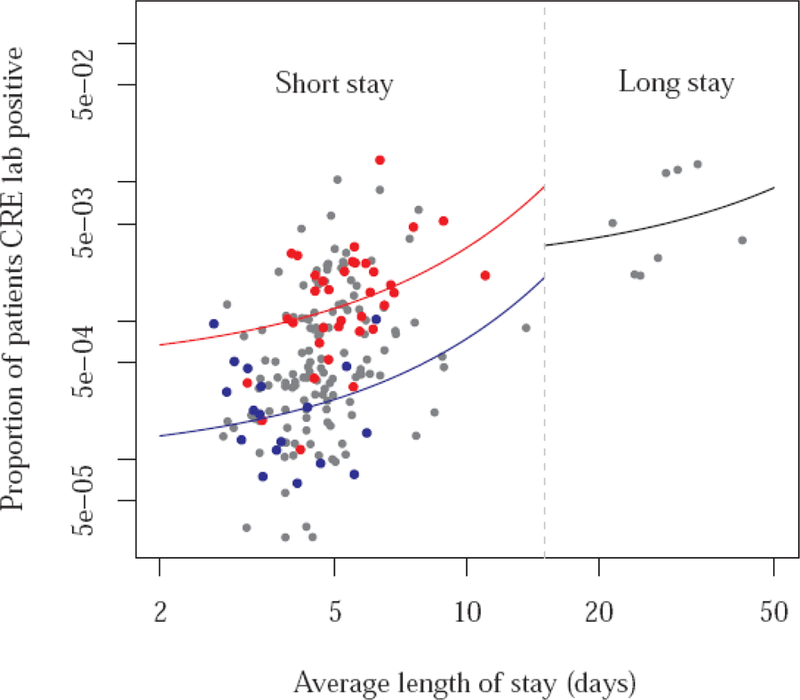
Proportion of patients with clinical isolates that were tested positive for CRE (NHSN laboratory results from 3 states, 2015) versus average length of stay, by reporting hospital. The continuous lines represent regression fits, used for estimation of transmissibility. The blue and red lines are fitted to the blue and red dots, respectively, representing short stay hospitals from two different exemplar HRRs.
Simulations
Simulations were run with transmissibility drawn randomly from probability distributions (one for short-stay, another for long-stay, hospitals), as well as for uniform transmissibility across each type of hospital (where transmissibility at each hospital takes on the mean value rather than one drawn from a distribution) (Figure 3). Heterogeneity in transmissibility results in more transmissions and, consequently, larger outbreaks. Across the heterogeneous simulations where enhanced infection control was implemented as a 20% reduction in β, 84 (IQR 77–89) of 160 hospitals had been targeted for intervention after three years into the outbreak. During the third year, 42% (IQR 37%−46%) of inpatient days (and, of patients on average) were under intervention. The reduction in the number of infectious persons three years into the outbreak was 76% (IQR 73%−77%) across simulations (Figure 3).
Figure 3.
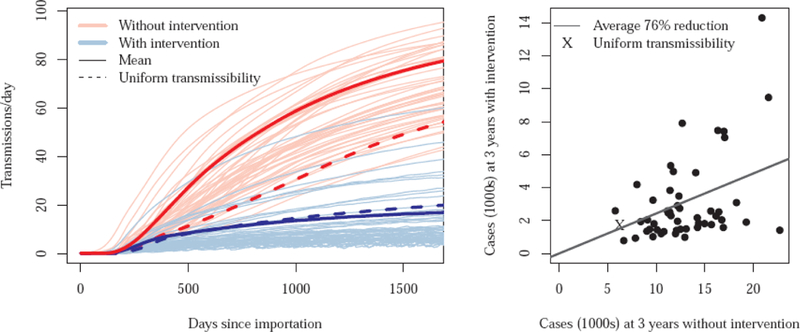
Left: Trajectories of simulated regional outbreaks with intervention that resulted in a 20% reduction in transmissibility at targeted hospitals, compared to the course of the outbreak with no intervention. Each pair of trajectories (blue and red, with and without containment, respectively) differs from other pairs in transmissibility. Right: Comparison of the number of prevalent cases three years into the outbreak, with and without containment. Each dot (and an “x” for the uniform draw) represents case counts at 3 years with and without intervention for a single draw from the two transmissibility probability distributions (short and long stay).
An additional set of simulations explored the effect of varying the reduction in the transmissibility, β, on the outbreak. As expected, the size of an outbreak decreases with increasing effectiveness of the intervention (Figure 4). As the effectiveness of the intervention increases, there is a diminishing return on the percent reduction in cases, with a plateau reached at about 40%. However, increased intervention effectiveness has the benefit of reducing the proportion of inpatient days that are subject to the intervention throughout the simulated intervention effectiveness range (Figure 5).
Figure 4.
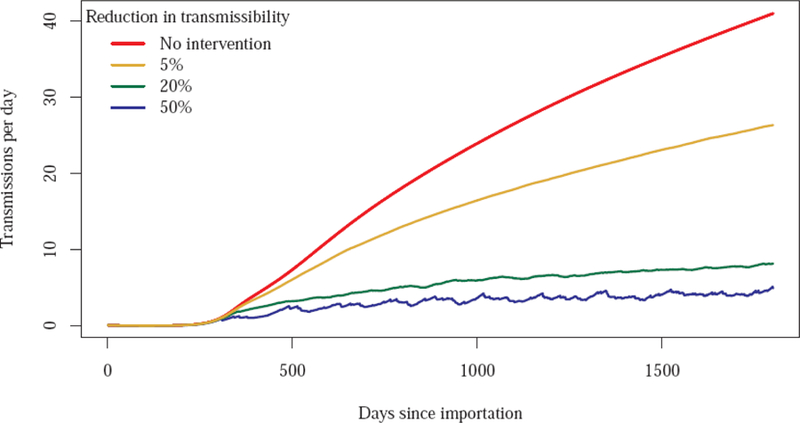
Course of outbreak without and with intervention, by intervention effectiveness (percent reduction in transmissibility). All four simulations assumed transmissibility were uniform within facility type (i.e., one uniform value for all short stay hospitals, and another uniform value for all long stay hospitals)..
Figure 5.
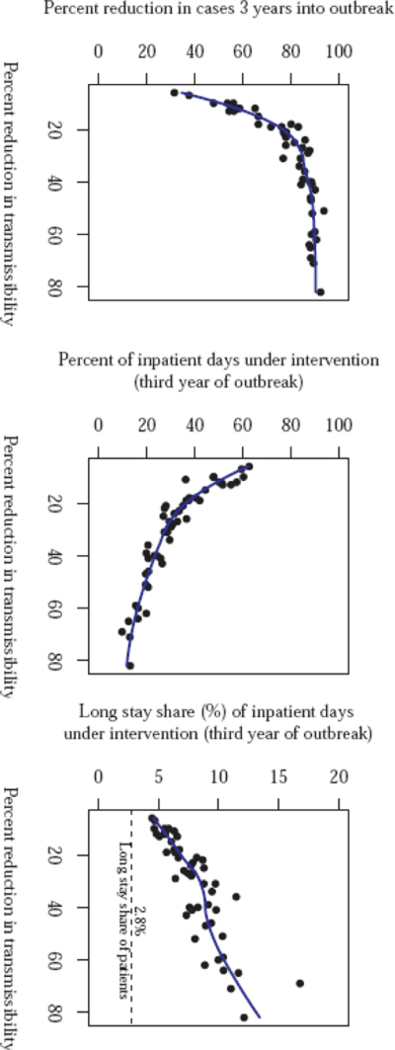
Relative reduction in prevalent case count three years into the outbreak (left), proportion of total inpatient days that were targeted for enhanced infection control measures (intervention) during the third year of the outbreak (center), and proportion of inpatient days under additional infection control measures that were at longer stay hospitals during the third year of the outbreak (right), versus reduction in transmissibility within targeted facilities due to intervention.
Discussion
This model indicates that implementation of the CDC guidance for containing MDROs, simulated on a state network of healthcare facilities interconnected through patient transfers, may have a substantial impact on the regional spread of a healthcare-associated MDRO. The simulation suggests that the impact is achievable even when intervening in a subset of all facilities, and with modest reductions in intra-facility transmission. For example, a 20% reduction within intervention facilities results in an estimated 76% drop in regional cases. The MDRO containment strategy parallels the successful ring vaccination strategy in smallpox eradication [10]. Ring vaccination followed up thorough investigation of confirmed smallpox patients with rapid vaccination of close contacts, as well as their close contacts. The containment strategy implements transmission risk reduction measures (akin to vaccination) at the facility, and then the neighboring facilities most connected through patient transfer (contacts) upstream and downstream. The success of ring vaccination for smallpox eradication exemplifies the judicious use of scarce resources. Similarly, the trade-off between the degree of reduction in transmissibility, and the duration and coverage of infection control measures in our results highlights opportunities for judicious allocation of resources by local health departments and hospitals to maximize impact. The diminishing returns on decrease in transmissibility, as well as the trade-off between decreasing transmissibility and duration of intervention, can be inputs to a planned cost analysis of the containment strategy.
Our results are consistent with successful demonstrations of MDRO containment through regional implementation of these types of responses in the past. After the 2006 introduction of a clonal outbreak of CRE in Israel, a national response aimed at preventing further CRE transmission was undertaken including hospital-level assessments of infection control and microbiology practices noting areas of improvement, contact isolation of CRE carriers, and monthly feedback on performance. These efforts resulted in a 71% decrease from peak incidence [11]. Smaller scale public health responses in Tennessee and Iowa have also been successful in containing the introduction of novel organisms and resistance mechanisms [7].
Our results are based on a mathematical transmission model of an emerging MDRO (CRE) parametrized through novel statistical analyses of NHSN data on clinical isolates. The analyses yield estimates of the epidemiologically important ratio of clinical infections to carriers, as well as of setting-specific transmissibility parameters. This is salient since test results of clinical isolates may be all that is available in the emergent phase of a novel MDRO. Our parameter estimates are similar to those reported elsewhere using other analytical methods and based on different datasets [9, 12]. The analytical steps presented here, from model parametrization to evaluation of a containment strategy, are therefore robust and can be adapted to other novel MDROs that are principally transmitted in healthcare settings.
The interventions described here require a level of capacity and expertise that, until recently, have not been widely available in the United States. Beginning in 2016, CDC has used funding provided to combat antibiotic resistance to begin to build the public health infrastructure needed to support such a response. CDC now funds all 50 states, Puerto Rico, DC and 5 other large cities in order to build expertise in state and local health departments in responding to prevent transmission of resistant pathogens and to detect carbapenemase-producing organisms. Health departments have the capacity to work with and across all facilities in a jurisdiction and are well-positioned to facilitate truly regional interventions. The Antibiotic Resistance Laboratory Network (ARLN) has increased early detection of emerging MDROs and provided free screening nationally (through seven regional laboratories) for new and emerging MDROs. This work has led to unprecedented levels of facility and patient level data that continues to inform response efforts including identifying more refined approaches to preventing transmission. These newly established laboratory and infection control resources have the potential to slow the spread and reduce the impact of antibiotic resistance in the United States.
There are a number of limitations of this study. The results are based on the network of hospitals in one U.S. state and may not be generalizable. In addition, nursing homes are not included in the model as data on them were either incomplete or unavailable. Longer stay post-acute care facilities were not included and the model might over-estimate the importance of facility connectedness as means to target facilities at high-risk for driving amplification of CP-CRE within a region over other characteristics like length of stay and acuity of care. Preliminary results from provisionally parametrized models suggest that inclusion of nursing homes yields broadly similar results for the public health impact (proportion of cases averted, for example) of the intervention. Further, our model treats each hospital as a homogeneous unit and is unable to capture the finer ward-level components of the MDRO containment strategy. While deterministic models with wards, or agent-based models, may be able to capture such details, the paucity of data to parametrize more granular models is a challenge. Note that this lack of intrafacility granularity in our model is expected to lead to an overestimation of the number of patient-days under enhanced infection control measures. Finally, the parametrization method we use assumes endemicity and is not, therefore, applicable to data from the emergent phase of an outbreak. However, the mathematical expressions remain good approximations even outside the strict bounds of endemicity, leading to reasonable parametric estimates. We note, too, that while simulations may be used to explore effects of variations in the containment protocol, a comprehensive examination of those effects is beyond the scope of this study. (Analyses of the effect of reducing start up delay from 30 to 7 days show very small public health impact.)
In conclusion, in this model of a strategy to contain the emergence of a targeted resistant pathogen, even modestly effective infection control interventions led to substantial reductions in transmission events. Further, targeting these interventions to a subset of facilities based on information on regional transfer of patients among hospitals, enables targeted and more efficient use of prevention resources during an outbreak response. Further work will be needed to identify if these findings hold in more complex models that more closely resemble the current U.S. healthcare system. In the meantime, use of models of this type, parameterized using local patient transfer data, might be a reasonable approach to guiding a public health response to containing MDROs in a region.
Supplementary Material
Footnotes
Publisher's Disclaimer: Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
No authors have any potential conflicts of interest to disclose.
References:
- 1.Oboho IK, Tomczyk SM, Al-Asmari AM, et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med 2015; 372(9): 846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowell G, Abdirizak F, Lee S, et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med 2015; 13: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guh AY, Bulens SN, Mu Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae in 7 US Communities, 2012–2013. JAMA 2015; 314(14): 1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guh AY, Limbago BM, Kallen AJ. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti Infect Ther 2014; 12(5): 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Tracking CRE Available at: https://www.cdc.gov/hai/organisms/cre/trackingcre.html. Accessed May 15, 2018.
- 6.Centers for Disease Control and Prevention. Interim guidance for a public health response to contain novel or targeted multidrug-resistant organisms (MDROs) In: US Department of Health and Human Services. Atlanta, GA, 2017. [Google Scholar]
- 7.Woodworth KR, Walters MS, Weiner LM, et al. Vital Signs: Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms - United States, 2006–2017. MMWR Morb Mortal Wkly Rep 2018; 67(13): 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Dartmouth Institute for Health Policy and Clinical Practice Available at: http://archive.dartmouthatlas.org/tools/downloads.aspx. Accessed February 26, 2019. [PubMed]
- 9.Toth DJA, Khader K, Slayton RB, et al. The Potential for Interventions in a Long-term Acute Care Hospital to Reduce Transmission of Carbapenem-Resistant Enterobacteriaceae in Affiliated Healthcare Facilities. Clin Infect Dis 2017; 65(4): 581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Ring Vaccination Available at: https://www.cdc.gov/smallpox/bioterrorism-response-planning/public-health/ring-vaccination.html. Accessed May 15, 2018.
- 11.Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011; 52(7): 848–55. [DOI] [PubMed] [Google Scholar]
- 12.Lee BY, Bartsch SM, Wong KF, et al. The Potential Trajectory of Carbapenem-Resistant Enterobacteriaceae, an Emerging Threat to Health-Care Facilities, and the Impact of the Centers for Disease Control and Prevention Toolkit. Am J Epidemiol 2016; 183(5): 471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


