Abstract
目的
探讨姜黄素对胶质瘤细胞的侵袭、迁徙能力的影响及相关机制。
方法
四甲基偶氮唑蓝实验筛选合适的姜黄素药物浓度; Transwell小室、Boyden小室及划痕实验检测姜黄素对胶质瘤细胞侵袭、迁移能力的影响; 蛋白免疫印迹实验和蛋白质免疫共沉淀实验检测相关蛋白质表达水平变化及相关蛋白质之间相互作用。
结果
终浓度为20 μmol/L的姜黄素培养基培养48 h作为实验组处理因素; 姜黄素能明显降低胶质瘤细胞的侵袭、迁移能力(P < 0.05);机制分析显示, 与空白对照组相比, 姜黄素处理组HDGF、N-cadherin、Vimentin、Snail、Slug蛋白表达水平下降, E-cadherin蛋白表达水平升高; 在姜黄素处理过的胶质瘤细胞中干扰和过表达HDGF可分别协同抑制和逆转上皮细胞-间充质转化(EMT)信号; 姜黄素可以明显降低HDGF与β-catenin的结合量。
结论
姜黄素通过抑制HDGF/β-catenin复合物, 降低了EMT信号, 从而抑制了胶质瘤细胞侵袭、迁移能力。
Keywords: 姜黄素, 胶质瘤, 细胞侵袭, HDGF, β-catenin
Abstract
Objective
To investigate the effect of curcumin on the invasion and migration of human glioma cells in vitro and explore the molecular mechanisms.
Methods
MTT assay was used for screening the optimal curcumin concentrations. The effects of curcumin on the invasion and metastasis of human glioma cell lines U251 and LN229 were tested using Transwell assay, Boyden assay and wound-healing assays. The expression of the related proteins and their interactions were determined using Western blotting and coimmunoprecipitation assay.
Results
Curcumin at the concentration of 20 μmol/L for 48 h was used as the optimal condition for subsequent cell treatment. In the two glioma cell lines, curcumin significantly suppressed the invasion and migration of the cells (P < 0.05) and lowered the expressions of hepatoma-derived growth factor (HDGF), Ncadherin, vimentin, Snail and Slug, but increased the expression of E-cadherin. Interference of HDGF in curcumin-treated glioma cells synergistically inhibited the epithelial-mesenchymal transition (EMT) signals, while overexpression of HDGF significantly reversed the inhibitory effect of curcumin on EMT; curcumin treatment could significantly reduce the binding of HDGF to β-catenin.
Conclusion
Curcumin suppresses EMT signal by reducing HDGF/β-catenin complex and thereby lowers the migration and invasion abilities of human glioma cells in vitro.
Keywords: curcumin, glioma, cell invasion, hepatoma-derived growth factor, β-catenin
胶质瘤来源于神经上皮, 是中枢神经系统最常见的原发性脑肿瘤, 具有生长快、预后差等临床特征[1]。低级胶质瘤的平均生存期约为5年, 而高级胶质瘤大约只有1年[2]。尽管手术、放疗、化疗、免疫治疗等治疗方式不断进步, 胶质瘤患者的预后仍然很差[3-4]。
姜黄素是从姜黄根茎提取的天然多酚, 具有抗动脉粥样硬化、抗炎、抗氧化、抗肿瘤等功效[5-7]。姜黄素可以通过调控多条信号通路抑制肿瘤细胞的增殖、转移和血管生成, 从而发挥抗肿瘤作用[8-9]。姜黄素可以通过诱导细胞凋亡途径抑制胶质瘤细胞增殖[10]。小鼠模型结果提示姜黄素可以抑制VEGF/Ang-2/TSP-1介导的血管生成来抑制肿瘤生长[11]。尽管研究越来越深入, 姜黄素对胶质瘤的生物学作用和分子机制仍不明确。
肝癌衍生生长因子(HDGF)是一种从肝癌细胞纯化而来的肝素结合生长因子, 在血管生成中起到了重要作用[12]。HDGF在部分肿瘤中的表达较周围正常组织明显升高, 并且与肿瘤的侵袭性有关[13]。最近的研究结果表明, AGAP2-AS1-miR-15a/b-5p轴通过上调HDGF从而激活Wnt/β-catenin信号促进胶质瘤的发生[14]。HDGF可以作为肿瘤靶标从而抑制胶质瘤细胞的恶性表型转变[15]。然而, HDGF在胶质瘤细胞侵袭中的详细作用机制仍不清楚。
β-连环蛋白(β-catenin)是Wnt信号通路的关键组分, Wnt信号的持续激活产生级联反应, 导致核内β- catenin大量累积, 从而促进肿瘤进展[16]。β-catenin介导了miR-92b对胶质瘤细胞生长、转移的促进[17]。而去泛素化后, β-catenin增加可以促进胶质瘤细胞的增殖[18]。
在本次研究中, 我们发现姜黄素可以抑制HDGF/β- catenin复合物引起的上皮细胞-间充质转化(EMT), 从而降低胶质瘤的侵袭性, 这为姜黄素治疗胶质瘤患者提供了新的实验支持证据。
1. 材料和方法
1.1. 细胞培养及传代
U251细胞和LN229细胞从中国科学院细胞库购买, 常规使用10%胎牛血清的DMEM培养基(ExCell), 放入37 ℃、5% CO2浓度的孵箱中培养; 当细胞密度达到培养皿面积80%时用胰蛋白酶消化, 传至两个培养皿(Nest Biotech)。
1.2. 四甲基偶氮唑蓝(MTT)实验
弃培养皿中的培基, 常规消化细胞后计数, 每个96孔板(Nest Biotech)接种5×103细胞; 10~12 h后, 弃96孔板培基, 将终浓度为0、5、10、20、40、80μmol/L姜黄素培养基分别加入200μL; 48 h后避光每孔加入20μL MTT, 敷箱中放置4 h; 吸出孔内液体, 每孔加入150 μL DMSO, 吹打、混匀后获取490 nm处光密度值(A490nm);
1.3. 瞬转si-HDGF及HDGF质粒由广州锐博生物公司设计和合成
取对数生长期细胞向六孔板((Nest Biotech))接种2×104个细胞; 待细胞贴壁后每孔用Lipofectamine-2000 (Invitro-gen Biotechnolog)进行转染; 8 h后换液, 72 h后提取蛋白质。si-HDGF序列信息:Sense5'CcggtgCCG TGAAATCAACAGCCAACTCGAGTTGGCTGTTGA TTTCACGGCATTTTTg3';Antisense5'aattcaaaaatgCC GTGAAATCAACAGCCAACTCGAGTTGGCTGTTG ATTTCACGGCA 3'。
1.4. Transwell小室实验
常规消化细胞后用无血清DMEM培养基(ExCell)洗涤细胞两次后加入小室(Millipore), 上室加入100 μL总数为1×105细胞的无血清培养基, 下室加入500 μL含10%胎牛血清的DMEM培养基, 放置敷箱培养; 显微镜下观察到下室有10个细胞穿过时取出上室, 用棉球及Dhanks清洗后将小室放入500μL甲醇中固定10 min; 取出上室, 晾干, 用结晶紫溶液染色3 min。
1.5. Boyden小室实验
上室基底膜均匀加入50 μL基质胶, 移入24孔板, 于培养箱中凝固30 min; 余下同Transwell小室实验。
1.6. 划痕实验
常规消化细胞后铺入6孔板(Nest Biotech), 待10~ 12 h贴壁后用10μL枪头在六孔板背面均匀划直线, 每孔划3条线, 用D-hanks清洗后放入敷箱中培养; 分别于0、12、24 h后在倒置显微镜下拍照。
1.7. 蛋白免疫印迹实验
提取细胞蛋白, 变性; 每个泳道加入30 mg蛋白, 依次进行电泳、转膜、封闭、敷抗体、曝光。电泳条件:80 V, 30 min; 100 V, 60 min。转膜条件:350 mA, 2 h。封闭条件:5%脱脂奶粉, 1 h。抗体:HDGF, β-catenin, E-cadherin, N-cadherin, Vimentin, Snail, Slug, β-actin均从广州锋硕生物购买, 以1:1000稀释。图片由ChemiDocTM CRSþ Molecular Imager(Bio-Rad)捕获。
1.8. 蛋白质免疫共沉淀
按照蛋白质免疫共沉淀试剂盒推荐步骤进行实验(Thermos Scientific)。抗体固定化; 提取蛋白, 纯化; 向上柱中加入1000μg蛋白质, 分别加入10 μg抗β-catenin抗体和10μg抗IgG抗体, 在4 ℃摇床孵育过夜; 洗脱和β-catenin结合的蛋白质, 变性蛋白后进行蛋白免疫印迹实验; 抗IgG抗体作为阴性对照。
1.9. 统计学分析
应用SPSS19.0进行数据分析, 定量资料以均数±标准差表示, 两组之间比较采用独立样本t检验, 检验水准α=0.05。
2. 结果
2.1. MTT法筛选合适姜黄素药物浓度
以0μmol/L姜黄素培养基作为空白对照, U251细胞和LN229细胞药物半抑制浓度(IC50)都在10~ 20 μmol/L之间(图 1), 故使用20μmol/L姜黄素培养基培养48 h作为实验组处理因素。
1.
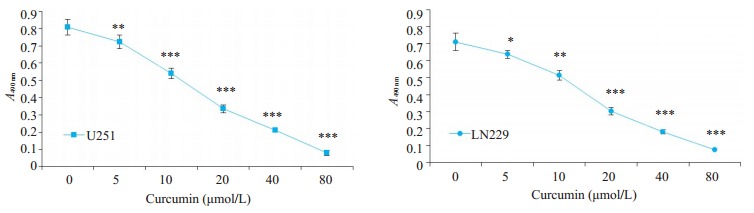
MTT法测定姜黄素对胶质瘤细胞U251和LN229的药物半抑制浓度
MTT assay for determining IC50 of curcumin in U251 and LN229 cells (Mean±SD). *P < 0.05, **P < 0.01, **P < 0.001 by Student-t test
2.2. 姜黄素抑制胶质瘤侵袭、迁移能力
划痕实验结果显示, 20 μmol/L姜黄素培养基培养48 h的胶质瘤细胞U251和LN229迁移距离明显短于对照组(图 2)。Transwell小室(图 3)、Boyden小室(图 4)结果显示, 与对照组相比, 姜黄素可以明显降低胶质瘤细胞U251和LN229的穿透数(P < 0.05)。
2.
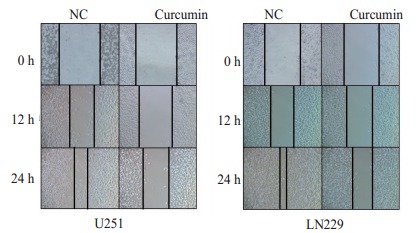
划痕实验测定姜黄素作用后0、12和24 h胶质瘤U251细胞和LN229细胞迁移情况
Wound-healing assays for assessing the migration ability of U251 and LN229 cells at 0, 12 and 24 h after curcumin treatment (Original magnification: ×100)
3.
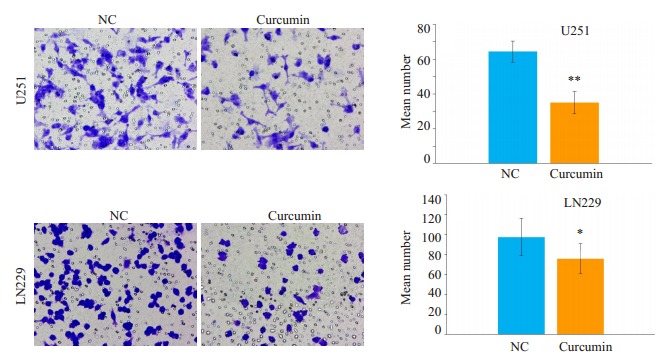
Transwell小室实验检测姜黄素对胶质瘤细胞U251和LN229侵袭的影响
Effect of curcumin on the migration of glioma cells assessed by Transwell assay (×400; Mean±SD). *P < 0.05, **P < 0.01 by Student-t test
4.
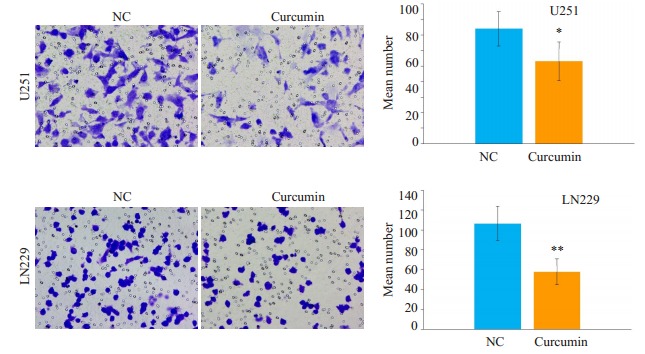
Boyden小室实验检测姜黄素对胶质瘤细胞U251和LN229侵袭的影响
Effect of curcumin on the invasion of glioma cell lines U251 and LN229 assessed by Boyden assay (× 400, 0.02 Mean±SD). *P < 0.05, **P < 0.01 by Student-t test
2.3. 姜黄素通过抑制HDGF降低胶质瘤的侵袭性
为了探索姜黄素抑制胶质瘤细胞游走和侵袭能力的机制, 我们行蛋白免疫印迹分析发现, 姜黄素处理组HDGF、N-cadherin、Vimentin、Snail、Slug蛋白水平下降, E-cadherin蛋白水平上升(图 5)。我们随后将HDGF质粒和si-HDGF分别转染用姜黄素处理过的胶质瘤细胞U251和LN229中, 结果显示, 在过表达HDGF之后, 姜黄素的EMT信号抑制作用被逆转(图 6), 而敲低HDGF则可以与姜黄素协同抑制EMT信号(图 7)。
5.
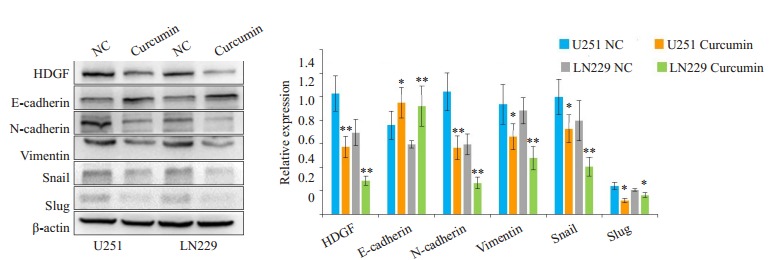
姜黄素作用48 h后胶质瘤细胞U251和LN229中相关蛋白表达水平的变化
Changes of related protein expressions in U251 and LN229 cells after curcumin treatment for 48 h
6.
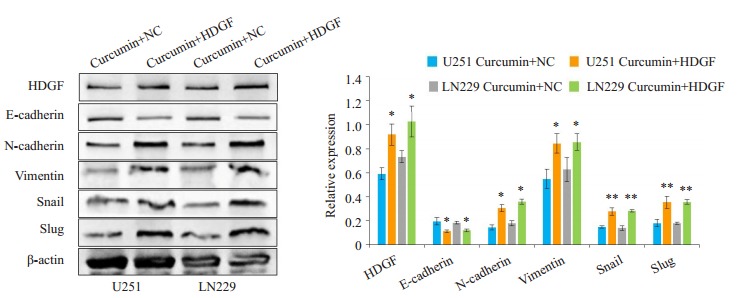
过表达HDGF对姜黄素抑制EMT作用的影响
Effect of HDGF overexpression on the inhibitory effect of curcumin on EMT
7.
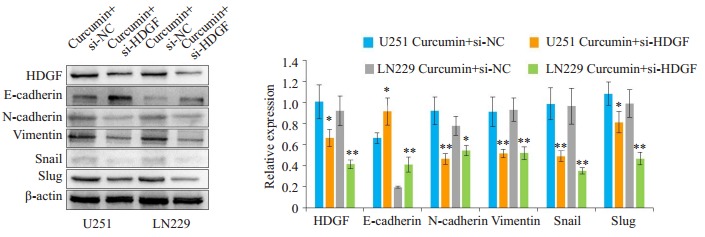
干扰HDGF对姜黄素抑制EMT作用的影响
Effect of HDGF interference on the inhibitory effect of curcumin on EMT
2.4. HDGF与β-catenin相互作用
已有研究证明Wnt信号通路可以促进上皮细胞-间充质转化, β-catenin可以和多种蛋白质结合参与肿瘤发生发展[19-20], 为了进一步探索姜黄素抗肿瘤的分子机制, 我们通过蛋白免疫共沉淀实验来检测β- catenin与HDGF在胶质瘤中是否存在相互作用。结果显示, 在胶质瘤细胞U251和LN229中, HDGF可以与β-catenin结合。与对照组相比, 姜黄素处理之后HDGF与β-catenin的结合量显著降低(图 8)。
8.
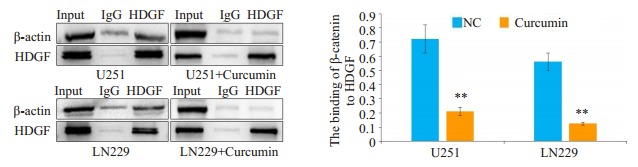
姜黄素对HDGF与β-catenin结合水平的影响
Effect of curcumin on the binding level of HDGF with β-catenin
3. 讨论
胶质瘤恶性程度高, 侵袭性强, 常呈浸润性生长, 因此手术难以完全切除, 导致复发性高[21], 胶质瘤患者的预后仍无明显改善, 还需探索更好的治疗策略[22-24]。
姜黄素可以靶向转录因子、生长因子、促炎性细胞因子、促癌激酶等, 从而抑制多种肿瘤细胞生长[25-26]。此外, 姜黄素在非小细胞肺癌中通过诱导自噬相关的细胞死亡能克服吉非替尼原发性化疗抵抗[27]。姜黄素通过促进Connexin 43蛋白降解以及他莫昔芬诱导的肿瘤细胞凋亡[28]。姜黄素可以降低E3泛素连接酶NEDD4, 导致Notch1和pAKT表达减少, 从而抑制胶质瘤的转移[29]。此外, 姜黄素在胶质瘤中还能通过抑制SKP2信号, 从而抑制细胞的生长和侵袭[10]。以上研究表明, 姜黄素在肿瘤中发挥了重要的抗肿瘤活性, 这其中也包括了胶质瘤。然而, 关于姜黄素抑制胶质瘤游走和侵袭的分子机制一直有待进一步的揭示。
本研究发现, 姜黄素处理过的胶质瘤U251与LN229细胞穿过基底膜的数量和迁徙的距离明显受到抑制, 这表明姜黄素可以降低胶质瘤细胞的侵袭和迁移能力。该结果与文献[28-29]类似, 提示姜黄素可以作为有效的抗胶质瘤药物。
EMT是上皮细胞受到刺激转化为具有间质表型细胞的生物学过程。在肿瘤中, EMT信号的转化增加常常促进肿瘤游走、侵袭和转移能力的增加。TRPM7能激活PI3K/AKT信号, 进而诱导EMT信号转化, 从而促进卵巢癌游走、侵袭和转移[30]。CASP1通过诱导Snail介导的上皮间质转化从而促进大肠癌转移[31]。姜黄素可以通过抑制c-Met/PI3K/Akt//mTOR信号通路降低EMT相关蛋白的表达, 从而降低肺癌的侵袭性, 表明姜黄素可以失活EMT信号通路抑制肿瘤转移[32]。为了明确姜黄素抑制胶质瘤细胞游走和侵袭的机制, 我们使用姜黄素处理了胶质瘤细胞, 随后发现与对照组相比, 姜黄素处理胶质瘤细胞组HDGF, N-cadherin、Vimentin、Snail、Slug表达量明显降低, E-cadherin蛋白水平上升, 提示姜黄素对胶质瘤的抗肿瘤作用可能是通过HDGF抑制上皮细胞-间充质转化相关。
HDGF作为肝素结合生长因子, 其过表达促进了多种肿瘤增殖、侵袭、转移和化疗抵抗[12-14]。HDGF与β-catenin能形成复合物, 从而增加β-catenin诱导EMT信号, 促进肿瘤的转移[12]。在姜黄素处理肺癌细胞株A549后, β-catenin表达明显降低, 这表明Wnt/β-catenin信号参与了姜黄素对癌细胞的抑制作用[33]。在本次研究中, 我们观察到姜黄素能明显抑制HDGF和β-catenin表达, 并降低两者的结合能力。在抑制HDGF表达后, 胶质瘤细胞中的N-cadherin、Vimentin、Snail、Slug蛋白水平下降, E-cadherin蛋白水平上升。在姜黄素处理的胶质瘤细胞中导入HDGF质粒后, 胶质瘤细胞的EMT转化信号明显被逆转。以上的结果表明, 姜黄素能通过抑制HDGF, 降低与β-catenin结合, 进而抑制其介导的EMT信号, 从而抑制了胶质瘤细胞的游走和侵袭, HDGF是姜黄素抗胶质瘤新的有效靶点。
因此, 姜黄素通过下调HDGF表达, 降低与β-catenin结合, 从而失活β-catenin诱导的EMT信号, 最终抑制胶质瘤细胞的游走和侵袭, 姜黄素可以作为潜在胶质瘤治疗药物。
Biography
罗起胜,博士,副教授,副主任医师,E-mail: 850198155@qq.com
Funding Statement
国家自然科学基金(81760450);广西自然科学基金面上项目(2017GXNSFAA198322)
Supported by National Natural Science Foundation of China (81760450)
Contributor Information
罗 起胜 (Qisheng LUO), Email: 850198155@qq.com.
唐 乾利 (Qianli TANG), Email: htmgx@163.com.
References
- 1.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meel MH, Kaspers GJL and Hulleman E. Preclinical therapeutic targets in diffuse midline glioma. Drug Resist Updat. 2019;44:15–25. doi: 10.1016/j.drup.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Miyauchi JT, Tsirka SE. Advances in immunotherapeutic research for glioma therapy. J Neurol. 2018;265(4):741–56. doi: 10.1007/s00415-017-8695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang RY, Lin YH, Lin SY, et al. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics. 2019;9(8):2411–23. doi: 10.7150/thno.29326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Momtazi-Borojeni AA, Haftcheshmeh SM, Johnston TP, et al. Curcumin: a natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev. 2018;17(2):125–35. doi: 10.1016/j.autrev.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Yuan J, Liu RP, Ma YH, et al. Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-B signaling and COX-2 in cigarette Smoke-Induced COPD mice. Inflammation. 2018;41(5):1804–14. doi: 10.1007/s10753-018-0823-6. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q, Zhang J, Gao L, et al. Dickkopf3 upregulation mediates the cardioprotective effects of curcumin on chronic heart failure. Mol Med Rep. 2018;17(5):7249–57. doi: 10.3892/mmr.2018.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva G, Lima FT, Seba V, et al. Curcumin analog CH-5 suppresses the proliferation, migration, and invasion of the human gastric cancer cell line HGC-27. Molecules. 2018;23(2):279. doi: 10.3390/molecules23020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang LH, Yang GL, Zhang RY, et al. Curcumin inhibits cell proliferation and motility via suppression of TROP2 in bladder cancer cells. Int J Oncol. 2018;53(2):515–26. doi: 10.3892/ijo.2018.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LX, Ye XT, Cai XM, et al. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cellsE. Oncotarget. 2015;6(20):18027–37. doi: 10.18632/oncotarget.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Li C, Tan Q, et al. Curcumin suppresses tumor growth and angiogenesis in human glioma cells through modulation of vascular endothelial growth factor/angiopoietin-2/thrombospondin- 1 signaling. CNS Neurol Disord Drug Targets. 2017;16(3):346–50. doi: 10.2174/1871527315666160902144513. [DOI] [PubMed] [Google Scholar]
- 12.Fu QF, Song X, Liu Z, et al. miRomics and proteomics reveal a miR-296-3p/PRKCA/FAK/Ras/c-Myc feedback loop modulated by HDGF/DDX5/beta-catenin complex in lung adenocarcinoma. Clinical Cancer Research. 2017;23(20):6336–50. doi: 10.1158/1078-0432.CCR-16-2813. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Ren H, Yuan P, et al. Down-regulation of hepatomaderived growth factor inhibits anchorage-independent growth and invasion of non-small cell lung cancer cells. Cancer Res. 2006;66(1):18–23. doi: 10.1158/0008-5472.CAN-04-3905. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YH, Lu SM, Xu YH, et al. Long non-coding RNA AGAP2- AS1 promotes the proliferation of glioma cells by sponging miR- 15a/b-5p to upregulate the expression of HDGF and activating Wnt/ beta-catenin signaling pathway. Int J Biol Macromol. 2019;128:521–30. doi: 10.1016/j.ijbiomac.2019.01.121. [DOI] [PubMed] [Google Scholar]
- 15.Zhang A, Long W, Guo Z, et al. Downregulation of hepatomaderived growth factor suppresses the malignant phenotype of U87 human glioma cells. Oncol Rep. 2012;28(1):62–8. doi: 10.3892/or.2012.1768. [DOI] [PubMed] [Google Scholar]
- 16.Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98(18):10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Wang X, Zou J, et al. miR-92b controls glioma proliferation and invasion through regulating Wnt/beta-catenin signaling via nemo-like kinase. Neuro Oncol. 2013;15(5):578–88. doi: 10.1093/neuonc/not004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan M. Muzumdar D and shiras a.attenuation of tumor suppressive function of FBXO16 ubiquitin ligase activates Wnt signaling in glioblastoma[J]. Neoplasia, 2019, 21(1): 106-16.
- 19.Valenta T. Hausmann G and basler K.the many faces and functions of beta-catenin[J]. EMBO J, 2012, 31(12): 2714-36.
- 20.Zhu C, Yamaguchi K, Ohsugi T, et al. Identification of FERM domain-containing protein 5 as a novel target of -catenin/TCF7L2 complex. Cancer Sci. 2017;108(4):612–9. doi: 10.1111/cas.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin AJ, Campian JL, Hui C, et al. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol. 2018;136(2):403–11. doi: 10.1007/s11060-017-2668-5. [DOI] [PubMed] [Google Scholar]
- 22.Rajesh Y, Pal I, Banik P, et al. Insights into molecular therapy of glioma: current challenges and next Generation blueprint. Acta Pharmacol Sin. 2017;38(5):591–613. doi: 10.1038/aps.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett JA, Cai HL, Miao J, et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System (R) (RTS (R)) gene Switch as gene therapy for the treatment of glioma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9b018e11720631a5d5d3833551a2cb45. Cancer Gene Ther. 2018;25(5/6):106–16. doi: 10.1038/s41417-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer DA, Auffinger BM, Murphy JP, et al. Hitting a moving target: glioma stem cells demand new approaches in glioblastoma therapy. Curr Cancer Drug Targets. 2017;17(3):236–54. doi: 10.2174/1568009616666161215161924. [DOI] [PubMed] [Google Scholar]
- 25.Shanmugam MK, Rane G, Kanchi MM, et al. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015;20(2):2728–69. doi: 10.3390/molecules20022728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima FT, Seba V, Silva G, et al. The curcumin analog CH-5 exerts anticancer effects in human osteosarcoma cells via modulation of transcription factors p53/Sp1. Int J Mol Sci. 2018;19(7):E1909. doi: 10.3390/ijms19071909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen P, Huang HP, Wang Y, et al. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J Exp Clin Cancer Res. 2019;38(1):254. doi: 10.1186/s13046-019-1234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang BR, Tsai CH, Chen CC, et al. Curcumin promotes connexin 43 degradation and Temozolomide-Induced apoptosis in glioblastoma cells. American Journal of Chinese Medicine. 2019;47(3):657–74. doi: 10.1142/S0192415X19500344. [DOI] [PubMed] [Google Scholar]
- 29.Weissenberger J, Priester M, Bernreuther C, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1, 2/STAT3 signaling pathway. Clin Cancer Res. 2010;16(23):5781–95. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Wu NY, Wang Y, et al. TRPM7 promotes the epithelialmesenchymal transition in ovarian cancer through the calciumrelated PI3K/AKT oncogenic signaling. J Exp Clin Cancer Res. 2019;38(1):106. doi: 10.1186/s13046-019-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao GX, Xu YY, Weng SQ, et al. CAPS1 promotes colorectal cancer metastasis via Snail mediated epithelial mesenchymal transformation. Oncogene. 2019;38(23):4574–89. doi: 10.1038/s41388-019-0740-7. [DOI] [PubMed] [Google Scholar]
- 32.Jiao DM, Wang J, Lu W, et al. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/ Akt/mTOR signaling pathways in lung cancer. Mol Ther-Oncoly. 2016;3:16018. doi: 10.1038/mto.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JY, Wang X, Wang XJ, et al. Curcumin inhibits the growth via Wnt/beta-catenin pathway in non-small-cell lung cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(21):7492–9. doi: 10.26355/eurrev_201811_16290. [DOI] [PubMed] [Google Scholar]


