Abstract
Purpose:
To report patients who demonstrated an alteration in the clinical and optical coherence tomography (OCT) features of neovascular age-related macular degeneration (AMD) following resolution of endophthalmitis.
Methods:
Retrospective case series of the subsequent changes in the macula and need for anti-VEGF therapy in patients with neovascular AMD who developed endophthalmitis following intravitreal injection.
Results:
The study included 7 eyes of 7 patients with follow-up ranging between 3 months and 11 years. The vitreous cultures (n=7) prior to intravitreal antibiotic injection were the following: culture negative (4) and coagulase-negative Staphylococcus (3). Initial treatment included vitreous tap and injection (4) and pars plana vitrectomy (PPV, 3). In 5/7 eyes, the OCT showed resolution of subretinal fluid and serous pigment epithelial detachment and there was no additional anti-VEGF treatment administered.
Conclusion:
After successful treatment of endophthalmitis in patients with neovascular AMD, there was relative involution of the maculopathy and reduced anti-VEGF treatment burden in this series.
Keywords: Neovascular Age-Related Macular Degeneration, Endophthalmitis
INTRODUCTION
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy has greatly improved outcomes in neovascular age-related macular degeneration (AMD), and a number of other retinal vascular diseases. Though infrequent, a serious potential complication of intravitreal injection is endophthalmitis, estimated to occur at a rate of 0.05% or 1 in 2,000.1 Outcomes are often poor, especially with cases of Streptococcus, though coagulase-negative Staphylococcus and culture negative cases are often less virulent.2 Though some eyes with neovascular AMD may respond to a finite duration of treatment, life-long therapy is needed in the vast majority of eyes to preserve visual acuity. Different dosing regimens may be used including monthly, pro re nata (PRN), and treat and extend approaches, each with relatively comparable results.3,4
In the current case series, patients are described who developed endophthalmitis during the course of treatment for neovascular AMD, and later exhibited decreased exudation of choroidal neovascularization.
METHODS
This is a multi-center, retrospective, observational case series review of clinical and imaging findings of 7 patients with neovascular AMD who developed endophthalmitis following intravitreal injection and later had changes in the status of their macula and their need for anti-VEGF therapy. The study was granted exemption by the Western Institutional Review Board (IRB, Puyallup, Washington, Study Number 19913291) given its retrospective design.
CASE REPORTS
Summary of Cases
Four women and 3 men, ranging in age from 72-91 years old, were receiving routine treatment for neovascular AMD. In all patients, the diagnosis of neovascular AMD was made by clinical examination, OCT, and sometimes fluorescein or indocyanine green angiography. All patients were treated with a “treat and extend” approach. Three patients were treated with intravitreal bevacizumab, two with intravitreal ranibizumab, one with ranibizumab and aflibercept, and one with PDT plus intravitreal triamcinolone (Table 1). The patients had received a total of 1 to 33 treatments over a course of 3 months to 5 years and had a baseline VA of 20/50-20/60. Following their last treatment, the patients presented with clinical signs and symptoms of infectious endophthalmitis and were treated with vitreous tap and injection of antibiotics or PPV. Four of the seven patients were diagnosed with culture negative endophthalmitis, and three had cultures positive for coagulase-negative Staphylococcus. One patient had two episodes of post-injection endophthalmitis, requiring intravitreal bevacizumab after her first episode of endophthalmitis, but permitting discontinuation of therapy after her second diagnosis of endophthalmitis 4.5 years later. All seven patients were eventually able to discontinue neovascular AMD treatment after their last episode of endophthalmitis without recurrences.
Table:
Summary of cases.
| Case # | Age (Years) | Sex | Agent | # Prior Inj. | Vitreous Culture | Pre-infection VA | Post-infection VA | Follow-up Post-infection | # Inj. Post-infection |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 87 | Female | Ranibizumab | 33 | Negative | 20/50 | 20/70 | 4 years | 0 |
| 2 | 89 | Male | Bevacizumab | 7 | Coagulase-negative Staphylococcus | 20/60 | 20/60 | 22 months | 0 |
| 3 | 72 | Female | Bevacizumab | 4 | Dry Tap | 20/50 | 20/30 | 18 months | 0 |
| 4 | 81 | Male | Triamcinolone with PDT | 3 | Negative | 20/60 | 20/30 | 11 years | 0 |
| 5 | 75 | Male | Bevacizumab | 30 | Negative | 20/50 | 20/400 | 2 years | 0 |
| 6 | 91 | Female | Bevacizumab/Aflibercept | 10/15 | Coagulase-negative Staphylococcus | 20/60 | 20/70 | 3 months | 0 |
| 7 | 89 | Female | Ranibizumab | 3 | Coagulase-negative Staphylococcus | NA | 20/80 | 6 years | 2 |
Case 1
An 87-year-old woman with a history of neovascular AMD treated with 33 prior intravitreal injections of ranibizumab in the right eye over the course of 5 years. She had previously been treated with a treat and extend protocol at a maximal interval of 2 months. Prior to that injection, her visual acuity was 20/50. Seven days after her final injection, she presented with blurred vision and pain. Visual acuity was 3/200. Slit lamp biomicroscopy revealed 2-3+ vitreous haze without hypopyon. The patient was treated with a PPV and intravitreal injection of 1 mg vancomycin, 0.25 mg ceftazidime, and 400 mcg dexamethasone. Vitreous culture was negative. After resolution of endophthalmitis, the patient has not required another intravitreal injection for her neovascular AMD, and her vision has remained stable at 20/70 over the last 4 years of follow-up (Figure 1).
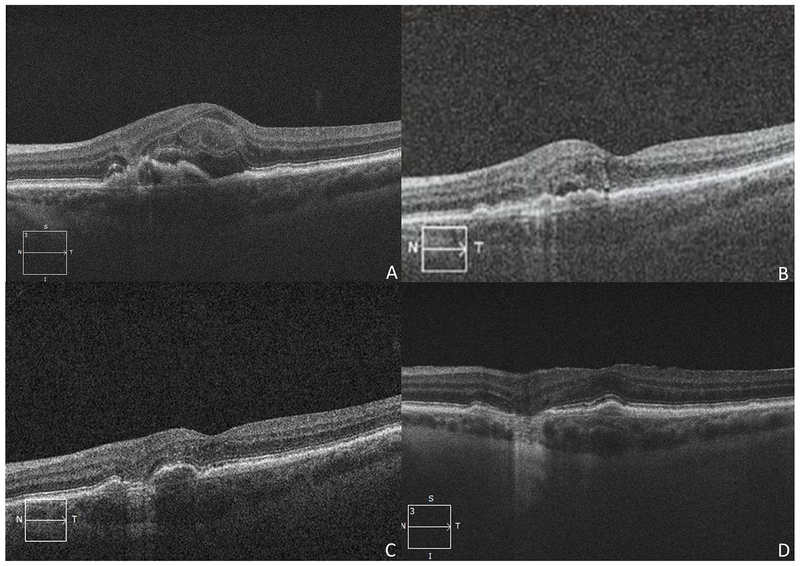
Figure 1:
Regression of active exudation in an 87-year-old woman in neovascular age-related macular degeneration after post-injection endophthalmitis (Case 1). A) Optical coherence tomography (OCT) of the right eye two years prior to endophthalmitis shows a hyperreflective vascular pigment epithelial detachment and intraretinal fluid as well as a small epiretinal membrane. B) OCT of the right eye 4 years after post-injection endophthalmitis shows trace subretinal and intraretinal fluid, resolution of the neovascular membrane, and atrophy.
Case 2
An 89-year-old man with a history of neovascular AMD treated previously with 7 injections of intravitreal bevacizumab had stable visual acuity of 20/60 over the course of 19 months of therapy using a PRN regimen ranging from 6 weeks to 5-month intervals. Two days after the patient’s last injection, the patient developed blurred vision, pain, and an afferent pupillary defect. Visual acuity was reduced to hand motions. Slit lamp biomicroscopy demonstrated corneal edema, hypopyon, and dense vitritis. The patient was treated with vitreous tap and injection of 1 mg vancomycin and 2.25 mg ceftazidime. Culture was positive for coagulase-negative Staphylococcus. Three months after resolution of his infection, the patient’s vision returned to 20/60 and was maintained at 20/60 without injections 12 months later. OCT demonstrated RPE scarring and persistent mild CME without subretinal fluid until 22 months later when subretinal fluid and hemorrhage were identified. The patient refused any additional injections.
Case 3
A 72-year-old woman with a history of exudative AMD had received 4 intravitreal bevacizumab injections for over 5 months. She was treated with a monthly protocol for the first injections and received her fourth injection at an interval of 2 months. At the time of her last injection, visual acuity was 20/50. Four days later, she developed pain, redness, and blurred vision. Visual acuity was 20/400. Slit lamp biomicroscopy revealed 1+ cells in the anterior chamber and vitritis but no hypopyon. A vitreous tap was attempted but was dry. Following the tap, the eye received intravitreal injection of 1 mg vancomycin and 2.25 mg ceftazidime. Three months later, the patient’s vision recovered to 20/50. She then developed recurrent exudation and required continued treatment with intravitreal bevacizumab for 4.5 more years. After having received 41 additional injections, she developed a second episode of endophthalmitis. Vitreous tap was culture-negative, and the patient was again treated with 1 mg vancomycin and 2.25 mg ceftazidime. One and a half years since the second episode of endophthalmitis; the patient’s visual acuity is 20/30 and the neovascular regressed, eliminating the need for additional injections (Figure 2).
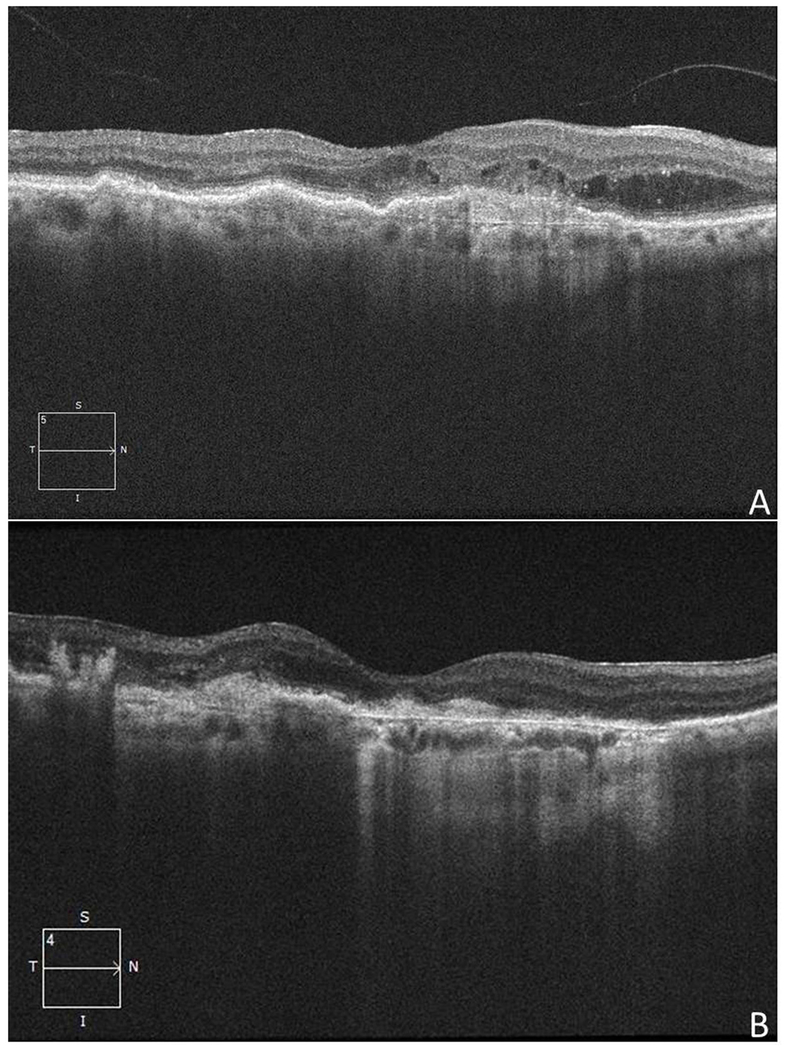
Figure 2:
Regression of choroidal neovascularization in a 72-year-old woman with a history of neovascular age-related macular degeneration (Case 3). A) Optical coherence tomography (OCT) 5 months prior to the first episode of endophthalmitis discloses a vascular pigment epithelial detachment with subretinal and intraretinal fluid. B) OCT 3 months after the first episode of endophthalmitis shows reduced intraretinal and subretinal fluid. C) OCT 20 months prior to the second episode of endophthalmitis shows a moderate pigment epithelial detachment with adjacent trace subretinal fluid. D) OCT 18 months after the second episode of endophthalmitis reveals resolved macular edema with a focal area of geographic atrophy.
Case 4
An 81-year-old man with a history of neovascular AMD in his right eye was treated with 3 sessions of photodynamic therapy (PDT) and intravitreal triamcinolone (prior to the availability of anti-VEGF therapy). At his last treatment, his visual acuity was 20/60. One day after injection, the patient developed blurred vision. Best-corrected visual acuity was 20/400. Slit lamp biomicroscopy showed 2+ cells in the anterior chamber and 4+ vitreous haze. The patient was treated with PPV and injection of 1 mg vancomycin, 2.25 mg ceftazidime, and 400 mcg dexamethasone. Culture results were negative. The patient has not required treatment for neovascular AMD following resolution of his infection and has been followed for 11 years with last visual acuity 20/30.
Case 5
A 75-year-old man with a history of neovascular AMD received 30 intravitreal injections of bevacizumab in his right eye over 5 years every 4-8 weeks based on a treat and extend regimen. The patient developed pain and redness 4 days after his last injection with vision reduced to count fingers. Prior to this injection, his visual acuity was stable at 20/150. Slit lamp biomicroscopy revealed corneal edema, hypopyon, and vitritis. Vitreous tap was negative for organisms. The patient was treated with intravitreal injections of 1 mg vancomycin and 2.25mg ceftazidime. Visual acuity decreased to 20/400 after endophthalmitis due to development of a dense posterior subcapsular cataract though his last OCT showed nearly a 50% reduction in central thickness. No additional treatment for neovascular AMD was required after endophthalmitis until the patient’s death two years later.
Case 6
A 91-year-old woman with a history of neovascular AMD received 10 bevacizumab and 15 aflibercept injections every 6-8 weeks over the course of 4 years in her right eye. Visual acuity prior to her last injection was 20/60. Two days after treatment, she developed decreasing vision and pain. Slit lamp biomicroscopy revealed 4+ cells in the anterior chamber and 2+ vitreous haze. Vitreous tap was positive for coagulase-negative Staphylococcus. The patient was treated with injection of 1 mg vancomycin and 2.25 mg ceftazidime. The next day, PPV was performed. The patient has been followed for 3 months post-endophthalmitis and has a VA of 20/70 without the need for further injections.
Case 7
An 89-year-old woman was treated with 3 ranibizumab injections for neovascular AMD in the left eye over the course of 3 months. After her last injection, she developed pain, redness, and decreased vision. Visual acuity was light perception. Slit lamp biomicroscopy showed corneal edema, hypopyon, and vitritis. A vitreous tap returned positive for coagulase-negative Staphylococcus, and the patient was treated with intravitreal injections of 1 mg vancomycin, 2.25 mg ceftazidime, and 0.4 mg/mL dexamethasone. After resolution of her infection, the patient received 2 additional ranibizumab injections at 3 months and 5 months post-endophthalmitis. Following these injections, no treatment for neovascular AMD was required. The patient’s current visual acuity, 6 years post-endophthalmitis, is 20/80.
DISCUSSION
Endophthalmitis is a serious adverse event that often results in poor visual outcomes.5 The eyes described in this case report had resolution of their infections with concomitant regression of their exudative neovascular membranes. Of note, all of the eyes in this series were infected with either coagulase-negative Staphylococcus or had negative taps, and there were no eyes infected with Streptococcus which has been associated with less favorable visual outcomes.6
In 2010 , two patients were described with neovascular AMD that developed endophthalmitis following intravitreal anti-VEGF therapy.7 Both eyes were treated with PPV and injection of intravitreal antibiotics and had negative cultures. Subsequent examinations demonstrated regression of neovascularization and no further need for treatment with anti-VEGF. The first case maintained favorable visual acuity and the choroidal neovascular membrane (CNVM) resolved leaving behind geographic atrophy. In the second case, the patient developed poor visual acuity, but the CNVM also resolved, also leaving behind a large area of atrophy. Of note, both of these patients were treated with PPV, and the authors postulated that removal of the vitreous may have contributed to the regression of neovascularization via reduction of VEGF levels, and improvement in intravitreal oxygenation.8
In the current series, most patients were treated with vitreous tap alone but still had a similar result. In a separate study by Yoon et al, it was reported that eyes treated with triamcinolone that then developed post-injection endophthalmitis had less macular edema after resolution of the infection though this study was limited to noninfectious endophthalmitis.9 More recently, Kally et al. described a case of resolution of subretinal fluid after post-injection endophthalmitis in a patient with neovascular AMD. The authors postulated that upregulation of complement factor H, which has been associated with a protective effect on the development of AMD, during the infection could be a biochemical explanation for the regression in exudation.10
The mechanisms for regression of exudation following infectious endophthalmitis are unclear. It is believed than an angiostatic inflammatory state can be reached in cases of chronic inflammation where there are high levels of pro-inflammatory cytokines such as IFNα/γ, TNFα, and IL-1α/β.11 These cytokines induce endothelial cells to secrete guanylate binding protein-1 (GBP-1), which exerts anti-angiogenic effects such as inhibition of endothelial cell proliferation, migration, and invasion. Thus, it is possible that the induction of an anti-angiogenic retinal environment during the active phase of endophthalmitis contributed to a decreased and persistent anti-angiogenic effect though this hypothesis is highly speculative. It is unknown what effect the actual microbe may have had on the vascular membrane. It is well known, however, that chronic inflammatory diseases12 may be complicated by the development of choroidal neovascularization and even infectious conditions such as endogenous endophthalmitis13–15, can lead to neovascularization, so the mechanism of regression in this case series is unclear.
Thus a key area for future investigation is whether our observation is more driven by the effect of the infectious process on scar formation or its effect on exudation. Optical coherence tomography angiography may be a useful adjunct to study these types of cases to determine whether there are also changes in lesion size or regression of choroidal neovascularization or whether this is simply an arrest in exudation without a change in vascular anatomy. In many cases where neovascular AMD arrests, vision may ultimately be limited by progression of atrophy or disciform scar. The patients in this series did not appear to develop more prominent scarring, yet many did develop atrophy, limiting the visual acuity. Given its observational nature, it is not possible from to determine the effect of the infectious process on the development of atrophy.
A significant limitation to this study is its retrospective nature, small sample size, author selection bias, and lack of a comparison group. It may be possible that that the regression on neovascularization simply represented the natural progression of these cases of neovascular AMD. Although less common, some patients may exhibit long-term stability after just a short duration of intravitreal anti-VEGF therapy.3 Prospective or retrospective comparative studies are needed to investigate this observation in the future.
CONCLUSIONS
Some patients who develop post-procedure endophthalmitis may experience regression of choroidal neovascularization after successful treatment and resolution of endophthalmitis. The mechanisms involved in this regression are poorly understood, but these patients may require less rigid schedules for intravitreal injection. These cases are intriguing, and further investigation of the mechanisms of may provide insight into future treatment approaches to this disease.
Summary Statement:
After successful treatment of endophthalmitis in patients with neovascular AMD, there may be a relative involution of the maculopathy and reduced anti-VEGF treatment burden in select patients.
Acknowledgments
Funding: This study is supported in part by NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Footnotes
Financial Disclosure: The authors have no financial disclosures pertaining to this manuscript.
Competing Interests: The authors have no competing interests pertaining to this manuscript.
REFERENCES
- 1.Gregori NZ, Flynn HW Jr, Schwartz SG et al. Current Infectious Endophthalmitis Rates After Intravitreal Injections of Anti-Vascular Endothelial Growth Factor Agents and Outcomes of Treatment. Ophthalmic Surg Lasers Imaging Retina. 2015June;46(6):643–8. [DOI] [PubMed] [Google Scholar]
- 2.Moshfeghi AA, Rosenfeld PJ, Flynn HW Jr, et al. Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: a six-year experience at a university referral center. Retina. 2011;31(4):662–8. [DOI] [PubMed] [Google Scholar]
- 3.CATT Research Group Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011. May 19;364(20):1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J; TREND Study Group. Treat-and-Extend versus Monthly Regimen in Neovascular Age-Related Macular Degeneration: Results with Ranibizumab from the TREND Study. Ophthalmology. 2017. September 8. pii: S0161-6420(17)31025-4. [DOI] [PubMed] [Google Scholar]
- 5.Fileta JB, Scott IU, Flynn HW Jr. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014Mar-Apr;45(2):143–9. [DOI] [PubMed] [Google Scholar]
- 6.Chen E, Lin MY, Cox J, Brown DM. Endophthalmitis after intravitreal injection: the importance of viridans streptococci. Retina. 2011. September;31(8):1525–33. [DOI] [PubMed] [Google Scholar]
- 7.Emoto H, Emoto Y, Lim JI, Sadun AA, Sebag J. Regression of choroidal neovascularization after vitrectomy for postinjection endophthalmitis. Retin Cases Brief Rep. 2010Fall;4(4):312–6. [DOI] [PubMed] [Google Scholar]
- 8.Stefánsson E Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006. Jul-Aug;51(4):364–80. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SJ, Rhee DY, Marx JL, Blaha GR, Rogers AH, Baumal CR, Reichel E, Duker JS. Anatomic and visual outcomes of noninfectious endophthalmitis after intravitreal triamcinolone. Am J Ophthalmol. 2009June;147(6):1031–6. [DOI] [PubMed] [Google Scholar]
- 10.Kally PM, Sidikaro Y, McCannel CA. Resolution of Treatment-Resistant Subretinal Fluid in a Patient with Exudative Age-Related Macular Degeneration Following Endophthalmitis. Retin Cases Brief Rep. 2017. Fall;11(4):316–8. [DOI] [PubMed] [Google Scholar]
- 11.Hammon M, Herrmann M, Bleiziffer O, et al. Role of guanylate binding protein-1 in vascular defects associated with chronic inflammatory diseases. J Cell Mol Med. 2011July;15(7):1582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouvas A, Petrou P, Douvali M, et al. Intravitreal ranibizumab for the treatment of inflammatory choroidal neovascularization. Retina. 2011. May;31(5):871–9. [DOI] [PubMed] [Google Scholar]
- 13.Fecko AM, Ho LY, Walsh MK, Williams GA. Intravitreal bevacizumab and ranibizumab for choroidal neovascularization secondary to endogenous endophthalmitis. Retin Cases Brief Rep. 2011. Summer;5(3):229–32. [DOI] [PubMed] [Google Scholar]
- 14.Fong AH, Li KK, Jon HC, Lai WW, Wong D. Choroidal neovascularaization secondary to Klebsiella pneumoniae endogenous abscess and endophthalmitis. Clin Exp Ophthalmol. 2009. March;37(2):239–40. [DOI] [PubMed] [Google Scholar]
- 15.Tedeschi M, Varano M, Schiano Lomoriello D, Scassa C, Parisi V. Photodynamic therapy outcomes in a case of macular choroidal neovascularization secondary to Candida endophthalmitis. Eur J Ophthalmol. 2007. Jan-Feb;17(1):124–7. [DOI] [PubMed] [Google Scholar]