Abstract
目的
研究鼠双微体2癌基因结合蛋白(MTBP)对人前列腺癌细胞迁移及侵袭能力的影响及其作用机制。
方法
用Western blot检测MTBP在22RV1、DU145及Lncap三种不同前列腺癌细胞中的表达水平。采用siRNA及MTBP质粒分别转染细胞,转染48 h后,用Western blot检测MTBP蛋白的表达量,划痕实验检测细胞的平行迁移能力,Transwell实验检测细胞的垂直迁移及侵袭能力。用Western blot检测细胞上皮间质转化(EMT)标志分子E-cadherin蛋白表达量的变化。
结果
在前列腺癌细胞中,MTBP在转移性前列腺癌细胞DU145中表达最高,Lncap细胞次之,局限性前列腺癌细胞22RV1中最低,MTBP的表达水平与前列腺癌细胞的转移侵袭能力呈正相关。siRNA干扰及MTBP质粒转染细胞分别能显著降低或提高前列腺癌细胞中MTBP的蛋白表达水平。划痕实验结果显示抑制MTBP的表达后,前列腺癌细胞的迁移能力降低,而MTBP过表达能显著促进前列腺癌细胞的迁移(P < 0.01)。Transwell实验发现抑制MTBP的表达可降低前列腺癌细胞的迁移侵袭能力,而MTBP过表达后,能显著促进前列腺癌细胞的迁移侵袭能力(P < 0.01);Western blot结果显示抑制MTBP的表达可使前列腺癌细胞的Ecadherin蛋白表达水平升高,而MTBP过表达的前列腺癌细胞中E-cadherin蛋白表达水平降低。
结论
MTBP可促进前列腺癌细胞的迁移和侵袭,其作用机制可能与诱导EMT有关。
Keywords: MTBP, 前列腺癌, 迁移, 侵袭, 上皮间质转化
Abstract
Objective
To investigate the role of MTBP in regulating the migration and invasion of human prostate cancer cells.
Methods
The baseline expressions of MTBP in 3 different human prostate cancer cells lines (22RV1, DU145 and Lncap) were detected using Western blotting. The cells were transfected with a small interfering RNA (siRNA) for MTBP knockdown or MTBP plasmid for MTBP overexpression, and 48 h later, the cells were examined for MTBP expression with Western blotting; the changes in the migration abilities of the cells were evaluated using wound healing assay and Transwell assay, and the cell invasiveness was assessed using Matrigel Transwell assay. The expression of E-cadherin protein, a marker of epithelial mesenchymal transition (EMT), was detected using Western blotting.
Results
MTBP expression was the highest in DU145 cells followed by Lncap cells, and was the lowest in 22RV1 cells, indicating a positive correlation of MTBP expression with the level of malignancy of human prostate cancer cells. Transfection of the cells with siRNA or MTBP plasmids efficiently lowered or enhanced the expressions of MTBP in human prostate cancer cells. Wound healing assay showed that inhibition of MTBP expression decreased the migration ability of the prostate cancer cells, and MTBP overexpression significantly promoted the migration of the cells (P < 0.01). Transwell assay showed that MTBP knockdown significantly lowered the migration and invasion ability of the cells, while MTBP overexpression markedly increased the number of migrating and invading cells (P < 0.01); Western blotting results showed that MTBP knockdown increased the expression of E-cadherin protein, and MTBP overexpression decreased E-cadherin expression in the prostate cancer cells.
Conclusion
MTBP overexpression promotes the migration and invasion of human prostate cancer cells possibly relation to the induction of EMT.
Keywords: MTBP, prostate cancer, migration, invasion, epithelial mesenchymal transition
前列腺癌是欧美国家男性最常见的恶性肿瘤,其死亡率仅次于肺癌[1]。随着饮食结构的改变、人口老年化和医疗水平的提高,我国前列腺癌的发病率和检出率呈逐年升高趋势[2]。前列腺癌发病隐匿,多数患者初次就诊时就已经发生转移,丧失根治性手术或根治性放疗的机会。国内的一项研究结果显示,新诊断的前列腺癌患者中,有54%的患者在诊断时就已经发生远处转移,未转移患者的5年相对生存率为80%,而发生远处转移患者的5年相对生存率降至30%[3],其无进展生存时间也只有未转移患者的一半[4]。肿瘤转移是前列腺癌患者的首要死亡原因,所以早期准确预测前列腺癌转移和阻断转移信号通路是目前前列腺癌研究的难点和热点,极具临床意义和研究价值[5]。
鼠双微体2癌基因结合蛋白(MTBP)是鼠双微体2(MDM 2)的结合蛋白,它能够通过抑制MDM 2的自身泛素化来维持MDM 2的稳定[6-8],而MDM2是肿瘤抑制基因P53的负向调控因子[9]。目前,已有多项研究[10-17]表明MTBP在肿瘤的发生发展过程中扮演了重要角色,且MTBP的高表达与恶性肿瘤的转移侵袭及临床不良预后密切相关。Lu等[11]发现MTBP的表达水平与肝癌患者的不良预后呈正相关,上调MTBP的表达可显著增强肝癌细胞的转移侵袭能力。Grieb等[12]的研究结果提示MTBP在乳腺癌中高表达,且其表达水平与患者的不良预后密切相关,MTBP表达的下调可抑制肿瘤的增殖。相关研究报道[10, 18],在前列腺癌组织中MTBP的表达明显高于癌旁组织,且MTBP的高表达与肿瘤的恶性程度和临床预后有关,提示MTBP可能参与前列腺癌的发生及进展。然而,MTBP在前列腺癌细胞中的生物学功能和作用机制目前国内外尚未见报道。本文以两种转移能力不同的前列腺癌细胞为研究对象,通过调控MTBP的表达水平,探讨MTBP对前列腺癌细胞迁移及侵袭能力的影响及其作用机制,为深入研究探讨前列腺癌的转移机制提供重要的理论基础与实验依据。
1. 材料和方法
1.1. 细胞株
前列腺癌细胞株22RV1、DU145及Lncap均购自美国典型物保藏中心。
1.2. 主要试剂和耗材
RPMI 1640培养基、胎牛血清和胰蛋白酶购自BI公司;Opti-MEM培养基,Lipofentamine2000,总蛋白提取试剂盒,蛋白浓度测定试剂盒,蛋白上样缓冲液5xloading buffer,PVDF膜,蛋白分子marker,MTBP兔多克隆抗体,GAPDH鼠单克隆抗体,辣根过氧化物酶标记的羊抗兔抗体,辣根过氧化物酶标记的羊抗鼠抗体,ECL化学发光液;BD Biocoat Matrigel基质;磷酸盐缓冲液(PBS),盐酸,甲醇,乙醇,TBS,吐温等试剂均为国产分析纯,所有缓冲液均用ddH2O水配制。
1.3. 前列腺癌细胞培养、siRNA干扰以及MTBP转染
前列腺癌细胞培养于含10%胎牛血清,100 U/mL青霉素和100 μg/mL链霉素的RPMI 1640培养基中,于37 ℃、5% CO2温育箱中培养,取对数生长期的细胞用于实验。转染前1 d将2~3×105细胞接种于6孔板中,当细胞融合度达50%~60%时,按Lipofectamine2000脂质体说明书转染细胞。每次实验设3个组:空白对照组(control)、阴性对照组(si-NC/p-NC)、实验组(si-MTBP/p-MTBP)。其中,si-MTBP正义链为5'-CCATGTACCATTAGTAA CA-3',转染6~8 h后更换培养液。转染48 h后,提取各组细胞的蛋白质,检测各项相关指标。
1.4. Western blot检测蛋白表达
设置分组,提取各组细胞总蛋白,加入上样缓冲液,95 ℃下变性15 min,取等量蛋白进行10%聚丙烯酰胺凝胶电泳(80 V,30 min;100 V,60 min)分离蛋白,湿法(200 mA,120 min)转印至PVDF膜;用含5%牛血清白蛋白(BSA)封闭液室温封闭l h;一抗兔抗人MTBP多克隆抗体1:1000、鼠抗人GAPDH单克隆抗体1:10 000,分别置于4 ℃孵育过夜;TBST液洗膜3次,每次洗10 min;二抗羊抗兔IgG 1:10 000、羊抗鼠IgG 1:10 000分别室温孵育1 h;TBST液洗膜5次,每次洗5 min;ECL发光试剂盒显影;Bio.Rad凝胶成像系统获取图像。
1.5. 划痕实验检测细胞平行迁移能力
收集各组细胞,分别以5×105/孔接种至6孔板,细胞汇合度接近100%时,用200 μL枪头比着直尺在6孔板内画横竖两条直线,宽度保持一致,PBS洗3次,清除漂浮细胞,加入无血清培养基,放入37 ℃、5% CO2温育箱中培养,于40倍倒置显微镜下观察,并分别于0、24 h在同一部位拍照,计算划痕愈合率,划痕愈合率(%)=(0 h划痕面积-24 h划痕面积)/0 h划痕面积×100%,实验重复3次。
1.6. Transwell实验检测细胞垂直迁移及侵袭能力
迁移实验:将各组细胞消化,制成细胞悬液,将含1×105细胞的200 μL悬液加入上室,下室加入750 μL含10% FBS的1640培养基,每组设3个复孔,放入37 ℃、5% CO2温育箱中培养,48 h后取出小室,吸弃上室培养基,用棉签轻拭去上室细胞,4%多聚甲醛固定15 min,结晶紫染色10 min,PBS洗涤3次,于100倍倒置显微镜下随机选取5个视野拍照计数。侵袭实验:预先用无血清1640培养基将50 mg/L的Matrigel 1:4稀释后,包被Transwell小室底部膜,4 ℃风干,水化基底膜。其余步骤同迁移实验。
1.7. 统计学分析
应用SPSS 22.0统计软件进行统计学检验,计量数据采用均数±标准差表示,组间比较用两样本t检验,以P < 0.05认为差异有统计学意义。图片数据的采集及处理用Graphpad+Prism5和Image J软件进行。每次实验重复3次以上。
2. 结果
2.1. MTBP在不同转移能力的前列腺癌细胞中的基础表达
Western blot分析显示,MTBP在局限性前列腺癌细胞22RV1中有较低水平的表达,而在转移性前列腺癌细胞DU145和Lncap中的表达显著高于22RV1细胞(图 1),提示MTBP表达水平与前列腺癌细胞的转移能力呈正相关,尤其在分化程度低,转移侵袭能力强的DU145细胞中表达最高。
1.
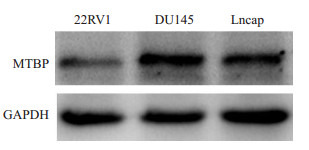
不同前列腺癌细胞中MTBP的表达水平
Protein expression of MTBP in different prostate cancer cells detected by Western blotting
2.2. siRNA干扰及MTBP质粒转染前列腺癌细胞后MTBP蛋白的表达水平
Western blot分析显示,siRNA干扰前列腺癌DU145细胞,能显著降低细胞中MTBP的蛋白表达水平(图 2);而MTBP质粒转染22RV1细胞,能显著提高细胞中MTBP的蛋白表达水平(图 2)。
2.
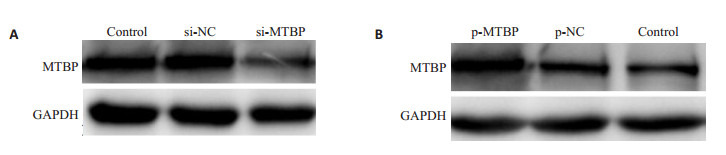
siRNA和MTBP质粒对前列腺癌细胞内MTBP表达的影响
Cellular expression of MTBP in prostate cancer cells after transfection with si-MTBP and p-MTBP. A: Effect of si-MTBP on MTBP expression in DU145 cells; B: Effect of p-MTBP on MTBP expression in 22RV1 cells
2.3. 沉默MTBP对前列腺癌DU145细胞迁移能力的影响
划痕实验结果显示,转染siRNA48 h后,沉默组siMTBP细胞划痕面积愈合百分比与control组及si-NC组相比均明显减少(P < 0.01,图 3)。同样,Transwell迁移实验显示,转染siRNA 48 h后,沉默组si-MTBP细胞从上室穿过小室膜至下室的细胞数较control组及si-NC组也明显减少(P < 0.01,图 3)。
3.
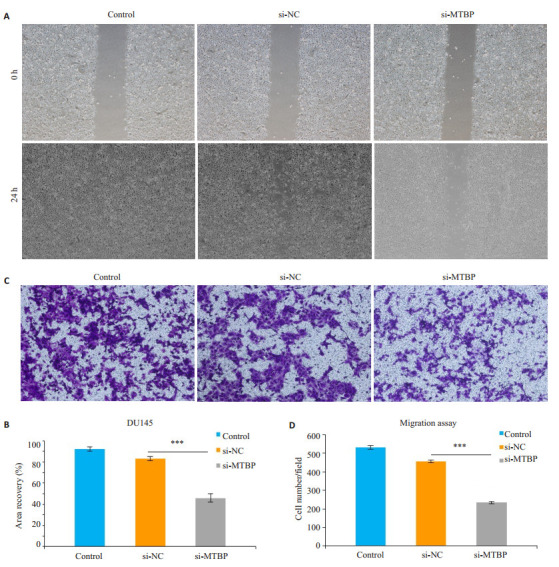
划痕实验和Transwell迁移实验观察沉默MTBP基因对DU145细胞迁移能力的影响
Migration ability of DU145 cells after si-MTBP transfection shown by wound healing assay (Original magnification: ×40) and Transwell assay (×100). A: Wound healing assay; B: Quantitative analysis of wound healing area (***P < 0.01); C: Transwell assay; D: Quantitative analysis of cell migration (***P < 0.01)
2.4. 沉默MTBP对前列腺癌DU145细胞侵袭能力的影响
Transwell侵袭实验显示,转染siRNA 48 h后,沉默组si-MTBP细胞从上室穿过小室膜至下室的细胞数较control组及si-NC组明显减少(P < 0.01,图 4)。
4.
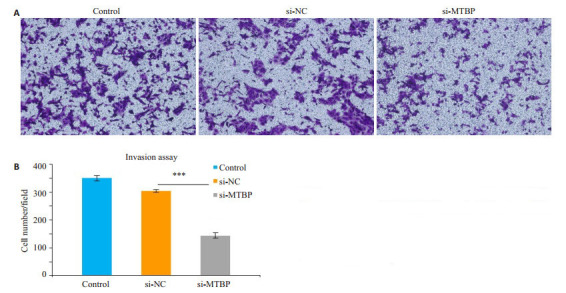
Transwell侵袭实验观察沉默MTBP基因对DU145细胞侵袭能力的影响
Changes in invasion ability of DU145 cells after si-MTBP transfection shown by Matrigel-coated Transwell assay (×100). A: Matrigel-coated Transwell assay; B: Quantitative analysis of cell invasion (***P < 0.01)
2.5. 过表达MTBP对前列腺癌22RV1细胞迁移能力的影响
划痕实验结果显示,转染MTBP质粒48 h后,过表达组p-MTBP组细胞划痕面积愈合百分比与control组及p-NC组相比均明显增加(P < 0.01,图 5)。同样,Transwell迁移实验显示,转染MTBP质粒48 h后,过表达组p-MTBP组细胞从上室穿过小室膜至下室的细胞数较control组及p-NC组也明显增加(P < 0.01,图 5)。
5.
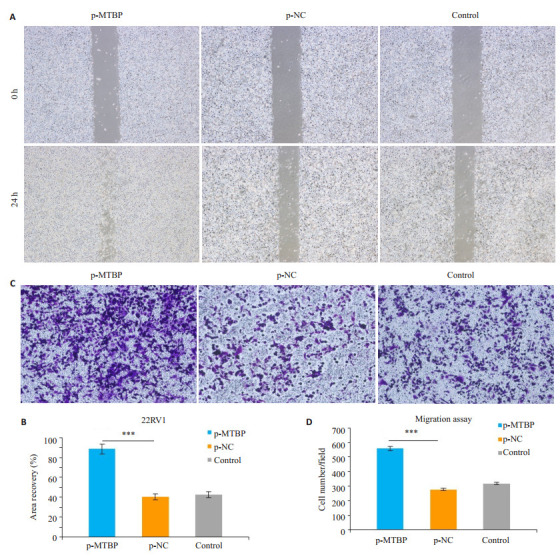
划痕实验和Transwell迁移实验观察过表达MTBP基因对22RV1细胞迁移能力的影响
Migration ability of 22RV1 cells after p-MTBP transfection shown by wound healing assay (×40) and Transwell assay (×100). A: Wound healing assay; B: Quantitative analysis of wound healing area (***P < 0.01); C: Transwell assay; D: Quantitative analysis of cell migration (***P < 0.01)
2.6. 过表达MTBP对前列腺癌22RV1细胞侵袭能力的影响
Transwell侵袭实验显示,转染MTBP质粒48 h后,过表达组p-MTBP组细胞从上室穿过小室膜至下室的细胞数较control组及p-NC组明显增加(P < 0.01,图 6)。
6.
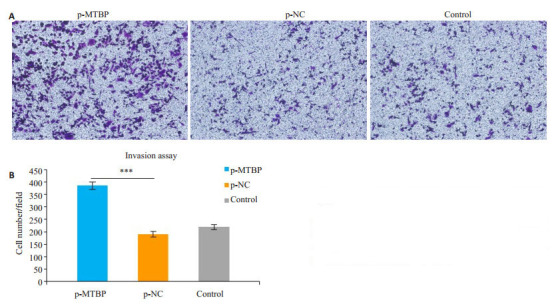
Transwell侵袭实验观察过表达MTBP基因对22RV1细胞侵袭能力的影响
Invasion ability of 22RV1 cells after p-MTBP transfection shown by Matrigel-coated Transwell assay (×100). A: Matrigel-coated Transwell assay; B: Quantitative analysis of cell invasion (***P < 0.01)
2.7. MTBP对E-cadherin蛋白表达水平的影响
Western blot结果显示,抑制MTBP的表达可使前列腺癌DU145细胞的E-cadherin蛋白表达水平升高(图 7),而过表达的前列腺癌22RV1细胞中E-cadherin蛋白表达水平降低(图 7)。
7.
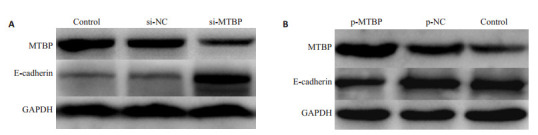
Western blotting实验检测沉默和过表达MTBP基因对前列腺癌细胞上皮间质转化分子标志蛋白E-cadherin蛋白的影响
Expression of E-cadherin detected by Western blotting in prostate cancer cells after si-MTBP and p-MTBP transfection. A: Expression of E- cadherin detected by Western blotting in DU145 cells after si-MTBP transfection; B: Expression of E-cadherin detected by Western blotting in 22RV1 cells after p-MTBP transfection
3. 讨论
众所周知,发生转移的肿瘤比未发生转移的肿瘤更难治疗[19-20]。肿瘤发生转移是前列腺癌进展及治疗失败的重要原因,探索前列腺癌转移及进展的机制是当前研究的热点和难点。目前,已有多项研究显示MTBP在前列腺癌组织中高表达,并与前列腺癌的恶性程度和临床预后不良有着密切联系[10, 18]。本研究检测了MTBP在3种转移能力不同的前列腺癌细胞22RV1,DU145以及Lncap中的表达水平,发现MTBP在转移性前列腺癌细胞DU145中表达最强,Lncap细胞次之,局限性前列腺癌细胞22RV1中则表达最低,提示MTBP的高表达与前列腺癌细胞的转移能力呈正相关。然而,目前并不清楚MTBP在前列腺癌中的表达增高是前列腺癌发生发展的结果,还是增强前列腺癌转移侵袭能力的重要分子生物学机制?这有助于进一步探讨MTBP用于前列腺癌的预后评估,还是将其作为治疗的分子靶点。近年来的多项研究[10-13]显示,MTBP在乳腺癌以及肝细胞癌等多种恶性肿瘤中高表达,是肿瘤转移侵袭的促进因子。因此,我们猜测MTBP的高表达可能是前列腺癌发生转移侵袭的重要分子学机制。为了进一步探究MTBP对前列腺癌细胞转移侵袭能力的影响,我们采用siRNA干扰技术下调DU145细胞MTBP的表达,质粒转染上调22RV1细胞MTBP的表达,通过划痕实验、Transwell迁移实验以及Transwell侵袭实验,发现下调MTBP的表达后,DU145细胞的迁移能力及侵袭能力均明显下降,而上调MTBP的表达可以显著增强22RV1细胞的迁移侵袭能力,表明MTBP可能参与促进前列腺癌细胞的迁移及侵袭。
那么,MTBP到底是如何促进前列腺癌细胞的迁移及侵袭呢?
研究表明,肿瘤细胞发生上皮细胞间充质转化(EMT)对肿瘤转移及进展起着决定性作用[21-26]。在上皮细胞间充质转化的过程中,具有极性的上皮细胞转化为运动能力增强的间质细胞,从而更易于在细胞外基质中自由移动,导致细胞的转移能力增强[27-30]。其中,E-钙黏着蛋白(E-cadherin)的减少或丢失是EMT最重要的标志性变化[31],也是肿瘤细胞获得转移侵袭能力的重要特征[32-33]。Lu等[11]的研究结果表明MTBP可通过促进MDM 2介导的E-Cadherin降解从而增强肝癌细胞的转移侵袭能力。而我们的研究也发现,抑制MTBP的表达可使DU145细胞的E-cadherin蛋白表达水平升高,而MTBP过表达的22RV1细胞中E-cadherin蛋白表达水平降低。由此推测,MTBP可能是通过诱导EMT从而促进前列腺癌细胞的转移侵袭。
综上所述,本研究证实了MTBP的表达水平与前列腺癌细胞的转移侵袭能力相关,以及MTBP对肿瘤细胞生物学行为能力的影响可能是其促进肿瘤转移侵袭的重要病理生理学机制之一,表明针对MTBP靶点的基因治疗可能成为治疗前列腺癌的潜在手段。但本实验的局限之处在于缺少体内试验,以及MTBP介导上皮间质转化促进前列腺癌细胞迁移及侵袭的具体机制仍有待进一步研究。
Biography
肖卓裕,在读硕士研究生,E-mail: 492084014@qq.com
Funding Statement
国家自然科学基金(81772257)
Supported by National Natural Science Foundation of China (81772257)
Contributor Information
肖 卓裕 (Zhuoyu XIAO), Email: 492084014@qq.com.
刘 存东 (Cundong LIU), Email: cundongliu@163.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65(1): 5-29.] [DOI] [PubMed] [Google Scholar]
- 2.Chen MK, Luo Y, Zhang H, et al. Investigation of optimal prostate biopsy schemes for Chinese patients with different clinical characteristics. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=27f445f610fa128f0816261c055c1df8. Urol Int. 2012;89(4):425–32. doi: 10.1159/000341694. [Chen MK, Luo Y, Zhang H, et al. Investigation of optimal prostate biopsy schemes for Chinese patients with different clinical characteristics[J]. Urol Int, 2012, 89(4): 425-32.] [DOI] [PubMed] [Google Scholar]
- 3.Siegel RA. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [Siegel RA. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018, 68(1): 7-30.] [DOI] [PubMed] [Google Scholar]
- 4.Ross RW, Xie WL, Regan MM, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate. Cancer. 2008;112(6):1247–53. doi: 10.1002/(ISSN)1097-0142. [Ross RW, Xie WL, Regan MM, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate[J]. Cancer, 2008, 112(6): 1247-53.] [DOI] [PubMed] [Google Scholar]
- 5.Psutka SP, Frank I, Karnes RJ. Risk stratification in hormonesensitive metastatic prostate cancer: more questions than answers. Eur Urol. 2015;68(2):205–6. doi: 10.1016/j.eururo.2014.10.007. [Psutka SP, Frank I, Karnes RJ. Risk stratification in hormonesensitive metastatic prostate cancer: more questions than answers [J]. Eur Urol, 2015, 68(2): 205-6.] [DOI] [PubMed] [Google Scholar]
- 6.Brady MM. Regulation of p53 and MDM2 activity by MTBP. Mol Cell Biol. 2005;25(2):545–53. doi: 10.1128/MCB.25.2.545-553.2005. [Brady MM. Regulation of p53 and MDM2 activity by MTBP[J]. Mol Cell Biol, 2005, 25(2): 545-53.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd MT, Vlatkovic N, Haines DS. A novel cellular protein (MTBP) binds to MDM2 and induces a G(1) arrest that is suppressed by MDM2. J Biol Chem. 2000;275(41):31883–90. doi: 10.1074/jbc.M004252200. [Boyd MT, Vlatkovic N, Haines DS. A novel cellular protein (MTBP) binds to MDM2 and induces a G(1) arrest that is suppressed by MDM2[J]. J Biol Chem, 2000, 275(41): 31883-90.] [DOI] [PubMed] [Google Scholar]
- 8.Ammer AG, Weed SA. Cortactin branches out: Roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65(9):687–707. doi: 10.1002/cm.v65:9. [Ammer AG, Weed SA. Cortactin branches out: Roles in regulating protrusive actin dynamics[J]. Cell Motil Cytoskeleton, 2008, 65(9): 687-707.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwakuma TG. MDM2, an introduction. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ026765908/ Mol Cancer Res. 2003;1(14):993–1000. [Iwakuma TG. MDM2, an introduction[J]. Mol Cancer Res, 2003, 1 (14): 993-1000.] [PubMed] [Google Scholar]
- 10.Grieb BC, Gramling MW, Arrate MP, et al. Oncogenic protein MTBP interacts with MYC to promote tumorigenesis. Cancer Res. 2014;74(13):3591–602. doi: 10.1158/0008-5472.CAN-13-2149. [Grieb BC, Gramling MW, Arrate MP, et al. Oncogenic protein MTBP interacts with MYC to promote tumorigenesis[J]. Cancer Res, 2014, 74(13): 3591-602.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu S, Zhou W, Wei HY, et al. MTBP promotes the invasion and metastasis of hepatocellular carcinoma by enhancing the MDM2- Mediated degradation of E-Cadherin. Dig Dis Sci. 2015;60(12):3681–90. doi: 10.1007/s10620-015-3824-4. [Lu S, Zhou W, Wei HY, et al. MTBP promotes the invasion and metastasis of hepatocellular carcinoma by enhancing the MDM2- Mediated degradation of E-Cadherin[J]. Dig Dis Sci, 2015, 60(12): 3681-90.] [DOI] [PubMed] [Google Scholar]
- 12.Grieb BC, Chen X, Eischen CM. MTBP is overexpressed in TripleNegative breast cancer and contributes to its growth and survival. Mol Cancer Res. 2014;12(9):1216–24. doi: 10.1158/1541-7786.MCR-14-0069. [Grieb BC, Chen X, Eischen CM. MTBP is overexpressed in TripleNegative breast cancer and contributes to its growth and survival[J]. Mol Cancer Res, 2014, 12(9): 1216-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao Y, Tian M, Pan B, et al. Hyper expression of MTBP May be an adverse signal for the survival of some malignant tumors. Medicine. 2018;97(35):e12021. doi: 10.1097/MD.0000000000012021. [Mao Y, Tian M, Pan B, et al. Hyper expression of MTBP May be an adverse signal for the survival of some malignant tumors[J]. Medicine, 2018, 97(35): e12021.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barretina J, Caponigro G, Stransky N, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. doi: 10.1038/nature11003. [Barretina J, Caponigro G, Stransky N, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity[J]. Nature, 2012, 483(7391): 603-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.李 根英, 张 云艳. MTBP与癌症的研究进展. http://d.old.wanfangdata.com.cn/Periodical/syzlxzz201606023. 实用肿瘤学杂志. 2016;30(6):560–2. [李根英, 张云艳. MTBP与癌症的研究进展[J].实用肿瘤学杂志, 2016, 30(6): 560-2.] [Google Scholar]
- 16.Venkatesan T, Alaseem A, Chinnaiyan AA, et al. MDM2 overexpression modulates the angiogenesis-related gene expression profile of prostate cancer cells. Cells. 2018;7(5):41. doi: 10.3390/cells7050041. [Venkatesan T, Alaseem A, Chinnaiyan AA, et al. MDM2 overexpression modulates the angiogenesis-related gene expression profile of prostate cancer cells[J]. Cells, 2018, 7(5): 41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Chen Z, Jin JJ, et al. MDM2 binding protein as a predictor of metastasis and a novel prognostic biomarker in patients with gastric cancer. https://www.spandidos-publications.com/10.3892/ol.2017.7031. Oncol Lett. 2017;14(6):6409–16. doi: 10.3892/ol.2017.7031. [Wang W, Chen Z, Jin JJ, et al. MDM2 binding protein as a predictor of metastasis and a novel prognostic biomarker in patients with gastric cancer[J]. Oncol Lett, 2017, 14(6): 6409-16.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Pan XL, Wu CJ, et al. An in vivo screen identifies PYGO2 as a driver for metastatic prostate cancer. Cancer Res. 2018;78(14):3823–33. doi: 10.1158/0008-5472.CAN-17-3564. [Lu X, Pan XL, Wu CJ, et al. An in vivo screen identifies PYGO2 as a driver for metastatic prostate cancer[J]. Cancer Res, 2018, 78(14): 3823-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [Gupta GP, Massagué J. Cancer metastasis: building a framework[J]. Cell, 2006, 127(4): 679-95.] [DOI] [PubMed] [Google Scholar]
- 20.Pandya P, Orgaz JL, Sanz-Moreno V. Modes of invasion during tumour dissemination. Mol Oncol. 2017;11(1):5–27. doi: 10.1002/1878-0261.12019. [Pandya P, Orgaz JL, Sanz-Moreno V. Modes of invasion during tumour dissemination[J]. Mol Oncol, 2017, 11(1): 5-27.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christofori G. New signals from the invasive front. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a80329596886800cb6579b79884ae78e. Nature. 2006;441(792):444–50. doi: 10.1038/nature04872. [Christofori G. New signals from the invasive front[J]. Nature, 2006, 441(792): 444-50.] [DOI] [PubMed] [Google Scholar]
- 22.Huber MA, Kraut N, Beuq H. Molecular requirements for epithelialmesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58. doi: 10.1016/j.ceb.2005.08.001. [Huber MA, Kraut N, Beuq H. Molecular requirements for epithelialmesenchymal transition during tumor progression[J]. Curr Opin Cell Biol, 2005, 17(5): 548-58.] [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–81. doi: 10.1083/jcb.200601018. [Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease[J]. J Cell Biol, 2006, 172(7): 973-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [Thiery JP. Epithelial-mesenchymal transitions in tumour progression [J]. Nat Rev Cancer, 2002, 2(6): 442-54.] [DOI] [PubMed] [Google Scholar]
- 25.Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007;101(4):816–29. doi: 10.1002/(ISSN)1097-4644. [Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment[J]. J Cell Biochem, 2007, 101(4): 816-29.] [DOI] [PubMed] [Google Scholar]
- 26.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis[J]. Cancer Res, 2006, 66(17): 8319-26.] [DOI] [PubMed] [Google Scholar]
- 27.Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5(2):17. doi: 10.3390/jcm5020017. [Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance[J]. J Clin Med, 2016, 5(2): 17.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelialmesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15(1):18. doi: 10.1186/s12943-016-0502-x. [Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelialmesenchymal transition through epigenetic and post-translational modifications[J]. Mol Cancer, 2016, 15(1): 18.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett. 2013;341(1):9–15. doi: 10.1016/j.canlet.2013.02.037. [Nakaya Y, Sheng G. EMT in developmental morphogenesis[J]. Cancer Lett, 2013, 341(1): 9-15.] [DOI] [PubMed] [Google Scholar]
- 30.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. [Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits[J]. Nat Rev Cancer, 2009, 9(4): 265-73.] [DOI] [PubMed] [Google Scholar]
- 31.张 可华, 宋 建国. EMT与肿瘤. 生命的化学. 2008;28(5):523–6. doi: 10.3969/j.issn.1000-1336.2008.05.001. [张可华, 宋建国. EMT与肿瘤[J].生命的化学, 2008, 28(5): 523-6.] [DOI] [Google Scholar]
- 32.Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–76. doi: 10.1158/1078-0432.CCR-05-2722. [Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition[J]. Clin Cancer Res, 2006, 12 (18): 5369-76.] [DOI] [PubMed] [Google Scholar]
- 33.Alonso SR, Tracey L, Ortiz PA, et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007;67(7):3450–60. doi: 10.1158/0008-5472.CAN-06-3481. [Alonso SR, Tracey L, Ortiz PA, et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis[J]. Cancer Res, 2007, 67(7): 3450-60.] [DOI] [PubMed] [Google Scholar]


