Abstract
Background:
Recent research suggests that latent phase of labor may terminate at 6 rather than 4 centimeters of cervical dilation. The objectives of this study were to: 1) characterize duration of the latent phase of labor among term, low-risk, U.S. women in spontaneous labor using the women’s self-identified onset; and 2) quantify associations between demographic and maternal/newborn health characteristics and the duration of the latent phase.
Methods:
This prospective study (n = 1281) described the duration latent phase in hours, stratified by parity at the mean, median, 80th, 90th, and 95th percentiles. The duration of the latent phase was compared for each characteristic using t-tests or Wilcoxon Rank-Sum tests and regression models that controlled for confounders.
Results:
In this sample of predominantly white, healthy women, duration of the latent phase was longer than described in previous studies: The median duration was 9.0 hours and mean duration was 11.8 hours in nulliparous women. The median duration was 6.8 hours and mean duration was 9.3 hours in multiparous women. Among nulliparous women, longer duration was seen in women whose fetus was in a malposition. Among multiparous women, longer durations were noted in women with chorioamnionitis and those who gave birth between 41 - 41+6 weeks gestation (vs. 40 – 40+6 weeks gestation).
Conclusion:
The latent phase of labor may be longer than previously estimated. Contemporary estimates of latent phase of labor duration will help woman and providers accurately anticipate, prepare, and cope during spontaneous labor.
Keywords: Labor, Latent phase, Labor progress, Chorioamnionitis, Fetal malposition, Postdates
Introduction
The latent phase of labor is the time between the beginning of labor symptoms and the onset of the active phase.1 The latent phase has been described as highly variable both in duration and with regard to women’s experiences.2–4 Clinical guidelines for the care of healthy, low-risk women recommend remaining at home during latent phase.5,6 While most women describe contractions as the primary harbinger of latent labor onset, other symptoms such as gastrointestinal changes may be the first sign of latent phase.2,7–11 The latent phase usually begins outside of the hospital, and the time of onset is determined by the woman’s first recognition of symptoms. For these reasons, defining spontaneous latent phase onset by the timing of hospital admission likely truncates estimates of latent phase duration.13
Four published studies have characterized the latent phase and/or analyzed the association between the duration of latent phase and outcomes among cohorts in the United States.4,13–15 In 3 of these studies, latent phase onset was either unspecified,4,13 or was defined by hospital admission.14 The 1 study that defined labor onset by the woman’s report of symptom onset did not examine the association between demographic or health characteristics and duration of the latent phase.15
Relationships among factors that initiate and propagate spontaneous labor are complex, and we are early in our understanding of these processes.16–18 Although a growing body of recent research has characterized duration of the active phase and associated outcomes, suggesting that latent phase may terminate at 6 rather than 4 centimeters of cervical dilation,3,19 the entire duration of the latent phase and associations have not yet been fully explored.12,20 Women will likely benefit from accurate estimates of labor duration to help them anticipate, prepare, and cope with the experience of labor. As well, the duration of the latent phase may affect perinatal outcomes, and longer durations may herald abnormal labor progress.14 It is important to differentiate which women will derive greater benefit from supportive care versus judicious intervention during the latent phase of labor.5,21,22
Informed by these considerations, the purposes of this study were to: 1) characterize the duration of latent phase among low-risk, U.S. childbearing women in spontaneous labor using the women’s self-identification of labor onset to define the beginning of latent phase; and 2) quantify associations between demographic and maternal/newborn health characteristics at 5 thresholds of the duration of the latent phase distribution (mean; median; and 80th, 90th, and 95th percentiles).
Materials and Methods
Data
The Oregon Health & Science University (OHSU) certified nurse-midwifery (CNM) practice has been at OHSU for 43 years. CNMs in this practice attend approximately 600 women during birth annually and are licensed, independent practitioners who consult as needed with other providers. Since 2012, the OHSU CNM faculty has been collecting observational data about women receiving care with this practice. This data collection was initiated for quality improvement and received OHSU Institutional Review Board (IRB) approval. Information is recorded at 3 points of clinical care: 1) the initial outpatient prenatal visit, 2) inpatient labor, birth, and postpartum care, 3) and the 6-week postpartum visit.
Data set questions are phrased to elicit unambiguous information, with most questions requiring a yes/no response such as ‘was epidural analgesia used for labor and/or birth?’ Non-binary questions require more information; e.g., ‘vaginal examination at time of amniotomy’ elicits response regarding effacement, dilation, and station (on a −1 to +5 scale). Questions were designed to gather a level of detail regarding midwifery clinical care processes and outcomes not easily captured by administrative or electronic health record data. For example, when the midwife indicates that an induction was initiated, she is prompted to specify all methods used for induction (i.e., acupuncture, amniotomy, Cervidil, Foley bulb, misoprostol, Pitocin, prostaglandin gel, herbs, castor oil).
All data forms collected for this prospective cohort are reviewed bi-weekly by a data entry team. CNM students, trained in data entry and verification, transfer data into the Research Electronic Data Capture (REDCap) system. Uncertainties or missing data were highlighted and addressed by the faculty who provided clinical care. Additionally, the CNM director compares a convenience sample (generally 10 cases) to the data recorded in the women’s electronic medical records twice monthly. This is accomplished both for ongoing quality assessment and to resolve any uncertainties or discrepancies.
Upon OHSU IRB approval to use this data repository for the current study, a de-identified sample was obtained. Ten percent of the sample was randomly selected for double-verification to assess accuracy between paper data collection forms, electronic medical records, and REDCap database. This process determined 99% data accuracy. All women in the study received antepartum and intrapartum care with the CNMs and met the following inclusion criteria: age 21 years or older, 37 or more weeks gestation, and in spontaneous labor with a singleton, non-anomalous, live, fetus in vertex presentation. We excluded women with a prior cesarean birth (Figure 1).
Figure 1.
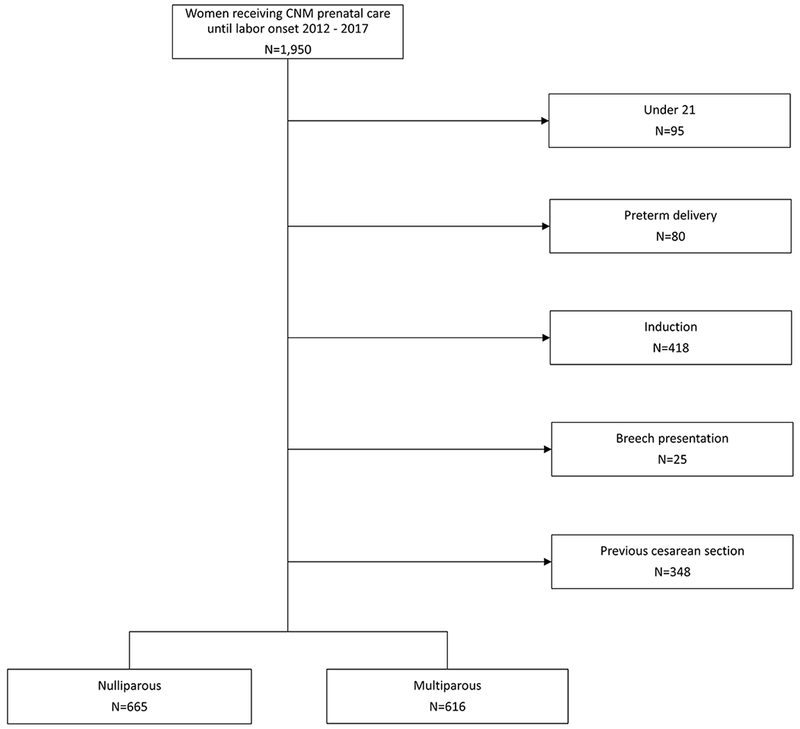
Exclusion chart for nulliparous and multiparous samples, CNM Database, Portland, OR, 2012-2017
Providers recorded the date and time (in hours, minutes) when a woman self-reported onset of the latent phase. Providers also recorded the date and time (in hours, minutes) of active phase onset; this time point was used to define the termination of the latent phase. While cervical examination is frequently performed to determine active phase onset, cervical examination was not required for identifying transition to active phase. Within this practice, providers may rely on women’s symptoms or behavior, e.g., shaking, emesis, increased expression or reports of pain,7,23,24 to identify the transition from the latent to the active phase of labor. Active phase signs and symptoms are frequently more prominent when women labor without analgesia,25 and 71% of women in this dataset did not use epidural anesthesia. Since the median years of practice of CNM providers was 31 years, the CNM team collecting data for this study was experienced in recognizing the transition from latent to active labor and also experienced in caring for a population of women in whom this transition is not masked by epidural use.
Analysis
We first described the duration of latent phase by parity. We focused unadjusted analyses on 2 measures of central tendency (mean, median) as well as 3 points of distribution marking longer latent phase (80th, 90th, and 95th percentiles). This approach was selected to facilitate comparison with existing estimates of the duration of the latent phase.4,14,15 We compared the length of women’s latent phase by each characteristic (e.g., did vs. did not receive a diagnosis of chorioamnionitis during labor) at each point of the duration of latent phase distribution (e.g., latent labor duration < mean vs. ≥ mean) using t-tests and Wilcoxon Rank-Sum tests. Our primary exposure variables were maternal demographic (e.g., age), health (e.g., pregnancy weight gain), and fetal characteristics (e.g., position at birth). Continuous variables (e.g., gestational age) were transformed into clinically-relevant categorical variables (e.g., early term vs. term gestational age). Our primary outcome was duration of the latent phase of labor.
Multivariate logistic regression models were fit to examine the adjusted association between latent phase that terminated or continued at and beyond the mean, 80th, 90th, and 95th percentiles of the duration of the latent phase and variables of interest that included: partner status, maternal age, maternal height, Body Mass Index category (normal, overweight, obese), excess pregnancy weight gain, Group Beta streptococcus vaginal colonization, gestational diabetes, pre-labor rupture of membranes, gestational age at birth, birth weight, chorioamnionitis, and fetal position at birth.
The mean was the point of central tendency selected for analysis. The logistic regression models compared women at each point of the duration of latent phase distribution (e.g., ≥mean duration of latent phase) to those below each distribution point (e.g., <mean duration of latent phase). All models controlled for maternal age, maternal height, BMI category (normal, overweight, obese), pregnancy weight gain, partner status, gestational diabetes, pre-labor rupture of membranes, GBS status, and neonatal birth weight (unless the variable was the outcome being examined). All analyses were conducted in Stata 15.
Results
After excluding data from women who did not meet eligibility criteria, our final sample included 665 nulliparous and 616 multiparous women (N = 1281). The women in this sample were predominantly married or partnered, white, and the majority gained gestational weight within Institute of Medicine (IOM) recommended guidelines (Table 1). Compared to nulliparous women, a higher percentage of multiparous women experienced shorter duration of latent phase (Figure 2). Among nulliparous women, the mean duration of the latent phase was 11.8 hours and the median was 9.0 hours. Among multiparous women, the mean duration of the latent phase was 9.3 hours and the median was 6.8 hours (Table II).
Table I.
Characteristics of low-risk women in spontaneous labor at term by parity, CNM Database, Portland, OR, 2012-2017
| Demographic Characteristics | Nulliparous (n=665) n(%) | Multiparous (n=616) n(%) |
|---|---|---|
| Race | ||
| Caucasian | 580 (89.1) | 541 (89.6) |
| African American | 7 (1.1) | 13 (2.2) |
| Asian | 46 (7.1) | 35 (5.9) |
| Multiracial | 13 (2.0) | 8 (1.3) |
| Native American | 1 (0.2) | 4 (0.7) |
| Native Hawaiian | 49 (0.6) | 3 (0.5) |
| Married or Partnered | 641 (97.7) | 597 (97.1) |
| ≥ 35 years of age | 148 (22.8) | 164 (27.0) |
| Hypertension or preeclampsia | 19 (2.9) | 20 (3.3) |
| Gestational diabetes | 51 (7.7) | 39 (6.3) |
| Body mass index | ||
| Underweight | 25 (3.8) | 23 (3.8) |
| Normal | 442 (66.6) | 392 (63.8) |
| Overweight | 131 (19.7) | 132 (21.5) |
| Obese | 66 (9.9) | 67 (10.9) |
| Excess pregnancy weight gain by Institute of Medicine guidelines | 19 (2.9) | 18 (3.0) |
| Group Beta streptococcus vaginal colonization | 154 (23.5) | 147 (24.3) |
| Pre-labor rupture of membranes | 21 (3.2) | 21 (3.4) |
| Chorioamnionitis | 37 (5.6) | 16 (2.6) |
| Gestational age at delivery | ||
| Early term (37 0/7– 38 6/7 weeks) | 84 (12.8) | 101 (16.6) |
| Term (39 0/7– 40 6/7 weeks) | 413 (62.7) | 371 (60.9) |
| Late term (41 0/7 – 41 6/7 weeks) | 143 (21.7) | 112 (18.4) |
| Postterm (42 0/7 + weeks) | 19 (2.9) | 25 (4.1) |
| Birthweight by gestational age* | ||
| Small for gestational age | 30 (5.2) | 25 (4.6) |
| At gestational age | 472 (82.4) | 460 (84.4) |
| Large for gestational age | 71 (12.4) | 60 (11.0) |
| Fetal position at birth | ||
| Occiput Anterior | 537 (90.9) | 544 (93.5) |
| Occiput Posterior/ Occiput Transverse | 54 (9.1) | 38 (6.5) |
Infants were designated small for gestational age if their birthweight was in the bottom 10th percentile for gestational age; infants were considered large for gestational age if their birthweight was at or above the 90th percentile for their gestational age
Figure 2.
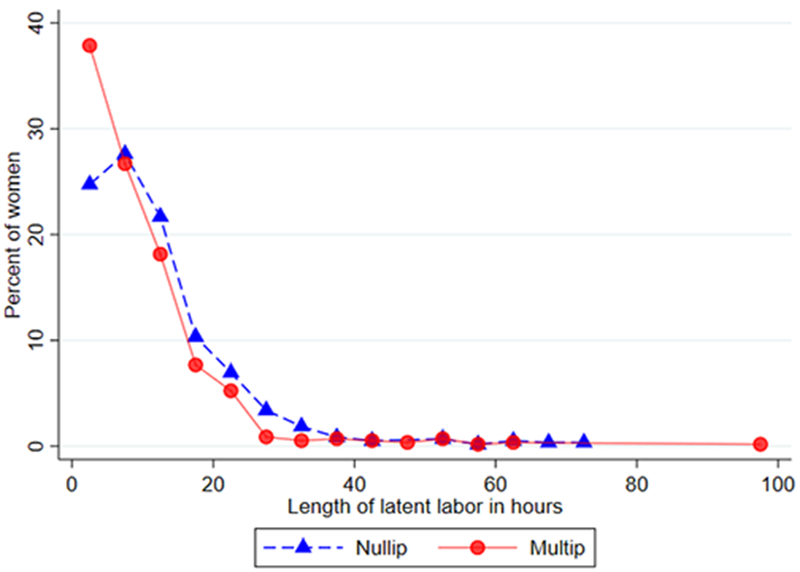
Latent phase distribution among nulliparous and multiparous women in spontaneous labor, CNM Database, Portland, OR, 2012-2017
Table II.
Duration of latent phase labor among low-risk women in spontaneous labor at term, CNM Database, Portland, OR, 2012-2017
| Nulliparous | Multiparous | |||
|---|---|---|---|---|
| Point of distribution | Duration latent phase (hours) | Number of women continuing latent phase beyond identified point of distribution (n) | Duration latent phase (hours) | Number of women continuing latent phase beyond identified point of distribution (n) |
| 50%/median | 9.0 | 305 | 6.8 | 287 |
| mean | 11.8 | 220 | 9.3 | 254 |
| 80% | 17.0 | 124 | 13.5 | 116 |
| 90% | 24.0 | 62 | 19.0 | 59 |
| 95% | 30.0 | 31 | 24.5 | 29 |
After adjusting for confounders, four variables remained significantly associated with longer latent phase at various distribution points. Nulliparous women whose fetus was in a malposition (occiput posterior or occiput transverse) either at birth or at the last cervical examination prior to birth had a duration of latent phase that was significantly longer when compared to women whose fetus was in an occiput anterior position [mean, 11.8 vs. 9.0 hours, P<0.05; median, 16.2 vs. 11.6 hours, P<0.01] (Figure 3). Multiparous women diagnosed with chorioamnionitis (defined as fever of ≥ 38C and one other symptom, e.g., tachycardia) had significantly longer duration of the latent phase than did multiparous women who did not have chorioamnionitis. When the median duration of latent phase for these women was evaluated it was more than 2-fold longer than the median duration of multiparous women not diagnosed with chorioamnionitis (mean, 18.3 vs. 9.1 hours, P<0.01; median, 15.9 vs. 6.5 hours, P<0.01) (Figure 3). Multiparous women who were not partnered or married also experienced longer latent phase (mean, 14.2 vs. 9.2 hours, P<0.05; median, 15.4 vs. 6.5 hours, P<0.05].
Figure 3.
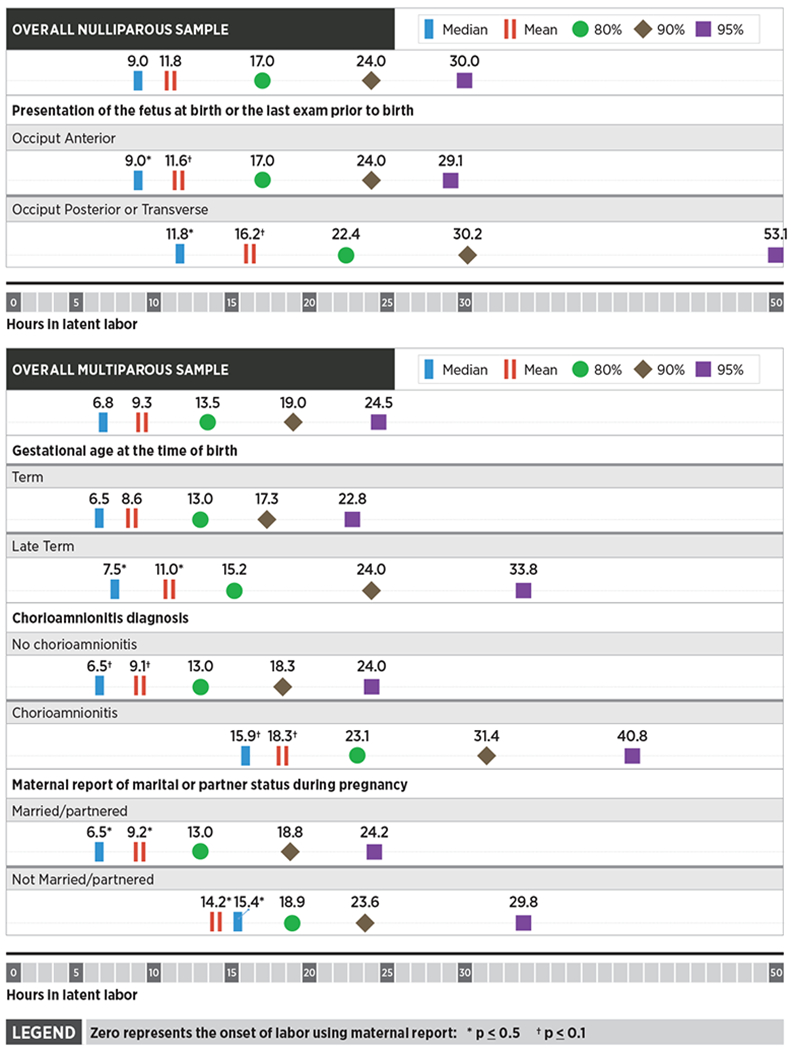
Results of unadjusted analyses comparing characteristics with significant differences of nulliparous and multiparous women and hours of spontaneous latent phase labor less than vs. at and beyond the mean, median, and 80th, 90th, and 95th percentiles, CNM Database Portland, OR, 2012-2017
Based on research that has evaluated active phase and second stage labor progress, we hypothesized that women with higher BMI, greater gestational weight gain, and longer gestational age at birth would have a longer duration of the latent phase.26–28 These comparisons were not statistically significant among nulliparous women. Findings were also non-significant among multiparous women with the exception that those who gave birth at late term (vs. term) gestational age had significantly longer duration of latent phase [mean: 7.5 vs. 6.5 hours, P<0.05; median 11 vs. 8.6 hours, P<0.05] (Figure 3). We found no additional relationships between the duration of the latent phase and other demographic or maternal/newborn health characteristics in this population.
During multivariate analysis after adjusting for partner status, maternal age, maternal height, Body Mass Index category (normal, overweight, obese), excess pregnancy weight gain, Group Beta streptococcus vaginal colonization, gestational diabetes, pre-labor rupture of membranes, gestational age at birth, birth weight, chorioamnionitis, and fetal position at birth. (unless the variable was the outcome being examined) we found that among nulliparous women, fetal malposition was significantly associated with duration of latent phase at and beyond the 95th percentile. Among multiparous women: a) chorioamnionitis was significantly associated with longer latent phase at and beyond the mean, 80th, and 90th percentiles, but not beyond the 95thpercentile; b) late term (vs. term) gestational age at birth was associated with significantly longer duration of latent phase at and beyond the 90th percentile; and c) those without (vs. with) partners experienced significantly longer latent phase only at the 80th percentile (Table III).
Table III.
Adjusted odds ratios for prolonged latent phase of labor by selected characteristics, with duration of latent phase at the mean, 80th, 90th, and 95th percentiles of duration of latent phase distribution, CNM database, Portland, OR, 2012-2017
| Adjusted odds of prolonged latent phase | |||||
|---|---|---|---|---|---|
| Parity | Mean (95% CI) | 80th percentile (95% CI) | 90th percentile (95% CI) | 95th percentile (95% CI) | |
| Occiput Posterior or Transverse | Nulliparous | 1.33 (0.61-2.90) | 2.14 (0.94-4.87) | 2.55 (0.95-6.87) | 3.35 (1.02-11.01) |
| Chorioamnionitis | Multiparous | 4.78 (1.46-15.62) | 17.18 (4.58-64.42) | 7.04 (2.02-24.52) | 4.57 (0.86, −24.27) |
| Late term delivery | Multiparous | 1.41 (0.91- 2.18) | 1.54 (0.90-2.62) | 2.09 (1.05-4.17) | 3.38 (1.36- 8.38) |
| Partner | Multiparous | 0.56 (0.18- 1.71) | 0.21 (0.06-0.69) | 0.41 (0.10-1.70) | 0.56 (0.06-4.89) |
Comparisons are as follows (referent category second):
Occiput Posterior or Transverse vs. Occiput Anterior, Chorioamnionitis present vs. no chorioamnionitis, Late term delivery vs. term delivery, Partnered vs. unpartnered
Models adjusted for:
partner status, maternal age, maternal height, Body Mass Index category (normal, overweight, obese), excess pregnancy weight gain, Group Beta streptococcus vaginal colonization, gestational diabetes, pre-labor rupture of membranes, gestational age at birth, birth weight, chorioamnionitis, and fetal position at birth (unless the variable was the outcome being examined)
Discussion
In this low-risk population of women in spontaneous labor, nulliparous women’s durations of the latent phase of labor were longer than the normal duration of this stage of labor identified in previous U.S. studies.4,14,15 By defining the onset of latent phase as beginning with the woman’s report of labor onset, this dataset allowed us to estimate the entire duration of spontaneous latent phase which commonly starts outside of a hospital setting. Women in this sample frequently chose and received labor care that can be characterized as non-interventional in the absence of complications (e.g., 76% of women in this practice experience spontaneous labor, 73% of labors proceed without augmentation, and 71% choose to labor without epidural). These factors create a unique opportunity to observe the natural history of contemporary spontaneous latent phase among low risk women who labor and give birth in a high-resource country.
Our duration of latent phase labor findings were similar to estimates observed in recent European samples. For example, a study of contemporary, low-risk Swedish women in spontaneous labor that used the woman’s self-report to identify latent phase onset, found mean duration of the latent phase to be 13.9 hours duration among nulliparous women and 10.8 hours among multiparous women.29 The study by Gross et al. conducted in Germany that assessed low-risk women’s perceptions of the duration of latent phase vs. their midwives’ perceptions of latent phase duration determined that women’s estimates were similar to our study findings (median time- nulliparous: 11 hours; multiparous: 6.5 hours).30 Notably, midwives in the Gross study provided duration of latent phase estimates that are similar to Friedman’s estimates.4,13,30 So while it is possible that our findings are indicative of actual changes in the duration of the latent phase of labor, potentially related to different health characteristics between current and prior samples, it is more likely that our findings reflect the difference in how latent phase duration was previously measured in U.S. samples.
Recent investigation regarding duration of the first stage of labor provided updated estimates of cervical dilation associated with active labor onset, suggesting that latent phase may last until 6 rather than 4 centimeters of cervical dilitation.3,19,31 This work has led to broad efforts to redefine the onset of active labor for clinical practice. Considered in light of our findings, the latent phase of labor may be much longer than previously estimated. Older latent labor time estimates truncated duration in two ways: 1) they did not consistently include latent labor duration prior to hospital admission, and 2) they did not include the time required for the cervix to dilate from 4 to 6 cm.
Pregnant women often seek information as they prepare for labor.32 Among the many pieces of information that may be of interest is what to expect for the duration of each phase of labor. For example, women may feel safe to continue daily activities and not transition to the hospital if they know that latent labor lasts on average 9-12 hours for nulliparous women and 7-9 hours for multiparous women, rather than Friedman’s older estimates of an average of 7-8 hours for nulliparous women and 4-5 hours for multiparous women.4,13 Use of more accurate estimates of the normal duration of the latent phase of labor could also assist maternity care providers in differentiating women who are in physiologic latent labor from those whose might benefit from additional support or intervention. Better estimates of the normal duration of this phase of labor can also inform health systems changes, such as improved antenatal preparation to support women with uncomplicated labors to remain home until active labor, or might inform facilities changes, such as creating a latent labor lounge.22,33
Our results also suggest there is association between fetal malposition and longer latent phase among nulliparous women. It is unclear if the association indicates that a malpositioned fetus from the onset of the latent phase leads to a longer latent phase or if longer duration of latent phase is dysfunctional or unable to accomplish the internal fetal rotation to occiput anterior. An additional possibility is whether women in this group may have pelvis types that encouraged both fetal malposition and a longer latent phase. Similar to prior research regarding active phase duration,34 these study findings further suggest that there is a positive association between chorioamnionitis and longer latent phase among multiparous women, though the temporal relationship of these variables also cannot be determined. Mediating factors might include the increased potential for more cervical examinations or physiologic changes related to longer labor that could render women more vulnerable to infection. It is also possible that the origin of factors that ultimately lead to chorioamnionitis during active or second stage labor may be present, but perhaps subclinical, during or prior to latent phase and may lengthen the latent phase. Alternately, infection may disrupt the cascade that propagates normal labor. If these study findings are replicated, increased caution with frequency of cervical examination and/or heightened attention to markers of chorioamnionitis may be appropriate among multiparous women with longer duration of the latent phase of labor.
Findings further indicate that multiparous women giving birth post-term may experience a longer latent phase than those delivering at term; a similar tendency was noted among nulliparous women, although the differences were not statistically significant. As greater knowledge develops regarding variation in labor processes and outcomes by gestational age, duration of latent phase should be evaluated as a potential driver or harbinger of gestational age differences in overall labor progress. Study findings also raise the question of whether support from a partner or spouse might decrease either the length of latent phase or delay women’s perceptions of latent phase onset. Given the small proportion of single women in this sample, these findings should be considered exploratory.
Women have reported that the latent phase of labor is a time of great uncertainty and anxiety.36 Revised estimates of the normal duration of the latent phase of labor may enhance women’s labor experiences, an important direction for future research. In addition, women’s perceptions of the latent phase drive several important decisions, including when to present to the hospital and/or request pain relief.36 Effectively helping low-risk women delay hospital admission until the active phase is established is a particularly important target because of robust evidence demonstrating that hospital admission during the latent phase is associated with more interventions without concomitant benefit,37–47 and lower cost-effectiveness.48
Future research should also investigate the latent phase of labor among other populations, including women with higher risk for poor outcomes, those receiving care from other maternity care providers, and those birthing in different settings. Additionally, future science should evaluate relationships between the duration of latent phase and labor/birth outcomes of active and second stages of labor as well as outcomes in the early postpartum. Finally, more information is needed about preparation women are given before the experience of labor,33 their symptoms and successful coping mechanisms during of latent phase,11 as well as how these factors may shape decision-making regarding when to transport to the hospital.49 This information will importantly inform efforts to decrease hospital admission during the latent phase of labor.2,50,51
Strengths and Limitations
Strengths of this study include the use of a data set that enables estimation of the entire duration of spontaneous labor experienced by women with an a priori low-risk for labor abnormalities who gave birth in a high resource setting. Using the laboring woman’s report of the onset of latent labor corresponds both with the definition of latent phase1 and recent recommendations for latent phase research.12 Since women in this sample were less likely to use common labor interventions such as pharmacologic augmentation or epidural analgesia,35 these study findings add to the sparse knowledge of the natural history of spontaneous latent labor.
While our results are valuable in that they contribute to a rarely studied area of women’s health, our sample is not representative of the U.S. population. The women who sought care at this location were predominantly white, partnered/married, with frequently normal BMIs, low levels of co-morbidities, and recommended gestational weight gain. In addition, the women in this sample self-selected midwifery-care and non-intervention during childbearing. Future research should include women more representative of the broader population of childbearing women in the U.S. Comparisons at the 80th, 90th, and 95th percentiles of duration of latent phase distribution necessarily include low sample sizes. Relationships that might be detected with a larger sample may have been missed with the smaller sample at these points of distribution.
Conclusions
Our study contributes new, contemporary estimates of the natural history of the duration of spontaneous latent phase. Importantly, we found that the latent phase of labor may be longer than previously estimated among healthy U.S. women with low-risk pregnancies in spontaneous labor. These new duration of latent phase estimates refine understanding of normal parameters for this portion of labor. Childbearing women, maternity care providers, and health systems might use this information to enhance anticipation, preparation, and coping during the spontaneous latent phase of labor.
Supplementary Material
Acknowledgments
Source of Funding:
Dr. Ellen Tilden receives support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institutes of Health Office of Research on Women’s Health, Oregon BIRCWH Scholars in Women’s Health Research across the Lifespan (K12HD043488-14). This source of funding had no involvement in any aspects of the research presented in this manuscript.
Dr. Jonathan M. Snowden and Mekhala Dissanayake are supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number R00 HD079658-03).
Dr. Julia C. Phillippi is supported by the Agency for Healthcare Research and Quality [grant number K08HS024733]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
This study was conducted at Oregon Health and Science University in Portland, Oregon in the department of Nurse-Midwifery in the School of Nursing.
The authors report no conflict of interest.
Presented at the American College of Nurse-Midwives Annual Meeting, May 20th – 24th, 2018, Savannah, GA.
REFERENCES
- 1.Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and problem pregnancies. 5th ed. Philadelphia: Churchill Livingston; 2007. [Google Scholar]
- 2.Gross MM, Haunschild T, Stoexen T, Methner V, Guenter HH. Women’s Recognition of the Spontaneous Onset of Labor. Birth. 2003;30(4):267–271 10.1046/j.1523-536X.2003.00257.x [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Troendle J, Mikolajczyk R, Sundaram R, Beaver J, Fraser W. The natural history of the normal first stage of labor. Obstetrics and gynecology. 2010;115(4):705–710 [DOI] [PubMed] [Google Scholar]
- 4.Friedman E Primigravid labor: A geographicostatistical analysis. Obstetrics & Gynecology. 1955:567–589 [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. Committee opinion no. 687: approaches to limit intervention during labor and birth. Obstet Gynecol. 2017;129:e20–e28 [DOI] [PubMed] [Google Scholar]
- 6.Rhoades JS, Cahill AG. Defining and Managing Normal and Abnormal First Stage of Labor. Obstetrics and Gynecology Clinics of North America. 2017;44(4):535–545 10.1016/j.ogc.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 7.Neal JL, Ryan SL, Hunter LA The first stage of labor, pp 816–817. In: King TL, Brucker MC, Kriebs JM, Fahey JO, eds. Varney’s Midwifery. Jones & Bartlett Publishers; 2013. [Google Scholar]
- 8.Gross MM, Hecker H, Matterne A, Guenter HH, Keirse MJNC. Does the way that women experience the onset of labour influence the duration of labour? BJOG: An International Journal of Obstetrics and Gynaecology. 2006;113(3):289–294 10.1111/j.1471-0528.2006.00817.x [DOI] [PubMed] [Google Scholar]
- 9.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. William’s Obstetrics. 23rd ed. New York: McGraw Hill; 2010. [Google Scholar]
- 10.Gross MM, Petersen A, Hille U, Hillemanns P. Association between women’s self-diagnosis of labor and labor duration after admission. J Perinat Med. 2010;38(1):33–38 [DOI] [PubMed] [Google Scholar]
- 11.Edmonds JK, Zabbo G. Women’s Descriptions of Labor Onset and Progression Before Hospital Admission. Nursing for Women’s Health. 2017;21(4):250–258 10.1016/j.nwh.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Hanley GE, Munro S, Greyson D, et al. Diagnosing onset of labor: a systematic review of definitions in the research literature. BMC Pregnancy Childbirth. 2016;16:71.PMC4818892. 10.1186/s12884-016-0857-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman EA. Labor in Multiparas: A graphicostatistical analysis. Obstetrics and gynecology. 1956;8(6):686–703 [PubMed] [Google Scholar]
- 14.Chelmow D, Kilpatrick SJ, Laros RK. Maternal and neonatal outcomes after prolonged latent phase. Obstetrics and gynecology. 1993;81(4):486–491 [PubMed] [Google Scholar]
- 15.Peisner DB, Rosen MG. Latent phase of labor in normal patients: A reassessment. Obstetrics and gynecology. 1985;66(5):644–648 [PubMed] [Google Scholar]
- 16.Buckley S Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care. Childbirth Connection Programs, National Partnership for Women & Families;2015. [Google Scholar]
- 17.Norwitz ER, Robinson JN, Challis JRG. The control of labor. New England Journal of Medicine. 1999;341(9):660–666 10.1056/NEJM199908263410906 [DOI] [PubMed] [Google Scholar]
- 18.Vannuccini S, Bocchi C, Severi FM, Challis JR, Petraglia F. Endocrinology of human parturition. Annales d’Endocrinologie. 2016;77(2):105–113 10.1016/j.ando.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstetrics and gynecology. 2010;116(6):1281–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abalos E, Oladapo OT, Chamillard M, et al. Duration of spontaneous labour in ‘low-risk’ women with ‘normal’ perinatal outcomes: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2018;223:123–132.PMC5884320. 10.1016/j.ejogrb.2018.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen PA, Weissinger S. Women’s perception of pre-hospital labour duration and obstetrical outcomes: a prospective cohort study. BMC Pregnancy Childbirth. 2014;14:182.PMC4060864. 10.1186/1471-2393-14-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul JA, Yount SM, Breman RB, et al. Use of an Early Labor Lounge to Promote Admission in Active Labor. Journal of Midwifery and Women’s Health. 2017;62(2):204–209 10.1111/jmwh.12591 [DOI] [PubMed] [Google Scholar]
- 23.Avery MD. Supporting a Physiologic Approach to Pregnancy and Birth: A Practical Guide. Oxford, UK: Wiley-Blackwell; 2013. [Google Scholar]
- 24.Klein S A Book for Midwives. Palo Alto, CA: Hesperian; 1995. [Google Scholar]
- 25.Moir DD, Willocks J. Epidural analgesia in British obstetrics. British Journal of Anaesthesia. 1968;40(2):129–138 10.1093/bja/40.2.129 [DOI] [PubMed] [Google Scholar]
- 26.Kominiarek MA, Zhang J, Vanveldhuisen P, Troendle J, Beaver J, Hibbard JU. Contemporary labor patterns: The impact of maternal body mass index. American Journal of Obstetrics and Gynecology. 2011;205(3) 10.1016/j.ajog.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young TK, Woodmansee B. Factors that are associated with cesarean delivery in a large private practice: the importance of prepregnancy body mass index and weight gain. American Journal of Obstetrics & Gynecology. 2002;187(2):312–318 [DOI] [PubMed] [Google Scholar]
- 28.Olesen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to postterm delivery: A national register-based study, 1978–1993. American Journal of Obstetrics and Gynecology. 2003;189(1):222–227 10.1067/mob.2003.446 [DOI] [PubMed] [Google Scholar]
- 29.Ängeby K, Wilde-Larsson B, Hildingsson I, Sandin-Bojö AK. Prevalence of Prolonged Latent Phase and Labor Outcomes: Review of Birth Records in a Swedish Population. Journal of Midwifery and Women’s Health. 2018;63(1):33–44 10.1111/jmwh.12704 [DOI] [PubMed] [Google Scholar]
- 30.Gross MM, Burian RA, Frömke C, Hecker H, Schippert C, Hillemanns P. Onset of labour: Women’s experiences and midwives’ assessments in relation to first stage duration. Archives of Gynecology and Obstetrics. 2009;280(6):899–905 10.1007/s00404-009-0990-7 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. American Journal of Obstetrics & Gynecology. 2002;187(4):824–828 [DOI] [PubMed] [Google Scholar]
- 32.Hollins Martin CJ, Robb Y. Women’s views about the importance of education in preparation for childbirth. Nurse Education in Practice. 2013;13(6):512–518 10.1016/j.nepr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 33.Tilden EL, Emeis CL, Caughey AB, Weinstein SR, Futernick SB, Lee CS. The Influence of Group Versus Individual Prenatal Care on Phase of Labor at Hospital Admission. Journal of Midwifery and Women’s Health. 2016;61(4):427–434 10.1111/jmwh.12437 [DOI] [PubMed] [Google Scholar]
- 34.Edwards RK. Chorioamnionitis and labor. Obstetrics and Gynecology Clinics of North America. 2005;32(2):287–296 10.1016/j.ogc.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 35.Declerqc ER, Sakala C, Corry MP, Applebaum S, Herrlich A. Listening to Mothers III: Pregnancy and Birth. New York, NY: 2013. [Google Scholar]
- 36.Eri TS, Bondas T, Gross MM, Janssen P, Green JM. A balancing act in an unknown territory: A metasynthesis of first-time mothers[U+05F3] experiences in early labour. Midwifery. 2015;31(3):e58–e67 10.1016/j.midw.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Bailit JL, Dierker L, Blanchard MH, Mercer BM. Outcomes of women presenting in active versus latent phase of spontaneous labor. Obstet Gynecol. 2005;105(1):77–79 10.1097/01.AOG.0000147843.12196.00 [DOI] [PubMed] [Google Scholar]
- 38.Boyle A, Reddy UM. Epidemiology of cesarean delivery: The scope of the problem. Seminars in perinatology. 2012;36(5):308–314 10.1053/j.semperi.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Davey M, LMcLachlan HL, Forster D, Flood M. Influence of timing of admission in labour and management of labour on method of birth: Results from a randomised controlled trial of caseload midwifery (COSMOS trial). Midwifery. 2013;29(12):1297–1302 10.1016/j.midw.2013.05.014i [DOI] [PubMed] [Google Scholar]
- 40.Gharoro EP, Enabudso EJ. Labour management: An appraisal of the role of false labour and latent phase on the delivery mode. Journal of Obstetrics and Gynaecology. 2006;26(6):534–537 [DOI] [PubMed] [Google Scholar]
- 41.Hemminki E, Simukka R. The timing of hospital admission and progress of labour. Eur J Obstet Gynecol. 1986;22:85–94 [DOI] [PubMed] [Google Scholar]
- 42.Holmes P, Oppenheimer LW, Wen SW. The relationship between cervical dilatation at initial presentation in labour and subsequent intervention. BJOG. 2001;108(11):1120–1124 [DOI] [PubMed] [Google Scholar]
- 43.Klein MC, Kelly A, Kaczorowski J, Grzybowski S. The effect of family physician timing of maternal admission on procedures in labour and maternal and infant morbidity. J Obstet Gynaecol Can. 2004;26(7):641–645 [DOI] [PubMed] [Google Scholar]
- 44.Lundgren I, Andren K, Nissen E, Berg M. Care seeking during the latent phase of labour - Frequencies and birth outcomes in two delivery wards in Sweden. Sex Reprod Healthc. 2013;4:141–146 [DOI] [PubMed] [Google Scholar]
- 45.McNiven PS, Williams JI, Hodnett E, Kaufman K, Hannah ME. An early labor assessment program: A randomized, controlled trial. Birth. 1998;25(1):5–10 [DOI] [PubMed] [Google Scholar]
- 46.Rahnama P, Ziaei S, Faghihzadeh S. Impact of early admission in labor on method of delivery. International Journal of Gynaecology & Obstetrics. 2006;92(3):217–220 [DOI] [PubMed] [Google Scholar]
- 47.Wood AM, Frey HA, Tuuli MG, et al. Optimal Admission Cervical Dilation in Spontaneously Laboring Women. American journal of perinatology. 2015;33(2):188–194 10.1055/s-0035-1563711 [DOI] [PubMed] [Google Scholar]
- 48.Tilden E, Allen A, Caughey A. Cost-effectiveness analysis latent labor hospital admission versus active labor hospital admission; Poster presentation Society for Maternal Fetal Medicine; 2013; San Francisco, CA. [Google Scholar]
- 49.Edmonds JK, Miley K, Angelini KJ, Shah NT. Decision Making about Hospital Arrival among Low-Risk Nulliparous Women after Spontaneous Labor Onset at Home. Journal of Midwifery and Women’s Health. 2018;63(4):455–461 10.1111/jmwh.12741 [DOI] [PubMed] [Google Scholar]
- 50.Reducing Primary Cesareans: Birth Tools for Optimizing the Outcomes of Labor Safely. American College of Nurse-Midwives; 2015; http://birthtools.org/HBI-Reducing-Primary-Cesareans. Accessed May, 3, 2016. [Google Scholar]
- 51.ACOG Committee Opinion Number 766 Approaches to Limit Intervention During Labor and Birth. 2019. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


