Abstract
目的
探讨甘草酸对MRL/lpr小鼠狼疮肾炎是否具有保护作用及其机制。
方法
MRL/lpr狼疮小鼠随机分为MRL/lpr组、MRL/lpr+地塞米松(1.5 mg/kg)组,MRL/lpr+甘草酸(20 mg/kg)组,MRL/lpr+甘草酸(40 mg/kg)组,每组10只;野生型正常对照组C57BL/6小鼠10只。采用ELISA法检测各组小鼠血清中尿酸(UA)与肌酐(Cr)含量,各组小鼠血清及肾脏组织中IL-1β、IL-6、TNF-α炎症因子水平;采用HE染色法检测肾脏病理改变;采用Western blot技术检测各组小鼠脾脏NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、NF-κB、p-IκBα、IκBα蛋白的表达。
结果
甘草酸能显著降低血清尿酸、肌酐含量;减少血清及肾脏IL-1β、IL-6、TNF-α炎症因子表达,改善肾脏病理改变;并能显著抑制NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、p-IκBα表达。
结论
甘草酸对MRL/lpr狼疮小鼠肾脏起到保护作用。
Keywords: 系统性红斑狼疮, 甘草酸, 肾脏, 炎症
Abstract
Objective
To investigate protective effect of glycyrrhizic acid (GA) against lupus nephritis in MRL/lpr mice and explore its underlying mechanisms.
Methods
Forty MRL/lpr mice were randomized equally into blank control group, dexamethasone (1.5 mg/kg) group, GA (20 mg/kg) group, and GA (40 mg/kg) group with corresponding treatments for 7 days, with 10 wild-type mice as the control group. Serum levels of uric acid and creatinine and inflammatory cytokines in the serum and kidney were tested after the treatments using enzyme-linked immunosorbent assays (ELISA). The pathological changes in the kidneys were detected using HE staining, and the protein expressions of NLRP3, ASC, caspase-1, IL-1β, p-NF-κB, NF-κB, p-IκBα, and IκBα were detected with Western blotting.
Results
GA obviously decreased serum levels of uric acid and creatinine, decreased inflammatory cytokines in the serum and kidney, ameliorated renal pathologies and inhibited the expressions of NLRP3, ASC, caspase-1, IL-1β, p-NF-κB, and p-IκBα proteins in MRL/lpr mice.
Conclusion
GA has protective effects against lupus nephritis in MRL/lpr mice.
Keywords: systemic lupus erythematosus, glycyrrhizinic acid, kidney, inflammation
系统性红斑狼疮(SLE)是一个累及多系统的慢性自身免疫性疾病。肾脏是最常受累的器官之一[1]。由于治疗上取得的进步,目前SLE患者5年生存率达到94%,10年生存率也达到了89%,但是SLE导致的肾脏炎症仍是造成患者死亡的主要原因之一[2]。经过糖皮质激素如地塞米松等和免疫制剂的积极治疗,肾炎缓解,肾功能恢复,但仍有相当一部分狼疮肾炎患者最终出现终末期肾病,导致死亡[3-4]。因此积极寻找其他有效的狼疮肾炎药物仍是必要的。
甘草酸(GA)是从草本植物甘草中提取的活性成份,能够缓解辐射诱导的肺损伤[5]。甘草酸通过抑制炎症、凋亡和氧化应激阻止脓毒血症诱导的急性肾损伤[6],也可以保护高糖诱导的肾小管上皮细胞损害[7]。因此甘草酸能抑制炎症,但甘草酸是否对狼疮肾炎有保护作用,目前不清楚。NLRP3炎症小体是最具特征性的炎症小体,它积极参与了百草枯诱导的急性肾损伤[8],也参与了慢性肾脏疾病中的慢性炎症[9]。NLRP3炎症小体同样和狼疮肾炎有关[10]。NF-κB信号通路也与狼疮肾炎的发病机制密切相关[11],本文拟研究甘草酸对NLRP3炎症小体和NF-κB信号通路是否有抑制作用,对狼疮肾炎是否有治疗作用。从而为临床上治疗狼疮肾炎提供新的思路。
1. 材料和方法
1.1. 材料
1.1.1. 实验动物
MRL/lpr狼疮小鼠及野生型C57BL/6小鼠,雌性,体质量18~22 g,购自南京大学模式动物研究中心。
1.1.2. 主要试剂
甘草酸(纯度>98%)购自中国食品药品检定研究院,地塞米松(纯度>98%),购自江苏先声大药房,采用0.9%生理盐水配置地塞米松。UA、Cr、IL-1β、IL-6、TNF-α炎症因子ELISA试剂盒为武汉Elabscience公司产品,NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、NF-κB、p-IκBα、IκBα等抗体试剂盒购自南京建成生物有限公司。
1.2. 主要方法
1.2.1. 分组及给药
MRL/lpr狼疮小鼠20只随机分成4组:MRL/lpr组,MRL/lpr +地塞米松(1.5 mg/kg)组,MRL/lpr +甘草酸(20 mg/kg)组,MRL/lpr +甘草酸(40 mg/kg)组,每组10只。正常对照组野生型C57BL/ 6小鼠10只。MRL/lpr+地塞米松(1.5 mg/kg)组、MRL/ lpr+甘草酸(20 mg/kg)组,MRL/lpr+甘草酸(40 mg/kg)组小鼠给药7 d,第8天取所有小鼠血清及肾脏组织测定各项指标。
1.2.2. 小鼠血清UA、检测
小鼠血清中UA、Cr检测方法严格按照试剂盒说明书进行检测。
1.2.3. ELISA检查炎症因子
取小鼠血清与肾脏,按照试剂盒说明书进行炎症因子检测。
1.2.4. Western blotting
将小鼠肾脏组织置于冰冷裂解液收集裂解物总蛋白,组织蛋白行SDS-PAGE并转印至PVDF膜;室温封闭2 h,分别与一抗孵育,膜经漂洗后再与辣根过氧化物酶偶联的二抗反应,增强化学发光法(ECL)发光试剂显影,灰度成像软件(UVP, UK)测定主带的光密度值以计算各蛋白表达水平。
1.3. 数据分析
采用SPSS11.5统计软件进行数据分析。数据以均数±标准差表示。多组间比较采用方差分析,两两比较用q检验,P < 0.05表示差异有统计学意义。
2. 结果
2.1. 小鼠血清UA、Cr含量
与野生型组相比,MRL/lpr小鼠血清中UA、Cr含量显著升高(P < 0.01),甘草酸各剂量组能显著降低血清中UA、Cr含量(P < 0.01,表 1)。
1.
甘草酸对MRL/lpr小鼠血清UA、Cr的影响
Effect of GA on serum levels of uric acid and creatinine in MRL/lpr mice (Mean±SD, n=10)
| Group | Dosage (mg/kg) | UA (mg/L) | Cr (μmol/L) |
| GA: Glycyrrhizic acid, UA: Uric acid; Cr: Creatinine. ##P < 0.01 vs wild type group; **P < 0.01 vs MRL/lpr group. | |||
| Wild type | - | 1.87±0.32 | 19.77±5.06 |
| MRL/lpr | - | 3.41±0.22## | 35.10±3.44## |
| MRL/lpr+DEX | 1.5 | 2.80±0.21** | 22.38±4.43** |
| MRL/lpr+GA | 20 | 2.72±0.21** | 22.68±3.35** |
| 40 | 2.69±0.14** | 21.92±4.13** | |
2.2. 小鼠血清中炎症因子
与野生型组相比,MRL/lpr小鼠血清中IL-1β、IL-6、TNF-α显著升高(P < 0.01);甘草酸各剂量组能显著降低血清中IL-1β、IL-6、TNF-α(P < 0.01,表 2)。
2.
各组小鼠血清中IL-1β、IL-6、TNF-α水平
Serum levels of IL-1β, IL-6 and TNF-α in the mice in different groups (Mean±SD, n=10)
| Group | IL-1β (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) |
| ##P < 0.01 vs. wild type group; **P < 0.01 vs. MRL/lpr group. | |||
| Wild type | 11.13±2.69 | 21.06±3.29 | 39.52±10.82 |
| MRL/lpr | 31.63±1.59## | 93.29±9.26## | 386.30±25.14## |
| MRL/lpr+DEX (1.5 mg/kg) | 21.38±1.54** | 24.65±7.65** | 114.48±16.11** |
| MRL/lpr+GA (20 mg/kg) | 28.03±1.28** | 50.99±7.25** | 214.41±19.13** |
| MRL/lpr+GA (40 mg/kg) | 23.66±2.05** | 27.44±6.05** | 151.45±34.69** |
2.3. 小鼠肾脏组织中炎症因子
与野生型组相比,MRL/lpr小鼠肾脏组织中IL-1β、IL-6、TNF-α显著升高(P < 0.01);甘草酸各剂量组能显著降低肾脏组织中IL-1β、IL-6、TNF-α(P < 0.01,表 3)。
3.
各组小鼠肾脏组织中IL-1β、IL-6、TNF-α水平
Expression of IL-1β, IL-6 and TNF-α in the kidney of the mice in different groups (Mean±SD, n=10)
| Group | IL-1β (pg/mg) | IL-6 (pg/mg) | TNF-α (pg/mg) |
| ##P < 0.01 vs wild type group; **P < 0.01 vs MRL/lpr group. | |||
| Wild type | 2.15±0.61 | 19.35±6.00 | 46.57±15.97 |
| MRL/lpr | 11.99±1.77## | 68.13±9.90## | 224.66±19.59## |
| MRL/lpr+DEX (1.5 mg/kg) | 7.31±1.28** | 25.15±7.04** | 100.99±6.09** |
| MRL/lpr+GA (20 mg/kg) | 8.13±0.69** | 43.28±7.85** | 181.32±14.09** |
| MRL/lpr+GA (40 mg/kg) | 7.72±1.09** | 38.71±5.06** | 147.89±7.03** |
2.4. 肾脏病理
与野生型组相比,MRL/lpr小鼠肾脏组织炎症细胞浸润;甘草酸各剂量组能显著缓解炎症细胞浸润(图 1)。
1.
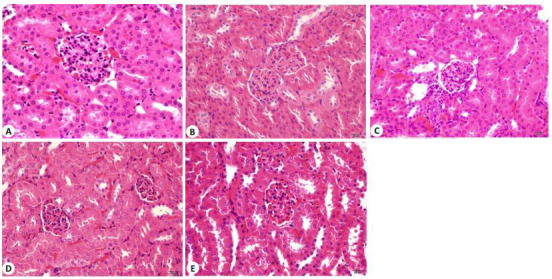
各组小鼠肾组织结构的变化
Histological changes of the kidney of the mice in different groups (HE staining, original magnification: ×200). A: Wild type group; B: MRL/lpr group; C: MRL/lpr+DEX(1.5 mg/kg) group; D: MRL/lpr+GA(20 mg/kg)group; E: MRL/lpr +GA(40 mg/kg) group.
2.5. Western blotting
Western blotting结果显示,与野生型组相比,MRL/lpr小鼠肾脏组织中NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、p-IκBα蛋白水平显著升高(P < 0.01);甘草酸各剂量组能降低MRL/lpr小鼠脾脏组织中NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、p-IκBα蛋白水平(P < 0.01,图 2、表 4)。
2.
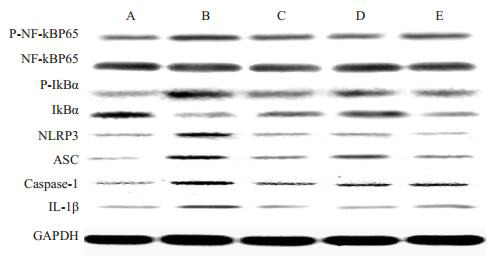
各组小鼠肾脏组织中NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、p-IκBα蛋白表达
Western blotting for expressions of NLRP3, ASC, caspase-1, IL-1β, p-NF-κB and p-IκBα in the kidney of the mice in different groups. A: Wild type group; B: MRL/lpr group; C: MRL/lpr+ DEX(1.5 mg/kg) group; D: MRL/lpr+GA (20 mg/kg) group; E: MRL/lpr+GA(40 mg/kg)group.
4.
各组小鼠肾脏组织中NLRP3、ASC、Caspase-1、IL-1β、p-NF-κB、p-IκBα蛋白表达
Quantitative analysis of NLRP3, ASC, caspase-1, IL-1β, p-NF-κB and p-IκBα in the kidney of the mice in different groups (Mean± SD, n=10)
| Group | NLRP3/GAPDH | ASC/GAPDH | Caspase-1/GAPDH | IL-1β/GAPDH | P-P65/P65 | p-IκBα/I κBα |
| ##P < 0.01 vs wild type group; **P < 0.01 vs MRL/lpr group. | ||||||
| Wild type | 0.45±0.14 | 0.57±0.12 | 0.43±0.16 | 0.12±0.03 | 0.18±0.05 | 0.45±0.12 |
| MRL/lpr | 0.84±0.13## | 1.43±0.18## | 0.87±0.12## | 0.45±0.04## | 0.56±0.09## | 0.95±0.15## |
| MRL/lpr+DEX (1.5 mg/kg) | 0.56±0.16** | 0.79±0.12** | 0.57±0.12** | 0.23±0.05** | 0.25±0.07** | 0.62±0.12** |
| MRL/lpr+GA (20 mg/kg) | 0.65±0.07** | 1.09±0.09** | 0.68±0.15** | 0.31±0.03** | 0.41±0.06** | 0.78±0.18** |
| MRL/lpr+GA (40 mg/kg) | 0.57±0.11** | 1.01±0.05** | 0.56±0.04** | 0.25±0.05** | 0.33±0.07** | 0.69±0.11** |
3. 讨论
MRL/lpr狼疮鼠是较适合研究狼疮肾炎的动物模型[12],其肾脏损害表现为血清尿素氮和肌酐的升高[11]。GA能降低脓毒血症诱导的急性肾损害所导致的血清肌酐和尿素氮的升高[6, 13]。本研究中发现MRL/lpr狼疮鼠的血清肌酐和尿素氮升高,经过地塞米松和GA治疗后均能明显降低。在狼疮肾炎的发病机制中,NF-κB信号通路的活化起着十分重要的作用[14]。MRL/lpr狼疮鼠的肾脏组织中NF-κB活化[15],而且NF-κB活化后分泌的炎症细胞因子IL-1β、IL-6、TNF-α均参与了狼疮肾炎的发病机制[16]。在脓毒血症诱导的急性肾损害中,GA抑制肾脏组织中的NF-κB活化,并减少肾脏中炎症细胞因子IL-1β、IL-6、TNF-α的分泌,从而缓解肾脏损害[13]。本研究中,MRL/lpr狼疮鼠血清和肾组织中炎症细胞因子表达均增加,而且肾组织中磷酸化的NF-κB和IкB量均增加。这些提示在MRL/lpr狼疮鼠肾脏中由于IkB活化,导致NF-κB的活化以及其下游产物炎症细胞因子IL-1β、IL-6、TNF-α分泌增加。GA和地塞米松一样能够抑制MRL/lpr狼疮鼠肾脏组织中IкB/NF-kB/炎症细胞因子通路。
NLRP3炎症小体复合物是由NLRP3、ASC、procaspase-1、IL-1β和IL-18组成。NLRP3炎症小体复合物与狼疮肾炎的发病机制相关[7, 19]。在MRL/lpr狼疮鼠肾组织中发现NLRP3/ASC/Caspase-1/IL-1β表达增加[19],阻断MRL/lpr狼疮鼠中NLRP3/IL-β信号轴,能减轻蛋白尿、自身抗体的产生、促炎症细胞因子的分泌以及肾损害[17-18]。在SLE患者中也发现NLRP3 rs10754558 C/G多态性与SLE易感性相关[20]。SLE患者血清中的抗dsDNA抗体通过结合TLR4活化单核/巨噬细胞中的NLRP3炎症小体[21]。NLRP3炎症小体复合物的重要产物IL-18也与SLE密切相关[22]。SLE患者血清中的IL-18与狼疮病情活动、狼疮肾炎的炎症程度有明显相关性[22]。这些研究说明NLRP3炎症小体在SLE肾脏中活化并通过产生炎症细胞因子引起肾损害。我们的研究也发现MRL/lpr狼疮鼠肾组织中NLRP3/ ASC/Caspase-1/IL-1β表达明显增加,地塞米松和GA能明显减少狼疮鼠肾脏中NLRP3/ASC/Caspase-1/IL-1β表达量。NLRP3在活化之前必须被各种刺激所启动,NF-kB活化刺激能诱导NLRP3蛋白的表达增加,进而引起NLRP3炎症小体的形成[23]。在MRL/lpr狼疮鼠肾组织中NLRP3的形成和活化是否由NF-kB信号通路控制还需要进一步研究。
GA能减轻脓毒血症诱导的肾脏组织中炎症细胞的浸润[6, 13],与此类似,我们的研究发现GA同样能够降低MRL/lpr狼疮鼠血清中肌酐、尿素氮水平并且减轻肾组织中炎症细胞的浸润。我们认为GA有可能是通过抑制NLRP3炎症小体复合物和NF-kB信号通路的活化,从而减轻狼疮肾炎反应,达到保护MRL/lpr狼疮鼠肾脏目的。
Biography
王元元,硕士,讲师,E-mail: wangyuanyuantcm@126.com
Funding Statement
安徽省自然科学基金(1608085MH215);安徽省高等学校省级自然科学研究重点项目(KJ2016A475);安徽省高等学校省级自然科学研究项目(KJ2015B009by); 蚌埠医学院科技发展基金(Bykf13A28);安徽省大学生创新创业项目(201610367028);安徽省蚌埠医学院研究生科技创新项目(Byycx1609)
Contributor Information
王 元元 (Yuanyuan WANG), Email: wangyuanyuantcm@126.com.
谢 长好 (Changhao XIE), Email: uglboy2002@126.com.
References
- 1.Imran TF, Yick F, Verma S, et al. Lupus nephritis: an update. Clin Exp Nephrol. 2016;20(1):1–13. doi: 10.1007/s10157-015-1179-y. [Imran TF, Yick F, Verma S, et al. Lupus nephritis: an update[J]. Clin Exp Nephrol, 2016, 20(1): 1-13.] [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Wang Y, Zhu R, et al. Long-term survival and death causes of systemic lupus erythematosus in China: a systemic review of observational studies. Medicine. 2015;94(17):e794. doi: 10.1097/MD.0000000000000794. [Wang Z, Wang Y, Zhu R, et al. Long-term survival and death causes of systemic lupus erythematosus in China: a systemic review of observational studies[J]. Medicine, 2015, 94(17): e794.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh SV, Rovin BH. Current and Emerging Therapies for Lupus Nephritis. J Am Soc Nephrol. 2016;27(10):2929–39. doi: 10.1681/ASN.2016040415. [Parikh SV, Rovin BH. Current and Emerging Therapies for Lupus Nephritis[J]. J Am Soc Nephrol, 2016, 27(10): 2929-39.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faurschou M, Dreyer L, Kamper AL, et al. Long-Term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken) 2010;62(6):873–80. doi: 10.1002/acr.20116. [Faurschou M, Dreyer L, Kamper AL, et al. Long-Term mortality and renal outcome in a cohort of 100 patients with lupus nephritis[J]. Arthritis Care Res (Hoboken), 2010, 62(6): 873-80.] [DOI] [PubMed] [Google Scholar]
- 5.Chen JM, Zhang WJ, Zhang LR, et al. Glycyrrhetinic acid alleviates radiation-induced lung injury in mice. J Radiat Res. 2017;58(1):41–7. doi: 10.1093/jrr/rrw091. [Chen JM, Zhang WJ, Zhang LR, et al. Glycyrrhetinic acid alleviates radiation-induced lung injury in mice[J]. J Radiat Res, 2017, 58(1): 41-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao HY, Liu ZN, Shen HT, et al. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur J Pharmacol. 2016;781:92–9. doi: 10.1016/j.ejphar.2016.04.006. [Zhao HY, Liu ZN, Shen HT, et al. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress[J]. Eur J Pharmacol, 2016, 781: 92-9.] [DOI] [PubMed] [Google Scholar]
- 7.Hou SZ, Zheng FF, Li Y, et al. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int J Mol Sci. 2014;15(9):15026–43. doi: 10.3390/ijms150915026. [Hou SZ, Zheng FF, Li Y, et al. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose[J]. Int J Mol Sci, 2014, 15(9): 15026-43.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Wang X, Wang Y, et al. NLRP3 inflammasome activation regulated by NF-κB and DAPK contributed to paraquat-induced acute kidney injury. Immunol Res. 2017;65(3):687–98. doi: 10.1007/s12026-017-8901-7. [Liu Z, Wang X, Wang Y, et al. NLRP3 inflammasome activation regulated by NF-κB and DAPK contributed to paraquat-induced acute kidney injury[J]. Immunol Res, 2017, 65(3): 687-98.] [DOI] [PubMed] [Google Scholar]
- 9.Yu G, Bai Z, Chen Z, et al. The NLRP3 inflammasome is a potential target of ozone therapy aiming to ease chronic renal inflammation in chronic kidney disease. Int Immunopharmacol. 2017;43:203–9. doi: 10.1016/j.intimp.2016.12.022. [Yu G, Bai Z, Chen Z, et al. The NLRP3 inflammasome is a potential target of ozone therapy aiming to ease chronic renal inflammation in chronic kidney disease[J]. Int Immunopharmacol, 2017, 43): 203-9.] [DOI] [PubMed] [Google Scholar]
- 10.Masood H, Che R, Zhang A. Inflammasomes in the pathophysiology of kidney diseases. Kidney Dis(Basel) 2015;1(3):187–93. doi: 10.1159/000438843. [Masood H, Che R, Zhang A. Inflammasomes in the pathophysiology of kidney diseases[J]. Kidney Dis(Basel), 2015, 1(3): 187-93.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You YW, Qin YE, Lin X, et al. Methylprednisolone attenuates lipopolysaccharide-induced Fractalkine expression in kidney of Lupus-prone MRL/lpr mice through the NF-kappaB pathway. BMC Nephrol. 2015;16:148. doi: 10.1186/s12882-015-0145-y. [You YW, Qin YE, Lin X, et al. Methylprednisolone attenuates lipopolysaccharide-induced Fractalkine expression in kidney of Lupus-prone MRL/lpr mice through the NF-kappaB pathway[J]. BMC Nephrol, 2015, 16: 148.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Model Mech. 2014;7(9):1033–46. doi: 10.1242/dmm.016451. [Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus[J]. Dis Model Mech, 2014, 7(9): 1033-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao HY, Zhao M, Wang Y, et al. Glycyrrhizic acid attenuates Sepsis-Induced acute kidney injury by inhibiting NF-kappa B signaling pathway. http://www.ncbi.nlm.nih.gov/pubmed/26904148. Evid Based Complement Alternat Med. 2016;2016:8219287. doi: 10.1155/2016/8219287. [Zhao HY, Zhao M, Wang Y, et al. Glycyrrhizic acid attenuates Sepsis-Induced acute kidney injury by inhibiting NF-kappa B signaling pathway[J]. Evid Based Complement Alternat Med, 2016, 2016: 8219287.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Du Y, Zhao FS, et al. Reagent-and separation-free measurements of urine creatinine concentration using stamping surface enhanced Raman scattering (S-SERS) Biomed Opt Express. 2015;6(3):849–58. doi: 10.1364/BOE.6.000849. [Li M, Du Y, Zhao FS, et al. Reagent-and separation-free measurements of urine creatinine concentration using stamping surface enhanced Raman scattering (S-SERS)[J]. Biomed Opt Express, 2015, 6(3): 849-58.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu QY, Yang CX, Wang Q, et al. Demethylzeylasteral (T-96) treatment ameliorates mice lupus nephritis accompanied by inhibiting activation of NF-kappa B pathway. PLoS One. 2015;10(7):e0133724. doi: 10.1371/journal.pone.0133724. [Hu QY, Yang CX, Wang Q, et al. Demethylzeylasteral (T-96) treatment ameliorates mice lupus nephritis accompanied by inhibiting activation of NF-kappa B pathway[J]. PLoS One, 2015, 10(7): e0133724.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. https://www.researchgate.net/publication/283967174_Pathogenic_Inflammation_and_Its_Therapeutic_Targeting_in_Systemic_Lupus_Erythematosus. Front Immunol. 2015;6:550. doi: 10.3389/fimmu.2015.00550. [Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus[J]. Front Immunol, 2015, 6: 550.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Zhang H, Huang Y, et al. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NFkappaB activation. Int Immunopharmacol. 2013;17(1):116–22. doi: 10.1016/j.intimp.2013.05.027. [Zhao J, Zhang H, Huang Y, et al. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NFkappaB activation[J]. Int Immunopharmacol, 2013, 17(1): 116-22.] [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Wang H, Huang Y, et al. Lupus nephritis: glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice. Arthritis Rheumatol. 2015;67(4):1036–44. doi: 10.1002/art.v67.4. [Zhao J, Wang H, Huang Y, et al. Lupus nephritis: glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice[J]. Arthritis Rheumatol, 2015, 67(4): 1036-44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao JJ, Wang HY, Dai C, et al. P2X(7) blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65(12):3176–85. doi: 10.1002/art.38174. [Zhao JJ, Wang HY, Dai C, et al. P2X(7) blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway[J]. Arthritis Rheum, 2013, 65(12): 3176-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YH, Bae SC. Association between functional NLRP3 polymorphisms and susceptibility to autoimmune and inflammatory diseases: a meta-analysis. Lupus. 2016;25(14):1558–66. doi: 10.1177/0961203316644336. [Lee YH, Bae SC. Association between functional NLRP3 polymorphisms and susceptibility to autoimmune and inflammatory diseases: a meta-analysis[J]. Lupus, 2016, 25(14): 1558-66.] [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Fu R, Guo CH, et al. Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/ macrophages. J Transl Med. 2016;14(1):156. doi: 10.1186/s12967-016-0911-z. [Zhang H, Fu R, Guo CH, et al. Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/ macrophages[J]. J Transl Med, 2016, 14(1): 156.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CY, Yang HY, Yao TC, et al. Serum IL-18 as biomarker in predicting long-term renal outcome among pediatric-onset systemic lupus erythematosus patients. Medicine. 2016;95(40):e5037. doi: 10.1097/MD.0000000000005037. [Wu CY, Yang HY, Yao TC, et al. Serum IL-18 as biomarker in predicting long-term renal outcome among pediatric-onset systemic lupus erythematosus patients[J]. Medicine, 2016, 95(40): e5037.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo HT, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. doi: 10.1038/nm.3893. [Guo HT, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics[J]. Nat Med, 2015, 21(7): 677-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]


