Abstract
目的
探索关节腔注射抗坏血酸-氯化铁混合液(AA/FeCl3)对骨性关节炎大鼠关节退变的影响。
方法
将30只成年雄性骨性关节炎大鼠随机等分为两组,自第3周起分别予每周1次关节腔注射生理盐水(对照组)或AA/FeCl3(实验组)。分别于第6、9、12周随机处死每组5只大鼠,使用X线评估关节内骨改变,标本肉眼观、番红/固绿染色和OARSI评分体系评估关节软骨退变,Ⅱ型胶原免疫组化观察细胞外基质变化。
结果
9周时仅对照组X线下见关节面不平整,12周时对照组关节线消失,实验组出现轻微关节面不平;对照组9周标本肉眼可见明显软骨溃疡,12周见大面积软骨缺损,实验组9周软骨表面粗糙,12周见小范围软骨溃疡;番红/固绿及OARSI评分示第9、12周实验组软骨退变显著轻于对照组(9周对照组vs实验组:18.67±0.67 vs 12.17± 2.75;12周:20.11±1.84 vs 13.77±0.40,P < 0.05),第6、9周实验组软骨层Ⅱ型胶原含量显著高于对照组(6周对照组vs实验组:0.36±0.039 vs 0.49±0.029;9周:0.25±0.041 vs 0.38±0.040,P < 0.05)。
结论
早期关节腔注射抗坏血酸-氯化铁混合液能有效延缓骨性关节炎大鼠的软骨退变。
Keywords: 骨性关节炎, 抗坏血酸, 氯化铁, 关节腔注射, 芬顿反应, 潜伏TGFβ1
Abstract
Objective
To assess the effect of ascorbic acid/ferric chloride (AA/FeCl3) in attenuating cartilage damage in rats with osteoarthritis.
Methods
Thirty adult male Wistar rats with surgically induced osteoarthritis were randomized into 2 groups for treatment with intra-articular injection of saline (control group) or AA/FeCl3 mixture (AA group) once a week starting from the third week after the operation. At 6, 9, and 12 weeks after the operation, 5 rats from each group were sacrificed for observing subchondral bone changes on X-ray films and evaluation of cartilage degeneration in the right knee joints using safranin-O/Fast green staining and a modified OARSI scoring system. The degradation of the cartilage matrix was observed by immunohistochemical staining for type Ⅱ collagen.
Results
X-ray examination in saline control group revealed the presence of osteophytes and narrowing of the joint space at 9 weeks, and the joint line disappeared at 12 weeks after the surgery; only slight irregularity of the articular surface was observed in the AA group at 9 and 12 weeks. OARSI scores were significantly lower in AA group than in the control group at 9 weeks (18.67±0.67 vs 12.17±2.75; P < 0.05) and 12 weeks (20.11±1.84 vs 13.77± 0.40; P < 0.05) but not at 6 weeks after the surgery. The content of type 2 collagen in AA group was significantly higher than that in the control group at 6 weeks (0.36±0.039 vs 0.49±0.029; P < 0.05) and 9 weeks after the surgery (0.25±0.041 vs 0.38±0.040; P < 0.05). Conclusions Early intra-articular injection of AA/FeCl3 can effectively delay the progression of post-traumatic osteoarthritis in rats.
Keywords: osteoarthritis, ascorbic acid, ferric chloride, intra-articular injection, fenton reaction, latent TGFβ1
骨性关节炎(OA)是最普遍的慢性关节疾病,近期研究表明中国45岁以上人群中有症状骨性关节炎患者占8.1% [1-2],随着人口老龄化这一比例还将增大。OA最常累及髋、膝、指间关节,表现为关节疼痛和活动障碍,以关节活动严重受限和人工关节置换为结局[3-4],严重降低患者生活质量并产生高额的医疗开销。
OA的病理改变以软骨退变为主,成年的关节软骨为无血管组织,且软骨细胞仅占总体积5%,因此软骨损伤一旦发生难以自发修复[5-6]。目前临床上OA的非手术治疗方式以控制症状为主,尚不能有效延缓病理发展。口服对乙酰氨基酚或NSAIDs类药物能缓解轻中度疼痛,但不能延缓或逆转关节软骨退变的结局,且部分药物具有一定肝毒性或易出现胃肠反应,因此不宜长期服用[7-8]。关节腔注射糖皮质激素能缓解口服药物无法控制的中、重度关节痛,但长期疗效较安慰剂并无明显差异[9],富血小板血浆(PRP)因制备方式不同而疗效差异大[10-11]。
转化生长因子β家族(TGFβs)被认为是维持关节稳态,防止软骨细胞退变,促进软骨组织再生的重要生长因子[12-14]。然而直接向关节腔注射外源性TGFβ耗费巨大,且浓度过高会增加骨赘形成风险[15]。TGFβs在人体微环境包括关节组织内以一种潜伏形式富集(latentTGFβs),在人类滑膜液中检测到平均1.84 ng/mL的latent-TGFβ1,软骨基质中也有平均68.5 ng/mL的latent-TGFβ1 [16-17]。因此将内源性Latent-TGFβ1激活至治疗浓度,从而延缓软骨退变是有可能的。
抗坏血酸(AA)是一种还原性维生素,细胞学实验证实AA能有效降低软骨细胞受到的氧化应激损伤[18]。此外AA可与氯化铁(FeCl3)通过芬顿化学反应产生低毒性过氧化物从而激活latent-TGFβ1 [19]。其他研究发现对碘酸盐诱导的OA大鼠每日喂食AA100 mg/kg能延缓其关节软骨退变[20]。然而长期口服治疗量的AA可能引起胃肠反应,血色素沉着症甚至尿路结石[21-22]。以手术方式增加关节不稳定性而建立的大鼠模型是研究OA药物的重要工具。其中前交叉韧带横断联合内侧半月板摘除(ACLT/pMM)因成功率高,不直接损伤关节软骨,造模周期短而被广泛应用[21-22]。目前尚无研究探索关节腔注射AA/FeCl3混合剂对任何OA模型包括ACLT/pMM大鼠的关节退变的影响。综上所述我们提出假设,关节腔注射AA/FeCl3能激活滑膜液和软骨基质中内源性latent-TGFβ1,使其达到治疗浓度。同时抗坏血酸可保护软骨细胞,减轻氧化应激损伤,最终延缓关节软骨退变。本实验通过观察ACLT/pMM大鼠关节腔注射AA/FeCl3后的关节退变情况验证上述假设。
1. 材料和方法
1.1. 实验动物
本实验选用30只SPF级雄性Wistar大鼠,10~12周龄,体质量300~350 g(南方医科大学南方医院实验动物中心提供)。
1.2. 动物造模和分组
大鼠购回后适应饲养环境1周。术前称重,按体质量予腹腔注射3%戊巴比妥(1 mL/kg),联合左后肢肌注速眠新注射液(0.1 mL/kg)。观察角膜反射、趾反射,消失后进行右后肢备皮,碘伏-酒精消毒,铺无菌手术单(图 1A)。手术方式采用改良Hulth法[23]。沿右膝关节髌腱内缘依次切开皮肤、筋膜、肌腱,向外侧脱位髌骨。屈曲膝关节暴露关节腔,直视下剪断前交叉韧带,前抽屉实验阳性后摘除内侧半月板(图 1B)。术毕逐层缝合切口,酒精消毒切口及周围皮肤(图 1C)。术后大鼠被随机分入生理盐水对照组和AA/FeCl3实验组,每组15只。
1.
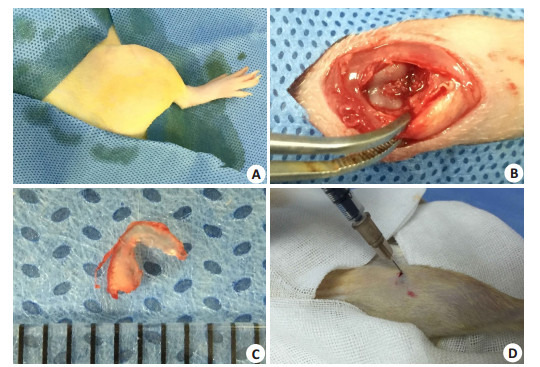
动物手术和关节腔注射
Surgical procedure and intra-articular injection. A: Disinfection; B: Exposure and disconnection of the anterior cruciate ligament; C: Medial meniscus; D: Intraarticular injection.
1.3. 关节腔注射
术后第3周末开始,在戊巴比妥麻醉下按体质量对实验组大鼠关节腔注射AA/FeCl3混合液(0.3 mL/kg)每周1次,对照组注射生理盐水。具体做法由右膝髌骨外上方向关节腔进针,感受到镂空感后立即推注,注射完毕活动关节。AA/FeCl3混合液成分为AA 2 mmol/L,FeCl3 200 μmol/L,溶解于注射用生理盐水,配制后0.5 h内注射。AA和FeCl3从美国SIGMA购买。
1.4. X线检查
术后第6、9、12周末,各组分别随机选取5只大鼠,以戊巴比妥麻醉后送至南方医科大学南方医院影像科,进行右膝曲屈位摄片观察关节面及软骨下骨改变。
1.5. 标本取材与组织学处理
拍摄X线后在麻醉下以颈椎脱臼法处死大鼠。剥离皮肤肌肉,将右侧膝关节从股骨髁上1 cm至胫骨平台下1 cm完整取下,小心暴露关节腔,剔除剩余肌肉组织。PBS冲洗标本2次,拍摄关节面情况。所有标本被置于4%多聚甲醛中固定48 h,随后用0.5 mol/L EDTA脱钙4周。脱钙结束将标本依次浸入60%、70%、80%乙醇、正丁醇,各4 h。脱水完成后进行常规石蜡包块制作,最终以5 μm厚度在缺损最严重部位连续切片。
1.6. 番红O/固绿染色与OARSI评分
切片脱蜡、复水后依次浸染铁苏木素5 min,0.02%固绿1 min,1%冰醋酸30 s,1%番红30 min,随后脱水、透明、封片。基于番红O/固绿染色切片,使用国际骨关节炎研究协会OARSI推荐方法(总分0~24)对软骨退变进行评估[24-25]。简单来说是将内侧胫骨平台软骨损伤深度和范围分别划分为6和4个等级,级数相乘即为得分,每个样本由3位观察者独立评分后取均值。
1.7. Ⅱ型胶原免疫组化及半定量分析
切片脱蜡、复水、抗原修复后,在4 ℃环境中敷大鼠Ⅱ型胶原单克隆抗体(Santa Cruz)14~18 h。次日将切片复温,并按说明书操作DAB免疫组化试剂盒(R & D system),结束后脱水封片。使用Image Pro Plus 6.0软件计算内侧胫骨平台软骨层的累计阳性积分IOD,以及检测区域面积Area,以IOD/Area代表Ⅱ型胶原阳性程度。
1.8. 统计方法
所有数据经SPSS 21.0软件分析,首先进行方差齐性和正态性检验,再使用单因素方差分析进行组间比较,方差齐用LSD检验,不齐用DunnettT3检验,P < 0.05认为差异具有统计学意义。文中数据表述为均值±标准差。
2. 结果
2.1. 动物术后观察
所有大鼠造模术后2 h苏醒,次日反应、进食良好,切口未见异常,右膝关节稍肿胀。术后第3天肿胀消失,大鼠步态恢复正常,术后第14天切口基本愈合。每次关节腔注射后0.5 h大鼠苏醒,次日右后肢能正常活动,所有大鼠未出现明显关节感染。
2.2. 右膝关节X线检查
屈曲位X线检查显示,生理盐水对照组和AA/ FeCl3实验组6周时均无明显改变(图 2A、D)。9周时对照组关节面不平整,可见骨赘,部分大鼠出现脱位。实验组仍未见明显骨关节炎改变(图 2B、E)。两组晚期样本均出现关节线消失,骨赘形成(图 2C、F)。
2.
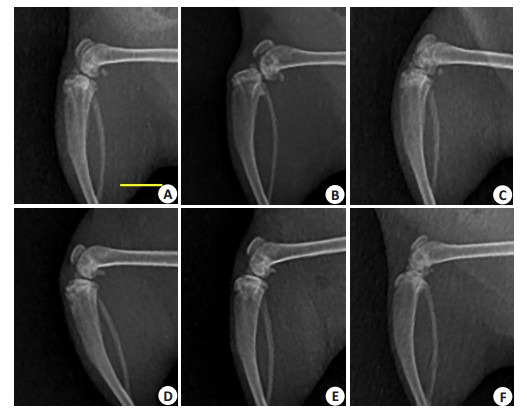
各组大鼠右膝曲屈位X线影像
X-ray films of the right knee (Scale bar=10 mm). A-C: Saline group at 6th, 9th, and 12th weeks, respectively; D-F: AA/FeCl3 group.
2.3. 右膝标本肉眼观
6周时2组内侧关节面均光滑完整(图 3A、D)。9周时对照组股骨内髁出现小面积软骨溃疡(箭头所示),内侧胫骨平台表面粗糙;实验组内侧胫骨平台表面粗糙,未见明显缺损(图 3B、E)。12周时对照组内侧股骨髁、内侧胫骨平台均出现软骨缺损,深及骨板,外侧关节面粗糙;实验组胫骨平台出现明显溃疡(图 3C、F)。
3.
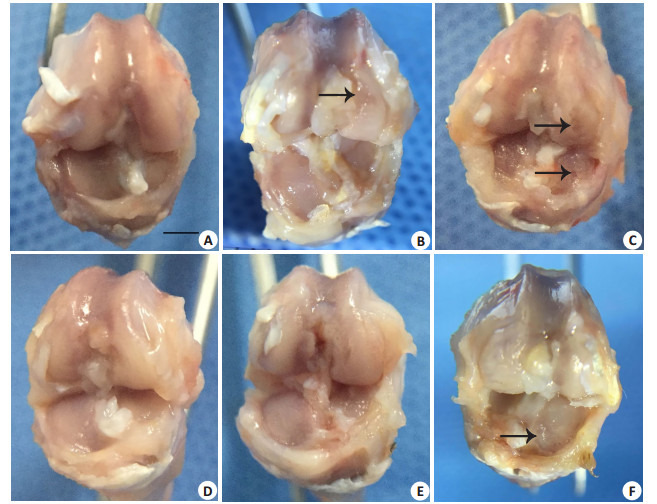
各组大鼠右膝标本
Right knee joint samples (Scale bar=5 mm). A-C: Saline group at 6th, 9th, and 12th weeks, respectively; D-F: AA/FeCl3 group. Black arrows indicate cartilage defects.
2.4. 组化情况及OARSI评分
番红O染色图片可见,6周时对照组内侧胫骨平台出现深及中层软骨的小范围缺损(15%~20%);实验组内侧胫骨平台表层软骨缺损,面积约30%~50%(图 4A、D)。9周时对照组内侧胫骨平台软骨缺损达到深层软骨,面积60%~80%;实验组缺损伤及中层软骨,面积约50%(图 4B、E)。晚期两组均有大面积缺损,对照组深至钙化软骨层,实验组波及深层软骨(图 4C、F)。9周和12周时实验组OARSI评分显著低于对照组(6周18.67±0.67 vs 12.17±2.75,P < 0.05;12周20.11±1.83 vs 13.77±0.40,P < 0.05)。
4.
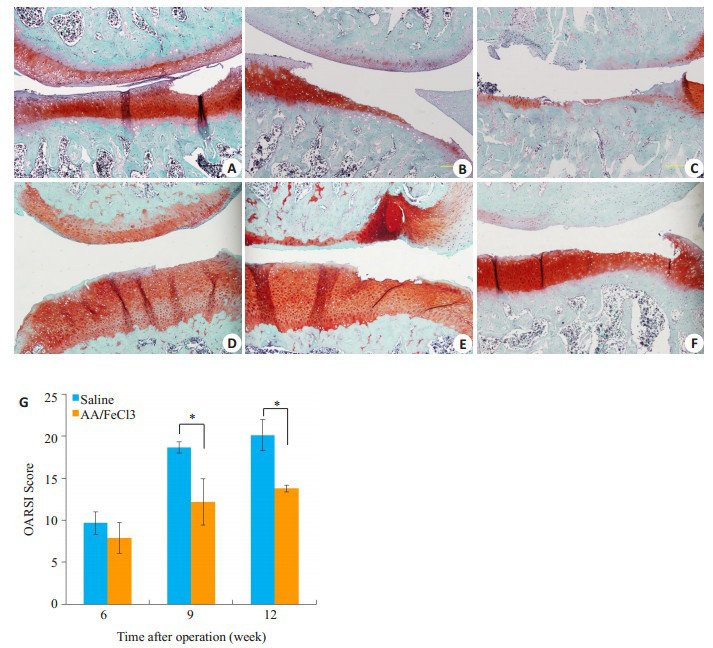
番红O/固绿染色与OARSI评分
Safranin-O/Fast green staining and OARSI scores. A-C: Safranin-O/Fast green staining of saline group at 6th, 9th, 12th weeks. D-F: AA/FeCl3 group. Scale bar=100 μm. G: OARSI scores of each group (*P < 0.05).
2.5. 软骨层Ⅱ型胶原改变
相比实验组,第6、9周对照组胫骨平台软骨层Ⅱ型胶原丢失明显,12周时两组无明显区别(图 5A~F)。半定量结果示6、9周两组间单位软骨面积内Ⅱ型胶原含量有显著差异,实验组多于对照组(6周0.366±0.039 vs 0.493±0.029,P < 0.05;9周0.256±0.041 vs 0.382±0.040,P < 0.05)。
5.
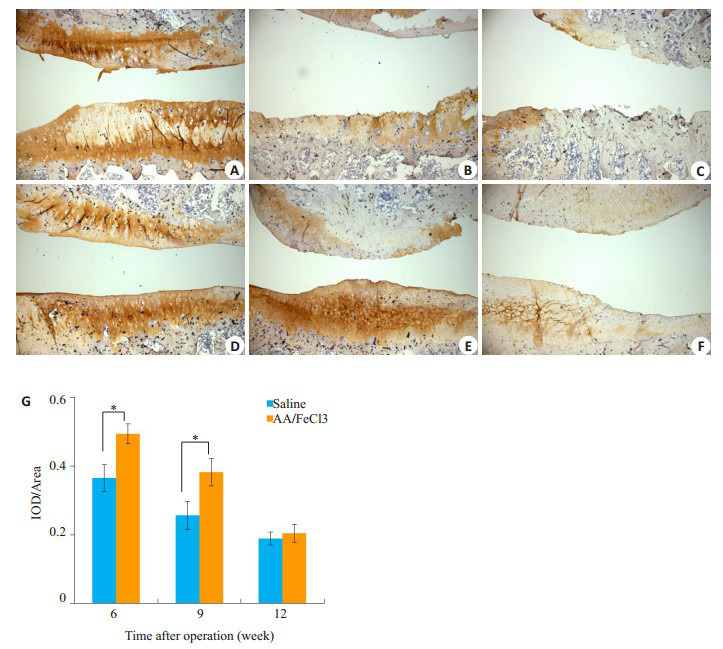
二型胶原染色与半定量分析
Immunohistochemistry of collagen type Ⅱ. A-C: Saline group at 6th, 9th, 12th weeks. D-F: AA/FeCl3 group. (Scale bar=100 μm). G: Semi-quantitative results (*P < 0.05)
3. 讨论
OA的一般病理改变为关节软骨退变(软骨细胞凋亡增加,细胞外基质丢失,软骨表面纤维化),关节边缘骨赘形成,软骨下骨吸收-重构紊乱[26-27]。高龄、关节创伤、肥胖和遗传易感性都被证实是OA的危险因素。OA的分子机制非常复杂,TGFβ1信号通路在OA中有着多重作用。构建了一种关节软骨细胞(AC)特异性的TGFβRⅡ基因敲除鼠,并发现这些小鼠AC高表达OA相关基因如Runx2、Mmp13、Col10以及Adamts5,最终导致自发性OA [28]。在体外利用慢病毒转染使关节软骨细胞高表达TGFβ1蛋白,随后筛选表达稳定且免疫原性低的细胞移植入软骨缺损来治疗OA,目前该治疗技术及产品已通过FDA临床Ⅲ期试验[29]。这些研究表明TGFβ通路能维持AC稳态,延缓软骨退变。而另一方面发现激活ACLT小鼠软骨下骨中骨髓间充质干细胞(BM-MSC)的TGFβ1通路促进骨赘形成,相反抑制该通路能减轻OA的软骨下骨紊乱[30]。可见TGFβ通路在OA中的作用是多重的,TGFβ相关的OA治疗应该针对特异的靶器官或细胞。在本实验中,治疗的起始时间为术后第3周,软骨的退变仍处于非常早期的状态,软骨厚度无显著改变(图 4A、E),因此AA/FeCl3主要激活范围为软骨层,激活软骨层TGFβ通路延缓OA是可能的(图 4G)。另一方面,6周、9周实验组X线影像并未见明显骨改变(图 2D、E),相反对照组可见骨赘形成(图 2B),侧面反映了AA/FeCl3激活范围是安全的。如果以9周、12周为起始点进行治疗,混合液激活范围深至软骨下骨,那么加重软骨下骨紊乱是有可能的,但这需要进一步实验探索。
AA作为还原性维生素对软骨细胞氧化损伤有很好的保护作用[18],有研究表明口服AA有预防OA软骨退变的作用[20, 31]。然而也有学者指出口服AA不能有效预防OA。对几内亚猪喂食AA(2.5~150 mg/d),发现喂食后自发性OA严重程度与计量正相关,并且在新生的骨赘中观察到活性TGFβ1富集[32]。对膝关节创伤患者进行跟踪研究发现,血清高浓度AA是发展为影像学OA的危险因素[33]。这些研究表明口服AA对OA的预防效果并不明确,相反有可能促进关节内骨赘形成,其原因可能与广泛的激活了TGFβ通路有关。在本研究中,关节腔注射的AA总量非常低(0.117 mg/kg),但因为在关节腔内直接接触软骨组织,因此对Latent TGFβ1及其通路的激活更加特异和高效,避免因全身用药引起的不良反应。
成年关节软骨的主要细胞外基质成分是胶原,尤其是Ⅱ型和Ⅲ型,它们很好的维持了软骨弹性。OA软骨退变时Ⅱ型胶原减少,X型胶原增多,提示软骨细胞终末分化。AA可通过调节转录因子SVCT2,提高Ⅱ型胶原在关节软骨细胞的转录和转录后表达水平[34]。我们的研究也发现,直接关节腔注射AA能显著降低OA大鼠软骨中Ⅱ型胶原丢失,尤其在早、中期(图 5)。
虽然本实验观察到AA/FeCl3混合液能延缓ACLT/ pMM模型大鼠的软骨退变,但在转化应用前还有许多问题需要阐明。在未来研究中我们将深入探索该治疗涉及的分子机制,从而获得更加安全有效的成分配比,尝试其他OA造模方法,并在大动物关节内进行验证。同时将细化不同阶段OA对该混合液的反应,并将软骨下骨和滑膜的改变纳入观察范围。
Biographies
廖哲霆,硕士,医师,E-mail: ztliao92@163.com
邢祯全,本科,副主任医师,E-mail: laogao12@189.cn
Funding Statement
国家自然科学基金(31328008);广东省自然科学基金(2014A030313275);广东省自然科学基金(s2013010014253)
Supported by National Natural Science Foundation of China (31328008)
Contributor Information
廖 哲霆 (Zhenting LIAO), Email: ztliao92@163.com.
邢 祯全 (Zhenquan XING), Email: laogao12@189.cn.
赵 亮 (Liang ZHAO), Email: lzhaonf@126.com.
References
- 1.Tang X, Wang S, Zhan S, et al. The Prevalence of Symptomatic Knee Osteoarthritis in China: Results From the China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68(3):648–653. doi: 10.1002/art.39465. [Tang X, Wang S, Zhan S, et al. The Prevalence of Symptomatic Knee Osteoarthritis in China: Results From the China Health and Retirement Longitudinal Study[J]. Arthritis Rheumatol, 2016, 68 (3): 648-653.] [DOI] [PubMed] [Google Scholar]
- 2.Li Z. A new look at rheumatology in China—opportunities and challenges. Nat Rev Rheumatol. 2015;11:313–7. doi: 10.1038/nrrheum.2014.218. [Li Z. A new look at rheumatology in China—opportunities and challenges[J]. Nat Rev Rheumatol, 2015, 11: 313-7.] [DOI] [PubMed] [Google Scholar]
- 3.Oliveria S, Felson D, Reed J, et al. Incidence of Symptomatic Hand, Hip, and Knee Osteoarthritis among Patients in a Health Maintenance Organization. Arthritis Rheumatol. 1995;38(8):1134–41. doi: 10.1002/(ISSN)1529-0131. [Oliveria S, Felson D, Reed J, et al. Incidence of Symptomatic Hand, Hip, and Knee Osteoarthritis among Patients in a Health Maintenance Organization[J]. Arthritis Rheumatol, 1995, 38(8): 1134-41.] [DOI] [PubMed] [Google Scholar]
- 4.Bijlsma J, Berenbaum F, Lafeber F, et al. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115. doi: 10.1016/S0140-6736(11)60243-2. [Bijlsma J, Berenbaum F, Lafeber F, et al. Osteoarthritis: an update with relevance for clinical practice[J]. Lancet, 2011, 377(9783): 2115.] [DOI] [PubMed] [Google Scholar]
- 5.Boushell MK, Hung CT, Hunziker EB, et al. Current strategies for integrative cartilage repair. Connect Tissue Res. 2017;58(5):393–406. doi: 10.1080/03008207.2016.1231180. [Boushell MK, Hung CT, Hunziker EB, et al. Current strategies for integrative cartilage repair[J]. Connect Tissue Res, 2017, 58(5): 393-406.] [DOI] [PubMed] [Google Scholar]
- 6.Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage[J]. Nat Rev Rheumatol, 2015, 11: 21-34.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiodt F, Rochling F, Casey D, et al. Acetaminophen Toxicity in an Urban County Hospital. N Engl J Med. 1997;337:1112–7. doi: 10.1056/NEJM199710163371602. [Schiodt F, Rochling F, Casey D, et al. Acetaminophen Toxicity in an Urban County Hospital[J]. N Engl J Med, 1997, 337: 1112-7.] [DOI] [PubMed] [Google Scholar]
- 8.Garcia RL, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal antiinflammatory drugs. Lancet. 1994;343(8900):769–772. doi: 10.1016/S0140-6736(94)91843-0. [Garcia RL, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal antiinflammatory drugs[J]. Lancet, 1994, 343(8900): 769-772.] [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part Ⅱ: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62. doi: 10.1016/j.joca.2007.12.013. [Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part Ⅱ: OARSI evidence-based, expert consensus guidelines[J]. Osteoarthritis Cartilage, 2008, 16(2): 137-62.] [DOI] [PubMed] [Google Scholar]
- 10.Weinberg ME, Kaplan DJ, Pham H, et al. Injectable biological treatments for osteoarthritis of the knee. JBJS Rev. 2017;5(4):e2. doi: 10.2106/JBJS.RVW.16.00028. [Weinberg ME, Kaplan DJ, Pham H, et al. Injectable biological treatments for osteoarthritis of the knee[J]. JBJS Rev, 2017, 5(4): e2.] [DOI] [PubMed] [Google Scholar]
- 11.Macmahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists1. radiology. 2009;252(3):647. doi: 10.1148/radiol.2523081929. [Macmahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists1[J]. radiology, 2009, 252(3): 647.] [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Li S, Chen D. TGF-βsignaling and the development of osteoarthritis. Bone Res. 2014;2:14002. doi: 10.1038/boneres.2014.2. [Shen J, Li S, Chen D. TGF-βsignaling and the development of osteoarthritis[J]. Bone Res, 2014, 2: 14002.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C, Cook J, Mendelson A. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–48. doi: 10.1016/S0140-6736(10)60668-X. [Lee C, Cook J, Mendelson A. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study [J]. Lancet, 2010, 376: 440-48.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Tao H, Jin C, et al. Transforming growth factor-β1 induces type Ⅱ collagen and aggrecan expression via activation of extracellular signal -regulated kinase 1/2 and Smad2/3 signaling pathways. Mol Med Rep. 2015;12(4):5573–9. doi: 10.3892/mmr.2015.4068. [Zhu Y, Tao H, Jin C, et al. Transforming growth factor-β1 induces type Ⅱ collagen and aggrecan expression via activation of extracellular signal -regulated kinase 1/2 and Smad2/3 signaling pathways[J]. Mol Med Rep, 2015, 12(4): 5573-9.] [DOI] [PubMed] [Google Scholar]
- 15.van Beuningen HM, Glansbeek HL, van der Kraan PM, et al. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-βinjections. Osteoarthritis Cartilage. 2000;8(1):25–33. doi: 10.1053/joca.1999.0267. [van Beuningen HM, Glansbeek HL, van der Kraan PM, et al. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-βinjections[J]. Osteoarthritis Cartilage, 2000, 8(1): 25-33.] [DOI] [PubMed] [Google Scholar]
- 16.Albro MB, Cigan AD, Nims RJ, et al. Shearing of synovial fluid activates latent TGF-β. Osteoarthritis Cartilage. 2012;20(11):1374–82. doi: 10.1016/j.joca.2012.07.006. [Albro MB, Cigan AD, Nims RJ, et al. Shearing of synovial fluid activates latent TGF-β[J]. Osteoarthritis Cartilage, 2012, 20(11): 1374-82.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albro MB, Nims RJ, Cigan AD, et al. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-β. J Biomech. 2013;46(8):1433–9. doi: 10.1016/j.jbiomech.2013.03.006. [Albro MB, Nims RJ, Cigan AD, et al. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-β[J]. J Biomech, 2013, 46(8): 1433-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Z, Huo L, Li P, et al. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol Med Rep. 2015;12(5):7086–92. doi: 10.3892/mmr.2015.4231. [Chang Z, Huo L, Li P, et al. Ascorbic acid provides protection for human chondrocytes against oxidative stress[J]. Mol Med Rep, 2015, 12(5): 7086-92.] [DOI] [PubMed] [Google Scholar]
- 19.Jobling MF, Mott JD, Finnegan MT. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat Res. 2006;166(6):839–48. doi: 10.1667/RR0695.1. [Jobling MF, Mott JD, Finnegan MT. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species[J]. Radiat Res, 2006, 166(6): 839-48.] [DOI] [PubMed] [Google Scholar]
- 20.Chiu P, Hu Y, Huang T, et al. Vitamin C protects chondrocytes against monosodium iodoacetate-induced osteoarthritis by multiple pathways. http://onlinelibrary.wiley.com/doi/10.1002/art.1780390417/full. Int J Mol Sci. 2017;18(1):38. doi: 10.3390/ijms18010038. [Chiu P, Hu Y, Huang T, et al. Vitamin C protects chondrocytes against monosodium iodoacetate-induced osteoarthritis by multiple pathways[J]. Int J Mol Sci, 2017, 18(1): 38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003;2(1):7. doi: 10.1186/1475-2891-2-7. [Naidu KA. Vitamin C in human health and disease is still a mystery? An overview[J]. Nutr J, 2003, 2(1): 7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook J, Reddy M. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet1, 2. http://jn.nutrition.org/content/136/3/576.long. Am J Clin Nutr. 2001;73(1):93–8. doi: 10.1093/ajcn/73.1.93. [Cook J, Reddy M. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet1, 2[J]. Am J Clin Nutr, 2001, 73 (1): 93-8.] [DOI] [PubMed] [Google Scholar]
- 23.王 云峰, 白 人骁, 张 扬, et al. 改良Hulth模型复制膝不同时期骨关节炎的实验研究. http://www.docin.com/p-1127350667.html. 天津医科大学学报. 2009;(3):400–4. [王云峰, 白人骁, 张扬, 等.改良Hulth模型复制膝不同时期骨关节炎的实验研究[J].天津医科大学学报, 2009(3): 400-4.] [Google Scholar]
- 24.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. https://www.sciencedirect.com/science/article/pii/S1063458405001974. Osteoarthritis Cartilage. 2006;(14):13–29. doi: 10.1016/j.joca.2005.07.014. [Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging[J]. Osteoarthritis Cartilage, 2006(14): 13-29.] [DOI] [PubMed] [Google Scholar]
- 25.Gerwin N, Bendele AM, Glasson S. The OARSI histopathology initiative e recommendations for histological assessments of osteoarthritis in the rat. https://www.sciencedirect.com/science/article/pii/S1063458410002384. Osteoarthritis Cartilage. 2010;(18) doi: 10.1016/j.joca.2010.05.030. [Gerwin N, Bendele AM, Glasson S. The OARSI histopathology initiative e recommendations for histological assessments of osteoarthritis in the rat[J]. Osteoarthritis Cartilage, 2010(18): S24-S34.] [DOI] [PubMed] [Google Scholar]
- 26.Karsenty G. An aggrecanase and osteoarthritis. N Engl J Med. 2005;353(5):522–3. doi: 10.1056/NEJMcibr051399. [Karsenty G. An aggrecanase and osteoarthritis[J]. N Engl J Med, 2005, 353(5): 522-3.] [DOI] [PubMed] [Google Scholar]
- 27.Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis. 2010;2(6):349–59. doi: 10.1177/1759720X10378373. [Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis [J]. Ther Adv Musculoskelet Dis, 2010: 2(6): 349-59.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen J, Li J, Wang B, et al. Deletion of the transforming growth factor β receptor type Ⅱ gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheumatol. 2013;65(12):3107–19. doi: 10.1002/art.38122. [Shen J, Li J, Wang B, et al. Deletion of the transforming growth factor β receptor type Ⅱ gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice[J]. Arthritis Rheumatol, 2013, 65(12): 3107-19.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho J, Kim T, Park Y, et al. Invossa TM (Tissuegene-C) in patients with osteoarthritis: A phase Ⅲ trial. Osteoarthritis Cartilage. 2016;24:S190. [Cho J, Kim T, Park Y, et al. Invossa TM (Tissuegene-C) in patients with osteoarthritis: A phase Ⅲ trial[J]. Osteoarthritis Cartilage, 2016, 24: S190.] [Google Scholar]
- 30.Zhen G, Wen C, Jia X, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–12. doi: 10.1038/nm.3143. [Zhen G, Wen C, Jia X, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis[J]. Nat Med, 2013, 19(6): 704-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Zeng C, Wei J, et al. Associations between dietary antioxidants intake and radiographic knee osteoarthritis. Clin Rheumatol. 2016;35(6):1585–92. doi: 10.1007/s10067-016-3177-1. [Li H, Zeng C, Wei J, et al. Associations between dietary antioxidants intake and radiographic knee osteoarthritis[J]. Clin Rheumatol, 2016, 35(6): 1585-92.] [DOI] [PubMed] [Google Scholar]
- 32.Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheumatol. 2004;50(6):1822–31. doi: 10.1002/(ISSN)1529-0131. [Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model [J]. Arthritis Rheumatol, 2004, 50(6): 1822-31.] [DOI] [PubMed] [Google Scholar]
- 33.Chaganti RK, Tolstykh I, Javaid MK, et al. High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190–6. doi: 10.1016/j.joca.2013.11.008. [Chaganti RK, Tolstykh I, Javaid MK, et al. High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis[J]. Osteoarthritis Cartilage, 2014, 22(2): 190-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mcnulty AL, Vail TP, Kraus VB. Chondrocyte transport and concentration of ascorbic acid is mediated by SVCT2. Biochim Biophys Acta. 2005;1712(2):212–21. doi: 10.1016/j.bbamem.2005.04.009. [Mcnulty AL, Vail TP, Kraus VB. Chondrocyte transport and concentration of ascorbic acid is mediated by SVCT2[J]. Biochim Biophys Acta, 2005, 1712(2): 212-21.] [DOI] [PubMed] [Google Scholar]


