Abstract
目的
探究沉默Yes相关蛋白1(YAP1)对鼻咽癌细胞增殖、迁移及侵袭能力的影响。
方法
采用实时荧光定量PCR和Western blot方法检测YAP1在鼻咽癌细胞系中的表达水平;在鼻咽癌细胞S26和S18中转染3条合成的小干扰RNA:si-NC, siYAP1#1和si-YAP1#2,以转染阴性对照si-NC的细胞作为对照组,转染si-YAP1#1和si-YAP1#2的细胞作为实验组,实时荧光定量PCR和Western blot检测转染后的干扰效率;通过细胞计数、平板克隆形成等方法评估转染si-YAP1#1和si-YAP1#2后的实验组和转染si-NC的对照组对鼻咽癌细胞增殖能力的影响;用划痕和Transwell方法分析各组细胞迁移与侵袭能力的变化;Western blot检测各组细胞中c-myc、上皮性钙黏蛋白(E-cadherin)、神经性钙黏蛋白(N-cadherin)和波形蛋白(Vimentin)等与细胞增殖和上皮间质转化相关蛋白的表达变化。
结果
YAP1在鼻咽癌细胞系中高表达;与阴性对照组相比,转染si-YAP1#1和siYAP1#2的实验组细胞中YAP1的mRNA及蛋白水平均显著降低(P < 0.05);生长计数、平板克隆、划痕及Transwell等实验结果均显示转染si-YAP1#1和si-YAP1#2的实验组细胞的生长和克隆能力、划痕愈合能力及侵袭能力较阴性对照组细胞明显下降(P < 0.01);且相较阴性对照组,实验组细胞中c-myc、N-cadherin和Vimentin等蛋白的表达水平降低,E-cadherin的表达量增加。
结论
YAP1在鼻咽癌细胞系中高表达,在鼻咽癌细胞中利用siRNA靶向敲低YAP1的表达后可以抑制鼻咽癌细胞的增殖、迁移及侵袭能力,提示YAP1可以作为一个潜在的鼻咽癌临床治疗干预靶点。
Keywords: Yes相关蛋白1, 鼻咽癌, 细胞增殖, 细胞迁移, 细胞侵袭
Abstract
Objective
To investigate the effects of Yes-associated protein 1 (YAP1) knockdown on the proliferation, migration and invasion in human nasopharyngeal carcinoma (NPC) cells.
Methods
We detected the expression of YAP1 mRNA and protein in different NPC cell lines and an immortalized nasopharyngeal epithelial cell line using RT-PCR and Western blotting. Two YAP1-targeting small interfering RNAs (siRNA) were transfected into NPC cell lines S26 and S18, and the knockdown efficiency was confirmed by RT-PCR and Western blotting. The effect of YAP1 knockdown on the proliferation of the NPC cells was determined by cell counting and colony formation assay; wound healing assay and Transwell assay were used to analyze the changes in the cell migration and invasion abilities in each group. Western blotting was used to analyze the changes in the expressions of c-myc, E-cadherin, N-cadherin and vimentin in the NPC cells after YAP1 knockdown.
Results
YAP1 was highly expressed in the NPC cell lines. Compared with the negative control group, the NPC cell lines with YAP1 knockdown showed significantly lowered YAP1 expressions at both the mRNA and protein levels (P < 0.05). YAP1 knockdown significantly suppressed the growth, cloning formation, migration and invasion of the NPC cells as compared with control cells (P < 0.01). YAP1 knockdown obviously decreased the expression levels of c-myc, N-cadherin and vimentin and increased E-cadherin expression in the NPC cells.
Conclusions
YAP1 knockdown via siRNA suppresses the proliferation, migration and invasion of NPC cells in vitro, suggesting that YAP1 may serve as a therapeutic target for NPC.
Keywords: Yes-associated protein 1, nasopharyngeal carcinoma, cell proliferation, cell migration, cell invasion
鼻咽癌(NPC)是起源于鼻咽被覆上皮的一种恶性肿瘤,其发病具有明显的地区聚集性,在我国南方以及东南亚一些国家高发[1]。其发病因素包括EB病毒感染、遗传因素、环境因素等[2]。目前鼻咽癌主要治疗方式是以放疗为主的综合治疗。由于鼻咽癌恶性程度较高,进展较快,多数鼻咽癌患者在就诊时已处于中晚期,患者整体预后较差[3]。随着基因靶向治疗研究的不断深入,探寻有效的靶基因对改善鼻咽癌患者的预后意义重大[4]。研究表明,Yes相关蛋白(YAP1)与细胞增殖、器官发育以及肿瘤的发生发展相关[5]。YAP1在结肠癌、卵巢癌、乳腺癌等肿瘤中表达水平异常,且其表达水平与预后相关[6-7]。下调YAP1表达后对乳腺癌、结肠癌等肿瘤细胞的增殖、迁移有抑制作用并调控肿瘤细胞的凋亡[8-9]。但YAP1与鼻咽癌相关的研究目前未见明确报导。本研究通过siRNA靶向下调鼻咽癌细胞S26和S18中YAP1的表达水平,从而明确YAP1在鼻咽癌细胞增殖、迁移及侵袭方面的作用,为鼻咽癌的靶向治疗提供新的理论依据。
1. 材料和方法
1.1. 材料
1.1.1. 细胞株
人鼻咽永生化上皮细胞NP69及鼻咽癌细胞株HONE1、HK-1、S26和S18由实验室保存提供。
1.1.2. 主要试剂
DMEM培养基、胎牛血清、0.25%胰蛋白酶、OPTI-MEM(Gibco),Lipofectamine2000(Invitrogen),YAP1小干扰RNA(si-YAP1#1和siYAP1#2)及随机合成的阴性对照(si-NC)(吉玛),RNA提取试剂盒(Qiagen),RNA逆转录及荧光定量PCR试剂盒(TAKARA),RIPA蛋白裂解液及BCA蛋白定量试剂盒(碧云天),YAP1抗体(Sigma);β-actin抗体(TransGen)。
1.2. 方法
1.2.1. 细胞培养
人鼻咽癌细胞株HONE1、HK-1、S26和S18培养于含有10%胎牛血清、双抗浓度为100 U/mL的DMEM培养基中,人鼻咽永生化上皮细胞NP69培养于含10%胎牛血清的Keratinocyte-SFM中,置于37 ℃,5% CO2培养箱常规培养。在细胞生长至约80%时,用胰蛋白酶消化、传代,选取对数生长期细胞进行进一步实验。
1.2.2. 细胞转染
6孔板中每孔接种约2×105鼻咽癌细胞S26,S18,12~16 h后待细胞密度约30%,参照Lipofectamine2000转染试剂盒方法转染si-NC作为阴性对照组,转染si-YAP1#1和si-YAP1#2作为实验组。分别于48、72 h后收集各组细胞以检测mRNA和蛋白水平的干扰效果(干扰序列见表 1)。
1.
YAP1 siRNA序列
siRNAsequences for YAP1 knockdown
| RNA | Sequences |
| Si-YAP1#1 | |
| F | 5'-GCGUAGCCAGUUACCAACATT-3' |
| R | 5'-UGUUGGUAACUGGCUACGCTT-3' |
| Si-YAP1#2 | |
| F | 5'- GUGGGACUCAAAAUCCAGUTT-3' |
| R | 5'- ACUGGAUUUUGAGUCCCACTT-3' |
| si-NC | |
| F | 5'-UUCUCCGAACGUGUCACGUTT-3' |
| R | 5'-ACGUGACACGUUCGGAGAATT-3' |
1.2.3. 荧光定量PCR检测YAP1的表达水平
参照RNA提取试剂盒的方法提取人鼻咽癌细胞(HONE1、HK-1、S26、S18)及人鼻咽永生化上皮细胞NP69或经过si-NC及si-YAP1#1和si-YAP1#2处理后的鼻咽癌细胞S26、S18总RNA,对提取的RNA进行浓度测定,存放于-80 ℃。参照Takara逆转录试剂盒合成cDNA,应用SYBR Green的方法检测YAP1的相对表达水平,反应条件为:95 ℃预变性5 min,95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸10 s,共40个循环,然后72 ℃延伸10 min(引物序列见表 2)。
2.
PCR引物序列
Primer sequences for PCR
| RNA | Sequences |
| YAP1 | |
| F | 5'-ACCCACAGCTCAGCATCTTC-3' |
| R | 5'-GCTGTGACGTTCATCTGGGA-3' |
| β-ACTIN | |
| F | 5'-CCCACACTGTGCCCATCTAC-3' |
| R | 5'-GGAACCGCTCATTGCCAATG-3' |
1.2.4. Western blot检测YAP1及增殖、上皮间质转化(EMT)相关蛋白表达水平
使用RIPA裂解液提取指定细胞的总蛋白,通过BCA蛋白定量试剂盒测定蛋白浓度,取约50 μg总蛋白上样,10%SDS-PAGE胶、110 V恒压电泳约1.5 h后转膜,5%脱脂牛奶室温封闭1 h,一抗(YAP1、c-myc、E-cadherin、N-cadherin、Vimentin)4 ℃孵育过夜,TBST洗膜3次每次10 min,二抗室温孵育1 h,TBST洗膜3次每次10 min,采用HRP- ECL法通过Biorad化学发光仪显影。
1.2.5. 细胞计数法测定细胞生长能力
在鼻咽癌细胞S26,S18中转染si-NC作为阴性对照组细胞,转染siYAP1#1和si-YAP1#2作为实验组细胞,待转染48 h后在12孔板每孔接种约1×103各组细胞,每组设3个复孔,于37℃、5% CO2培养箱中常规培养分别1~3 d后,用胰蛋白酶消化,离心后1 mL培养基重悬,取10 μL细胞悬液使用细胞计数板于显微镜下计数。
1.2.6. 平板克隆测定细胞克隆形成能力
6孔板中接种约2000个转染siRNA 48 h后的阴性对照组(si-NC)及实验组(si-YAP1#1和si-YAP1#2)细胞,于37 ℃、5% CO2培养箱中培养10~14 d。弃掉培养基后PBS洗涤1次,4%多聚甲醛固定10 min,结晶紫染色10 min后用水清洗掉结晶紫,置于空气中干燥。计数细胞克隆数目。
1.2.7. 细胞划痕和Transwell实验
将S26,S18中转染48 h后的阴性对照组(si-NC)及实验组(si-YAP1#1和si-YAP1#2)细胞调整到密度为6×105,充分混匀后取70 μL细胞悬液置于划痕器中,约16~20 h后取去划痕器并更换无血清培养基,分别于0 h,36 h观察细胞的迁移情况。在小室上室加入(或不加)200 μL稀释的Matrigel,干燥过夜。将8×104个siRNA处理后的各组细胞制成200 μL无血清细胞悬液接种于小室的上层,小室下层给予700 μL常规培养基,于37 ℃、5% CO2培养箱中培养24 h后取出小室,PBS轻柔洗涤1次,4%多聚甲醛固定10 min,结晶紫染色10 min,然后用棉签擦净上层小室中未穿过滤膜的细胞,显微镜下拍照、计数。
1.2.8. 统计学分析
采用SPSS 20.0统计软件分析,数据以均数±标准差表示,采用两样本t检验统计学方法,P < 0.05为差异有统计学意义。
2. 结果
2.1. YAP1在鼻咽癌组织及细胞系中的表达
课题组前期通过对87例NPC患者组织及10例正常对照组织的转录组测序(RNA-Seq)数据分析,结果显示,与对照组织比较,YAP1在鼻咽癌组织中的表达上调。在本项研究中,我们通过荧光定量PCR及Western blot技术检测人鼻咽永生化上皮细胞NP69及鼻咽癌细胞系HONE1、HK-1、S26、S18中YAP1的表达水平,发现相较人鼻咽永生化上皮细胞NP69,YAP1在鼻咽癌细胞HONE1、S26和S18中相对高表达(图 1),后续选取S26和S18进行进一步的功能实验。
1.
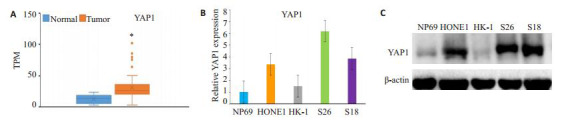
YAP1在鼻咽癌组织(A)和细胞系(B-C)中的表达水平
YAP1 expression in NPC tissues (A) and cell lines (B, C). NP69 as a normal control in cell line expression.*P < 0.05 vs Normal group as control.
2.2. YAP1干扰效率验证
采用荧光定量PCR及Western blot技术检测鼻咽癌细胞S26,S18在转染siRNA后细胞中YAP1表达水平的变化。结果显示,相对si-NC处理的阴性对照组细胞而言,转染si-YAP1#1和si-YAP1#2后的实验组细胞YAP1的mRNA及蛋白水平均明显降低,差异有统计学意义(P < 0.05),证实了siRNA的有效性(图 2)。
2.
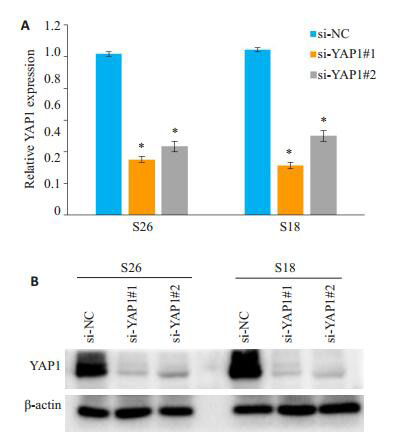
荧光定量PCR、Western blot检测YAP1干扰前后阴性对照组及实验组细胞中mRNA和蛋白的表达变化
YAP1 mRNA (A) and protein (B) expressions detected by RT-PCR and Western blotting in negative control group (si-NC) and experimental groups (si-YAP1#1 and si-YAP1#2).*P < 0.05 vs si-NC as control group.
2.3. YAP1对鼻咽癌细胞生长能力和克隆形成能力的影响
鼻咽癌细胞S26,S18分别转染si-NC、si-YAP1#1和si-YAP1#2后在指定时间点进行细胞计数,相比对照组(si-NC),实验组(si-YAP1#1和si-YAP1#2)细胞的生长受到明显抑制,差异有统计学意义(P < 0.01)。平板克隆形成实验结果显示,相较阴性对照组(si-NC),实验组(si-YAP1#1和si-YAP1#2)细胞的克隆形成能力显著下降,差异有统计学意义(P < 0.01,图 3)。
3.
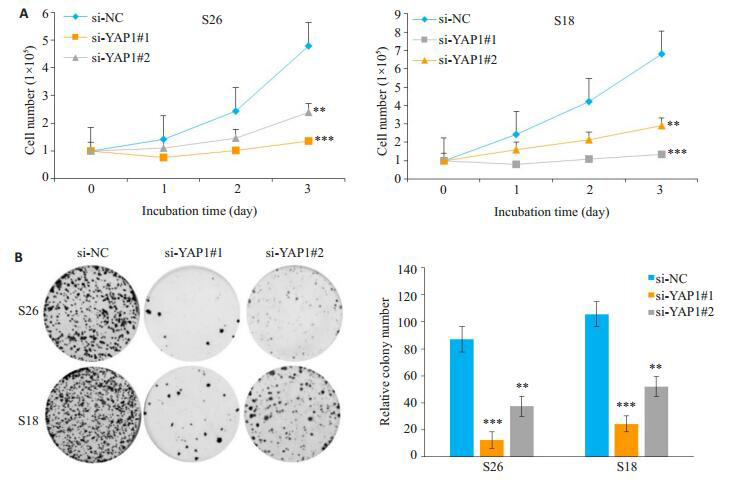
敲低YAP1抑制鼻咽癌细胞的生长能力(A)和平板克隆形成能力(B)
YAP1 knockdown inhibited the growth (A) and colony formation ability (B) of NPC cells.**P < 0.01 vs si-NC as control group, ***P < 0.001 vs si-NC as control group.
2.4. 敲低YAP1对鼻咽癌细胞迁移及侵袭能力的影响
鼻咽癌细胞S26, S18中转染si-NC、si-YAP1#1和si-YAP1#2后进行划痕实验及Transwell实验,划痕实验结果显示相比对照组(si-NC),实验组(si-YAP1#1和siYAP1#2)细胞的划痕愈合能力明显减弱;Transwell结果显示实验组(si-YAP1#1和si-YAP1#2)细胞的迁移及侵袭细胞数相对阴性对照组(si-NC)明显减少,差异具有统计学意义(P < 0.01,图 4)。
4.
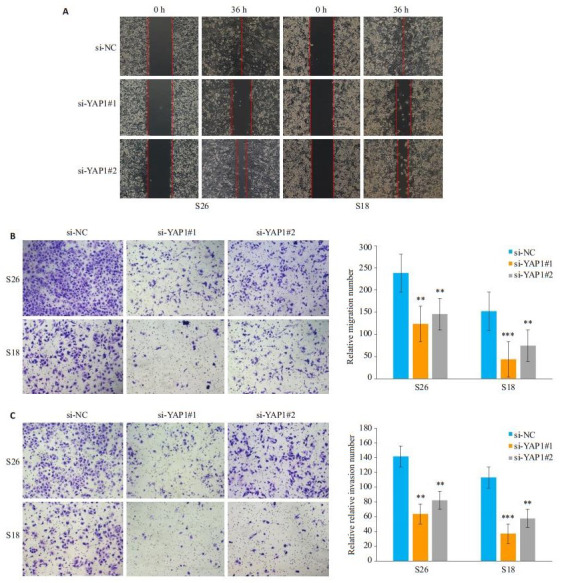
敲低YAP1后抑制鼻咽癌细胞的迁移及侵袭能力
YAP1 knockdown suppresses the migration (A, B) and invasion (C) of NPC cells in vitro.**P < 0.01 vs si-NC as control group, ***P < 0.001 vs si-NC as control group.
2.5. 敲低YAP1对增殖、上皮间质转化(EMT)相关蛋白的影响
Western blot结果显示,与阴性对照组(si-NC)相比,实验组(si-YAP1#1和si-YAP1#2)细胞增殖相关蛋白c-myc水平显著降低,迁移、侵袭相关的间质标志物N-cadherin和Vimentin表达量降低,上皮标志物Ecadherin表达量增加(图 5)。
5.
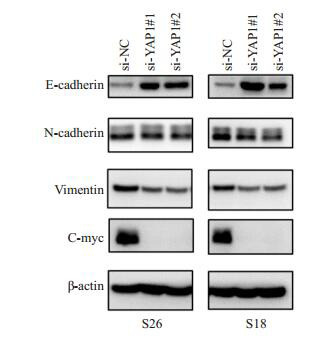
敲低YAP1后,c-myc、Vimentin和N-cadherin蛋白表达量降低,E-cadherin表达量增加
YAP1 knockdown reduced the expression of c-myc, vimentin and N-cadherin and increased the expression of E-cadherin in the NPC cell lines S26 and S18 in vitro.
3. 讨论
鼻咽癌在我国南方地区尤其是广东有着非常高的发病率,其发病部位隐匿,早期临床表现不典型,多数鼻咽癌患者在就诊时病情已处于中晚期[10-12],以放疗为主的综合治疗方式疗效虽相对显著,但癌细胞的远处转移仍使得部分患者无法治愈[13],晚期鼻咽癌患者的5年生存率为61%~66% [14]。鼻咽癌高侵袭转移的特征是其影响鼻咽癌晚期患者不良预后的重要原因[15]。基于鼻咽癌的病情特征及其治疗现状,找寻鼻咽癌发生发展过程中有效的关键分子作为治疗靶点对于改善晚期鼻咽癌患者的不良预后具有重要意义。
人YAP1基因编码Hippo信号通路下游的效应分子,通过与细胞核内的有关因子结合影响基因的转录、表达,参与细胞的发育、生长等过程[16]。Hippo信号通路的失活会导致YAP1去磷酸化并聚集在细胞核中,继而调控与细胞增殖、抗凋亡、EMT及干细胞活性等相关基因的表达[17-18]。根据已有研究表明,YAP1在乳腺癌、非小细胞肺癌、胰腺癌等肿瘤中表达上调且影响肿瘤的发展进程及患者预后[19-22]。另有研究表明,YAP1对一些抗肿瘤药物如埃罗替尼、西妥昔单抗等的继发耐药也发挥一定的作用[21, 23-25]。由此看来,YAP1作为Hippo信号通路的转录调节因子,可以作为癌症治疗的潜在靶标。
在本研究中,课题组前期通过对87例NPC患者组织及10例对照组织的转录组测序数据分析发现YAP1在NPC患者组织中的表达水平较对照组织增高,荧光定量PCR及Western blot检测到YAP1在NPC细胞系中表达上调,提示其可能作为癌基因在鼻咽癌的发展过程中起作用。随后通过荧光定量PCR及western blot验证siRNA干扰效果的有效性,结果显示转染si-YAP1#1和si-YAP1#2后细胞YAP1的表达水平相比si-NC显著下降。在确认了siRNA的干扰效率后,我们进一步进行了生长计数及平板克隆实验以评估YAP1对鼻咽癌细胞增殖能力的影响,实验结果表明,相比阴性对照组(si-NC),实验组(si-YAP1#1和si-YAP1#2)细胞的生长能力及克隆形成能力受到明显抑制,且实验组细胞中与增殖有关的标记物c-myc的蛋白水平也较对照组显著下降,说明YAP1的表达水平对鼻咽癌细胞的增殖有重要的影响。
此外,肿瘤细胞的迁移及侵袭是鼻咽癌患者预后不良的重要因素,在明确YAP1的表达对鼻咽癌细胞增殖能力的影响后,我们继续探究了YAP1对鼻咽癌细胞迁移及侵袭能力的影响。划痕实验结果表示,与阴性对照组(si-NC)相比,实验组(si-YAP1#1和si-YAP1#2)细胞的划痕愈合能力明显减弱;Transwell迁移和侵袭中的实验组(si-YAP1#1和si-YAP1#2)的穿膜细胞数比阴性对照组(si-NC)明显减少。这些实验表明,敲低YAP1使鼻咽癌细胞的迁移及侵袭能力受到了显著抑制。
EMT是肿瘤细胞获得迁移及侵袭能力的重要途径,是肿瘤细胞突破基底膜发生侵袭-转移级联反应的第一步[26]。其主要表现为上皮细胞极性丧失和黏附性下降使得细胞的活动性增强并逐步向间质细胞转化[27-28]。在肿瘤发展过程中,E-cadherin向N-cadherin转化,意味着细胞发生了EMT,增加了细胞的活动性及迁移、侵袭能力,E-cadherin与N-cadherin的表达量呈负相关[29]。Vimentin是一种维持细胞间质特性的中间纤维,是间质细胞的标志物。细胞黏附分子E-cadherin表达减少时,角蛋白为主的细胞骨架转变为波形蛋白Vimentin为主的细胞骨架,进而影响细胞的形态及活动性[30]。基于YAP1影响鼻咽癌细胞迁移和侵袭的表型,我们进一步采用Western blot检测了EMT相关的分子标志物E-cadherin、N-cadherin和Vimentin等。Western blot结果显示,与阴性对照组(si-NC)比较,实验组(si-YAP1#1和si-YAP1#2)细胞的E-cadherin表达增加,N-cadherin和Vimentin表达降低。由此可见,YAP1的表达水平对鼻咽癌细胞EMT以及迁移侵袭发挥了重要的作用。
综上所述,YAP1的表达在鼻咽癌细胞的增殖、迁移及侵袭过程中有着重要的作用,提示YAP1作为鼻咽癌发展过程中的一个有效关键分子,有可能成为鼻咽癌治疗中新的潜在治疗靶点。但本研究目前只在体外证实了YAP1对鼻咽癌细胞的功能影响,其体内功能的验证及其在鼻咽癌发展中起作用的机制有待进一步研究。
Biography
周雅青,在读硕士研究生,E-mail: zhouyq@sysucc.org.c
Funding Statement
国家自然科学基金青年基金(81802719);广东省医学科学技术研究基金(A2017433)
Supported by National Natural Science Foundation of China (81802719)
Contributor Information
周 雅青 (Yaqing ZHOU), Email: zhouyq@sysucc.org.c.
马 刚 (Gang MA), Email: magang@sysucc.org.cn.
References
- 1.Kuang KR, W ei, Rong RS, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer. 2014;36(8):381–7. doi: 10.5732/cjc.014.10086. [Kuang KR, Wei, Rong RS, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010[J]. Chin J Cancer, 2014, 36 (8): 381-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su L, Zhang MW, Zhang WJ, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(11):e6364. doi: 10.1097/MD.0000000000006364. [Su L, Zhang MW, Zhang WJ, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma A systematic review and meta-analysis[J]. Medicine (Baltimore), 2017, 96(11): e6364.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC metaanalysis. Lancet Oncol. 2015;16(6):645–55. doi: 10.1016/S1470-2045(15)70126-9. [Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC metaanalysis[J]. Lancet Oncol, 2015, 16(6): 645-55.] [DOI] [PubMed] [Google Scholar]
- 4.Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25(1):91–101. doi: 10.1016/j.ccr.2013.12.015. [Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy[J]. Cancer Cell, 2014, 25(1): 91-101.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moroishi T HC, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–9. doi: 10.1038/nrc3876. [Moroishi T HC, Guan KL. The emerging roles of YAP and TAZ in cancer[J]. Nat Rev Cancer, 2015, 15(2): 73-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2013;11(1):31. doi: 10.1186/1478-811X-11-31. [Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells[J]. Cell Commun Signal, 2013, 11(1): 31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oku Y, Nishiya N, Shito T, et al. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio. 2015;5:542–9. doi: 10.1016/j.fob.2015.06.007. [Oku Y, Nishiya N, Shito T, et al. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers[J]. FEBS Open Bio, 2015, 5: 542-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua K, Yang W, Song H, et al. Up-regulation of miR-506 inhibits cell growth and disrupt the cell cycle by targeting YAP in breast cancer cells. Int J Clin Exp Med. 2015;8(8):12018–27. [Hua K, Yang W, Song H, et al. Up-regulation of miR-506 inhibits cell growth and disrupt the cell cycle by targeting YAP in breast cancer cells[J]. Int J Clin Exp Med, 2015, 8(8): 12018-27.] [PMC free article] [PubMed] [Google Scholar]
- 9.Wm K, J r, Kyler SL, et al. Wnt/beta-catenin signaling regulates Yesassociated protein(YAP)gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287(15):11730–9. doi: 10.1074/jbc.M111.327767. [Wm K, Jr, Kyler SL, et al. Wnt/beta-catenin signaling regulates Yesassociated protein(YAP)gene expression in colorectal carcinoma cells[J]. J Biol Chem, 2012, 287(15): 11730-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and Trends-- An update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and Trends-- An update[J]. Cancer Epidemiol Biomarkers Prev, 2016, 25(1): 16-27.] [DOI] [PubMed] [Google Scholar]
- 11.Niu M, Gao D, Wen Q, et al. MiR-29c regulates the expression of miR-34c and miR-449a by targeting DNA methyltransferase 3a and 3b in nasopharyngeal carcinoma. BMC Cancer. 2016;16:218. doi: 10.1186/s12885-016-2253-x. [Niu M, Gao D, Wen Q, et al. MiR-29c regulates the expression of miR-34c and miR-449a by targeting DNA methyltransferase 3a and 3b in nasopharyngeal carcinoma[J]. BMC Cancer, 2016, 16: 218.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersson F. Nasopharyngeal carcinoma: A review. Semin Diagn Pathol. 2015;32(1):54–73. doi: 10.1053/j.semdp.2015.02.021. [Petersson F. Nasopharyngeal carcinoma: A review[J]. Semin Diagn Pathol, 2015, 32(1): 54-73.] [DOI] [PubMed] [Google Scholar]
- 13.Jiang F, Jin T, Feng XL, et al. Long-term outcomes and failure patterns of patients with nasopharyngeal carcinoma staged by magnetic resonance imaging in intensity-modulated radiotherapy era: the Zhejiang cancer Hospital's experience. J Cancer Res Ther. 2015;11(6, S):C179–84. doi: 10.4103/0973-1482.168181. [Jiang F, Jin T, Feng XL, et al. Long-term outcomes and failure patterns of patients with nasopharyngeal carcinoma staged by magnetic resonance imaging in intensity-modulated radiotherapy era: the Zhejiang cancer Hospital's experience[J]. J Cancer Res Ther, 2015, 11(6, S): C179-84.] [DOI] [PubMed] [Google Scholar]
- 14.He W, Zou C, Tian Z, et al. Nasopharyngeal carcinoma treated with bevacizumab combined with paclitaxel liposome plus cisplatin: a case report and literature review. Onco Targets Ther. 2017;10:67–72. doi: 10.2147/OTT.S122238. [He W, Zou C, Tian Z, et al. Nasopharyngeal carcinoma treated with bevacizumab combined with paclitaxel liposome plus cisplatin: a case report and literature review[J]. Onco Targets Ther, 2017, 10: 67-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Li G, Yang N, et al. miR-324-3p suppresses migration and invasion by targeting WNT2B in nasopharyngeal carcinoma. Cancer Cell Int. 2017;17:2. doi: 10.1186/s12935-016-0372-8. [Liu C, Li G, Yang N, et al. miR-324-3p suppresses migration and invasion by targeting WNT2B in nasopharyngeal carcinoma[J]. Cancer Cell Int, 2017, 17: 2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d2c7c2ca58276b0fd16250e86a354b96. J Hepatol. 2015;62(2):S479. doi: 10.1016/j.jhep.2015.04.011. [Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation[J]. J Hepatol, 2015, 62(2): S479.] [DOI] [PubMed] [Google Scholar]
- 17.Nishio M, Maehama T, Goto H, et al. vs. crab: tissue-specific functions of the mammalian hippo pathway. Genes to Cells. 2017;22(1):6–31. doi: 10.1111/gtc.2017.22.issue-1. [Nishio M, Maehama T, Goto H, et al. Hippo vs. crab: tissue-specific functions of the mammalian hippo pathway[J]. Genes to Cells, 2017, 22(1): 6-31.] [DOI] [PubMed] [Google Scholar]
- 18.Mo Js PH, Guan KL. The hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15(6):642–56. doi: 10.15252/embr.201438638. [Mo Js PH, Guan KL. The hippo signaling pathway in stem cell biology and cancer[J]. EMBO Rep, 2014, 15(6): 642-56.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Benedetto A, Mottolese M, Sperati F, et al. The hippo transducers TAZ/YAP and their target CTGF in male breast cancer. Oncotarget. 2016;7(28):43188–98. doi: 10.18632/oncotarget.9668. [Di Benedetto A, Mottolese M, Sperati F, et al. The hippo transducers TAZ/YAP and their target CTGF in male breast cancer[J]. Oncotarget, 2016, 7(28): 43188-98.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou XX, Su JN, Feng SY, et al. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7(48):79062–74. doi: 10.18632/oncotarget.12596. [Zhou XX, Su JN, Feng SY, et al. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells[J]. Oncotarget, 2016, 7(48): 79062-74.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget. 2016;7(32):51922–33. doi: 10.18632/oncotarget.10458. [Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells[J]. Oncotarget, 2016, 7(32): 51922-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su LL, Ma WX, Yuan JF, et al. Expression of yes-associated protein in non-small cell lung cancer and its relationship with clinical pathological factors. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhcmj201222018. Chin Med J (Engl) 2012;125(22):4003–8. [Su LL, Ma WX, Yuan JF, et al. Expression of yes-associated protein in non-small cell lung cancer and its relationship with clinical pathological factors[J]. Chin Med J (Engl), 2012, 125(22): 4003-8.] [PubMed] [Google Scholar]
- 23.Zhang MC, Zeng J, Zhao ZW, et al. Loss of MiR-424-3p, not miR- 424-5p, confers chemoresistance through targeting YAP1 in nonsmall cell lung cancer. Mol Carcinog. 2017;56(3):821–32. doi: 10.1002/mc.v56.3. [Zhang MC, Zeng J, Zhao ZW, et al. Loss of MiR-424-3p, not miR- 424-5p, confers chemoresistance through targeting YAP1 in nonsmall cell lung cancer[J]. Mol Carcinog, 2017, 56(3): 821-32.] [DOI] [PubMed] [Google Scholar]
- 24.Lee KW, Lee SS, Kim SB, et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res. 2015;21(2):357–64. doi: 10.1158/1078-0432.CCR-14-1374. [Lee KW, Lee SS, Kim SB, et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients[J]. Clin Cancer Res, 2015, 21(2): 357-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–87. doi: 10.1038/nrc.2016.36. [Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy [J]. Nat Rev Cancer, 2016, 16(5): 275-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyer B VA, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60(8):1091–9. doi: 10.1016/S0006-2952(00)00427-5. [Boyer B VA, Edme N. Induction and regulation of epithelial-mesenchymal transitions[J]. Biochem Pharmacol, 2000, 60(8): 1091-9.] [DOI] [PubMed] [Google Scholar]
- 27.Li L, Li W. Epithelial-mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [Li L, Li W. Epithelial-mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation[J]. Pharmacol Ther, 2015, 150: 33-46.] [DOI] [PubMed] [Google Scholar]
- 28.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206. doi: 10.1101/gad.225334.113. [Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis[J]. Genes Dev, 2013, 27(20): 2192-206.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–83. doi: 10.1002/(ISSN)1097-4652. [Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression[J]. J Cell Physiol, 2007, 213(2): 374-83.] [DOI] [PubMed] [Google Scholar]
- 30.Ye Y, Xiao Y, Wang W, et al. ERalpha signaling through slug regulates E-cadherin and EMT. Oncogene. 2010;29(10):1451–62. doi: 10.1038/onc.2009.433. [Ye Y, Xiao Y, Wang W, et al. ERalpha signaling through slug regulates E-cadherin and EMT[J]. Oncogene, 2010, 29(10): 1451-62.] [DOI] [PubMed] [Google Scholar]


