Abstract
目的
探讨桑根酮C诱导雄激素非依赖型前列腺癌PC3细胞凋亡的作用及其可能机制。
方法
MTT法检测桑根酮C对PC3细胞増殖的抑制作用:实验分6组,分别加入不同浓度的桑根酮C(0、1、5、20、50、100 μmol/L),作用24 h检测细胞生长抑制率;20 μmol/L桑根酮C加入PC3细胞,分别于0、6、12、24、48 h收集细胞,MTT法检测生长抑制率。流式细胞技术检测细胞凋亡率:20 μmol/L桑根酮C加入PC3细胞,分别于0、12、24、48 h收集细胞检测凋亡率。荧光分光光度计检测caspase 3的活性:20 μmol/L桑根酮C作用PC3细胞,分别在0、6、12、18、24、48 h收集细胞,检测caspases 3的活性。MTT法检测caspase 3、caspase 8及caspase 9抑制剂对桑根酮C抑制PC3细胞增殖的影响:实验分4组,分别加入DMSO、z-DEVD-fmk、z-LEHD-fmk及z-IETD-fmk,1 h加入桑根酮C,24 h收集细胞,MTT法计算细胞生长抑制率。
结果
桑根酮C能抑制PC3细胞的增殖,呈剂量、时间依赖性,桑根酮C 1、5、20、50、100 μmol/L作用PC3细胞24 h的增值抑制率分别为:4.86%、12.92%、52.34%、82.05%、87.45%,1 μmol/L组与对照组相比差异无统计学意义(P>0.05),其他组与对照组相比差异有统计学意义(P<0.05)。半数有效抑制浓度IC50为18.76 μmol/L。20 μmol/L桑根酮C作用PC3细胞,分别在6、12、24、48、72 h检测增值抑制率为:10.57%、27.09%、51.88%、80.73%、87.99%,与对照组相比差异均有统计学意义(P<0.05)。20 μmol/L桑根酮C可诱导PC3细胞的凋亡,在12、24、48 h的凋亡率分别为:7.43%、20.91%、37.56%,与对照组相比差异有统计学意义(P<0.05)。检测caspases 3的活性18 h比对照组增加了7.6倍,应用caspase抑制剂后发现caspase 3及caspase 9的抑制剂可显著逆转桑根酮C对PC3细胞增殖的抑制作用,抑制率从52.48%降到24.29%及28.81%,与对照组相比差异有统计学意义(P<0.01)。而caspase8抑制剂作用不明显,与对照组相比差异没有统计学意义(P>0.05)。
结论
桑根酮C可通过激活caspase 3及caspase 9途径诱导PC3细胞的凋亡。
Keywords: 桑根酮C, 前列腺癌, PC3细胞, caspase, 凋亡
Abstract
Objective
To investigate the effects of Sanggenon C in inducing apoptosis of prostate cancer PC3 cell line andexplore the underlying mechanism.
Methods
The proliferation of PC3 cells treated for 24 h with 1, 5, 20, 50, and 100 μmol/Lsanggenon C or treated with 20 μmol/L Sanggenon C for 0, 6, 12, 24 and 48 h was evaluated using MTT assay. Flow cytometrywas performed for analysis of apoptosis of PC3 cells after exposure to sanggenon C with different treatment protocols, and theactivity of caspase 3 was detected using spectrofluorometry. The inhibitory effect of sanggenon C on PC3 cells pretreated withDMSO, z-DEVD-fmk, z-LEHD-fmk or z-IETD-fmk for 1 h was detected by MTT assay.
Results
Sanggenon C inhibited theproliferation of PC3 cells in a dose-and time-dependent manner (P < 0.05 except for 1 μmol/L group) with a 24-h IC50 of18.76 μmol/L. Sanggenon C at 20 μmol/L caused inhibition rates of PC3 cells of 10.57%, 27.09%, 51.88%, 80.73% and 87.99%after treatment for 6, 12, 24, 48, and 72 h, respectively (P < 0.05), and resulted in apoptosis rates of 7.43%, 20.91% and 37.56% at12 h, 24 h and 48 h, respectively. Sanggenon C significantly increased caspase-3 activity in the cells, and its effect on PC3 cellproliferation was partially reversed by caspase 3 and caspase 9 inhibitors.
Conclusion
Sanggenon C can dose-dependentlyinduce growth inhibition and apoptosis of PC3 cells possibly by activating caspase 9 and caspase 3 pathways.
Keywords: sanggenon C, prostate cancer, PC3 cells, caspases, apoptosis
前列腺癌是男性常见的恶性肿瘤之一,早期局限性前列腺癌的治疗一般采用手术及局部放疗的方式,但由于前列腺癌早期常无症状,大多数发现时已属中晚期,错过了根治性治疗的时机,中晚期癌症患者主要采取睾丸切除联合内分泌治疗,在治疗过程中极易发展成为雄激素非依赖型前列腺癌,目前,对于雄激素非依赖型前列腺癌还没有非常有效的治疗方法[1-3]。桑根酮C是从桑科植物中提取的黄酮类化合物,主要存在于桑白皮中[4-6]。桑白皮为桑科植物桑的干燥根皮,具有抗癌、抗炎、降血压、降血糖、和抗动脉粥样硬化作用,临床主要用于治疗前列腺癌、高血压,高血脂和糖尿病等[7-11]。近年研究发现从桑科植物分离纯化的黄酮类化合物(quercetin-3-O-β-D-glucopyranoside和quercetin-3-7-diO-β-D-glu copyranoside可抑制人早幼粒白血病细胞HL60的生长并诱导其分化,表达CD66B和CD14抗原[12]。桑白皮的甲醇提取物morusin可通过抑制NF-ΚB的衰减诱导人宫颈癌HeLa细胞的凋亡,并能靶向杀灭肿瘤干细胞[13]。桑根酮C是桑白皮黄酮类化合物中最有活性的成分,它可以作为一个候选抗癌剂来使用。目前对于桑根酮C的生物学作用实验研究非常少,多停留在纯化提取方面,鲜有在抗氧化和抗炎等方面作用的报道[14-15]。目前尚未有研究探讨桑根酮C对前列腺癌的作用,本研究以雄激素非依赖型前列腺癌细胞PC3细胞为研究对象,通过观察桑根酮C对PC3细胞的增殖和凋亡的影响,并探讨相关机制,以期为桑根酮C的抗癌治疗提供新的实验依据。
1. 材料和方法
1.1. 材料
PC3细胞株购于中国科学院上海生命科学研究院细胞库;桑根酮C购自上海经科化学科技有限公司;甲基噻唑蓝(MTT)、DMEM F12培养基、胰蛋白酶、胎牛血清、二甲基亚砜(DMSO)均购自Gibco;流式细胞检测试剂盒、Ac-DEVD-AMC、z-IETD-fmk、z-DEVD-fmk及z-LEHD-fmk均购于BioVision;caspase 3检测试剂盒购自中国碧云天公司。
1.2. 方法
1.2.1. 细胞培养
人前列腺癌细胞PC3在含10%胎牛血清的DMEM F12培养基中,置于37 ℃、CO2体积分数为5%的饱和湿度培养箱中培养,隔天换液,融合度达80%时传代。
1.2.2. MTT法检测细胞抑制率
细胞按5000/孔的密度接种于96孔板,培养24 h后,分别加入不同浓度的桑根酮C(0、1、5、20、50、100 μmol/L),其中对照组只加DMSO,每组设5个复孔。加药培养24 h后每孔加MTT(5 g/L)20 μL继续培养4 h后每孔加入150 μL DMSO,置摇床上低速振荡10 min后在酶标仪A490 nm处测量各孔的吸光值A,计算抑制率(%)=[1-(药物组吸光度值/对照组吸光度值)]×100%,各药重复试验3次。
1.2.3. 流式细胞仪检测细胞凋亡
细胞按5×108/L的密度接种在6孔板,对数生长期加入20 μmol/L的桑根酮C,分别培养12、24、48 h。离心收集细胞(1~5)× 105。加入300 μL Binding buffer悬浮细胞。再加入5 μL FITC标记的Annexin V,室温混匀并避光30 min,然后加入PI,避光反应5 min后。上流式细胞仪进行检测。
1.2.4. Caspase 3活性检测
桑根酮C 20 μmol/L作用PC3细胞,分别在时间点0、6、12、18、24 h收集细胞。取1×106个细胞,加入100 L细胞裂解液,反复吹打并震荡混匀,室温作用30 min后离心取上清,加入AC-DEVD AMC 10 μL(浓度为1 g/L)和900 μL Hepes缓冲液,37 ℃水浴1 h。用荧光分光光度计检测荧光强度(激发波长360 nm、发射波长440 nm),实验重复3次。
1.2.5. Caspase抑制剂对桑根酮C抑制PC3细胞增殖的影响
PC3细胞接种于96孔板,培养24 h后分别加入20 μmol/L z-DEVD-fmk(caspase-3抑制剂)、z-LEHD fmk(caspase-8抑制剂)及z-IETD-fmk(caspase-9抑制剂),1 h后各孔加入20 μmol/L的桑根酮C,每组设5个复孔,作用24 h后按1.2.2项方法进行MTT检测。
1.3. 统计学处理
所有实验结果数据均以均数±标准差表示,采用SPSS17.0统计软件对数据进行处理,分析方法采用方差分析,组间两两比较用LSD法,以P<0.05为差异有统计学意义。
2. 结果
2.1. 桑根酮C对PC3细胞生长的抑制作用
阴性对照组PC3细胞生长活跃,经1、5、20、50、100 μmol/L桑根酮C处理24 h后,PC3细胞呈不同程度的生长抑制,抑制率为(4.86 ± 0.88)%、(12.92 ± 2.13)%、(52.34 ± 1.88)%、(82.05 ± 2.08)%、(87.45 ± 2.16)%,各组间比较除对照组和1 μmol/L组及50 μmol/L与100 μmol/L组之间P>0.05外,其他组间比较差异均有统计学意义,半数有效抑制浓度为:18.76±1.53 μmol/L。20 μmol/L桑根酮C作用PC3细胞6、12、24、48、72 h后,抑制率分别为:(10.57 ± 1.54)%、(27.09 ± 1.35)%、(51.88±2.21)%、(80.73±2.96)%、(87.99±2.14)%,组间比较除48 h与72 h组间没有明显差异外,其他均有统计学意义。结果表明随着剂量的增加、时间的延长,抑制率明显增加(图 1A,B)。
1.
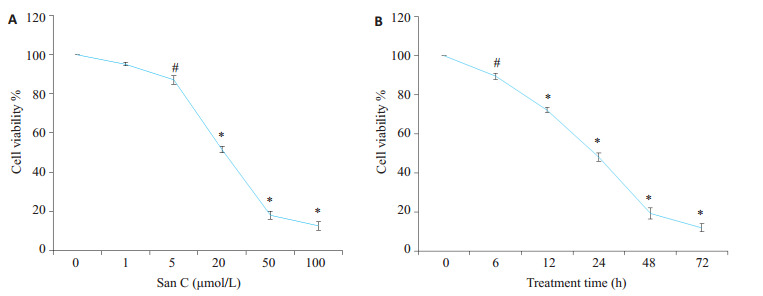
桑根酮C对PC3细胞增殖抑制作用
Inhibitory effect of Sanggenon C on the proliferation of PC3 cells detected by MTT assay. A: PC-3 cells treated with indicated concentrations of Sanggenon C for 24 h; B: PC-3 cells treated with 20 μmol/L Sanggenon C for up to 72 h. #P < 0.05, *P < 0.01 vs control group.
2.2. 桑根酮C诱导PC3细胞凋亡的作用注
桑根酮C 20 μmol/L作用PC3细胞12、24、48 h后,流式细胞仪检测细胞凋亡率分别为:(7.43±0.97)%、(20.91±2.34)%、(37.56±1.03)%,与阴性对照组(0.78± 0.23)%间比较差异有统计学意义(P<0.05),各组间比较差异有统计学意义(P<0.05,图 2)。
2.
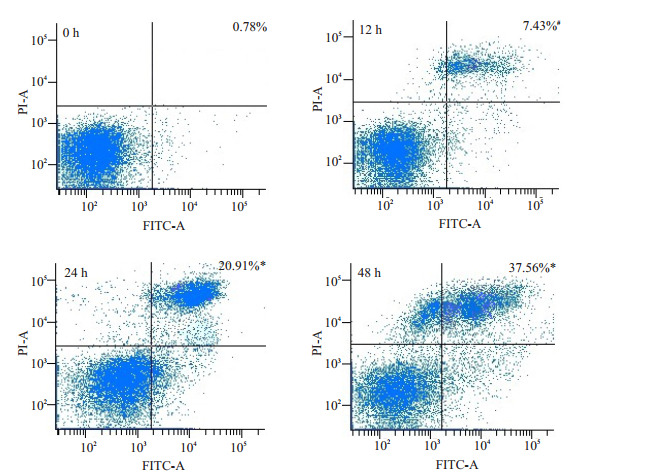
20 μmol/L桑根酮C在不同时间点诱导PC3细胞凋亡作用
Apoptotic rates of PC-3 cells treated with 20 μmol/L Sanggenon C for up to 48 h measured with flow cytometry. #P < 0.05, *P < 0.01 vs control group.
2.3. 桑根酮C对PC3细胞Caspases 3活性的影响
桑根酮C 20 μmol/L作用PC3细胞后,分别在6、12、18、24 h检测活性,发现随着时间的延长caspase 3的活性先增加,在18 h达高峰后逐渐下降(图 3)。
3.
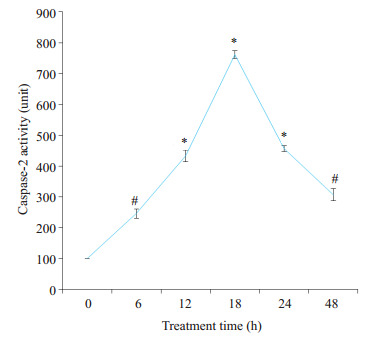
桑根酮C对PC3细胞caspases 3活性的影响
Effect of Sanggenon C on caspases 3 activity in PC3 cells. #P < 0.05, *P < 0.01 vs control group.
2.4. Caspase抑制剂对桑根酮C抑制PC3细胞增殖的影响
Caspase 3抑制剂z-DEVD-fmk及Caspase 9抑制剂z-LEHD-fmk,能有效逆转桑根酮C对PC3细胞的增殖抑制作用,细胞生长抑制率由(52.48±1.17)%分别降至(24.29±2.24)%及(28.81±1.52)%(P<0.05);Caspase 8抑制剂z-IETD-fmk的几乎没有作用,细胞生长抑制率由52.48%降至(47.95±3.73)%(P>0.05)。
3. 讨论
前列腺癌是西方国家男性发病率最高、死亡率居第2位的实体肿瘤[16]。我国近年来前列腺癌的发病率呈“井喷式”增长,在上海地区发病率甚至排在男性泌尿系统恶性肿瘤的第1位[2]。中晚期前列腺癌多数发展成为雄激素非依赖型前列腺癌,主要以内分泌治疗为主,当前常规使用的抗癌药物不仅治疗效果不佳而且毒副作用大[17-18]。桑白皮是一种可食用的中草药,几乎无毒副作用,临床中药方剂中常用来治疗前列腺癌[5]。桑白皮可通过线粒体或死亡受体途径,减少抗凋亡蛋白Survivin的表达,上调促凋亡蛋白Bax的表达来诱导人白血病细胞HL60及人乳腺癌细胞MCF-7及MDA-MB-231的凋亡[19-20]。但目前还没有研究探讨其抗前列腺癌的机制。
4.
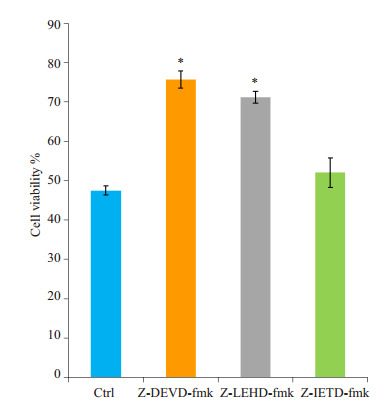
Caspase抑制剂对桑根酮C抑制PC3细胞增殖的影响
Effects of the caspase inhibitors on Sanggenon C-induced inhibition of PC3 proliferation. *P < 0.01 vs control.
桑根酮C是桑白皮中最有活性的黄酮类化合物,本实验室既往研究发现其可抑制蛋白酶体的活性引起小鼠肝癌细胞H22、小鼠白血病细胞P388及人慢性髓系白血病K562细胞的死亡,以坏死为主,也有一部分凋亡[21]。本研究发现桑根酮C可抑制雄激素非依赖型前列腺癌PC3细胞的增殖活性,呈时间、剂量依赖性,作用24 h后半数有效抑制浓度IC50为18.76 μmol/L;同时发现桑根酮C可引起PC3细胞的死亡,以凋亡为主,考虑其引起PC3细胞死亡的机制可能与之前不同。凋亡经典途径包括内源性线粒体细胞色素释放途径和外源性死亡受体途径,现有研究表明这两种通路并非独立,而是相互关联交错的,而这两种途径最终都将激活凋亡终末效应酶caspase 3[22-25]。本实验发现20 μmol/L桑根酮C作用PC3细胞12 h后细胞开始凋亡(凋亡率为7.43%,以早期凋亡为主),同时检测caspase 3的活性较对照组增加了4.3倍,且随着时间推移,caspase 3的活性逐渐增强,在18 h时达高峰增加了7.6倍,随后在24 h后活性逐渐减弱,考虑为18 h后细胞在桑根酮C的诱导下逐渐转入凋亡晚期或消亡期所致(与本实验凋亡检测结果相符)。为探讨其凋亡的机制,应用caspase抑制剂发现caspase 9及caspase 3抑制剂可明显逆转桑根酮C对PC3细胞的生长抑制作用;而caspase 8抑制剂几乎没有作用。其中caspase 8是外源性凋亡途径的执行者,caspase 9是内源性凋亡途径的执行者[26-29]。
综上所述,桑根酮C能有效抑制PC3细胞的增殖,并诱导其凋亡;这无疑显示了它在中晚期前列腺癌治疗中的潜在应用价值,目前只初步探讨了其诱导凋亡的信号转导通路是以caspase 9激活的线粒体通路为主,其他具体的分子机制以及药物作用的靶点有待深入探讨。
Biography
周萍,硕士,实验师,E-mail: 836738643@qq.com
Funding Statement
国家自然科学基金(81072091/H1619)
Supported by National Natural Science Foundation of China (81072091/H1619)
Contributor Information
周 萍 (Ping ZHOU), Email: 836738643@qq.com.
汤 平 (Ping TANG), Email: sytangping@yahoo.com.cn.
References
- 1.Adeola HA, Blackburn JM, Rebbeck TR, et al. Emerging pro teomics biomarkers and prostate cancer burden in Africa. http://europepmc.org/abstract/MED/28388542. Oncotarget. 2017;8(23):37991–8007. doi: 10.18632/oncotarget.16568. [Adeola HA, Blackburn JM, Rebbeck TR, et al. Emerging pro teomics biomarkers and prostate cancer burden in Africa[J]. Oncotarget, 2017, 8(23): 37991-8007.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng HH, Lin DW, Yu EY. Advanced clinical states in prostate cancer. Urol Clin North Am. 2012;39(4):561. doi: 10.1016/j.ucl.2012.07.011. [Cheng HH, Lin DW, Yu EY. Advanced clinical states in prostate cancer[J]. Urol Clin North Am, 2012, 39(4): 561.] [DOI] [PubMed] [Google Scholar]
- 3.徐 亚文, 郑 少波, 陈 玢屾, et al. 苯丁酸钠对多烯紫杉醇耐药前列腺癌细胞株的增值抑制和凋亡诱导作用. http://www.j-smu.com/Upload/html/201701130.html. 南方医科大学学报. 2017;37(1):130–4. doi: 10.3969/j.issn.1673-4254.2017.01.24. [徐亚文, 郑少波, 陈玢屾, 等.苯丁酸钠对多烯紫杉醇耐药前列腺癌细胞株的增值抑制和凋亡诱导作用[J].南方医科大学学报, 2017, 37 (1): 130-4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon DH, Cheon JM, Choi EO, et al. The immunomodulatory activity of Mori folium, the leaf of morus alba L, in RAW 264.7 macrophages in vitro. J Cancer Prev. 2016;21(3):144–51. doi: 10.15430/JCP.2016.21.3.144. [Kwon DH, Cheon JM, Choi EO, et al. The immunomodulatory activity of Mori folium, the leaf of morus alba L, in RAW 264.7 macrophages in vitro[J]. J Cancer Prev, 2016, 21(3): 144-51.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.高 学敏. 中药学. 北京: 中国中医药出版社; 2007. pp. 23–8. [高学敏.中药学[M]2版.北京:中国中医药出版社, 2007: 23-8.] [Google Scholar]
- 6.Kuk EB, Jo AR, Oh SI, et al. Anti-Alzheimer's disease activity of compounds from the root bark of morus alba L. Arch Pharm Res. 2017;40(3):338–49. doi: 10.1007/s12272-017-0891-4. [Kuk EB, Jo AR, Oh SI, et al. Anti-Alzheimer's disease activity of compounds from the root bark of morus alba L[J]. Arch Pharm Res, 2017, 40(3): 338-49.] [DOI] [PubMed] [Google Scholar]
- 7.Ann JY, Eo H, Lim Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015;10(6):46. doi: 10.1007/s12263-015-0495-x. [Ann JY, Eo H, Lim Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice[J]. Genes Nutr, 2015, 10(6): 46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo H, Xu Y, Huang W, et al. Kuwanon G preserves LPS-Induced disruption of gut epithelial barrier in vitro. Molecules. 2016;21(11):1597–611. doi: 10.3390/molecules21111597. [Guo H, Xu Y, Huang W, et al. Kuwanon G preserves LPS-Induced disruption of gut epithelial barrier in vitro[J]. Molecules, 2016, 21 (11): 1597-611.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J, Fan M, He J, et al. New cytotoxic and anti-inflammatory compounds isolate-ed from Morus alba L. Nat Prod Res. 2015;29(18):1711–8. doi: 10.1080/14786419.2014.999333. [Qin J, Fan M, He J, et al. New cytotoxic and anti-inflammatory compounds isolate-ed from Morus alba L[J]. Nat Prod Res, 2015, 29(18): 1711-8.] [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TD, Binh Xuan Phung Thi, Le Thi Phuong Quynh, et al. Cytotoxic prenylated flavonoids from Morus alba. Fitoterapia. 2010;81(8):1224–7. doi: 10.1016/j.fitote.2010.08.006. [Nguyen TD, Xuan Phung Thi Binh, Le Thi Phuong Quynh, et al. Cytotoxic prenylated flavonoids from Morus alba[J]. Fitoterapia, 2010, 81(8): 1224-7.] [DOI] [PubMed] [Google Scholar]
- 11.Eo HJ, Park JH, Park GH, et al. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement Altern Med. 2014;14(1):200. doi: 10.1186/1472-6882-14-200. [Eo HJ, Park JH, Park GH, et al. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark[J]. BMC Complement Altern Med, 2014, 14(1): 200.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SY, Gao JJ, Kang HK. Two flavonoids from the leaves of Morus alba induce differentiation of the human promyelocytic leukemia (HL-60) cell line. Biol Pharm Bull. 2000;23(4):451–5. doi: 10.1248/bpb.23.451. [Kim SY, Gao JJ, Kang HK. Two flavonoids from the leaves of Morus alba induce differentiation of the human promyelocytic leukemia (HL-60) cell line[J]. Biol Pharm Bull, 2000, 23(4): 451-5.] [DOI] [PubMed] [Google Scholar]
- 13.Kondylis V, Kumari S, Vlantis K, et al. The interplay of IKK, NF-κB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol Rev. 2017;277(1):113–27. doi: 10.1111/imr.2017.277.issue-1. [Kondylis V, Kumari S, Vlantis K, et al. The interplay of IKK, NF-κB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation[J]. Immunol Rev, 2017, 277(1): 113-27.] [DOI] [PubMed] [Google Scholar]
- 14.Li LC, Shen F, Hou Q, et al. Inhibitory effect and mechanism of action of sanggenon C on human polymorphonuclear leukocyte adhesion to human synovial cells. http://europepmc.org/abstract/MED/11866874. Acta Pharmacol Sin. 2002;23(2):138–42. [Li LC, Shen F, Hou Q, et al. Inhibitory effect and mechanism of action of sanggenon C on human polymorphonuclear leukocyte adhesion to human synovial cells[J]. Acta Pharmacol Sin, 2002, 23 (2): 138-42.] [PubMed] [Google Scholar]
- 15.Dat NT, Binh PT, Quynh le TP, et al. Sanggenon C and O inhibit NO production, iNOS expression and NF-κB activation in LPS induced RAW264.7 cells. Immunopharmacol Immunotoxicol. 2012;34(1):84–8. doi: 10.3109/08923973.2011.580755. [Dat NT, Binh PT, Quynh le TP, et al. Sanggenon C and O inhibit NO production, iNOS expression and NF-κB activation in LPS induced RAW264.7 cells[J]. Immunopharmacol Immunotoxicol, 2012, 34(1): 84-8.] [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(1): 7-30.] [DOI] [PubMed] [Google Scholar]
- 17.Rivera M, Ramos Y, Rodríguez-Valentín M, et al. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. http://pubmedcentralcanada.ca/pmcc/articles/PMC5476315/ PLoS One. 2017;12(6):e0179587. doi: 10.1371/journal.pone.0179587. [Rivera M, Ramos Y, Rodríguez-Valentín M, et al. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells[J]. PLoS One, 2017, 12(6): e0179587.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng X, Zhou P, Wei X, et al. Plasma proteasomal chymotrypsin like activity correlates with prostate cancer progression. Tumour Biol. 2015;36(6):4115–21. doi: 10.1007/s13277-015-3045-7. [Deng X, Zhou P, Wei X, et al. Plasma proteasomal chymotrypsin like activity correlates with prostate cancer progression[J]. Tumour Biol, 2015, 36(6): 4115-21.] [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi T, Nihei M, Nagai H, et al. Albanol a from the root bark of morus alba L. induces apoptotic cell death in HL60 human leukemia cell line. Chem Pharm Bull (Tokyo) 2010;58(4):568–71. doi: 10.1248/cpb.58.568. [Kikuchi T, Nihei M, Nagai H, et al. Albanol a from the root bark of morus alba L. induces apoptotic cell death in HL60 human leukemia cell line[J]. Chem Pharm Bull (Tokyo), 2010, 58(4): 568-71.] [DOI] [PubMed] [Google Scholar]
- 20.Kang S, Kim EO, Kim SH, et al. Morusin induces apoptosis by regulating expression of Bax and Survivin in human breast cancer cells. http://europepmc.org/abstract/MED/28599457. Oncol Lett. 2017;13(6):4558–62. doi: 10.3892/ol.2017.6006. [Kang S, Kim EO, Kim SH, et al. Morusin induces apoptosis by regulating expression of Bax and Survivin in human breast cancer cells[J]. Oncol Lett, 2017, 13(6): 4558-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Liu N, Zhao K, et al. Sanggenon C decreases tumor cell viability associated with proteasome inhibition. http://www.ncbi.nlm.nih.gov/pubmed/21622138. Front Biosci (Elite Ed) 2011;3(3):1315–25. doi: 10.2741/e335. [Huang H, Liu N, Zhao K, et al. Sanggenon C decreases tumor cell viability associated with proteasome inhibition[J]. Front Biosci (Elite Ed), 2011, 3(3): 1315-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duclos C, Lavoie C, Denault JB. Caspases rule the intracellular trafficking cartel. FEBS J. 2017;284(10):1394–420. doi: 10.1111/febs.14071. [Duclos C, Lavoie C, Denault JB. Caspases rule the intracellular trafficking cartel[J]. FEBS J, 2017, 284(10): 1394-420.] [DOI] [PubMed] [Google Scholar]
- 23.Qiu M, Chen L, Tan G, et al. JS-K promotes apoptosis by inducing ROS production in human prostate cancer cells. http://europepmc.org/abstract/MED/28454225. Oncol Lett. 2017;13(3):1137–42. doi: 10.3892/ol.2016.5535. [Qiu M, Chen L, Tan G, et al. JS-K promotes apoptosis by inducing ROS production in human prostate cancer cells[J]. Oncol Lett, 2017, 13(3): 1137-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YS, Kim SK, Park SJ. Apoptotic effect of demetho xyfumitremorgin C from Marine fungus Aspergillus fumigatus on PC3 human prostate cancer cells. http://europepmc.org/abstract/MED/28359723. Chem Biol Interact. 2017;269(269):18–24. doi: 10.1016/j.cbi.2017.03.015. [Kim YS, Kim SK, Park SJ. Apoptotic effect of demetho xyfumitremorgin C from Marine fungus Aspergillus fumigatus on PC3 human prostate cancer cells[J]. Chem Biol Interact, 2017, 269 (269): 18-24.] [DOI] [PubMed] [Google Scholar]
- 25.Lee JC, Shin EA, Kim B, et al. Auraptene induces apoptosis via myeloid cell leukemia 1-Mediated activation of caspases in PC3 and DU145 prostate cancer cells. Phytother Res. 2017;31(6):891–8. doi: 10.1002/ptr.5810. [Lee JC, Shin EA, Kim B, et al. Auraptene induces apoptosis via myeloid cell leukemia 1-Mediated activation of caspases in PC3 and DU145 prostate cancer cells[J]. Phytother Res, 2017, 31(6): 891-8.] [DOI] [PubMed] [Google Scholar]
- 26.Derakhshan A, Chen Z, Van Waes C. Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin Cancer Res. 2017;23(6):1379–87. doi: 10.1158/1078-0432.CCR-16-2172. [Derakhshan A, Chen Z, Van Waes C. Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways[J]. Clin Cancer Res, 2017, 23(6): 1379-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Najafov A, Py BF. Roles of caspases in necrotic cell death. Cell. 2016;167(7):1693–704. doi: 10.1016/j.cell.2016.11.047. [Yuan J, Najafov A, Py BF. Roles of caspases in necrotic cell death [J]. Cell, 2016, 167(7): 1693-704.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao YM, Jiang Z, Zeng Z, et al. Bcl-2 silencing attenuates hypoxia-induced apoptosis resistance in pulmonary microvascular endothelial cells. Apoptosis. 2016;21(1):69–84. doi: 10.1007/s10495-015-1184-3. [Cao YM, Jiang Z, Zeng Z, et al. Bcl-2 silencing attenuates hypoxia-induced apoptosis resistance in pulmonary microvascular endothelial cells[J]. Apoptosis, 2016, 21(1): 69-84.] [DOI] [PubMed] [Google Scholar]
- 29.Patmanathan SN, Wang W, Yap LF, et al. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. http://europepmc.org/abstract/MED/28302566. Cell Signal. 2017;34(34):66–75. doi: 10.1016/j.cellsig.2017.03.002. [Patmanathan SN, Wang W, Yap LF, et al. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer[J]. Cell Signal, 2017, 34(34): 66-75.] [DOI] [PubMed] [Google Scholar]


