Abstract
B细胞连接蛋白,是B细胞受体通路的关键接头蛋白。B细胞连接蛋白通过自身的结构特性及蛋白互作网络参与调控PLC-γ的活性和Ras通路激活,在B细胞受体信号转导中发挥重要作用,广泛参与调控B细胞的增殖、分化、凋亡及信号传导等过程,并与过敏性疾病、多发性硬化症、染色体组非整倍体、无丙种球蛋白血症、B淋巴细胞白血病、淋巴瘤的发生发展密切相关。本文对B细胞连接蛋白的结构、生物学功能及其在B细胞相关疾病中的作用进行综述:B细胞连接蛋白可与多个效应蛋白协作,激活B细胞受体信号通路,从而影响着B细胞的发育成熟及功能调节;B细胞连接蛋白发生功能性突变,会破坏B细胞稳态并影响B细胞发育成熟,从而导致B细胞相关疾病的发生。全面总结B细胞连接蛋白的生物学功能不仅有助于了解B细胞相关疾病的发生机制,也有望将B细胞连接蛋白作为相关疾病的治疗靶标提供新思路和突破口。
Keywords: B细胞连接蛋白, B细胞受体, 信号通路, 分化, B细胞相关疾病
Abstract
B cell linker (BLNK) is a key linker protein of B cell receptor (BCR) signaling pathway. BLNK participates in the regulation of PLC-γactivity and the activation of Ras pathway through its typical structure and interaction network with other proteins, and is thus widely involved in the regulation of B cell proliferation, differentiation, apoptosis and signal transduction. Furthermore, it is closely related to anaphylactic diseases, multiple sclerosis, chromosomal aneuploidy, aneuglobulinemia, B lymphocytic leukemia and lymphoma. Herein we review the structure and biological function of BLNK and its role in B cell-related diseases. BLNK can cooperate with a series of effective proteins to activate BCR signaling pathway, thereby regulating the development, maturation and function of B cells. The functional mutation of BLNK can destroy the homeostasis of B cells and affect the development and maturation of B cells, which leads to the occurrence of B cell related diseases. A comprehensive understanding of the biological functions of BLNK not only provides insights into the pathogenesis of B cell-related diseases, but also inspires new ideas and helps to find breakthroughs for the treatment of these diseases with BLNK as the therapeutic target.
Keywords: B-cell linker, B cell receptor, signaling pathway, differentiation, B cell-related diseases
B细胞连接蛋白(BLNK)又称SLP-65或BASH,典型的BLNK的基本结构包括N-末端亮氨酸拉链,其后是“酸性”区域,富含脯氨酸和关键区域Src同源结构域2(SH2)[1],亮氨酸拉链因其卷曲螺旋的特性,能与膜蛋白相互作用,使BLNK定位于质膜。BLNK具有多个受体酪氨酸激酶磷酸化位点,磷酸化的BLNK可以招募多种效应蛋白(包括Grb2、Sos、PLCγ和Nck),进而活化PLC-γ2、Ras和Rac1-JNK信号通路[2-3]。然而关于BLNK结构的文献报道较少,对蛋白质亚基组成、折叠方式等方面的认识尚且不足,也是今后BLNK结构研究亟待解决的问题。
1. BLNK生物学作用
1.1. BLNK在B细胞发育过程中的作用
BLNK在祖B细胞向前B细胞转化的进程中起关键作用。BLNK在骨髓细胞发育早期表达水平较高,而随着B细胞的发育成熟其表达水平逐渐降低。BLNK调控祖B细胞的发育依赖于B细胞受体(BCR)通路的活化[4]。BCR前体产生过程中发生μ重链基因重排、细胞表面标记物改变等事件,而BLNK在协调上述过程中发挥了巨大作用,其作为“衔接蛋白”将BCR相关激酶与多种信号蛋白连接起来,组成B细胞信号通路,影响着B细胞的发育成熟及功能调节。BCR前体和BLNK的缺陷,将导致B细胞的发育停滞在前B细胞阶段。研究表明,BLNK在整个B细胞个体发育过程中表达,并在B细胞发育周期中具有潜在的重要作用[5],而前B细胞分化为早期幼稚B细胞的过程同样需要BLNK的调控[6]。
1.2. BLNK在BCR信号通路中作用
BCR是B细胞表面重要的免疫球蛋白。高水平BLNK表达量与BCR的活化密切相关[7]。当BCR活化发生偶联后,激活至少3种蛋白酪氨酸激酶(包括Src-PTK、SYK、BTK)以调节下游因子的活化[8-9]。BCR的ITAM基序与蛋白酪氨酸激酶结合,使蛋白酪氨酸激酶发生酪氨酸磷酸化,磷酸化的蛋白酪氨酸激酶继而与BLNK相互作用,促进BLNK的酪氨酸磷酸化。磷酸化的BLNK可以招募下游的多个效应蛋白,激活相应信号通路(图 1)。BLNK的活性对BCR信号通路的关闭同样重要:激酶PI3K介导了下游AKT/PKB的激活,从而引起BTK(S51/T495)和SYK(S295/S297)的丝氨酸/苏氨酸位点磷酸化;同时,AKT和HPK1共同使BLNK的S285和T152发生磷酸化,导致BLNK蛋白发生泛素化降解,从而终止了BCR信号通路的传递[10-11]。
1.
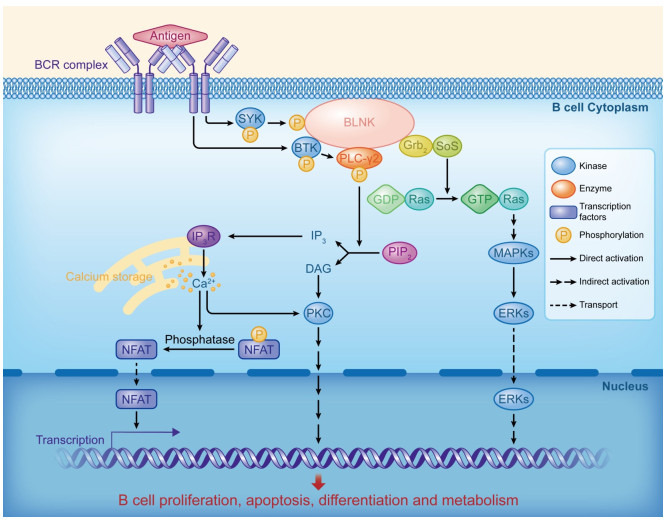
BLNK在BCR信号通路中的作用机制
Mechanism of BLNK in the BCR signaling pathway.
除了BCR通路外,BLNK的活性还与NOTCH通路的激活密切相关。在正常B细胞中,NOTCH通路的激活可以增强BCR对胞外信号的应答并提高BLNK的表达水平;利用特异性抗体封闭NOTCH受体后,NOTCH和BCR通路的活性同时显著降低,进而影响了NOTCH通路相关转录调控分子的表达以及B细胞的成熟[12]。
1.2.1. BLNK调控PLC-γ的活性
BLNK和PLC-γ是激活Ca2+信号介导的BCR信号通路的关键组分。BLNK通过结合SYK和BTK参与PLC-γ2分子的活化[13]。过表达BLNK促进PLC-γ的酪氨酸磷酸化、受体介导的Ca2+应答及NFAT的转录活化;反之,BLNK显性负突变体抑制PLC-γ磷酸化、Ca2+应答及NFAT的转录活化进程。PLC-γ磷酸化的具体机制如下:BCR复合物耦联后激活下游的SYK,SYK使BLNK发生酪氨酸磷酸化并促进BLNK从细胞质向细胞膜跃迁,BLNK与PLC-γ的SH2结构域结合并促进其磷酸化,同时将PIP2切割成三磷酸肌醇和二酰基甘油,导致游离Ca2+浓度增加和PKC的活化。三磷酸肌醇与其受体在内质网结合后,向细胞质释放Ca2+,游离的Ca2+可激活丝氨酸/苏氨酸、磷酸酶、钙调磷酸酶等多种钙依赖性酶,从而使NFAT发生去磷酸化,促进NFAT从细胞质转移到细胞核参与转录调控,进而促进B细胞的活化[14]。
E3泛素连接酶Cbl参与了SYK负调控PLC-γ2的生物学进程。BCR复合物的偶联活化能够促进Cbl的SH2结构域与BLNK结合,抑制BLNK与PLC-γ的结合,从而抑制PLC-γ2信号通路的激活;同时,Cbl的SH2结构域还能与SYK结合,抑制SYK的活化,进而抑制PLC-γ的磷酸化[15]。受体酪氨酸激酶BTK也参与了PLC-γ2通路的激活。BTK的PH结构域与Ptd Ins-3, 4, 5-P3结合,促进BTK向细胞膜迁移,随后,BTK的SH2结构域与BLNK结合,使BLNK发生磷酸化,磷酸化的BLNK为BTK和PLC-γ提供结合位点,从而激活PLC-γ2[16]。
1.2.2. BLNK参与Ras通路的激活
BLNK能够与Grb2的SH2及SH3结构域结合,使Grb2被招募到细胞膜上,同时Grb2的SH3结构域也能够与鸟苷释放蛋白Sos的脯氨酸富集区结合,Sos促进Ras释放GDP,结合GTP,使Ras蛋白由非性状态转变为活性状态;激活的Ras通过诱发酶促级联反应而激活MAPK/ERK信号通路[17-18]。
2. BLNK与B细胞相关疾病
BLNK与B细胞相关疾病的发生发展关系极为密切,是B细胞病变与疾病发生过程中的关键作用分子,BLNK在B细胞成熟的不同阶段可能发生功能性突变,破坏B细胞稳态并导致B细胞发育成熟障碍,从而导致B细胞相关疾病的发生。近年来人们对BLNK在各种B细胞相关疾病中的作用机制进行了诸多探索。
2.1. BLNK与过敏性疾病的关系
IgE抗体的异常产生会导致过敏性疾病的发生。正常情况下,IgE+B细胞极少分化为记忆B细胞或长寿命浆细胞,因为它们只是短暂地参与生发中心的合成。但膜IgE可通过BLNK-JNK/p38通路而触发浆细胞的快速分化和凋亡,并且这种浆细胞分化不依赖抗原和细胞外环境,反应涉及多个IgE结构域和SYK、BTK等关键分子。在BLNK-/-小鼠中生发中心IgE+B细胞增殖的抑制解除和IgE过度产生的进程被延长[19-20]。可见BLNK与过敏性疾病有密切关系。
2.2. BLNK与多发性硬化症的关系
多发性硬化症是中枢神经系统慢性炎症性脱髓鞘疾病。研究表明,B淋巴细胞去除药物利妥昔单抗在治疗复发-缓解型多发性硬化症患者时具有良好的临床效果,证实B细胞参与多发性硬化症的发病进程,实验性自身免疫性脑脊髓炎(EAE)是研究多发性硬化症的理想动物模型[21]。金桂花等[22]利用MOG 35-55多肽抗原配剂分别免疫野生型C57BL/6鼠与BLNK-/-鼠制备动物EAE模型,发现BLNK表达缺失可加重EAE反应,使EAE病程延长,提示BLNK参与了EAE的发生发展。该课题组还发现BLNK-/-鼠脾脏中调节性T细胞(Treg)数量较C57BL/6鼠显著减少[22],表明在EAE中,B细胞可能通过促进Treg的增殖维持其数量,使得Treg细胞发挥抑制EAE的作用,而BLNK的功能性缺失可能通过影响B细胞的生物学状态从而调节Treg在EAE中的作用。Ray等[23]认为B细胞依靠GITR配体与Treg表面表达的GITR结合,从而促进EAE中Treg的增殖以维持调节免疫的稳态;该课题组检测了EAE小鼠血清中的抗MOG 35-55特异性抗体,发现无论在发病的高峰期还是恢复期,BLNK-/-小鼠血清中的MOG特异性IgM和IgG水平始终较低,表明BLNK缺失能够显著抑制MOG特异性抗体的产生;另外,在BLNK-/-小鼠中,B细胞分泌的IL-10明显降低,而IL-2的表达增高,由B细胞分泌的IL-10可抑制巨噬细胞和树突状细胞分泌IL-12,并抑制T细胞增殖及分化,从而抑制由Th1型T细胞介导的疾病,提示BLNK可能通过调控B细胞分泌的Th1/Th2细胞因子而影响EAE炎症反应。T细胞的活化、抗体的升高、细胞因子的异常调控等事件的共同作用导致了EAE的发生。
2.3. BLNK与染色体组非整倍体的关系
染色体组的非整倍体特性是恶性肿瘤的典型标志。非整倍体可能是恶性肿瘤发生的主要原因。BLNK通过调控胞质分裂,参与调控p53基因的转录活性,从而维护染色体的完整性,阻止非整倍体的产生。p53基因在50%以上的人类癌症中均发生突变,是肿瘤复杂信号传导网络中的中枢调控分子。p53基因是BLNK的转录调控靶点,过表达BLNK能够增强p53基因的表达,从而促进p53介导的DNA损伤修复;另一方面,外源BLNK的异位表达能够抑制细胞分裂,通过调控p53稳定性而维持基因组的完整性,从而阻止了非整倍体的发生[24]。
2.4. BLNK与无丙种球蛋白血症的关系
先天性无丙种球蛋白血症是一种遗传性免疫系统紊乱疾病,主要由早期B细胞发育过程中的先天错误而导致机体无法正常产生抗体,临床表现为血液循环中B淋巴细胞数量减少,各类免疫球蛋白含量明显降低或缺乏。X-连锁无球蛋白血症是最主要的病理亚型,主要由BTK基因突变导致的B淋巴细胞分化发育障碍引起[25]。临床上还发现与X-连锁无球蛋白血症临床表现相似,但无X染色体关联性的疾病,称为非Btk基因突变的先天性无丙种球蛋白血症,它的发生可能由BLNK基因突变引起。Lagresle等[26]将BLNK基因进行移码突变,导致骨髓中的B细胞发育阻滞在pre-B1至pre-B2阶段。与健康B细胞相比,BLNK缺失突变细胞中的BLNK转录产物和蛋白质表达急剧减少,B淋巴细胞数量显著降低;另外,BLNK缺失突变还能破坏B细胞稳态并造成未成熟B细胞的异常扩增及B细胞分化发育异常,导致成熟B细胞和各种类型的免疫球蛋白缺乏[27-28]。
2.5. BLNK与B淋巴细胞白血病的关系
前B细胞白血病与BCR前体信号通路密切相关,BLNK和BTK作为BCR前体信号通路的关键蛋白,是前B细胞白血病常见的治疗靶点[29]。在BLNK-/-小鼠中,大约15%的小鼠在出生6月后自发形成前B细胞白血病。在免疫缺陷小鼠体内注射BLNK-/-的前B细胞,3~5周后可引发小鼠白血病。从机理上解释:BTK与BLNK发挥协同作用,终止BCR前体介导的前B细胞增殖扩张,从而阻止B细胞白血病的发生。
除了B细胞白血病,儿童急性淋巴细胞白血病(acute lymphoblastic leukemia, ALL)细胞中也往往缺乏BLNK蛋白表达,BLNK的表达与ALL患者的预后显著相关,表明BLNK是B细胞相关肿瘤的关键抑癌基因[30]。JAK3/STAT5信号通路的激活是驱动前B细胞白血病细胞增殖的主要动力,而该通路的激活在一定程度上需要IL-7的参与。在ALL中,BLNK可以抑制JAK3/STAT5通路活性及IL-7R的表达,JAK3活性的降低增强了细胞周期抑制基因p27kip1的表达水平,造成细胞周期阻滞,从而促进ALL细胞凋亡。BLNK对JAK3的抑制作用依赖于BLNK与JAK3的直接相互作用。BLNK的体细胞丢失和伴随的JAK3/STAT 5通路激活是导致B细胞白血病发生的主要原因[31]。
PAX5基因是儿童和成人ALL体细胞突变最常见的基因,其突变频率在儿童和成人ALL体细胞中分别为38.9%和34%。PAX5是BLNK转录激活必需的转录因子。PAX5的融合蛋白(如PAX5-PML)已被证实对PAX5转录活性有明显的负调控作用,其在B淋巴细胞中可抑制PAX5基因的表达,导致BLNK的表达水平被选择性地抑制。Imoto等[32]将PAX5-PML融合基因转入正常的小鼠祖B细胞中,导致细胞发育停滞在祖B细胞阶段,且小鼠在长时间饲养后可自发形成ALL。PAX5基因的突变抑制BLNK的表达,BLNK的选择性抑制对ALL的发生具有重要意义[33]。
在慢性淋巴细胞白血病中,BLNK和CD5高表达,而二者在正常B或T细胞中不表达。BLNK和CD5是STAT 3磷酸化所必需的。敲低BLNK或CD5可显著降低慢性淋巴细胞白血病细胞中STAT 3的丝氨酸水平,可见BLNK是STAT 3磷酸化状态的重要调控因子[34]。
2.6. BLNK与淋巴瘤的关系
致癌基因rel的持续性激活是许多肿瘤的标志事件之一。Gupta等[35]通过微阵列分析发现v-rel和c-rel是淋巴瘤细胞的关键转录因子,rel与BLNK基因的上游启动子结合,可负调控关键BCR元件及BLNK的转录活性。v-rel和c-rel的转录调控作用及BLNK的功能性抑制是淋巴细胞恶性转化的重要因素,且两者在多种淋巴瘤中的表达呈显著负相关。在原发性纵膈大B细胞淋巴瘤和霍奇金淋巴瘤中,c-rel蛋白发生明显的核积聚现象,导致BLNK及各种BCR通路信号分子表达下调;c-rel还可以诱导B细胞凋亡并增强B细胞肿瘤和其他肿瘤细胞对化疗的敏感性[35]。该课题组还发现,BLNK的过表达强烈拮抗由v-rel引起的淋巴细胞恶性转化,提示BLNK可能通过负反馈调控v-rel及其下游通路发挥抑癌基因作用[36]。
3. BLNK的研究前景
BLNK在BCR信号通路中发挥重要作用,通过多条信号通路的级联传递,最终影响特定基因的转录表达,参与B细胞的增殖、分化、凋亡等生物学进程。随着对BLNK的结构、功能及分子作用机制认识的不断深入,人们越来越意识到BLNK作用方式的多效性、复杂性和重要性。多项研究已证实由BLNK基因突变或缺失引起的B细胞功能紊乱可导致自身免疫性疾病的发生。因此,平衡BLNK的表达水平和维持B细胞功能稳定被认为是多种自身免疫性疾病的潜在治疗方向[37]。BLNK在特定疾病中还可能以融合基因的方式发挥生物学作用。在费城染色体样急性淋巴细胞白血病中,检测到BLNK的5’端与DNTT基因发生融合,这种不常见的融合方式可能为研发靶向BLNK的功能性抑制剂提供新的思路[38]。作者一直致力于BLNK在恶性肿瘤中的机制研究,目前已发现BLNK能够在体内和体外促进乳腺癌的增殖和转移(未发表),然而BLNK促进乳腺癌转移的作用靶点尚不清楚。最后,围绕BLNK的作用机制尚有一些问题亟待解决:BLNK如何影响B细胞的发育?BLNK在BCR信号通路中还有哪些未知的功能机制?BLNK如何影响B细胞相关疾病的发生发展?这些研究方向也将为自身免疫性疾病的治疗方案提供新的思路。
Biography
肖斌,博士,技师,E-mail: xiaobin2518@163.com
Funding Statement
军队后勤科研项目(CWH17C017);广州市科技计划项目(201804010186)
Contributor Information
肖 斌 (Bin XIAO), Email: xiaobin2518@163.com.
李 林海 (Linhai LI), Email: mature303@126.com.
References
- 1.Han Y, Xin L, Shi B, et al. Identification and characterisation of the immune response properties of Lampetra japonica BLNK. http://www.nature.com/articles/srep25308. Sci Rep. 2016;5(6):25308–16. doi: 10.1038/srep25308. [Han Y, Xin L, Shi B, et al.Identification and characterisation of the immune response properties of Lampetra japonica BLNK[J].Sci Rep, 2016, 5(6):25308-16.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94(3):193–205. doi: 10.1111/ejh.2015.94.issue-3. [Seda V, Mraz M.B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells[J].Eur J Haematol, 2015, 94(3):193-205.] [DOI] [PubMed] [Google Scholar]
- 3.Kusuyama J, Ai K, Changhwan S, et al. Spleen tyrosine kinase influences the early stages of multilineage differentiation of bone marrow stromal cell lines by regulating phospholipase C gamma activities. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=99bc8feab74c28608716d5aec55be57e. J Cell Physiol. 2018;233(3):118–29. doi: 10.1002/jcp.26130. [Kusuyama J, Ai K, Changhwan S, et al.Spleen tyrosine kinase influences the early stages of multilineage differentiation of bone marrow stromal cell lines by regulating phospholipase C gamma activities[J].J Cell Physiol, 2018, 233(3):118-29.] [DOI] [PubMed] [Google Scholar]
- 4.Szydlowski M, Jablonska E, Juszczynski P. FOXO1 transcription factor:a critical effector of the PI3K-AKT axis in B-Cell development. Int Rev Immunol. 2014;33(2):146–57. doi: 10.3109/08830185.2014.885022. [Szydlowski M, Jablonska E, Juszczynski P.FOXO1 transcription factor:a critical effector of the PI3K-AKT axis in B-Cell development[J].Int Rev Immunol, 2014, 33(2):146-57.] [DOI] [PubMed] [Google Scholar]
- 5.Bednarski JJ, Pandey R, Schulte E, et al. RAG-mediated DNA double-strand breaks activate a cell type-specific checkpoint to inhibit pre-B cell receptor signals. J Experim Med. 2016;213(2):209–23. doi: 10.1084/jem.20151048. [Bednarski JJ, Pandey R, Schulte E, et al.RAG-mediated DNA double-strand breaks activate a cell type-specific checkpoint to inhibit pre-B cell receptor signals[J].J Experim Med, 2016, 213(2):209-23.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelrasoul H, Werner M, Setz CS, et al. PI3K induces B-cell development and regulates B cell identity. http://europepmc.org/abstract/MED/29358580. Sci Rep. 2018;8(1):1327–36. doi: 10.1038/s41598-018-19460-5. [Abdelrasoul H, Werner M, Setz CS, et al.PI3K induces B-cell development and regulates B cell identity[J].Sci Rep, 2018, 8(1):1327-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polgarova K, Vaskova M, Fronkova E, et al. Quantitative expression of regulatory and differentiation-related genes in the key steps of human hematopoiesis:The LeukoStage Database. http://www.ncbi.nlm.nih.gov/pubmed/26674556. Differentiation. 2016;91(1/3):19–28. doi: 10.1016/j.diff.2015.11.003. [Polgarova K, Vaskova M, Fronkova E, et al.Quantitative expression of regulatory and differentiation-related genes in the key steps of human hematopoiesis:The LeukoStage Database[J].Differentiation, 2016, 91(1/3):19-28.] [DOI] [PubMed] [Google Scholar]
- 8.Woyach JA, Ruppert AS, Guinn D, et al. BTKC481S-Mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35(13):1437–45. doi: 10.1200/JCO.2016.70.2282. [Woyach JA, Ruppert AS, Guinn D, et al.BTKC481S-Mediated resistance to ibrutinib in chronic lymphocytic leukemia[J].J Clin Oncol, 2017, 35(13):1437-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndipagbornchi TG, Mauno V. Simulation of the dynamics of primary immunodeficiencies in B cells. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6082931/ Front Immunol. 2018;(9):1785–93. doi: 10.3389/fimmu.2018.01785. [Ndipagbornchi TG, Mauno V.Simulation of the dynamics of primary immunodeficiencies in B cells[J].Front Immunol, 2018, (9):1785-93.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budzyńska PM, Niemelä M, Sarapulov AV, et al. IRF4 deficiency leads to altered BCR signalling revealed by enhanced PI3K pathway, decreased SHIP expression and defected cytoskeletal responses. Scand J Immunol. 2015;82(5):418–28. doi: 10.1111/sji.2015.82.issue-5. [Budzyńska PM, Niemelä M, Sarapulov AV, et al.IRF4 deficiency leads to altered BCR signalling revealed by enhanced PI3K pathway, decreased SHIP expression and defected cytoskeletal responses[J].Scand J Immunol, 2015, 82(5):418-28.] [DOI] [PubMed] [Google Scholar]
- 11.Mohammad DK, Nore BF, Gustafsson MO, et al. Protein kinase B (AKT) regulates SYK activity and shuttling through 14-3-3 and importin. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f9f2b9bbd69b997f771d8876a013c5fa. Intern J Biochem Cell Biol. 2016;78(1):63–74. doi: 10.1016/j.biocel.2016.06.024. [Mohammad DK, Nore BF, Gustafsson MO, et al.Protein kinase B (AKT) regulates SYK activity and shuttling through 14-3-3 and importin[J].Intern J Biochem Cell Biol, 2016, 78(1):63-74.] [DOI] [PubMed] [Google Scholar]
- 12.Poe JC, Jia W, Su H, et al. An aberrant NOTCH2-BCR signaling axis in B cells from patients with chronic GVHD. Blood. 2017;130(19):2131–45. doi: 10.1182/blood-2017-05-782466. [Poe JC, Jia W, Su H, et al.An aberrant NOTCH2-BCR signaling axis in B cells from patients with chronic GVHD[J].Blood, 2017, 130(19):2131-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Sohn H, Sun G, et al. The autoinhibitory C-terminal SH2 domain of phospholipase C-γ2 stabilizes B cell receptor signalosome assembly. Sci Signal. 2014;7(343):e89–95. doi: 10.1126/scisignal.2005392. [Wang J, Sohn H, Sun G, et al.The autoinhibitory C-terminal SH2 domain of phospholipase C-γ2 stabilizes B cell receptor signalosome assembly[J].Sci Signal, 2014, 7(343):a89-95.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family:regulation and function. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3506428. Annu Rev Immunol. 1997;15(15):707–47. doi: 10.1146/annurev.immunol.15.1.707. [Rao A, Luo C, Hogan PG.Transcription factors of the NFAT family:regulation and function[J].Annu Rev Immunol, 1997, 15(15):707-47.] [DOI] [PubMed] [Google Scholar]
- 15.Patterson HC, Kraus M, Kim Y, et al. The B cell receptor promotes B cell activation and proliferation through a non-ITAM tyrosine in the Ig alpha cytoplasmic domain. Immunity. 2006;25(1):55–65. doi: 10.1016/j.immuni.2006.04.014. [Patterson HC, Kraus M, Kim Y, et al.The B cell receptor promotes B cell activation and proliferation through a non-ITAM tyrosine in the Ig alpha cytoplasmic domain[J].Immunity, 2006, 25(1):55-65.] [DOI] [PubMed] [Google Scholar]
- 16.Tsukada S, Baba Y, Dai W. Btk and BLNK in B cell development. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_2144513. Adv Immunol. 2001;77(5):123–37. doi: 10.1016/s0065-2776(01)77016-2. [Tsukada S, Baba Y, Dai W.Btk and BLNK in B cell development[J].Adv Immunol, 2001, 77(5):123-37.] [DOI] [PubMed] [Google Scholar]
- 17.Imamura Y, Oda A, Katahira T, et al. BLNK binds active H-Ras to promote B cell receptor-mediated capping and ERK activation. J Biol Chem. 2009;284(15):9804–13. doi: 10.1074/jbc.M809051200. [Imamura Y, Oda A, Katahira T, et al.BLNK binds active H-Ras to promote B cell receptor-mediated capping and ERK activation[J].J Biol Chem, 2009, 284(15):9804-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang HC, Wang X, Tan TH. MAP4K family kinases in immunity and inflammation. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9d52618b43ee5dce56034000e6faf0de. Adv Immunol. 2016;129(3):277–314. doi: 10.1016/bs.ai.2015.09.006. [Chuang HC, Wang X, Tan TH.MAP4K family kinases in immunity and inflammation[J].Adv Immunol, 2016, 129(3):277-314.] [DOI] [PubMed] [Google Scholar]
- 19.Haniuda K, Fukao S, Kodama T, et al. Autonomous membrane IgE signaling prevents IgE-memory formation. Nat Immunol. 2016;17(9):1109–17. doi: 10.1038/ni.3508. [Haniuda K, Fukao S, Kodama T, et al.Autonomous membrane IgE signaling prevents IgE-memory formation[J].Nat Immunol, 2016, 17(9):1109-17.] [DOI] [PubMed] [Google Scholar]
- 20.Yang ZY, Robinson MJ, Chen XJ, et al. Regulation of B cell fate by chronic activity of the IgE B cell receptor. http://pubmedcentralcanada.ca/pmcc/articles/PMC5207771/ Elife. 2016;(5):21238–46. doi: 10.7554/eLife.21238. [Yang ZY, Robinson MJ, Chen XJ, et al.Regulation of B cell fate by chronic activity of the IgE B cell receptor[J].Elife, 2016, (5):21238-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain RZ, Hayardeny L, Cravens PC, et al. Immune surveillance of the central nervous system in multiple sclerosis-Relevance for therapy and experimental models. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2bdb491b7290ffd79093167ae8e1f487. J Neuroimmunol. 2014;276(1/2):9–17. doi: 10.1016/j.jneuroim.2014.08.622. [Hussain RZ, Hayardeny L, Cravens PC, et al.Immune surveillance of the central nervous system in multiple sclerosis-Relevance for therapy and experimental models[J].J Neuroimmunol, 2014, 276(1/2):9-17.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.金 桂花, 李 雪, 藤 本学, et al. B细胞连接蛋白的表达抑制小鼠实验性自身免疫性脑脊髓炎. http://www.cnki.com.cn/Article/CJFDTotal-MYXZ201610004.htm. 免疫学杂志. 2016;(10):848–52. [金桂花, 李雪, 藤本学, 等.B细胞连接蛋白的表达抑制小鼠实验性自身免疫性脑脊髓炎[J].免疫学杂志, 2016(10):848-52.] [Google Scholar]
- 23.Ray A, Basu S, Williams CB, et al. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188(7):3188–98. doi: 10.4049/jimmunol.1103354. [Ray A, Basu S, Williams CB, et al.A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand[J].J Immunol, 2012, 188(7):3188-98.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamino H, Futamura M, Nakamura Y, et al. B-cell linker protein prevents aneuploidy by inhibiting cytokinesis. Cancer Sci. 2008;99(12):2444–54. doi: 10.1111/cas.2008.99.issue-12. [Kamino H, Futamura M, Nakamura Y, et al.B-cell linker protein prevents aneuploidy by inhibiting cytokinesis[J].Cancer Sci, 2008, 99(12):2444-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Arja RF, Chernin LR, Abusin G, et al. Successful hematopoietic cell transplantation in a patient with X-linked agammaglobulinemia and acute myeloid leukemia. Pediatr Blood Cancer. 2015;62(9):1674–6. doi: 10.1002/pbc.25554. [Abu-Arja RF, Chernin LR, Abusin G, et al.Successful hematopoietic cell transplantation in a patient with X-linked agammaglobulinemia and acute myeloid leukemia[J].Pediatr Blood Cancer, 2015, 62(9):1674-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagresle PC, Millili M, Luce S, et al. The BLNK adaptor protein has a nonredundant role in human B-cell differentiation. J Allergy Clin Immunol. 2014;134(1):145–56. doi: 10.1016/j.jaci.2013.12.1083. [Lagresle PC, Millili M, Luce S, et al.The BLNK adaptor protein has a nonredundant role in human B-cell differentiation[J].J Allergy Clin Immunol, 2014, 134(1):145-56.] [DOI] [PubMed] [Google Scholar]
- 27.Nasereddin A, Shamriz O, Keller B, et al. Enteroviral infection in a patient with BLNK adaptor protein deficiency. J Clin Immunol. 2015;35(4):356–60. doi: 10.1007/s10875-015-0164-2. [Nasereddin A, Shamriz O, Keller B, et al.Enteroviral infection in a patient with BLNK adaptor protein deficiency[J].J Clin Immunol, 2015, 35(4):356-60.] [DOI] [PubMed] [Google Scholar]
- 28.Nasrullayeva GM, Mammadova VR, Khalilova AV, et al. The novel patient with BLNK gene type of agammaglobulinemia. Open Access Library J. 2017;4(11):1–7. [Nasrullayeva GM, Mammadova VR, Khalilova AV, et al.The novel patient with BLNK gene type of agammaglobulinemia[J].Open Access Library J, 2017, 4(11):1-7.] [Google Scholar]
- 29.Hiratsuka T, Takei Y, Ohmori R, et al. ZFP521 contributes to pre-Bcell lymphomagenesis through modulation of the pre-B-cell receptor signaling pathway. Oncogene. 2016;35(25):3227–38. doi: 10.1038/onc.2015.385. [Hiratsuka T, Takei Y, Ohmori R, et al.ZFP521 contributes to pre-Bcell lymphomagenesis through modulation of the pre-B-cell receptor signaling pathway[J].Oncogene, 2016, 35(25):3227-38.] [DOI] [PubMed] [Google Scholar]
- 30.Ez-Enrã EC, Rcenas-Lã RA, Hidalgo-Miranda A, et al. Gene expression profiling of acute lymphoblastic leukemia in children with very early relapse. Arch Med Res. 2016;47(8):644–55. doi: 10.1016/j.arcmed.2016.12.005. [Ez-Enrã EC, Rcenas-Lã RA, Hidalgo-Miranda A, et al.Gene expression profiling of acute lymphoblastic leukemia in children with very early relapse[J].Arch Med Res, 2016, 47(8):644-55.] [DOI] [PubMed] [Google Scholar]
- 31.Katerndahl CD, Heltemes-Harris LM, Willette MJ, et al. Antagonism of B cell enhancer networks by STAT5 drives leukemia and poor patient survival. Nat Immunol. 2017;18(6):694–705. doi: 10.1038/ni.3716. [Katerndahl CD, Heltemes-Harris LM, Willette MJ, et al.Antagonism of B cell enhancer networks by STAT5 drives leukemia and poor patient survival[J].Nat Immunol, 2017, 18(6):694-705.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imoto N, Hayakawa F, Kurahashi S, et al. B cell linker protein (BLNK) is a selective target of repression by PAX5-PML protein in the differentiation block that leads to the development of acute lymphoblastic leukemia. J Biol Chem. 2016;291(9):4723–31. doi: 10.1074/jbc.M115.637835. [Imoto N, Hayakawa F, Kurahashi S, et al.B cell linker protein (BLNK) is a selective target of repression by PAX5-PML protein in the differentiation block that leads to the development of acute lymphoblastic leukemia[J].J Biol Chem, 2016, 291(9):4723-31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue K, Song JZ, Yang Y, et al. PAX5 promotes pre-B cell proliferation by regulating the expression of pre-B cell receptor and its downstream signaling. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cc42093878fc62a905781332bf5d949d. Mol Immunol. 2016;73(14):1–9. doi: 10.1016/j.molimm.2016.03.007. [Xue K, Song JZ, Yang Y, et al.PAX5 promotes pre-B cell proliferation by regulating the expression of pre-B cell receptor and its downstream signaling[J].Mol Immunol, 2016, 73(14):1-9.] [DOI] [PubMed] [Google Scholar]
- 34.Rozovski U, Harris DM, Li P, et al. Constitutive phosphorylation of STAT3 by the CK2-BLNK-CD5 complex. Molecul Cancer Res. 2017;15(5):610–8. doi: 10.1158/1541-7786.MCR-16-0291. [Rozovski U, Harris DM, Li P, et al.Constitutive phosphorylation of STAT3 by the CK2-BLNK-CD5 complex[J].Molecul Cancer Res, 2017, 15(5):610-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta N, Delrow J, Drawid A, et al. Repression of B-cell linker (BLNK) and B-cell adaptor for phosphoinositide 3-kinase (BCAP) is important for lymphocyte transformation by Rel proteins. Cancer Res. 2008;68(3):808–14. doi: 10.1158/0008-5472.CAN-07-3169. [Gupta N, Delrow J, Drawid A, et al.Repression of B-cell linker (BLNK) and B-cell adaptor for phosphoinositide 3-kinase (BCAP) is important for lymphocyte transformation by Rel proteins[J].Cancer Res, 2008, 68(3):808-14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuang ZX, Guo L, Li X. Identification of key genes and pathways associated with classical hodgkin lymphoma by bioinformatics analysis. Mol Med Rep. 2017;16(4):4685–93. doi: 10.3892/mmr.2017.7158. [Kuang ZX, Guo L, Li X.Identification of key genes and pathways associated with classical hodgkin lymphoma by bioinformatics analysis[J].Mol Med Rep, 2017, 16(4):4685-93.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dilillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann NY Acad Sci. 2010;1183(1):38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [Dilillo DJ, Matsushita T, Tedder TF.B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer[J].Ann NY Acad Sci, 2010, 1183(1):38-57.] [DOI] [PubMed] [Google Scholar]
- 38.Tasian SK, Loh ML, Hunger SP. Philadelphia chromosome-like acute lymphoblastic leukemia. Blood. 2017;130(19):2064–72. doi: 10.1182/blood-2017-06-743252. [Tasian SK, Loh ML, Hunger SP.Philadelphia chromosome-like acute lymphoblastic leukemia[J].Blood, 2017, 130(19):2064-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]


