Abstract
目的
筛选可用来指示宫颈癌从癌前病变到癌变的血浆蛋白标志物,并分析其潜在机制与功能。
方法
收集健康人(Control组),低度鳞状上皮内病变(LSIL)、高度鳞状上皮内病变(HSIL)、宫颈癌(CC)和治疗后患者血浆样本富集低丰度蛋白。LC-MS/MS检测样本的全多肽类型与序列,检测结果用Proteome Discoverer 2.2进行数据库检索,筛选差异蛋白类型与种类(要求Coverage≥20%,且Unique Peptides≥2蛋白质),LSIL、HSIL、CC组分别与Control组进行T检验比较,P≤0.05视为表达有显著差异的蛋白。通过观察差异蛋白在各组中的色谱图筛选出能提示宫颈病变及宫颈癌的潜在标志物及预后效能评估标记物,并通过靶向蛋白组学技术,在上述实验的基础上样本量对潜在蛋白标记物在各组的含量进行验证实验。进一步通过蛋白功能、功能富集及共表达分析明确差异蛋白的功能探讨其作为标志物的意义与病理机制。
结果
基于LC-MS/MS和蛋白组学分析发现LSIL、HSIL组相较于Control组分别存在9个异常表达蛋白,CC组相较于Control组存在5个差异蛋白。其中ORM2、HPR在LSIL组相对于Control组的表达存在一定差异,可能是指示宫颈癌癌前病变的潜在标志物;F9在LSIL、HSIL、CC组中相对于Control组,随着疾病的发展呈上升趋势,是一种潜在的指示宫颈病变程度的蛋白标记物;CFI、AFM蛋白在预后组和CC组差异明显,是一类潜在的评价疗效和预后的蛋白指标。各组中的差异蛋白在功能富集分析中发现与补体和凝血级联反应通路有关。
结论
F9、CFI、AFM、HPR、ORM2五种新型蛋白可能是指示宫颈病变程度、疗效和预后的潜在标志物,联合分子标志物来评估宫颈病变的发生发展可以增强诊断的灵敏度和特异性,具有一定的临床应用前景。
Keywords: 宫颈癌, LC-MS/MS, 蛋白组学, 血浆标志物
Abstract
Objective
To screen potential plasma protein biomarkers for the progression of cervical precancerous lesions into cervical carcinoma and analyze their functions.
Methods
Plasma samples obtained from healthy control subjects, patients with low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), cervical cancer (CC), and patients with CC after treatment were enriched for low-abundance proteins for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The MS data of the samples were analyzed using Discoverer 2.2 software, and the differential proteins (peptide coverage ≥20%, unique peptides≥2) were screened by comparison of LSIL, HSIL and CC groups against the control group followed by verification using target proteomics technology. Protein function enrichment and coexpression analyses were carried out to explore the role of the differentially expressed proteins as potential biomarkers and their pathological mechanisms.
Results
Compared with the control group, both LSIL group and HSIL group showed 9 differential proteins; 5 differentially expressed proteins were identified in CC group. The proteins ORM2 and HPR showed obvious differential expressions in LSIL and HSIL groups compared with the control group, and could serve as potential biomarkers for the progression of cervical carcinoma. The expression of F9 increased consistently with the lesion progression from LSIL to HSIL and CC, suggesting its value as a potential biomarker for the progression of cervical cancer. CFI and AFM protein levels were obviously decreased in treated patients with CC compared with the patients before treatment, indicating their predictive value for the therapeutic efficacy. Protein function enrichment analysis showed that all these differentially expressed proteins were associated with the complement system and the coagulation cascades pathway.
Conclusion
We identified 5 new protein biomarkers (F9, CFI, AFM, HPR, and ORM2) for cervical precancerous lesions and for prognostic evaluation of CC, and combined detection of these biomarkers may help in the evaluation of the development and progression of CC and also in improving the diagnostic sensitivity and specificity of cervical lesions.
Keywords: cervical carcinoma, liquid chromatographytandem mass spectrometry, proteomics, serum biomarkers
据世界卫生组织/国际癌症研究署数据显示,宫颈癌已经成为女性第四大恶性肿瘤,是发展中国家女性癌症死亡的主要原因之一[1]。2015年我国宫颈癌新发病例数达到9.89万,死亡病例数高达3.05万,发病率与死亡率总体呈快速上升趋势[2]。阴道镜检查、子宫颈活检是目前临床宫颈癌的主要诊断方法,对医务人员的主观性判断依赖性很强,存在诊断灵敏度与特异性不佳的问题[3-4],因此寻找准确、客观的诊断指标对宫颈病变和宫颈癌的发生发展状态进行预判,是非常有临床意义的研究方向[5]。近年来,对各种新型的宫颈癌早期筛选、诊断方法的探究热度不减,但临床应用效果一般。如,P16/ Ki67双染色检测CIN2、CIN3的特异性只有58.9%和56.9%[6];光电探测系统检测分别为74.4%、78.8%[7]。虽然MRI对宫颈癌分期诊断准确率为86%,对于发现宫旁侵犯方面明显优于临床,但对IA期宫颈癌的诊断价值有限[8-9]。因此,宫颈癌前病变的诊断灵敏度和特异性不高,是临床假阳性率高、漏检和误诊的根本原因。
相较于国内常用的阴道镜检查、子宫颈活检等诊断方法,通过分子标志物的鉴定建立非侵入式诊断方法在临床上具有重要意义。近年来,蛋白组学为肿瘤发生发展过程中的机制与诊断标志物研究开辟了新路径[10-11],即可直接检测到蛋白表达、鉴定到疾病发展相关的蛋白变化[12],又可基于潜在标志物为疾病的分子机制提供新视角[13]。一项来自马来西亚的研究,通过蛋白组学及生信分析明确了一系列蛋白可以诊断、区分CINⅢ(3级宫颈上皮内瘤样病变)、SCCⅠ和SCCⅡ(早期鳞状癌)、SCCⅢ和SCCⅣ(晚期鳞状癌)患者的血浆样本,具有不同阶段的诊断意义[10]。另一组来自荷兰的研究结合iTRAQ与靶向质谱技术定量分析CIN、早期和晚期宫颈癌患者和健康人群血浆蛋白,筛选发现6个差异表达蛋白在区分CIN和健康组显示了67%的灵敏度和88%的特异性[11]。
总之,现有少数的宫颈癌蛋白标志物探究报道中,基于蛋白组学的生信分析起到了重要的作用,也得到了一系列可喜的研究成果[10-14]。然而,这些研究基本集中在组织类样本的研究,这对最终的临床应用带来了一定的挑战。此外,我国现阶段宫颈癌蛋白组学的研究较少且也集中在组织样本[12-14],对宫颈癌血浆蛋白组学研究已有报道[15],但宫颈癌前病变潜在诊断蛋白组学研究目前未见报道。本研究拟采用LC-MS/MS技术检测中国南方广东地区人群中低度鳞状上皮内病变(LSIL)、高度鳞状上皮内病变(HSIL)、宫颈癌(CC)患者的血浆蛋白谱,筛选差异蛋白来寻找不同宫颈病变时期潜在的血浆蛋白诊断标志物,为宫颈癌前病变的筛选、诊断及宫颈癌治疗后预后评估,提高其检测的灵敏度和特异性提供一定的临床指导意义。
1. 资料和方法
1.1. 研究资料
收集2015年5月~2016年4月在广东省中医院妇科就诊的患者血浆标本76例,均排除妊娠期和月经期,根据TCT结果、阴道镜结果和病理活检结果将收集的样本分为低度鳞状上皮内病变组、高度鳞状上皮内病变组、宫颈癌组、宫颈癌治疗后组。另选取同期健康体检人群血浆样本作为健康对照组,该组纳入标准为:白带清洁度Ⅱ度以下,HPV阴性,TCT结果阴性,支原体、衣原体、淋球菌等均检测阴性。本研究已通过广东省中医院伦理委员会审查(批件号B2016-019-01)。抗凝管收集受试者血液样本,离心收集血浆于-20 ℃保存用于LC-MS/MS检测。
1.2. 血浆蛋白预处理
使用Thermo Fisher的血清高丰度蛋白去除试剂盒(PierceTM Top 12 Abundant Protein Depletion Spin Columns, 85165, Thermo fisher, US)去除血浆样本12种常见的高丰度蛋白。取200 μL血浆到1.5 mL离心管,1000 r/min离心10 min,吸10 μL上清到试剂盒柱子内,颠倒60 min摇匀,离心收集液体。使用Thermo Fisher蛋白质浓度检测试剂盒(PierceTM BCA Protein Assay Kit, 23227, Thermo fisher, US)进行蛋白质定量。除高丰度蛋白的样品直接取20 μL,未除高丰度蛋白的样品取2 μL加18 μL PBS缓冲液于96孔板孔中。将标准品分别按5、10、15、20、25、30、35 μg加入96孔板孔中。加190 μL的BCA试剂,混匀,37 ℃反应30 min。上机测定浓度,设定波长为562 nm。使用DTT和IAA对蛋白进行还原和封闭。使用胰蛋白酶(Trypsin, TPCK Treated, A003740, sangon, CHN)酶解蛋白质。更换新的收集管,在超滤管中加入胰蛋白酶,总量2~4 μL(与蛋白质量比1:50),加50 μL TEAB,37 ℃反应过夜(注意用封口膜封口,不能让样本蒸发)。次日瞬时离心一下,让管壁的液体甩到底部。加入按照说明书稀释后的Trypsin TPCK Treated(胰蛋白酶与蛋白质量比1:100),37 ℃反应4 h,反应后12 000 r/min离心20 min,酶解消化后的肽段溶液离心于收集管底部。在超滤管中加入50 μL 0.5 mol/L TEAB,12 000 r/min再次离心20 min,与上步合并,收集管底部共得到100 μL酶解后的样品。
1.3. 相液质联用LC-MS/MS检测蛋白谱
肽段用样品溶解液(0.1%甲酸、5%乙腈)溶解,充分振荡涡旋,13 500 r/min,4 ℃离心20 min,上清转移到上样管中,质谱仪Thermo Scientific Q Exactive进行Label-free quantification检测。Q Exactive方法设置,use lock masses: best, chrom. Peak width(FWHM): 30 s,Method duration: 90.00 min;实验设置,Runtime: 8 to 90 min, Polarity: Positive, In-source CID: 0.0 eV, Default change state: 2, Microscans: 1, Resolution: 70 000, AGC target: 3e6, Maximum IT: 60 ms, Number of scan ranges: 1, Scan range: 300 to 2000 m/z, Spectrum data type: Profile, Microscans: 1, Resolution: 17 500, AGC target: 1e6, Maximum IT: 50 ms, Loop count: 20, MSX count: 1, TopN: 20, Isolation window: 2.2 m/z, Isolation offset: 0.0 m/z, Scan range: 200 to 2000 m/z, Fixed first mass: 100.0 m/z, NCE/stepped NCE: 27, Underfill ratio: 1%, Intensity threshold: 2.0e5, Charge exclusion: 1, 7, 8, > 8, Peptide match: Preferred, Exclude isotopes: on, Dynamic exclusion: 30 s。流动相A:0.1%甲酸,2%乙腈水溶液;流动相B:0.1%甲酸,98%乙腈水溶液;流速:600 nL/min,每个组分分析时间:90 min。
1.4. 蛋白鉴定与筛选
采用Proteome Discoverer 2.2进行数据库检索,软件参数设定消化酶为Trypsin,鉴定的原始结果设置ms准确度10 ppm,MS / MS准确度0.02 Da,胰蛋白酶消化,允许两次错过切割,半胱氨酸的氨基甲酰基甲基固定修饰和氧化甲硫氨酸的可变修饰。筛选Coverage≥20%,且Unique Peptides≥2的蛋白质进行后续分析。Label-free quantification中质谱峰的面积代表了多肽量,通过比较不同样本同一肽段质谱峰面积可直接得到该肽段所代表蛋白的相对定量信息。LSIL、HSIL、CC组分别与Control组做T检验分析,P值≤0.05视为有显著差异蛋白,相对表达量 > 1视为上调,相对表达量 < 1视为下调,通过等级聚类分析各组样本的聚类情况及差异蛋白在各组的表达趋势。最终通过观察差异蛋白对应的多肽在各组中色谱图的表达量筛选出能预示宫颈癌前病变的潜在标志物,对应的多肽信息即视为该蛋白的唯一肽段。
1.5. 数据分析
WebGestaltR(<a href="http://www.webgestalt.org/option.php" target="_blank">http://www.webgestalt.org/option.php</a>)对差异蛋白进行GO功能和代谢通路富集分析,对蛋白参与的分子功能、生物学过程和细胞定位,及其参与的代谢网络及在代谢通路中的位置进行描述。采用string 10.5(<a href="https://string-db.org/cgi/input.pl" target="_blank">https://string-db.org/cgi/input.pl</a>)数据库进行差异显著蛋白共表达分析,明确蛋白间功能的相关性。验证差异蛋白分析,通过分组分析明确潜在蛋白标志物,利用靶向蛋白检测分析手段,重新验证测试样本(无额外样本)中的潜在标志物表达情况。
1.6. 统计学分析
数据采用SPSS20.0软件分析,正态分布的计量资料结果以均数±标准差表示,两组间比较采用t检验,两组以上的比较采用单因素方差分析,P < 0.05为差异有统计学意义。
2. 结果
2.1. 实验室血清学诊断信息
对纳入研究的受试者年龄、转氨酶(ALT)、转氨酶AST(U/L)、总蛋白(TP)、白蛋白(ALB)、尿素(Urea)、肌酐(Crea)、甲胎蛋白(AFP)、癌胚抗原(CEA)、CA125、CA153、CA199、SCC-Ag等实验室血清学信息进行了统计(表 1)。临床用于宫颈癌筛查的血清学标记物AFP、CEA、CA125、CA153、CA199和SCC-Ag并不能对宫颈病变和宫颈癌发生发展的各个阶段进行明确的区分和预示。
1.
全部受试患者实验室血清学诊断信息
Serological diagnostic data of all the samples
| Characteristics | Control | LSIL | HSIL | CC | Treatment |
| Compared with the control group, *P < 0.05, **P < 0.01. | |||||
| Age (year) | 51.25±9.32 | 44.5±3.54 | 46±4.24 | 47±5.66 | 57.5±0.71 |
| ALT (U/L) | 13.25±5.38 | 30±7.07* | 13.5±2.12 | 9±3.75* | 11±2.65 |
| AST (U/L) | 14.75±3.3 | 19.5±0.71 | 14.5±2.12 | 14±1.41 | 19.5±9.19 |
| TP(g/L) | 66.18±6.12 | 64.2±1.70 | 72.85±6.01 | 69.5±4.38 | 60.55±4.46 |
| ALB (g/L) | 40.88±2.26 | 41.7±0.85 | 46.2±2.24 | 44.45±2.48 | 37.95±3.6 |
| Urea (mmol/L) | 5.06±1.28 | 4.11±1.71 | 3.82±0.40 | 5.01±0.86 | 4.95±2.19 |
| Crea (μmol/L) | 57±11.58 | 50±7.07 | 57.5±9.19 | 53.5±10.61 | 70±1.41* |
| AFP (ng/mL) | 2.16±0.66 | 1.92±1.29 | 3.1±1.54 | 1.75±0.72 | 2.945±1.21 |
| CEA (ng/mL) | 1.41±1.01 | 1.685±1.41 | 1.47±1.06 | 2.12±1.21 | 3.18±3.11* |
| CA125 (U/mL) | 8.8±5.87 | 12.47±1.73 | 14.99±8.68* | 44.85±34.34** | 22.58±17.16* |
| CA153 (U/mL) | 9.14±5.39 | 14.2±4.8* | 10.07±2.46 | 9.77±4.41 | 9.58±0.18 |
| CA199 (U/mL) | 9.13±5.73 | 15.76±14.38* | 1.2±1.02** | 10.47±4.51 | 11.69±0.48 |
| SCC-Ag (ng/mL) | - | 0.5±0.05 | 0.5±0.02 | 3±2.11* | 0.8±0.42 |
2.2. 差异性蛋白鉴定与筛选
通过LC-MS/MS总共鉴定出2728个多肽,包含369个蛋白质,其中满足Coverage≥20%,且Unique Peptides≥2的蛋白质有172个。LSIL组相较于control组存在异常表达的蛋白有9个,HPR、ORM2、C4BPB、C8A、C8B、GPLD1、RBP4、C1RL等8个表达上调,C5表达下调。HSIL组相较于control组存在9个差异蛋白,其中表达上调的有8个,分别是ORM2、C4BPB、ORM1、APOD、LCAT、C1QB、RBP4、C1RL,表达下调的是CPN2。CC组相较于control组存在5个差异蛋白,其中F9、CFI、C8A、AFM表达上调,C1QC表达下调(表 2)。
2.
差异蛋白在各组的相对表达量
Differentially expressed proteins in LSIL, HSIL and CC groups compared with the control group
| Gene symbol | Protein | LSIL/control | HSIL/control | CC/control | |||||
| Average ratio | P | Average ratio | P | Average ratio | P | ||||
| HPR | Haptoglobin-related protein | 2.13 | 0.02 | 1.92 | 0.07 | 0.98 | 0.96 | ||
| ORM2 | Alpha-1-acid glycoprotein 2 | 1.72 | 0.03 | 2.65 | 0.001 | 1.68 | 0.17 | ||
| C4BPB | C4b-binding protein beta chain | 1.69 | 0.02 | 2.10 | 0.01 | 1.58 | 0.16 | ||
| C8A | Complement component C8 alpha chain | 1.44 | 0.03 | 1.26 | 0.05 | 1.30 | 0.02 | ||
| C8B | cDNA FLJ77947, highly similar to Human complement protein C8 beta subunit mRNA | 1.37 | 0.04 | 1.29 | 0.17 | 1.05 | 0.73 | ||
| GPLD1 | Phosphatidylinositol-glycan-specific phospholipase D | 1.27 | 0.01 | 1.01 | 0.87 | 0.82 | 0.06 | ||
| RBP4 | Retinol-binding protein 4 | 1.27 | 0.03 | 1.17 | 0.02 | 0.98 | 0.81 | ||
| C1RL | Complement C1r subcomponent-like protein | 1.20 | 0.01 | 1.14 | 0.03 | 1.41 | 0.16 | ||
| C5 | Complement C5 | 1.14 | 0.05 | 1.12 | 1.13 | 1.07 | 0.28 | ||
| ORM1 | Alpha-1-acid glycoprotein | 1.81 | 0.20 | 2.09 | 0.01 | 1.15 | 0.63 | ||
| APOD | Apolipoprotein D (Fragment) | 1.09 | 0.73 | 1.85 | 0.05 | 0.91 | 0.66 | ||
| LCAT | Testicular secretory protein Li 24 | 1.25 | 0.12 | 1.40 | 0.02 | 0.91 | 0.67 | ||
| C1QB | Complement component 1, q subcomponent, B chain, isoform CRA_a | 1.10 | 0.25 | 1.22 | 0.04 | 0.80 | 0.06 | ||
| CPN2 | Carboxypeptidase N subunit 2 | 0.91 | 0.56 | 0.76 | 0.04 | 0.86 | 0.08 | ||
| F9 | Coagulation factor Ⅸ | 0.87 | 0.68 | 1.44 | 0.42 | 2.32 | 0.02 | ||
| CFI | Heavy chain of factor I (Fragment) | 0.95 | 0.56 | 1.04 | 0.39 | 1.34 | 0.01 | ||
| AFM | Afamin | 0.96 | 0.64 | 0.95 | 0.50 | 1.28 | 0.02 | ||
| C1QC | Complement C1q subcomponent subunit C | 1.00 | 0.96 | 1.00 | 0.95 | 0.81 | 0.03 | ||
统计上述差异蛋白在各组中的相对表达量发现,HPR在LSIL与HSIL两组间的表达无明显差异,相较于control组表达上调2倍左右,当疾病进展到CC开始下调。ORM2、C4BPB、ORM1、APOD、LCAT的表达具有相同的趋势,都随着疾病的发展从LSIL到HSIL不断上调,疾病进展到CC开始下调。CFI、AFM在LSIL、HSIL组中的表达相较于control组无明显变化,但在CC组中表达开始上调。F9在LSIL、HSIL、CC组中相对于Control组,随着疾病的发展呈不断上升趋势(表 2)。等级聚类分析更形象的展示了上述9个差异调节蛋白在各组中的表达情况(图 1)。通过观察9个差异蛋白峰图发现F9、CFI、AFM、HPR、ORM2蛋白在control、LSIL、HSIL、CC组中表达存在一定差异,可能作为宫颈病变和宫颈癌的潜在标志物(图 2,表 3)。
1.
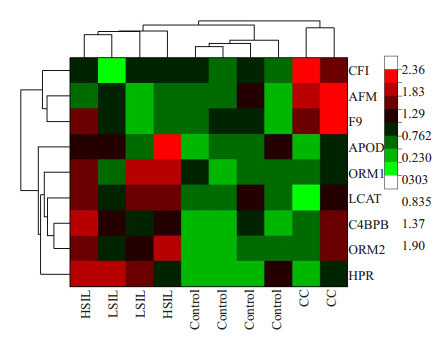
全部检测样本异常表达蛋白的等级聚类分析
Clustering analysis of all the differentially expressed proteins. The color depth in the diagram indicates the abundance of protein expression. A lighter green color indicates a lower protein expression level, and a darker red color indicates a higher protein expression level
2.
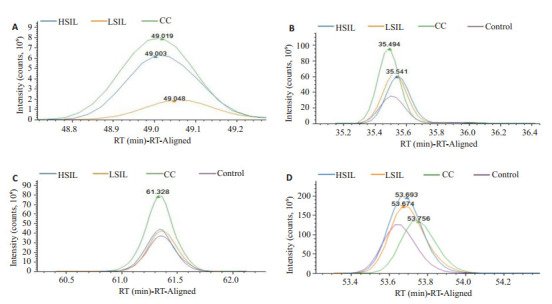
差异性蛋白F9、CFI、AFM、ORM2蛋白唯一性多肽在各组中的表达含量。F9在control组未检测出,HPR蛋白的唯一性多肽并未在各组中有明显的表达趋势
Differential expression of F9 (A), CFI (B), AFM (C) and ORM2 (D) proteins by unique polypeptides in each group. F9 was not detected in the control group, and the HPR protein had no unique polypeptide series
3.
差异蛋白相关组成肽段信息
Peptide information of the differentially expressed proteins
| Accession | Gene symbol | Sequence | MW(kDa) | calc. pI | Positions in protein | RT (min) | XCorr |
| F2RM37 | F9 | VDAFCGGSIVNEK | 51.7 | 5.47 | 248-260 | 48.4192 | 3.35 |
| Q6LAM1 | CFI | KVTYTSQEDLVEK | 35.9 | 7.59 | 1-13 | 35.2658 | 5.84 |
| P43652 | AFM | RHPDLSIPELLR | 69 | 5.9 | 361-372 | 60.4513 | 4.55 |
| P00739 | HPR | DIAPTLTLYVGK | 39 | 7.09 | 158-169 | 67.658 | 4.39 |
| P19652 | ORM2 | TEDTIFLR | 23.6 | 5.11 | 74-81 | 53.6119 | 3.13 |
2.3. 宫颈癌治疗后差异蛋白表达分析
LC-MS/MS鉴定结果发现宫颈癌患者经治疗后,F9的蛋白表达量无明显下降;CFI、AFM则显著下调;而HRP、ORM2蛋白在治疗后显著上调(图 3)。
3.
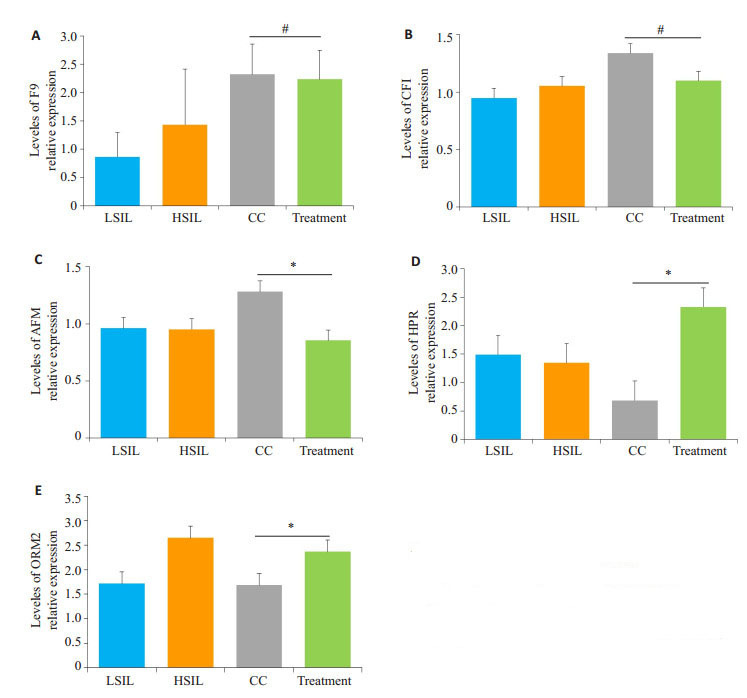
差异蛋白F9、CFI、AFM、HPR、ORM2在LSIL、HSIL、CC、Treatment组与Control组间的平均相对表达水平
Relative expressions of F9 (A), CFI (B), AFM (C), HPR (D) and ORM2 (E) in LSIL, HSIL, CC and treated CC groups against the control group(#P > 0.05, *P < 0.05)
2.4. 筛选的标记物在各组样本中的表达验证分析
根据临床数据及LC-MS/MS鉴定结果与分析,发现HRP、ORM2蛋白表达在宫颈病变组相较于对照组,出现显著上调;F9蛋白的表达在宫颈病变和宫颈癌过程中,随着疾病的发展显示出正相关的趋势。CFI、AFM蛋白表达随着病变程度也有轻微上升的趋势,且在宫颈癌治后能呈现下降趋势,尤其是AFM蛋白,可用于宫颈癌疗效和预后评估指标。从入选的76例样本中,各组随机选取10例样本,通过LC-MS/MS靶向蛋白组学技术对以上5种蛋白进行了验证(图 4)。
4.
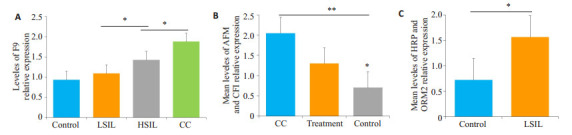
5种潜在血清蛋白标志物对宫颈病变发生发展恶性程度(A)、治疗及预后评估(B)及宫颈癌前病变(C)的靶向蛋白验证结果
Verification experiment results of serum protein markers for cervical precancerous lesion, cervical lesion development and therapy efficacy by target LC-MS/MS (n=10 in each group). A: Relative expression of F9 in control, LSIL, HSIL and CC groups; B: Mean levels of AFM and CFI in Control, CC and treated CC groups; C: Mean levels of HRP and ORM2 expression in control and LSIL groups. *P < 0.05, **P < 0.05
2.5. 蛋白功能分析
为了进一步了解以上差异表达蛋白的分子功能、参与的细胞组成和生物学过程等,我们进行了GO分析。结果显示,在生物过程分类中,大部分蛋白预测与生物调节(16/18)、应激反应(14/8)、代谢过程(13/18)相关。在细胞组成分类中,全部蛋白位于胞外空间(18/18),大部分存在于囊泡(17/18)、大分子复合物(9/18)。在分子功能分类中与蛋白结合(10/18)、水解酶活性(6/18)有关(图 5)。
5.
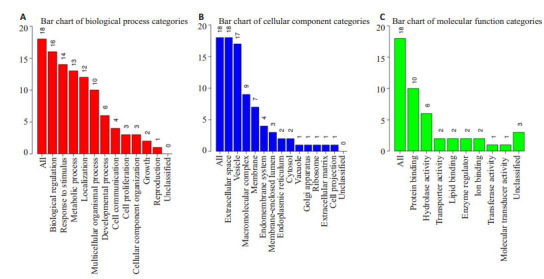
差异蛋白功能分类,差异蛋白注释到相关的分子功能、细胞组成和生物学过程
Functional classification, differential protein annotation to related molecular functions, cell composition and biological processes of the differentially expressed proteins. A: Differential protein annotation to related biological processes; B: Differential protein annotation to related cellular components; C: Differential protein annotation to related molecular functions
2.6. 蛋白共表达及功能富集分析
通过共表达分析预测蛋白功能的相关性。观察到F9与AFM、C4BPB、ORM1、CFI、ORM2、HPR蛋白表达之间的功能存在相关性(图 6)。进一步将差异蛋白注释到KEGG通路中,明显的富集通路为补体和凝血级联反应(hsa04610, P Value=6.06e-03, FDR=1.82e-02)(图 7),8个差异蛋白F9、CFI、C1QB、C1QC、C4BPB、C5、C8A、C8B参与到该通路。
6.
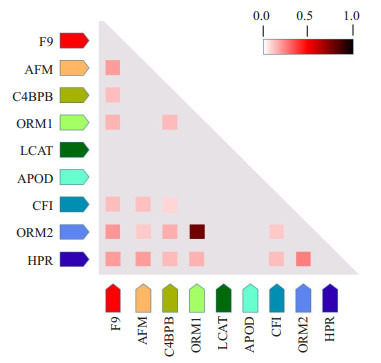
蛋白共表达分析。颜色的深浅表示两个蛋白间功能相关的可信度水平
Coexpression analysis of the differentially expressed proteins. The depth of the color indicates the level of trust related to the function between the two proteins
7.
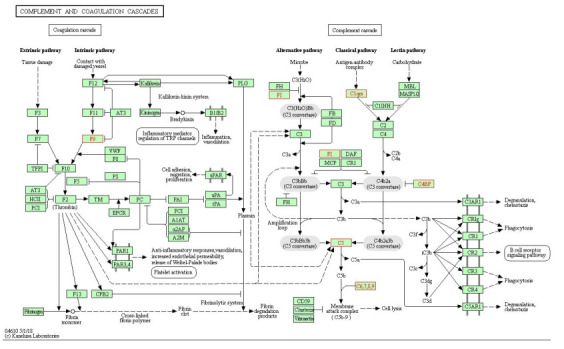
补体和凝血级联反应通路(hsa04610),有8个差异蛋白注释到该通路上
Pathway of complement and coagulation cascade reaction. Eight differentially expressed proteins were annotated to this pathway
3. 讨论
癌症的发展伴随着复杂的宿主系统性反应,通常反映在血浆蛋白质组中,因此血浆蛋白包含了大量癌症信息[17-18]。利用蛋白质组学技术筛查和监测血浆蛋白存在或丰度变化,对早期发现、诊断,监测复发或转移,指导治疗和判断宫颈癌预后具有重要意义[19-22]。本研究选取LSIL、HSIL、CC及治疗后的不同疾病阶段的宫颈病变患者进行血浆蛋白组学研究。在LSIL、HSIL中分别鉴定到9种差异表达蛋白,在CC中鉴定到5种差异蛋白,通过观察差异蛋白色谱图筛选出5个在Control、LSIL、HSIL、CC组中存在一定差异的蛋白,HPR、ORM2、F9、CFI、AFM可能是指示宫颈疾病进程的潜在标志物。GO分析上述差异蛋白的生物学功能表明大部分与生物调节、应激反应、代谢过程、蛋白结合、水解酶活性有关,并且参与到补体和凝血级联反应代谢通路。
本研究筛选出的触珠相关蛋白(HPR)是一种灵长类动物特异性蛋白,通过触珠蛋白(HPT)基因复制进化而来,是一种介导无细胞血红蛋白清除的急性期可溶性蛋白[23]。人ORM蛋白,也称为α-1-酸性糖蛋白,是一种急性期蛋白,属于免疫球蛋白亚家族,一组具有抗炎和免疫调节功能的小分子结合蛋白[24]。ORM2由ORM基因编码,是一种常见的血浆蛋白,占血浆总蛋白的1%~ 3%,主要在干细胞和脂肪细胞周围合成和分泌[25]。通常在感染、炎症、肿瘤、组织损伤和败血症这些病理条件下显著上升[26]。ORM2的抗炎和免疫调节功能主要在于它的抗嗜中性粒细胞和抗补体活性,同时也可以抑制细胞因子的分泌来保护机体健康[27]。ORM2在正常状态下维持较低的水平,但在急性期反应中,在炎症细胞因子和转录因子如C/EBPβ的诱导下,可促进ORM2的表达[28]。我们在研究中发现ORM2在LSIL、HSIL的表达量相对于Control组分别是1.72、2.65倍,HPR则分别是2.13、1.92倍,HPR、ORM2在LSIL、HSIL表达中上调可能与相关炎症反应有关,提示LSIL、HSIL的患者处于一种炎症状态。研究同样发现HPT在CIN、早期宫颈癌和晚期宫颈癌患者血清中存在差异,尤其是早期和晚期的宫颈癌,其表达量分别是健康对照组的3.24、4.2倍[11]。HPT具有抗炎和抗氧化的特性,在肝脏中合成的同时也可以在白细胞介素-6的刺激下由子宫内膜细胞合成[29]。此外,该研究同样发现其他5个急性期蛋白A1AT、A1AG1、VTDB、TRFE、FETUA在CIN、早期宫颈癌和晚期宫颈癌患者中存在显著差异[11]。在我们研究中观察到HPR、ORM2这2个急性期蛋白的表达水平在LSIL、HSIL患者中存在显著差异,因此推测ORM2可作为宫颈癌前病变的潜在标志物。
FXI(F9)水平升高导致静脉血栓形成,宫颈癌的血栓前状态与恶性程度有直接关系,该水平与临床分期呈正相关,与生存时间呈负相关[30]。有报道发现,促血栓形成状态与肿瘤密切相关,恶性肿瘤和促血栓形成状态的合并是血栓形成的根本原因,凝血状态随着肿瘤恶性程度的加重在不断上调。因此,高凝状态与恶性肿瘤的侵袭和转移有关[31]。通过分析胞外腺嘌呤核苷酸水解作用和血小板的聚集的变化来诊断不同阶段宫颈上皮内瘤变和子宫侵润性癌症,同样发现上皮内瘤变和子宫浸润癌与凝血功能紊乱有关[32]。与此一致的是,我们同样检测到凝血级联反应通路蛋白F9随着宫颈病变的进展,即从LSIL、HSIL到CC不断上调,相对于对照组其表达量分别是0.87、1.44、2.32倍。F9作为凝血级联反应通路中的关键蛋白,其表达量的上调预示着凝血状态的增强,促血栓状态的形成与肿瘤的恶性程度、侵袭和转移增加密切相关,因此我们推测F9可做为提示肿瘤疾病,尤其是类似宫颈癌类高转移肿瘤发生发展的潜在标志物。
在功能富集分析中,我们发现大部分差异蛋白富集在补体级联反应通路上。补体系统在激活先天免疫和后天免疫方面起着重要的作用,而CFI、AFM(afamin)蛋白在补体激活的调节中具有重要作用[33]。检测补体成分在皮肤鳞状细胞癌(cSCC)发展中的作用,用全转录组、定量反转录PCR、Western blotting发现补体因子Ⅰ(CFI)在cSCC细胞系中高表达,敲降CFI的过表达将抑制cSCC细胞系的生长。IHC分析发现CFI染色增强出现在cSCC侵袭阶段,CFI染色阴性或者较弱出现在成熟的正常表皮和良性表皮乳头瘤,因此提出CFI可以作为提示cSCC发展的生物标志物[34]。Kratzer等[33]在脑血管内皮细胞中发现afamin表达并证明afamin促进维生素E在合适的细胞培养模型中通过血脑屏障转运/转移,提示afamin在血脑屏障中调节维生素E的摄取和转运。许多类型的癌症研究中均发现了外周血AFM浓度的变化,包括胃癌、膀胱癌、结直肠癌、乳腺癌和宫颈癌[35-36]。有意思的是我们发现CFI、AFM在宫颈癌组中高表达,分别是对照组的1.34、1.28倍,治疗组中开始下调,尤其是后者。Ilambarthi同样在宫颈癌的血浆蛋白组学研究中发现2个差异表达蛋白富集在补体级联反应的代谢通路上,即complement factor H(CFH)和complement factor B(CFB),CFH在宫颈癌中的表达是癌症早期的1.13倍,但CFB的表达则呈现下调趋势[10]。补体级联反应与细胞死亡和炎症过程有关[37],在各种癌症中通过促进细胞的增殖来促进肿瘤的发生[37-38]。CFH已经证实了在卵巢癌[41]和肺癌[40]这些恶性肿瘤中高表达。我们同样在研究中发现了补体激活系统代谢通路相关蛋白含量的变化,进一步证明了宫颈癌的患病与补体代谢通路异常调节有关。扩大样本量,利用靶向蛋白组学技术的分析结果也验证了此猜想。从数据结果看此蛋白在指示宫颈癌及其治疗效果、预后判断上具有潜在价值和意义,因此作为标志物具有较高的可信度。
总之,由于种族的多样性和特异性使得蛋白标志物的表达存在差异,本文通过LC-MS/MS和蛋白组学分析首次报道了南方广东地区宫颈病变患者的血清蛋白标志物,筛选到五个新型蛋白(HPR、ORM2、F9、CFI、AFM)可作为宫颈病变和宫颈癌发生发展及疗效预后的诊断标志物。其中,ORM2、HPR急性期蛋白组合是一种潜在指示宫颈癌癌前病变的诊断标志物,在相关研究中同样有报道其它的急性期蛋白与癌前病变和宫颈癌的发展有关,推测ORM2、HPR蛋白组合在提示宫颈癌癌前病变阶段上具有较高的可信度。AFM、CFI蛋白组合是一种潜在宫颈癌疗效评估和预后的标记物,在其他研究中同样报道了宫颈癌患者补体激活系统代谢通路相关蛋白含量的变化,进一步证明了宫颈癌的患病与补体代谢通路异常调节有关。F9蛋白是我们首次发现的一种潜在评估宫颈疾病恶性化程度的诊断标记物,作为凝血级联反应通路中的关键蛋白,其表达量的上调预示着凝血状态的增强,促血栓状态的形成与肿瘤的恶性程度、侵袭和转移增加密切相关,因此我们推测F9可做为提示肿瘤疾病,尤其是类似宫颈癌类高转移肿瘤发生发展的潜在标志物。以上蛋白在宫颈病变过程中作用机制尚未见报道,有待进一步研究。
Biography
邱峰,博士,副主任技师,E-mail: QFSFL@126.com
Funding Statement
国家自然科学基金(81572088);广东省科技计划项目(2014A020212681)
Supported by National Natural Science Foundation of China (81572088)
Contributor Information
邱 峰 (Feng QIU), Email: QFSFL@126.com.
黄 宪章 (Xianzhang HUANG), Email: huangxz020@163.com.
References
- 1.Ferlay J, Soerjomataram I, Ervik M. FGLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancer base No.11 [Internet]. Lyon, France: International agency for research on cancer [OL]. 2013.Available from: <a href="http://globocan.iarc.fr" target="_blank">http://globocan.iarc.fr</a>, accessed on 15/5/2015.
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CACancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015 [J]. CACancer J Clin, 2016, 66(2): 115-32.] [DOI] [PubMed] [Google Scholar]
- 3.王 玉东, 孙 璐璐. 妊娠期宫颈癌筛查. http://d.old.wanfangdata.com.cn/Periodical/gwyx-fybj200906051. 中国实用妇科与产科杂志. 2016;32(5):421–5. [王玉东, 孙璐璐.妊娠期宫颈癌筛查[J].中国实用妇科与产科杂志, 2016, 32(5): 421-5.] [Google Scholar]
- 4.陈 晓琴, 王 晓银, 罗 晓菊, et al. 妊娠期妇女对宫颈癌筛查认知行为的调查研究. 中国计划生育和妇产科. 2018;28(2):67–71. doi: 10.3969/j.issn.1674-4020.2018.02.19. [陈晓琴, 王晓银, 罗晓菊, 等.妊娠期妇女对宫颈癌筛查认知行为的调查研究[J].中国计划生育和妇产科, 2018, 28(2): 67-71.] [DOI] [Google Scholar]
- 5.Stoler MH, Vichnin MD, Ferenczy A, et al. The accuracy of colposcopic biopsy: analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128(6):1354–62. doi: 10.1002/ijc.v128.6. [Stoler MH, Vichnin MD, Ferenczy A, et al. The accuracy of colposcopic biopsy: analyses from the placebo arm of the Gardasil clinical trials[J]. Int J Cancer, 2011, 128(6): 1354-62.] [DOI] [PubMed] [Google Scholar]
- 6.Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 dual stain cytology for detection of cervical precancer in HPV-Positive women. J Natl Cancer Inst. 2015;107(12):257. doi: 10.1093/jnci/djv257. [Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 dual stain cytology for detection of cervical precancer in HPV-Positive women [J]. J Natl Cancer Inst, 2015, 107(12): 257.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He XK, Luo XP, Mao LZ, et al. An optoelectronic cervical cancer screening system for screening cervical cancer: comparison with cervical cytology. https://www.ncbi.nlm.nih.gov/pubmed/20965832. J South Med Univ. 2010;30(10):2304–6. [He XK, Luo XP, Mao LZ, et al. An optoelectronic cervical cancer screening system for screening cervical cancer: comparison with cervical cytology[J]. J South Med Univ, 2010, 30(10): 2304-6.] [PubMed] [Google Scholar]
- 8.Li XC, Shang JB, Wu XM, et al. MRI findings of uterine cervical cancer and value of MRI in preoperative staging. https://www.ncbi.nlm.nih.gov/pubmed/17425990. J South Med Univ. 2007;27(3):352–4. [Li XC, Shang JB, Wu XM, et al. MRI findings of uterine cervical cancer and value of MRI in preoperative staging[J]. J South Med Univ, 2007, 27(3): 352-4.] [PubMed] [Google Scholar]
- 9.Guo X, Hao Y, Kamilijiang M, et al. Potential predictive plasma biomarkers for cervical cancer by 2D-DIGE proteomics and ingenuity pathway analysis. Tumour Biol. 2015;36(3):1711–20. doi: 10.1007/s13277-014-2772-5. [Guo X, Hao Y, Kamilijiang M, et al. Potential predictive plasma biomarkers for cervical cancer by 2D-DIGE proteomics and ingenuity pathway analysis[J]. Tumour Biol, 2015, 36(3): 1711-20.] [DOI] [PubMed] [Google Scholar]
- 10.Lokamani I, Looi ML, Ali SA, et al. Gelsolin and ceruloplasmin as potential predictive biomarkers for cervical cancer by 2D-DIGE proteomics analysis. Pathol Oncol Res. 2014;20(1):119–29. doi: 10.1007/s12253-013-9670-9. [Lokamani I, Looi ML, Ali SA, et al. Gelsolin and ceruloplasmin as potential predictive biomarkers for cervical cancer by 2D-DIGE proteomics analysis[J]. Pathol Oncol Res, 2014, 20(1): 119-29.] [DOI] [PubMed] [Google Scholar]
- 11.Boichenko AP, Govorukhina N, Klip HG, et al. A panel of regulated proteins in serum from patients with cervical intraepithelial neoplasia and cervical cancer. J Proteome Res. 2014;13(11):4995–5007. doi: 10.1021/pr500601w. [Boichenko AP, Govorukhina N, Klip HG, et al. A panel of regulated proteins in serum from patients with cervical intraepithelial neoplasia and cervical cancer[J]. J Proteome Res, 2014, 13(11): 4995-5007.] [DOI] [PubMed] [Google Scholar]
- 12.Liang Z, Hong Z, Li DJ, et al. Comparative proteomic study of cervical cancer with different radiotherapy sensitivity. http://www.cqvip.com/QK/90333A/200904/30046273.html. ChineseGerman J Clinic Oncol. 2009;8(4):219–24. [Liang Z, Hong Z, Li DJ, et al. Comparative proteomic study of cervical cancer with different radiotherapy sensitivity[J]. ChineseGerman J Clinic Oncol, 2009, 8(4): 219-24.] [Google Scholar]
- 13.Qing S, Tulake W, Ru MF, et al. Proteomic identification of potential biomarkers for cervical squamous cell carcinoma and human papillomavirus infection. http://pesquisa.bvsalud.org/portal/resource/en/mdl-28443473. Tumour Biol. 2017;39(4):1010428317697547. doi: 10.1177/1010428317697547. [Qing S, Tulake W, Ru MF, et al. Proteomic identification of potential biomarkers for cervical squamous cell carcinoma and human papillomavirus infection[J]. Tumour Biol, 2017, 39(4): 1010428317697547.] [DOI] [PubMed] [Google Scholar]
- 14.谢 松喜, 李 伟雄, 黄 玉娟, et al. 脑脊液蛋白质质谱在非小细胞肺癌脑转移诊断中的应用. http://www.j-smu.com/oa/darticle.aspx?type=view&id=201003498. 南方医科大学学报. 2010;30(3):498–501. [谢松喜, 李伟雄, 黄玉娟, 等.脑脊液蛋白质质谱在非小细胞肺癌脑转移诊断中的应用[J].南方医科大学学报, 2010, 30(3): 498-501.] [PubMed] [Google Scholar]
- 15.蔡 思娜, 刘 国炳, 郭 晓红, et al. 应用蛋白芯片-飞行质谱技术筛选宫颈癌血清差异蛋白质. http://www.j-smu.com/oa/darticle.aspx?type=view&id=20090132. 南方医科大学学报. 2009;29(1):32–5. doi: 10.3321/j.issn:1673-4254.2009.01.032. [蔡思娜, 刘国炳, 郭晓红, 等.应用蛋白芯片-飞行质谱技术筛选宫颈癌血清差异蛋白质[J].南方医科大学学报, 2009, 29(1): 32-5.] [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Jia HL, Huang JM, et al. Identification of biomarkers for lymph node metastasis in early-stage cervical cancer by tissue-based proteomics. Br J Cancer. 2014;110(7):1748–58. doi: 10.1038/bjc.2014.92. [Wang W, Jia HL, Huang JM, et al. Identification of biomarkers for lymph node metastasis in early-stage cervical cancer by tissue-based proteomics[J]. Br J Cancer, 2014, 110(7): 1748-58.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsubara J, Honda K, Ono M, et al. Identification of adipophilin as a potential plasma biomarker for colorectal cancer using label-free quantitative mass spectrometry and protein microarray. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2195–203. doi: 10.1158/1055-9965.EPI-11-0400. [Matsubara J, Honda K, Ono M, et al. Identification of adipophilin as a potential plasma biomarker for colorectal cancer using label-free quantitative mass spectrometry and protein microarray[J]. Cancer Epidemiol Biomarkers Prev, 2011, 20(10): 2195-203.] [DOI] [PubMed] [Google Scholar]
- 18.王 睿, 王 岚, 黎 明新, et al. 血清PARP-1蛋白高表达在胃癌中的临床意义. http://d.old.wanfangdata.com.cn/Periodical/zgazzz201512010. 中国癌症杂志. 2015;35(12):972–7. [王睿, 王岚, 黎明新, 等.血清PARP-1蛋白高表达在胃癌中的临床意义[J].中国癌症杂志, 2015, 35(12): 972-7.] [Google Scholar]
- 19.Lin YW, Lai HC, Lin CY, et al. Plasma proteomic profiling for detecting and differentiating in situ and invasive carcinomas of the uterine cervix. Int J Gynecol Cancer. 2006;16(3):1216–24. doi: 10.1111/ijg.2006.16.issue-3. [Lin YW, Lai HC, Lin CY, et al. Plasma proteomic profiling for detecting and differentiating in situ and invasive carcinomas of the uterine cervix[J]. Int J Gynecol Cancer, 2006, 16(3): 1216-24.] [DOI] [PubMed] [Google Scholar]
- 20.Xia T, Zheng ZG, Gao Y, et al. Application of SELDI-TOF serum proteome profiling in cervical squamous cell carcinoma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ez200803010. Ai Zheng. 2008;27(3):279–82. [Xia T, Zheng ZG, Gao Y, et al. Application of SELDI-TOF serum proteome profiling in cervical squamous cell carcinoma[J]. Ai Zheng, 2008, 27(3): 279-82.] [PubMed] [Google Scholar]
- 21.Looi ML, Karsani SA, Rahman MA, et al. Plasma proteome analysis of cervical intraepithelial neoplasia and cervical squamous cell carcinoma. J Biosci. 2009;34(6):917–25. doi: 10.1007/s12038-009-0106-3. [Looi ML, Karsani SA, Rahman MA, et al. Plasma proteome analysis of cervical intraepithelial neoplasia and cervical squamous cell carcinoma[J]. J Biosci, 2009, 34(6): 917-25.] [DOI] [PubMed] [Google Scholar]
- 22.Dae HJ, Hyoung KK, Abd-Ei BP, et al. Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2008;19(3):173–80. doi: 10.3802/jgo.2008.19.3.173. [Dae HJ, Hyoung KK, Abd-Ei BP, et al. Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix[J]. Gynecol Oncol, 2008, 19(3): 173-80.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceciliani F, Pocacqua V. The acute phase protein alpha 1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8(1):91–108. doi: 10.2174/138920307779941497. [Ceciliani F, Pocacqua V. The acute phase protein alpha 1-acid glycoprotein: a model for altered glycosylation during diseases[J]. Curr Protein Pept Sci, 2007, 8(1): 91-108.] [DOI] [PubMed] [Google Scholar]
- 24.Jo M, Kim JH, Song GJ, et al. Astrocytic orosomucoid-2 modulates microglial activation and neuroinflammation. J Neurosci. 2017;37(11):2878–94. doi: 10.1523/JNEUROSCI.2534-16.2017. [Jo M, Kim JH, Song GJ, et al. Astrocytic orosomucoid-2 modulates microglial activation and neuroinflammation[J]. J Neurosci, 2017, 37(11): 2878-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baraniuk JN, Casado B, Maibach H, et al. A chronic fatigue syndrome-related proteome in human cerebrospinal fluid. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_bb14a667dac1380c626ba2e066071694. BMC Neurol. 2005;5(1):22. doi: 10.1186/1471-2377-5-22. [Baraniuk JN, Casado B, Maibach H, et al. A chronic fatigue syndrome-related proteome in human cerebrospinal fluid[J]. BMC Neurol, 2005, 5(1): 22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestriner FL, Spiller F, Laure HJ, et al. Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc Natl Acad Sci USA. 2007;104(49):19595–600. doi: 10.1073/pnas.0709681104. [Mestriner FL, Spiller F, Laure HJ, et al. Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism[J]. Proc Natl Acad Sci USA, 2007, 104(49): 19595-600.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sai K, Kurose K, Koizumi T, et al. Distal promoter regions are responsible for differential regulation of human orosomucoid-1 and-2 gene expression and acute phase responses. Biol Pharm Bull. 2014;37(1):164–8. doi: 10.1248/bpb.b13-00551. [Sai K, Kurose K, Koizumi T, et al. Distal promoter regions are responsible for differential regulation of human orosomucoid-1 and-2 gene expression and acute phase responses[J]. Biol Pharm Bull, 2014, 37(1): 164-8.] [DOI] [PubMed] [Google Scholar]
- 28.Sun YH, Cui L, Chen J, et al. Analysis of relationships between prethrombotic states and cervical cancer. Asian Pac J Cancer Prev. 2015;16(14):6163–6. doi: 10.7314/APJCP.2015.16.14.6163. [Sun YH, Cui L, Chen J, et al. Analysis of relationships between prethrombotic states and cervical cancer[J]. Asian Pac J Cancer Prev, 2015, 16(14): 6163-6.] [DOI] [PubMed] [Google Scholar]
- 29.Piva M, Horowitz GM, Sharpe-Timms KL. Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotic cells in vitro: a model for endometrial-peritoneal interaction in endometriosis. http://d.old.wanfangdata.com.cn/NSTLQK/10.1210-jc.86.6.2553/ J Clin Endocrinol Metab. 2001;86(6):2553–61. doi: 10.1210/jcem.86.6.7613. [Piva M, Horowitz GM, Sharpe-Timms KL. Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotic cells in vitro: a model for endometrial-peritoneal interaction in endometriosis[J]. J Clin Endocrinol Metab, 2001, 86 (6): 2553-61.] [DOI] [PubMed] [Google Scholar]
- 30.Khosa F, Otero HJ, Prevedello LM, et al. Imaging presentation of venous thrombosis in patients with cancer. AJR Am J Roentgenol. 2010;194(4):1099–108. doi: 10.2214/AJR.09.2501. [Khosa F, Otero HJ, Prevedello LM, et al. Imaging presentation of venous thrombosis in patients with cancer[J]. AJR Am J Roentgenol, 2010, 194(4): 1099-108.] [DOI] [PubMed] [Google Scholar]
- 31.Maldonado PA, Pimentel VC, Negrini LA, et al. Role of the purinergic system in patients with cervical intraepithelial neoplasia and uterine cancer. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=58b8f4841a4bc837d4ee2c1ead4b053b. Biomed Pharmacother. 2012;66(1):6–11. doi: 10.1016/j.biopha.2011.09.007. [Maldonado PA, Pimentel VC, Negrini LA, et al. Role of the purinergic system in patients with cervical intraepithelial neoplasia and uterine cancer[J]. Biomed Pharmacother, 2012, 66(1): 6-11.] [DOI] [PubMed] [Google Scholar]
- 32.Dieplinger H, Ankerst DP, Burges AA, et al. Afamin and apolipoprotein A-Ⅳ: novel protein markers for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1127–33. doi: 10.1158/1055-9965.EPI-08-0653. [Dieplinger H, Ankerst DP, Burges AA, et al. Afamin and apolipoprotein A-Ⅳ: novel protein markers for ovarian cancer[J]. Cancer Epidemiol Biomarkers Prev, 2009, 18(4): 1127-33.] [DOI] [PubMed] [Google Scholar]
- 33.Kratzer I, Bernhart E, Wintersperger A, et al. Afamin is synthesized by cerebrovascular endothelial cells and mediates alpha-tocopherol transport across an in vitro model of the blood-brain barrier. J Neurochem. 2009;108(3):707–18. doi: 10.1111/jnc.2009.108.issue-3. [Kratzer I, Bernhart E, Wintersperger A, et al. Afamin is synthesized by cerebrovascular endothelial cells and mediates alpha-tocopherol transport across an in vitro model of the blood-brain barrier[J]. J Neurochem, 2009, 108(3): 707-18.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riihila P, Nissinen L, Farshchian M, et al. Complement factor I promotes progression of cutaneous squamous cell carcinoma. http://www.jidonline.org/article/S0022-202X(15)37113-X/references. J Invest Dermatol. 2014;134(2):S18. doi: 10.1038/jid.2013.346. [Riihila P, Nissinen L, Farshchian M, et al. Complement factor I promotes progression of cutaneous squamous cell carcinoma[J]. J Invest Dermatol, 2014, 134(2): S18.] [DOI] [PubMed] [Google Scholar]
- 35.Humphries JM, Penno MA, Weiland FA, et al. Identification and validation of novel candidate protein biomarkers for the detection of human gastric cancer. Biochim Biophys Acta. 2014;1844(5, SI):1051–8. doi: 10.1016/j.bbapap.2014.01.018. [Humphries JM, Penno MA, Weiland FA, et al. Identification and validation of novel candidate protein biomarkers for the detection of human gastric cancer[J]. Biochim Biophys Acta, 2014, 1844(5, SI): 1051-8.] [DOI] [PubMed] [Google Scholar]
- 36.Choi JW, Liu H, Shin DH, et al. Proteomic and cytokine plasma biomarkers for predicting progression from colorectal adenoma to carcinoma in human patients. Proteomic. 2013;13(15):2361–74. doi: 10.1002/pmic.v13.15. [Choi JW, Liu H, Shin DH, et al. Proteomic and cytokine plasma biomarkers for predicting progression from colorectal adenoma to carcinoma in human patients[J]. Proteomic, 2013, 13(15): 2361-74.] [DOI] [PubMed] [Google Scholar]
- 37.Rutkowski MJ, Sughrue ME, Kane AJ, et al. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 2010;59(11):897–905. doi: 10.1007/s00011-010-0220-6. [Rutkowski MJ, Sughrue ME, Kane AJ, et al. The complement cascade as a mediator of tissue growth and regeneration[J]. Inflamm Res, 2010, 59(11): 897-905.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutkowski MJ, Sughrue ME, Kane AJ, et al. Cancer and the complement cascade. Mol Cancer Res. 2010;8(11):1453–65. doi: 10.1158/1541-7786.MCR-10-0225. [Rutkowski MJ, Sughrue ME, Kane AJ, et al. Cancer and the complement cascade[J]. Mol Cancer Res, 2010, 8(11): 1453-65.] [DOI] [PubMed] [Google Scholar]
- 39.unnikkala S, Hakulinen J, Jarva H, et al. Secretion of soluble complement inhibitors factor H and factor H-like protein (FHL-1)by ovarian tumour cells. Br J Cancer. 2002;87(10):1119–27. doi: 10.1038/sj.bjc.6600614. [unnikkala S, Hakulinen J, Jarva H, et al. Secretion of soluble complement inhibitors factor H and factor H-like protein (FHL-1)by ovarian tumour cells[J]. Br J Cancer, 2002, 87(10): 1119-27.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ajona D, Castano Z, Garayoa M, et al. Expression of complement factor H by lung cancer cells: Effects on the activation of the alternative pathway of complement. Cancer Res. 2004;64(17):6310–8. doi: 10.1158/0008-5472.CAN-03-2328. [Ajona D, Castano Z, Garayoa M, et al. Expression of complement factor H by lung cancer cells: Effects on the activation of the alternative pathway of complement[J]. Cancer Res, 2004, 64(17): 6310-8.] [DOI] [PubMed] [Google Scholar]


