Abstract
目的
探讨金盏花苷E对脂多糖(LPS)诱导炎症反应的抑制作用及可能的分子机制。
方法
CCK-8实验检测不同浓度(0、2、4、6、8、10、20、25、30 μg/mL)的金盏花苷E对RAW264.7细胞活力的影响; 不同浓度的金盏花苷E(0、6、8、10 μg/mL)预处理RAW264.7细胞2 h, 然后用LPS(100 ng/mL)刺激细胞特定的时间, ELISA检测炎症因子TNF-α、IL-1β释放; Western blotting检测iNOS、COX-2的表达水平及JAK-stats、MAPKs及NF-кB信号途径的磷酸化; ROS检测试剂盒检测RAW264.7细胞内ROS含量; 激光共聚焦实验检测转录因子stat3的核转位。
结果
CCK-8结果显示, 金盏花苷E浓度在低于20 μg/mL时对RAW264.7细胞无明显毒性作用; 金盏花苷E浓度依赖性地下调LPS诱导的iNOS和COX-2的表达(P < 0.01 vs LPS组); 抑制LPS诱导的促炎细胞因子TNF-α及IL-1β的释放, 且1 0 μg/mL组抑制作用尤为显著(P < 0.01 vs LPS组); 抑制LPS诱导的JAK1-stat3信号途径激活及stat3的核转位; 降低LPS诱发的ROS产生(P < 0.01 vs LPS组)。
结论
金盏花苷E通过抑制ROS介导的JAK1-stat3信号途径, 抑制LPS诱导的炎症反应。
Keywords: 金盏花苷E, 脂多糖, RAW264.7细胞, 炎症, JAK1-stat3信号途径
Abstract
Objective
To investigate the effect of calenduloside E on lipopolysaccharide (LPS)-induced inflammatory response in RAW264.7 cells and explore the underlying molecular mechanism.
Methods
CCK-8 assay was used to examine the effect of different concentrations of calenduloside E (0-30 μg/mL) on the viability of RAW264.7 cells. The release of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in RAW264.7 cells in response to pretreatment with 6, 8, and 10 μg/mL calenduloside E for 2 h followed by stimulation with 100 ng/mL LPS was detected using enzyme-linked immunosorbent assay (ELISA). The expression levels of iNOS and COX-2 and the activation of JAK-stats, MAPKs and NF-кB signaling pathways in the treated cells were determined using Western blotting. A reactive oxygen species (ROS) detection kit was used to detect ROS production in the cells, and the nuclear translocation of the transcription factor stat3 was observed by laser confocal microscopy.
Results
Calenduloside E below 20 μg/mL did not significantly affect the viability of RAW264.7 cells. Calenduloside E dose-dependently decreased the expression levels of iNOS and COX-2 induced by LPS, inhibited LPS-induced release of TNF-α and IL-1β, and suppressed LPS-induced JAK1-stat3 signaling pathway activation and stat3 nuclear translocation. Calenduloside E also significantly reduced ROS production induced by LPS in RAW264.7 cells.
Conclusion
Calenduloside E inhibits LPS-induced inflammatory response by blocking ROS-mediated activation of JAK1-stat3 signaling pathway in RAW264.7 cells.
Keywords: calenduloside E, lipopolysaccharide, RAW264.7 cells, inflammation, JAK1-stat3 signaling pathway
炎症是机体组织针对有害刺激(如病原体, 受损细胞或刺激物)所产生的复杂生物反应, 是一种涉及免疫细胞, 血管和分子介质的保护性反应[1]。然而, 过度的炎症反应常常导致疾病的发生, 如关节炎及脓毒症[2-4]。脂多糖(LPS), 也称脂聚糖或内毒素, 存在于革兰氏阴性菌的外膜中, 通过与位于细胞表面的脂多糖结合蛋白(LBP)和CD14形成复合物激活靶细胞, 诱导胞内多条信号通路的活化, 从而引发多种促炎细胞因子释放和炎症相关蛋白的表达[5-6]。MAPKs、NF-κB及JAK-stats等信号途径参与LPS诱导的炎症反应[7-9]。反应活性氧(ROS)作为炎症反应的重要介质, 在炎症相关疾病的发生、发展中发挥非常重要的作用[10-12]。我们已有的研究发现, LPS刺激RAW264.7细胞, 诱导胞内ROS的生成增加, ROS作为上游信号分子介导NF-κB, JAK-stats, MAPKs等信号通路的激活, 诱发炎症的发生[7, 13-14]。
越来越多的研究表明, 天然活性产物可以拮抗LPS诱导的炎症反应[15-17]。金盏花苷E又称去葡萄糖竹节参皂苷, 从竹节参中提取的五环三萜类化合物。有文献报道金盏花苷E能够保护过氧化氢诱导的心肌细胞凋亡[18-19]。但金盏花苷E其它的生物学作用尚不清楚。我们的预实验结果发现, 金盏花苷E能够抑制LPS诱导的炎症因子释放, 提示其具有一定的抗炎作用, 但其发挥抗炎作用的分子机制尚不清楚。因此, 本研究拟进一步探究金盏花苷E的抗炎作用及可能的分子机制, 为金盏花苷E用于炎症相关疾病的治疗提供实验依据。
1. 材料和方法
1.1. 药物、试剂及抗体
金盏花苷(HPLC≥95%)(上海源叶生物), LPS(Sigma)。兔单克隆抗体iNOS, COX-2, β-actin, p-p38, p-ERK, p-JNK, p-JAK1, p-JAK2(CST), p-stat1, p-stat3 (Santa Cruz)。ROS检测试剂盒和WB细胞裂解液(碧云天生物), CCK-8试剂盒(凯基生物)。
1.2. 细胞培养及传代
小鼠单核巨噬细胞RAW264.7购买于中科院典型培养物保藏委员会昆明细胞库。细胞使用含10%胎牛血清(南美), 100 U/mL青霉素, 100 μg/mL链霉素的DMEM高糖培养基(美国Hyclone)常规培养, 每2 d传代1次。细胞培养条件为5% CO2, 37 ℃, 取对数生长期细胞用于实验研究。
1.3. 细胞活力检测
RAW264.7细胞接种于96孔细胞培养板, 细胞密度为1×104。不同浓度(2、4、6、8、10、20、25、30μg/mL)的金盏花苷E分别作用细胞24 h, 每组3个复孔。去除原培养基, PBS清洗1遍之后, 将CCK-8原液用培养基按照1:10的比例稀释, 每孔加入100 μL稀释液, 在细胞培养箱(37 ℃, 5% CO2)中继续孵育2 h。使用全波段酶标仪(美国Thermo)在450 nm波长处检测各组吸光度(A450 nm)。细胞存活率(%)=(A实验组-A空白组)/(A对照组-A空白组)×100%。
1.4. 促炎细胞因子检测
RAW264.7细胞接种于12孔细胞培养板, 分别用不同浓度的金盏花苷E(6、8、10μg/mL)预处理RAW264.7细胞2 h, 然后LPS(100 ng/mL)刺激细胞16 h。收集细胞培养液, 12 000×g离心5 min, 取上清用于ELISA实验。TNF-α、IL-1β ELISA检测试剂盒均为R & D公司产品。具体操作按照厂家提供的说明书进行, 每组3个复孔, 450 nm波长检测各组的吸光度。
1.5. Western blotting
RAW264.7细胞接种于12孔细胞培养板, 设置空白对照组、金盏花苷E组、LPS组和金盏花苷E与LPS联合作用组。不同浓度的金盏花苷E(6、8、10μg/mL)首先预处理RAW264.7细胞2 h, 然后用100 ng/mL的LPS继续刺激特定的时间。药物处理后的细胞用预冷的PBS洗涤两次, 然后加入含蛋白酶抑制剂的细胞裂解液放置在冰上裂解30 min, 4 ℃, 12 000×g离心10 min, 收集上清, 并进行蛋白定量。蛋白样品加入SDS上样缓冲液, 煮沸5~10 min, 取等量蛋白进行SDS-PAGE。电泳分离的蛋白转移至硝酸纤维素膜(PALL, USA), 然后用5%脱脂奶粉室温封闭2 h, TBST洗膜3次, 每次5 min。加入相应一抗4 ℃孵育过夜, TBST洗涤3次后, 加入相应荧光二抗(LI-COR Biosciences)室温继续孵育1 h。最后使用Odyssey双色红外激光成像系统(LI-COR)检测实验结果, Image J软件用于光密度分析。
1.6. 激光共聚焦显微镜观察stat3核转位
磷酸化的stat通过形成二聚体进入细胞核调控炎症相关基因的表达[20]。因此, 我们通过共聚焦实验检测金盏花苷E对LPS诱导stat3核转位的影响。RAW264.7细胞接种在共聚焦激光小皿中, 实验分为对照组, 金盏花苷E单独处理组, LPS组及金盏花苷E与LPS联合刺激组。药物处理后的细胞, 弃去细胞培养基, PBS洗涤后, 用4%多聚甲醛室温固定30 min, 然后加入含3%牛血清白蛋白的PBS封闭1 h, PBS洗涤3次之后加入stat3一抗(1:100稀释), 4 ℃孵育过夜。PBS洗涤之后加入Alexa Fluor® 555标记的二抗(1:200稀释), 室温避光孵育1 h。最后DAPI避光染色细胞核5 min。激光共聚焦显微镜(德国Leica)观察stat3的核转位(630×)。
1.7. ROS检测
ROS通过激活转录因子stat在LPS诱导的炎症反应中发挥关键作用, 且ROS的生成有助于JAK-stats信号途径的激活[21]。我们通过检测不同处理条件下RAW264.7细胞中ROS的变化情况, 探究金盏花苷E对LPS诱导ROS产生的影响作用。RAW264.7细胞接种于12孔细胞培养板, 实验分组同1.5所述。金盏花苷E预处理细胞2 h, 然后LPS刺激15 min。按照1:200的比例用无血清DMEM培养基稀释CM-H2DCFDA荧光探针(碧云天), 每组加入1 mL CM-H2DCFDA探针稀释液, 37 ℃, 5% CO2条件下孵育细胞30 min, 弃除细胞培养液, 用PBS清洗3次, 充分洗涤除去未进入细胞的游离探针。在488 nm激发波长, 525 nm发射波长处使用倒置荧光显微镜(Olympus)观察细胞内ROS荧光强度并拍照。
1.8. 统计分析
数据以均数±标准差表示。Prism 6.0软件(GraphPad Software, Inc., La Jolla, CA, 美国)分析实验结果。单因素方差分析(ANOVA)用于实验数据统计分析, P < 0.05认为组间差异有统计学意义。
2. 结果
2.1. 金盏花苷E对RAW264.7细胞活力的影响
金盏花苷E在小于20μg/mL时对RAW264.7细胞活力没有显著影响(图 1)。所以, 我们选择6、8、10μg/mL 3个剂量组用于后续实验研究。
1.
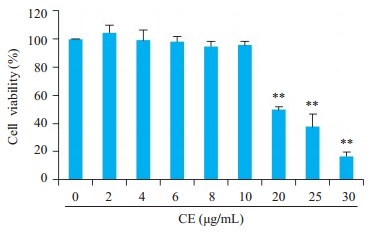
金盏花苷E对RAW264.7细胞活力的影响
Effect of different concentrations of calenduloside E (CE) on RAW264.7 cell viability. **P < 0.01 vs control group
2.2. 金盏花苷E抑制LPS诱导的iNOS、COX-2表达和促炎细胞因子释放
金盏花苷E明显下调LPS诱导的RAW264.7细胞中iNOS、COX-2蛋白的表达(图 2A), LPS诱导的促炎细胞因子TNF-α、IL-1β释放亦被显著抑制(图 2B-C), 且抑制作用呈现出浓度依赖性。
2.
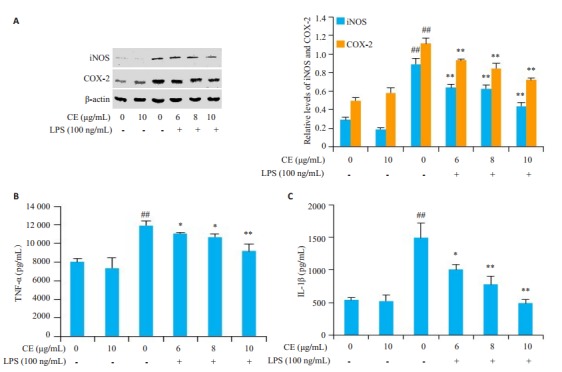
金盏花苷抑制LPS诱导炎症蛋白表达(A)及促炎因子释放(B和C)
Inhibitory effects of CE on LPS-induced inflammatory proteins (A) and inflammatory cytokines (B, C) in RAW264.7 cells. ##P < 0.01 vs control group; *P < 0.05, **P < 0.01 vs LPS group.
2.3. 金盏花苷E抑制LPS诱导的JAK1-stat3信号途径的活化
LPS刺激的RAW264.7细胞, p38 MAPK、ERK、JNK及IKK、IκB的磷酸化水平明显升高, 而且金盏花苷E对LPS诱导的上述信号途径的激活并无明显影响(图 3)。
3.
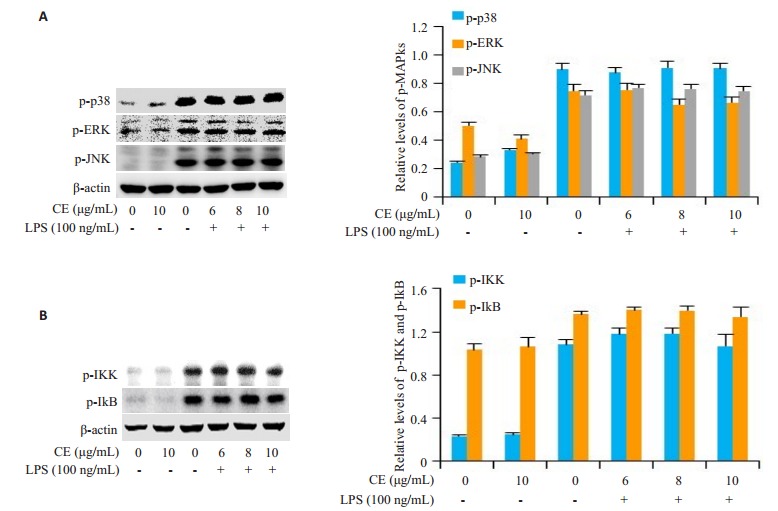
金盏花苷E对LPS诱导的MAPKs及NF-κB信号途径活化的影响
Effects of CE on LPS-induced activation of MAPKs and NF-κB signaling pathways in RAW264.7 cells
金盏花苷E明显下调了LPS诱导的JAK1磷酸化而对JAK2的磷酸化并无明显影响(图 4A), 金盏花苷E虽然对LPS诱导的stat1磷酸化并无显著影响, 却抑制了stat3的磷酸化(图 4B)。
4.
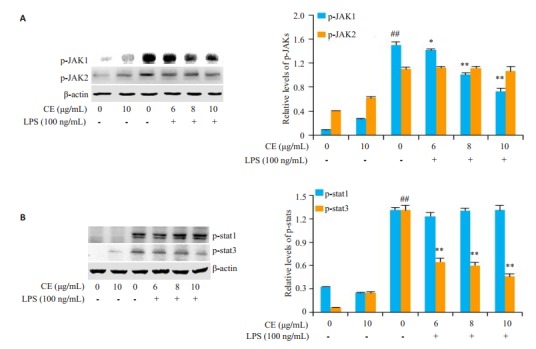
金盏花苷E对LPS诱导JAK-stats信号通路活化的影响
Effects of CE on LPS-induced activation of JAK-stats signaling pathway in RAW264.7 cells. ##P < 0.01 vs control group; *P < 0.05, **P < 0.01 vs LPS group
2.4. 金盏花苷E抑制LPS诱导stat3的核转位现象
金盏花苷E预处理RAW264.7细胞2 h, 然后LPS刺激4 h, 观察细胞质和细胞核中stat3的定位情况(图 5), 与对照组相比, 在LPS的刺激下, stat3明显向胞核转移, 而金盏花苷E(10μg/mL)预处理的细胞, LPS诱导的stat3核转位现象则被显著抑制。
5.
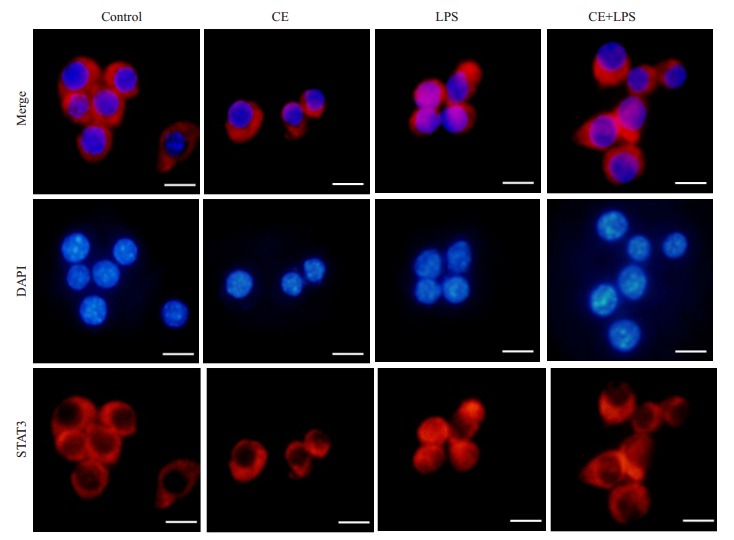
金盏花苷E抑制LPS诱导的stat3核转位
Inhibitory effects of CE on LPS-induced nuclear translocation of stat3 in RAW264.7 cells (Scale bar=25 μm)
2.5. 金盏花苷E抑制LPS诱导的ROS的产生
结果显示, 和对照组相比, LPS单独处理组RAW264.7细胞中ROS含量明显增多, 而金盏花苷E能够明显抑制LPS诱导的ROS生成, 尤其是8和10μg/mL组(图 6)。
6.
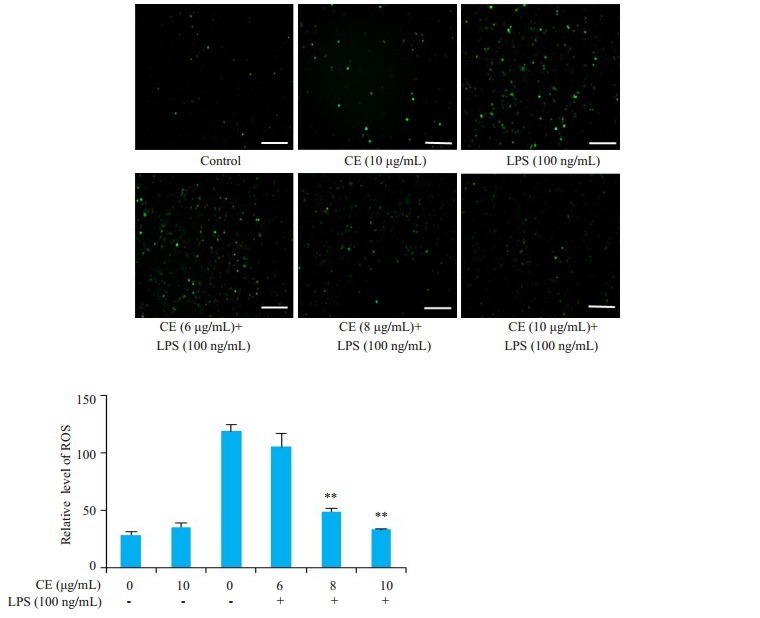
金盏花苷E抑制LPS诱导的ROS产生
Inhibitory effect of CE on LPS-induced ROS production in RAW264.7 cells (Scale bar= 100μm). **P < 0.01 vs LPS group
3. 讨论
CCK-8结果显示, 金盏花苷E在浓度低于20 μg/mL时对细胞无明显毒性效应。因此, 我们选择6、8、10μg/mL 3个浓度用于后续研究。炎症相关蛋白iNOS、COX-2及促炎细胞因子TNF-α、IL-1β与炎症的发生密切相关[22]。本研究通过WB和ELISA实验检测金盏花苷E对LPS诱导炎症蛋白表达及促炎细胞因子释放的影响。结果表明, 金盏花苷E明显下调LPS诱导的iNOS、COX-2的表达, 抑制TNF-α和IL-1β的释放, 且抑制效应呈现一定的浓度依赖性。由于MAPKs, NF-кB及JAK-stats信号通路参与炎症的发生[7-8, 20], 为了进一步探究金盏花苷E对抗LPS诱导炎症反应的分子机制是否与上述信号通路有关, 我们通过WB检测金盏花苷E对LPS诱导上述信号通路活化的影响。结果发现, 金盏花苷E对LPS诱导的MAPKs及NF-кB通路的激活无明显影响。
已有的研究表明, LPS刺激RAW264.7细胞, JAK1、JAK2的磷酸化水平约5 min开始升高, 10~15 min左右达到最高点; stat1和stat3磷酸化水平在LPS作用细胞2 h开始升高, 约4~6 h达到最高点[23-24]。根据以上实验结果, 我们选择LPS刺激细胞15 min和4 h的条件下, 分别检测金盏花苷E对JAK及stats磷酸化的影响。结果发现, 金盏花苷E能够明显降低LPS诱导的RAW264.7细胞中JAK1及其下游信号分子stat3的磷酸化, 并进一步抑制stat3的核转位现象。总之, 以上研究结果意味着, 金盏花苷E主要通过抑制JAK1-stat3信号通路的活化及stat3的核转位, 抑制LPS诱导的炎症反应。
研究发现, LPS刺激巨噬细胞促进ROS的产生[25-26], ROS还能够作为第二信使, 介导相关信号通路的活化和转录因子的激活, 进而调控炎症反应的发生、发展[27-28]。既然金盏花苷E能够抑制JAK1-stat3信号途径的激活, 我们不禁猜测, 该抑制效应的产生是否与金盏花苷E调控ROS的生成有关呢?因此, 我们继续深入探究金盏花苷E对ROS产生的影响作用。结果发现, 金盏花苷E确实能够有效抑制LPS诱导的RAW264.7细胞中ROS的产生。我们前期研究已证明, ROS清除剂NAC(N-乙酰-L-半胱氨酸)能够显著降低JAK-stat通路的磷酸化及iNOS、COX-2的表达[29-30]。综合以上研究结果, 金盏花苷E主要通过抑制ROS的产生及其介导的JAK1- stat3信号通路抑制LPS诱发的炎症反应。
然而, 金盏花苷E如何调控LPS诱导的胞内ROS的产生及下游信号途径的活化尚不清楚, 它是否通过影响细胞表面与LPS结合的受体, 或阻止LPS与LBP及CD14分子的结合能力来发挥生物学作用呢?以上假设也是我们将来探究的重点。
Biography
汤托,在读硕士研究生,E-mail: 1350902219@qq.com
Funding Statement
国家自然科学基金(81601380,81872371);安徽高校自然科学研究项目重大项目(KJ2016SD59);安徽省优秀青年人才支持计划重点项目(gxyqZD2016173);皖南医学院博士启动基金(WK2014RC05)
Supported by National Natural Science Foundation of China (81601380, 81872371)
Contributor Information
汤 托 (Tuo TANG), Email: 1350902219@qq.com.
戚 之琳 (Zhilin QI), Email: qizhilin9123@sohu.com.
References
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, et al. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1 beta Generation. Clin Exp Immunol. 2007;147(2):227–35. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Na HS, Song YR, Kim S, et al. Aloin inhibits interleukin (IL)-1β-stimulated IL-8 production in KB cells. J Periodontol. 2016;87:e108–115. doi: 10.1902/jop.2016.150447. [DOI] [PubMed] [Google Scholar]
- 3.YU, Qian, ZENG, et al. Ginsenoside Rk1 suppresses proinflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the Jak2/Stat3 pathway[J]. Chin J Nat Med, 2017(10): 751-7.
- 4.Achoui M, Appleton D, Abdulla MA, et al. In vitro and in vivo antiinflammatory activity of 17-Oacetylacuminolide through the inhibition of cytokines, NF-κB translocation and IKK β activity. PLoS One. 2010;5:e15105. doi: 10.1371/journal.pone.0015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweet MJ, Da HM. Endotoxin signal transduction in macrophages. J Leu Biol. 1996;60(1):8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Sanghera JS, Weinstein SL, Aluwalia M, et al. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156(11):4457–65. [PubMed] [Google Scholar]
- 7.Qi S, Xin Y, Guo Y, et al. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/ NF-κB signaling pathways. Int Immunopharmacol. 2012;12(1):278–87. doi: 10.1016/j.intimp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Lee SB, Lee WS, Shin JS, et al. Xanthotoxin suppresses LPSinduced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW264.7 macrophages. Int Immunopharmacol. 2017;49:21–29. doi: 10.1016/j.intimp.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Byung, Hy uk, H an, et al. Hwangryunhaedoktang exerts antiinflammation on LPS-induced NO production by suppressing MAPK and NF-κB activation in RAW264.7 macrophages. J Integr Med. 2017;15(4):326–36. doi: 10.1016/S2095-4964(17)60350-9. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra JD, Miao HZ, Zhang KZ, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105(47):18525–30. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitochondria LG, Species RO. Which role in physiology and Pathology. Adv Exp Med Biol. 2012;942(3):93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- 12.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive Oxygen species. Nat Rev Immunol. 2013;13(5):349–61. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi ZL, Qi SM, Ling LF, et al. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–71. doi: 10.1016/j.intimp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Ren J, Li LX, Wang YE, et al. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NFkappa B and MAPK activation to reduce inflammation in LPSactivated RAW264.7 cells. Biomed Pharmacother. 2019;109:555–62. doi: 10.1016/j.biopha.2018.10.112. [DOI] [PubMed] [Google Scholar]
- 15.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, et al. Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr Pharm Des. 2004;10(30):3813–33. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Wang Y, Yuan X, et al. Berberine inhibits lipopolysaccharideinduced expression of inflammatory cytokines by suppressing TLR4-mediated NF-ĸB and MAPK signaling pathways in rumen epithelial cells of Holstein calves. J Dairy Res. 2019;30:1–6. doi: 10.1017/S0022029919000323. [DOI] [PubMed] [Google Scholar]
- 17.Yang QX, Luo J, Lv HJ, et al. Pulegone inhibits inflammation via suppression of NLRP3 inflammasome and reducing cytokine production in mice. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/08923973.2019.1588292. Immunopharmacol Immunotoxicol. 2019;28:1–8. doi: 10.1080/08923973.2019.1588292. [DOI] [PubMed] [Google Scholar]
- 18.Tian Y, Wang S, Shang H, et al. The clickable activity-based probe of anti-apoptotic calenduloside E. Pharm Biol. 2019;57(1):133–9. doi: 10.1080/13880209.2018.1557699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian Y, Du YY, Shang H, et al. Calenduloside E analogues protecting H9c2 cardiomyocytes against H2O2-Induced apoptosis: design, synthesis and biological evaluation. Front Pharmacol. 2017;8:862. doi: 10.3389/fphar.2017.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler C, Levy DE, Decker T. JAK-STAT signaling: From interferons to cytokines. J Biol Chem. 2007;282(28):20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 21.Pan XL, Cao X, Li N, et al. Forsythin inhibits lipopolysaccharideinduced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm Res. 2014;63(7):597–608. doi: 10.1007/s00011-014-0731-7. [DOI] [PubMed] [Google Scholar]
- 22.Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563–82. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Tang T, Sheng L, et al. Aloin suppresses lipopolysaccharideinduced inflammation by inhibiting JAK1STAT1/3 activation and ROS production in RAW264.7 cells. Int J Mol Med. 2018;42(4):1925–34. doi: 10.3892/ijmm.2018.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.齐 世美, 李 强, 姜 琦, et al. 白杨素通过JAK-STATs信号通路抑制内毒素诱导的巨噬细胞炎症反应. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=201803243. 南方医科大学学报. 2018;38(3):243–50. doi: 10.3969/j.issn.1673-4254.2018.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han W, Li H, Cai J, et al. NADPH oxidase limits lipopolysaccharide- induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-κB activity. J Immunol. 2013;190(9):4786–94. doi: 10.4049/jimmunol.1201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon SW, Ahn CB, Oh Y, et al. Lotus(nelumbo nucifera)seed protein isolate exerts anti-inflammatory and antioxidant effects in LPS-stimulated RAW264.7 macrophages via inhibiting NF-κB and MAPK pathways, and upregulating catalase activity. Int J Biol Macromol. 18;134:791–7. doi: 10.1016/j.ijbiomac.2019.05.094. [DOI] [PubMed] [Google Scholar]
- 27.Zhang LL, Mu GG, Ding QS, et al. PTEN represses colon Cancer progression through inhibiting paxillin transcription via PI3K/AKT/ NF-kB pathway. J Biol Chem. 2015;290(24):15018–29. doi: 10.1074/jbc.M115.641407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawate S, Shen Q, Fan F, et al. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77(4):540–51. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 29.Qi ZL, Yin F, Lu LA, et al. Baicalein reduces lipopolysaccharideinduced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm Res. 2013;62(9):845–55. doi: 10.1007/s00011-013-0639-7. [DOI] [PubMed] [Google Scholar]
- 30.Qi S, Feng Z, Li Q, et al. Myricitrin modulates NADPH Oxidase- Dependent ROS production to inhibit Endotoxin-Mediated inflammation by blocking the JAK/STAT1 and NOX2/p47phox Pathways. Oxid Med Cell Longev. 2017:9738745. doi: 10.1155/2017/9738745. [DOI] [PMC free article] [PubMed] [Google Scholar]


