Abstract
目的
研究不同时间电刺激模拟不同强度运动对C2C12肌管线粒体氧化应激水平的影响,并进一步探讨5′-磷酸腺苷活化蛋白激酶(AMPK)信号通路在不同运动强度过程中所发挥的作用。
方法
C2C12肌管分化7 d后给予1次电刺激,刺激强度为15 V,30 ms,3 Hz,刺激时间分别为0、60、120和180 min。实验共4组,对照组(Con); 电刺激60 min组(E60),120 min(E120)和180 min(E180)。细胞电刺激处理后于倒置显微镜观察肌管形态; 试剂盒法检测脂质过氧化产物(MDA)和活性氧自由基(ROS); 流式细胞技术检测线粒体ROS和膜电位; 免疫蛋白印迹法检测PGC1、AMPK-Ser485、AMPK-Thr172、AMPK的蛋白表达。
结果
不同时间的电刺激后,C2C12肌管的形态无显著差异; 电刺激60 min,MDA、ROS、AMPK-Ser485和AMPK-Thr172显著上升(P < 0.05);电刺激120 min和180 min,MDA、ROS、线粒体ROS、AMPK-Ser485、PGC1显著上升(P < 0.05),线粒体膜电位显著下降(P < 0.05)。
结论
电刺激导致肌管氧化应激,且随时间延长,线粒体氧化应激程度越强烈,线粒体损伤越严重; AMPKThr172调控中长时间电刺激引起的氧化应激,而AMPK-Ser485及PGC1调控超长时间电刺激引起的氧化应激。
Keywords: 电刺激, 线粒体, 氧化应激, 5′-磷酸腺苷活化蛋白激酶
Abstract
Objective
To study effect of electrical stimulations of different intensities on mitochondrial oxidative stress in C2C12 myotubes and explore the molecular mechanisms.
Methods
After 7 days of differentiation, C2C12 myotubes were subjected to electrical stimulations (15 V, 3Hz, 30 ms) for 60, 120, or 180 min, and the morphological changes of muscular tubes were observed under inverted microscope. The levels of MDA and SOD activity of the cells were detected, and flow cytometry was used to detect mitochondrial reactive oxygen species (ROS) and membrane potential. Western blotting was used to detect the expression of PGC1, AMPK-Ser485, AMPK-Thr172, and AMPK in the cells.
Results
No significant changes occurred in the morphology of C2C12 myotubes in response to electrical stimulations. Electrical stimulation for 60 min resulted in significantly increased levels of MDA, AMPK-Ser485 and AMPK-Thr172 in the cells (P < 0.05); simulations of the cells for 120 and 180 min caused significantly increased MDA, ROS, mitochondrial ROS, AMPK-Ser485 and PGC1 along with marked reduction of mitochondrial membrane potential (P < 0.05).
Conclusion
Electrical stimulation significantly activates oxidative stress, and a longer stimulation time causes stronger mitochondrial oxidation. AMPK-Thr172 regulates oxidative stress induced by stimulations for a moderate time length, while AMPK-Ser485 and PGC1 function to modulate oxidative stress following prolonged stimulations.
Keywords: electrical stimulation, mitochondria, oxidative stress, AMPK
氧化应激是新陈代谢生成的活性氧(ROS)与机体的抗氧化系统之间失去平衡所导致的一系列应激反应。众所周知,适宜强度的运动可以延缓肌肉损伤及老化[1-2],而长时间力竭运动导致机体ROS水平急剧增加,加速肌肉的疲劳损伤及老化[3],严重影响运动员的成绩。从ROS的角度出发,通过调控ROS缓解氧化损伤可能是依赖运动处方进行抗疲劳损伤的重要方法之一,其中的机制值得我们去探讨。5′-磷酸腺苷活化蛋白激酶(AMPK)及过氧化物酶体增殖物激活受体辅助激活因子(PGC1),对于细胞内的氧化应激水平具有重要的调节效应[4-6],能减少活性氧自由基的生成,改善细胞的生存环境[7-8]。
运动训练可通过活化多种转录因子,增强肌肉代谢、提高运动能力[9]。AMPK是一种由催化亚基α、β和γ三个亚基组成的异源三聚体蛋白。运动过程中,ROS引起AMPK异源三聚体的结构异变[10],但不同ROS水平导致AMPK的活化位点目前没有定论。AMPKα可通过亚基Thr172和Ser485磷酸化发挥调节作用,目前研究最多的是Thr172[11-12],而关于Ser485的研究相对较少[13-14]。多数研究支持,骨骼肌中ROS的上升导致AMPKThr172磷酸化而激活AMPK[15-17],而AMPK-Ser485拮抗AMPK-Thr172[18-19]。也有研究显示,AMPK-Ser485与Thr172变化一致,其活性的调节作用取决于实验环境和组织[11]。因此,不同运动时间后,ROS与AMPKThr172及AMPK-Ser485的关系仍值得研究。
本文基于AMPKα的Thr172和Ser485,研究不同时间电刺激模拟不同强度运动对C2C12肌管线粒体氧化应激水平的影响,并进一步探讨AMPK信号通路在不同运动强度过程中所发挥的作用,以期对后续的运动处方开具提供更有力的分子靶标。C2C12细胞是小鼠骨骼肌肌母细胞株,分化的C2C12肌管可完成与在体骨骼肌细胞同样的收缩[20],同时避免了在体实验中其他器官、组织、细胞和激素的影响,能更实时的研究不同运动刺激对骨骼肌线粒体功能的影响,为运动损伤及恢复提供理论依据。
1. 材料和方法
1.1. 研究材料
C2C12细胞(南方医科大学解剖教研室);DMSO、DEPC、L-谷氨酰胺(sigma);4-羟乙基哌嗪乙磺酸(HEPES,MBCHEM公司);胎牛血清(四季青);DMEM培养基、马血清(GIBCO);MDA、SOD试剂盒(建成);AMPK、AMPK-Ser485、AMPK-Thr172、p-ULK(CST),PGC1(abcam);四甲基罗丹明甲酯TMRM染料(哈灵)。
1.2. 电刺激方案及分组
C2C12细胞常规培养,取生长状态好的细胞传代于35 cm的6孔板培养皿中,加入含10%胎牛血清的生长培养基培养,待细胞铺满皿底面积的90%以上时,加入含2%马血清的分化培养基分化。采用GRASS S48 Stimulator型电刺激器电刺激共分化7 d的C2C12肌管,强度为15 V,30 ms,3 Hz,电刺激时间为60、120、180 min。实验共4组,对照组(Con);电刺激60 min组(E60),120 min(E120)和180 min(E180)。
1.3. MDA和ROS检测
1.3.1. MDA测量
细胞收集后,按照试剂盒说明书加试剂,旋涡混匀器混匀。管口用保鲜膜扎紧,用针头刺一小孔,95 ℃水浴40 min,取出后流水冷却,3500 r/min离心10 min。移取200 μL至96T板中,532 nm处测定吸光度(A)。
1.3.2. ROS检测
细胞收集后悬浮于稀释好的DCFHDA(10 μmoL/L)中,37 ℃细胞培养箱内孵育20 min。每隔3~5 min颠倒混匀一下,使探针和细胞充分接触。用无血清细胞培养液洗涤细胞3次,以充分去除未进入细胞内的DCFH-DA。使用488 nm激发波长,525 nm发射波长,荧光酶标仪实时或逐时间点检测刺激前后荧光的强弱。
1.4. 免疫蛋白印迹
取处理后的6孔板,冰PBS冲洗2遍,每孔加入150μL的裂解抽提液比例,混合并匀浆。取上清2~4 μL,进行蛋白定量。取蛋白样品,加入上样缓冲液混匀,煮沸10 min后,上样,蛋白样品经8%或10%的聚丙烯酰胺凝胶电泳分离后,运用湿转法将凝胶上的蛋白条带转移至聚偏二氟乙烯(PVDF)膜。用3%的牛血清白蛋白(BSA)封闭PVDF膜,置于脱色摇床上,室温封闭2 h。加入相应的一抗,4 ℃孵育过夜。所用抗体分别为AMPK、AMPKSer485、AMPK-Thr172、PGC1;TBST冲洗,二抗敷育2 h,TBST冲洗,常规显色曝光。
1.5. 流式细胞仪测定肌管线粒体ROS
按照线粒体提取试剂盒说明提取线粒体,加入荧光探针10 μmol DCFH-DA避光染色30 min;11 000 g离心,10 min,4 ℃,弃上清;翘液清洗2遍,每次11 000 g离心,10 min,4 ℃,弃上清;加入翘液重悬,上机检测线粒体ROS。
1.6. 流式细胞仪测定线粒体膜电位
加入荧光探针为100 nmol TMRM,避光染色30 min;11 000 g离心,10 min,4 ℃,弃上清;翘液清洗2遍,每次11 000 g离心,10 min,4 ℃,弃上清;加入翘液重悬,上机检测线粒体膜电位。
1.7. 数据统计处理
研究数据用SPSS 16.0统计软件进行统计学处理,各数据以均数±标准差表示,并用单因素方差分析进行各组间的比较。P < 0.05为差异有统计学意义。
2. 结果
2.1. 电刺激对C2C12肌管形态的影响
分化7 d的C2C12肌管作为电刺激对象,此时的肌管已分化完全,给予外源性刺激后具有在体性质的收缩能力。不同时间的电刺激后,10×的每组图片中均含有大量C2C12肌管;40×放大后,可清晰看到骨骼肌肌管的精细形态,但肉眼无法辨别其显著差异(图 1)。
1.
电刺激对C2C12肌管形态的影响
Effects of electrical stimulation on C2C12 tube phenotypes.
2.2. 电刺激对C2C12肌管MDA和ROS的影响
分化7 d的C2C12肌管给予一次模拟在体运动模式的电刺激,电刺激时间为60 min,120 min和180 min,分别模拟在体中等时间、长时间及力竭运动模式。收集各组肌管后,分别检测MDA和ROS水平,评价氧化应激水平。电刺激60 min ROS及MDA上升(P < 0.05),电刺激120 min和180 min ROS及MDA亦上升(P < 0.01) (表 1)。
1.
电刺激对C2C12肌管MDA和ROS影响
Effect of electrical stimulations on MDA and ROS in C2C12 myotubes
| Control | E60 | E120 | E180 | |
| *P < 0.05; **P < 0.01 vs Control. | ||||
| MDA (nmol/mL) | 3.65±0.14 | 4.37±0.21* | 4.95±0.09** | 5.21±0.15** |
| ROS (U/mL) | 21.7±12.4 | 88.2±16.7* | 155.4±11.2** | 147.4±10.9** |
2.3. 电刺激对C2C12肌管线粒体ROS的影响
电刺激60 min线粒体ROS稍有上升,但无显著差异(P>0.05);电刺激120 min的情况下线粒体ROS显著上升(P < 0.05),电刺激180 min的情况下线粒体ROS稍有上升(图 2)。
2.
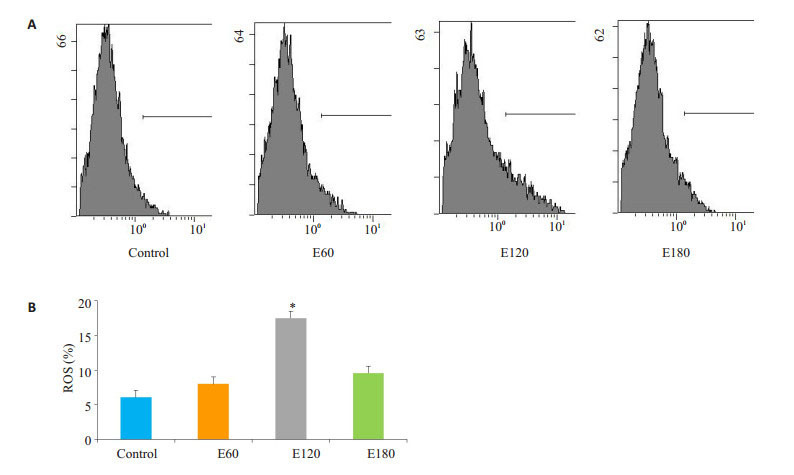
不同时间电刺激对C2C12肌管线粒体ROS影响
Effect of electrical stimulation on ROS in C2C12 myotubes (*P < 0.05 vs Control). A: Flow cytometry of DCFH; B: Quantification of the results.
2.4. 电刺激对C2C12肌管线粒体膜电位的影响
电刺激60 min线粒体膜电位稍有下降,但无显著差异(P>0.05);电刺激120 min的情况下线粒体膜电位下降(P < 0.05),电刺激180 min的情况下线粒体膜电位亦下降(P < 0.01,图 3)。
3.
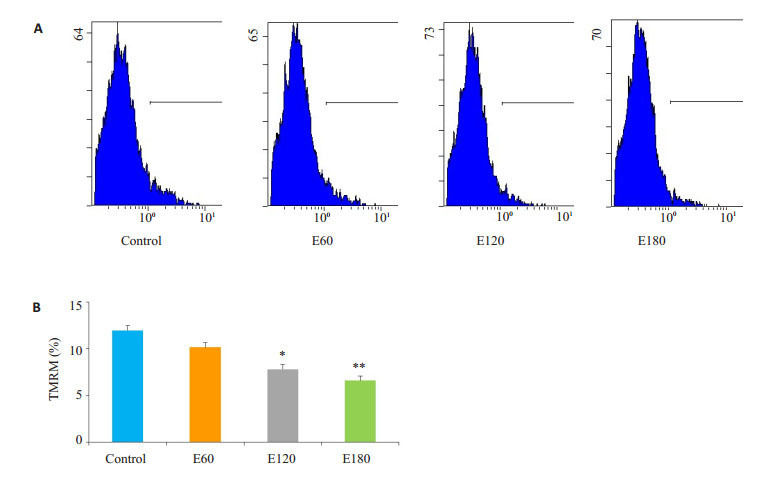
不同时间电刺激对C2C12肌管线粒体膜电位影响
Effect of electrical stimulation on TMRM in C2C12 myotubes (*P < 0.05; **P < 0.01 vs Control). A: Flow cytometry of the TMRM; B: Quantification of the results.
2.5. 电刺激对C2C12肌管机制蛋白的影响
电刺激60 min和120 min可以显著激活AMPKSer485(P < 0.05),而AMPK-Thr172仅在电刺激60 min显著活化(P < 0.01),PGC1在电刺激120 min显著上升(P < 0.01,图 4)。
4.
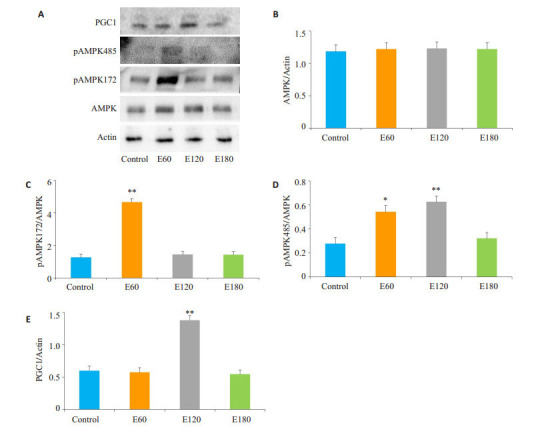
不同时间电刺激后C2C12肌管AMPK表达
Expression of AMPK proteins in C2C12 myotube after electrical stimulations (*P < 0.05; **P < 0.01 vs Control). A: Immunoblotting of the related proteins; B-E: Quantification of the results.
3. 讨论
众所周知,不同的运动方案引发不同的细胞和组织表型变化[21-23]。耐力运动的特点是高摄氧量、低收缩力和线粒体依赖功能,运动可刺激骨骼肌线粒体生物发生,促进线粒体融合与分裂[24-25]。此外,最近研究表明肌肉是运动中产生自由基的重要来源[26-27],因此耐力运动一定程度上提高了骨骼肌的氧化能力和线粒体功能[28-30]。本研究发现,随着电刺激时间的延长,虽然肌管的形态没有显著变化,但是肌管MDA、ROS和线粒体ROS都有上升,线粒体膜电位逐渐下降。表明本实验的电刺激强度模拟的是有氧耐力运动,而且成功导致运动氧化应激和线粒体功能下降。但是,不同的电刺激时间导致的运动氧化应激和线粒体功能的变化不同,因此需要开展其机制研究。
我们的实验结果显示,电刺激60 min,AMPKSer485显著上升,AMPK-Thr172极显著上升;电刺激120 min,只有AMPK-Ser485极显著上升,电刺激180 min,两者均无变化。分析结果可知,首先,中等时间电刺激后,虽然两个AMPK两个位点都被磷酸化,但AMPKThr172磷酸化占绝对优势,此时AMPK处于活化状态。因此,C2C12肌管电刺激60 min后,即使ROS造成了显著的氧化应激,但线粒体膜电位虽有下降,并无显著差异。其次,中长时间电刺激后,AMPK-Ser485磷酸化占绝对优势,AMPK活化受到抑制,此时ROS造成的氧化应激引起明显的线粒体膜损伤。最后,超长时间电刺激后氧化应激水平较高,肌管损伤非常严重,AMPK两个位点的磷酸化均未显著上升。
综上所述,不同时间的电刺激后导致肌管不同程度的氧化应激,且随时间延长,氧化应激程度越强烈,线粒体损伤越严重。不同时间电刺激引起的氧化应激由AMPK不同位点的磷酸化调控,AMPK-Thr172调控中长时间电刺激引起的氧化应激,而AMPK-Ser485及PGC1调控超长时间电刺激引起的线粒体氧化应激。本文研究了不同强度的运动训练对ROS及AMPK信号通路的影响,探索最新的分子靶点,为调控骨骼肌细胞稳态及运动后肌肉疲劳损伤及老化。
Biography
董合玲,博士,讲师,E-mail: dongyihe914@163.com
Funding Statement
广东省自然科学基金(2017A030310178);中央高校教育教学改革专项(82617058)
Contributor Information
董 合玲 (Heling DONG), Email: dongyihe914@163.com.
赵 军 (Jun ZHAO), Email: 870835676@qq.com.
徐 晓阳 (Xiaoyang XU), Email: xuxy@scnu.edu.cn.
References
- 1.Berlin K, Kruger T, Klenosky DB. A mixed-methods investigation of successful aging among older women engaged in sports- based versus exercise-based leisure time physical activities. J Women Aging. 2018;30(1):27–37. doi: 10.1080/08952841.2016.1259439. [Berlin K, Kruger T, Klenosky DB. A mixed-methods investigation of successful aging among older women engaged in sports- based versus exercise-based leisure time physical activities[J]. J Women Aging, 2018, 30(1): 27-37.] [DOI] [PubMed] [Google Scholar]
- 2.Seo DY, Lee SR, Kim N, et al. Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res. 2016;5(3):182–6. doi: 10.1016/j.imr.2016.07.003. [Seo DY, Lee SR, Kim N, et al. Age-related changes in skeletal muscle mitochondria: the role of exercise[J]. Integr Med Res, 2016, 5(3): 182-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, et al. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats. Dis Model Mech. 2016;9(10):1221–9. doi: 10.1242/dmm.026591. [Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, et al. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats[J]. Dis Model Mech, 2016, 9(10): 1221-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nylén C, Aoi W, Abdelmoez AM, et al. IL6 and LIF mRNA expression in skeletal muscle is regulated by AMPK and the transcription factors NFYC, ZBTB14 and SP1. https://doi.org/10.1152/ajpendo.00398.2017. Am J Physiol Endocrinol Metab. 2018, [Epub ahead of print] doi: 10.1152/ajpendo.00398.2017. [Nylén C, Aoi W, Abdelmoez AM, et al. IL6 and LIF mRNA expression in skeletal muscle is regulated by AMPK and the transcription factors NFYC, ZBTB14 and SP1[J]. Am J Physiol Endocrinol Metab, 2018, [Epub ahead of print].] [DOI] [PubMed] [Google Scholar]
- 5.Lee SG, Wu HM, Lee CG, et al. Binge alcohol intake after hypergravity stress sustainably decreases AMPK and transcription factors necessary for hepatocyte survival. Alcohol Clin Exp Res. 2017;41(1):76–86. doi: 10.1111/acer.13265. [Lee SG, Wu HM, Lee CG, et al. Binge alcohol intake after hypergravity stress sustainably decreases AMPK and transcription factors necessary for hepatocyte survival[J]. Alcohol Clin Exp Res, 2017, 41(1): 76-86.] [DOI] [PubMed] [Google Scholar]
- 6.Chang HW, Pisano S, Chaturbedi A, et al. Transcription factors CEP- 1/p53 and CEH-23 collaborate with AAK-2/AMPK to modulate longevity in Caenorhabditis elegans. Aging Cell. 2017;16(4):814–24. doi: 10.1111/acel.2017.16.issue-4. [Chang HW, Pisano S, Chaturbedi A, et al. Transcription factors CEP- 1/p53 and CEH-23 collaborate with AAK-2/AMPK to modulate longevity in Caenorhabditis elegans[J]. Aging Cell, 2017, 16(4): 814-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo VF, Carnio S, Vainshtein A, et al. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity. Autophagy. 2014;10(11):1883–94. doi: 10.4161/auto.32154. [Lo VF, Carnio S, Vainshtein A, et al. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity[J]. Autophagy, 2014, 10(11): 1883-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi L, Zhang T, Zhou Y, et al. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPKPGC-1α-Sirt3 signaling pathway. Endocrine. 2015;50(2):378–89. doi: 10.1007/s12020-015-0599-5. [Shi L, Zhang T, Zhou Y, et al. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPKPGC-1α-Sirt3 signaling pathway[J]. Endocrine, 2015, 50(2): 378-89.] [DOI] [PubMed] [Google Scholar]
- 9.Close GL, Hamilton DL, Philp A, et al. New strategies in sport nutrition to increase exercise performance. Free Radic Biol Med. 2016;98:144–58. doi: 10.1016/j.freeradbiomed.2016.01.016. [Close GL, Hamilton DL, Philp A, et al. New strategies in sport nutrition to increase exercise performance[J]. Free Radic Biol Med, 2016, 98: 144-58.] [DOI] [PubMed] [Google Scholar]
- 10.Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPKmediated signal transduction beyond energetic clues. http://jcs.biologists.org/content/125/9/2115.abstract. J Cell Sci. 2012;125(Pt 9):2115–25. doi: 10.1242/jcs.095216. [Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPKmediated signal transduction beyond energetic clues[J]. J Cell Sci, 2012, 125(Pt 9): 2115-25.] [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Song SE, Kim YW, et al. Adiponectin inhibits palmitateinduced apoptosis through suppression of reactive Oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010;207(1):35–44. doi: 10.1677/JOE-10-0093. [Kim JE, Song SE, Kim YW, et al. Adiponectin inhibits palmitateinduced apoptosis through suppression of reactive Oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase[J]. J Endocrinol, 2010, 207(1): 35-44.] [DOI] [PubMed] [Google Scholar]
- 12.Ohsaka Y, Nishino H, Nomura Y. Adipose cells induce phospho-Thr- 172 AMPK production by epinephrine or CL316243 in mouse 3T3- L1 adipocytes or MAPK activation and G protein-associated PI3K responses induced by CL316243 or Aluminum fluoride in rat white adipocytes. http://www.ncbi.nlm.nih.gov/pubmed/25152050. Folia Biol (Praha) 2014;60(4):168–79. doi: 10.14712/fb2014060040168. [Ohsaka Y, Nishino H, Nomura Y. Adipose cells induce phospho-Thr- 172 AMPK production by epinephrine or CL316243 in mouse 3T3- L1 adipocytes or MAPK activation and G protein-associated PI3K responses induced by CL316243 or Aluminum fluoride in rat white adipocytes[J]. Folia Biol (Praha), 2014, 60(4): 168-79.] [DOI] [PubMed] [Google Scholar]
- 13.Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281(9):5335–40. doi: 10.1074/jbc.M506850200. [Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491[J]. J Biol Chem, 2006, 281(9):5335-40.] [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Park M, Lee SK, et al. Phosphorylation of hypothalamic AMPK on serine(485/491) related to sustained weight loss by alphalipoic acid in mice treated with olanzapine. Psychopharmacology (Berl) 2014;231(20):4059–69. doi: 10.1007/s00213-014-3540-3. [Kim H, Park M, Lee SK, et al. Phosphorylation of hypothalamic AMPK on serine(485/491) related to sustained weight loss by alphalipoic acid in mice treated with olanzapine[J]. Psychopharmacology (Berl), 2014, 231(20): 4059-69.] [DOI] [PubMed] [Google Scholar]
- 15.Morales-Alamo D, Ponce-González JG, Guadalupe-Grau A, et al. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKα phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J Appl Physiol (1985) 2012;113(6):917–28. doi: 10.1152/japplphysiol.00415.2012. [Morales-Alamo D, Ponce-González JG, Guadalupe-Grau A, et al. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKα phosphorylation, in response to sprint exercise in severe acute hypoxia in humans[J]. J Appl Physiol (1985), 2012, 113(6): 917-28.] [DOI] [PubMed] [Google Scholar]
- 16.Miura S, Kai Y, Tadaishi M, et al. Marked phenotypic differences of endurance performance and exercise-induced Oxygen consumption between AMPK and LKB1 deficiency in mouse skeletal muscle: changes occurring in the diaphragm. Am J Physiol Endocrinol Metab. 2013;305(2):E213–29. doi: 10.1152/ajpendo.00114.2013. [Miura S, Kai Y, Tadaishi M, et al. Marked phenotypic differences of endurance performance and exercise-induced Oxygen consumption between AMPK and LKB1 deficiency in mouse skeletal muscle: changes occurring in the diaphragm[J]. Am J Physiol Endocrinol Metab, 2013, 305(2): E213-29.] [DOI] [PubMed] [Google Scholar]
- 17.Morales-Alamo D, Calbet JA. Free radicals and sprint exercise in humans. Free Radic Res. 2014;48(1):30–42. doi: 10.3109/10715762.2013.825043. [Morales-Alamo D, Calbet JA. Free radicals and sprint exercise in humans[J]. Free Radic Res, 2014, 48(1): 30-42.] [DOI] [PubMed] [Google Scholar]
- 18.Dagon Y, Hur E, Zheng B, et al. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab. 2012;16(1):104–12. doi: 10.1016/j.cmet.2012.05.010. [Dagon Y, Hur E, Zheng B, et al. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake[J]. Cell Metab, 2012, 16(1): 104-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley SA, Ross FA, Gowans GJ, et al. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. Biochem J. 2014;459(2):275–87. doi: 10.1042/BJ20131344. [Hawley SA, Ross FA, Gowans GJ, et al. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells[J]. Biochem J, 2014, 459(2): 275-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irving M. Regulation of contraction by the thick filaments in skeletal muscle. Biophys J. 2017;113(12):2579–94. doi: 10.1016/j.bpj.2017.09.037. [Irving M. Regulation of contraction by the thick filaments in skeletal muscle[J]. Biophys J, 2017, 113(12): 2579-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibb AA, Epstein PN, Uchida S, et al. Exercise-Induced changes in glucose metabolism promote physiological cardiac growth. Circulation. 2017;136(22):2144–57. doi: 10.1161/CIRCULATIONAHA.117.028274. [Gibb AA, Epstein PN, Uchida S, et al. Exercise-Induced changes in glucose metabolism promote physiological cardiac growth[J]. Circulation, 2017, 136(22): 2144-57.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short AK, Yeshurun S, Powell R, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry. 2017;7(5):e1114. doi: 10.1038/tp.2017.82. [Short AK, Yeshurun S, Powell R, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety[J]. Transl Psychiatry, 2017, 7(5): e1114.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown B, Somauroo J, Green DJ, et al. The complex phenotype of the athlete's heart: implications for preparticipation screening. Exerc Sport Sci Rev. 2017;45(2):96–104. doi: 10.1249/JES.0000000000000102. [Brown B, Somauroo J, Green DJ, et al. The complex phenotype of the athlete's heart: implications for preparticipation screening[J]. Exerc Sport Sci Rev, 2017, 45(2): 96-104.] [DOI] [PubMed] [Google Scholar]
- 24.Gioscia-Ryan RA, Battson ML, Cuevas LM, et al. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. http://www.aging-us.com/article/101099/text#! Aging (Albany NY) 2016;8(11):2897–914. doi: 10.18632/aging.101099. [Gioscia-Ryan RA, Battson ML, Cuevas LM, et al. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice[J]. Aging (Albany NY), 2016, 8(11): 2897-914.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpentieri A, Gamberi T, Modesti A, et al. Profiling carbonylated proteins in heart and skeletal muscle mitochondria from trained and untrained mice. J Proteome Res. 2016;15(10):3666–78. doi: 10.1021/acs.jproteome.6b00475. [Carpentieri A, Gamberi T, Modesti A, et al. Profiling carbonylated proteins in heart and skeletal muscle mitochondria from trained and untrained mice[J]. J Proteome Res, 2016, 15(10): 3666-78.] [DOI] [PubMed] [Google Scholar]
- 26.Chen PB, Yang JS, Park Y. Adaptations of skeletal muscle mitochondria to obesity, exercise, and polyunsaturated fatty acids. Lipids. 2018;53(3):271–8. doi: 10.1002/lipd.2018.53.issue-3. [Chen PB, Yang JS, Park Y. Adaptations of skeletal muscle mitochondria to obesity, exercise, and polyunsaturated fatty acids [J]. Lipids, 2018, 53(3): 271-8.] [DOI] [PubMed] [Google Scholar]
- 27.Maeda A, Shirao T, Shirasaya D, et al. Piperine promotes glucose uptake through ROS-Dependent activation of the CAMKK/AMPK signaling pathway in skeletal muscle. Mol Nutr Food Res. 2018;62(11):e1800086. doi: 10.1002/mnfr.v62.11. [Maeda A, Shirao T, Shirasaya D, et al. Piperine promotes glucose uptake through ROS-Dependent activation of the CAMKK/AMPK signaling pathway in skeletal muscle[J]. Mol Nutr Food Res, 2018, 62(11): e1800086.] [DOI] [PubMed] [Google Scholar]
- 28.Sadeghi A, Rostamirad A, Seyyedebrahimi S, et al. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. https://link.springer.com/article/10.1007/s10787-018-0466-0. Inflammopharmacology. 2018, [Epub ahead of print] doi: 10.1007/s10787-018-0466-0. [Sadeghi A, Rostamirad A, Seyyedebrahimi S, et al. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production[J]. 2018, Inflammopharmacology, [Epub ahead of print].] [DOI] [PubMed] [Google Scholar]
- 29.Zhou T, Prather ER, Garrison DE, et al. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int J Mol Sci. 2018;19(2):E417. doi: 10.3390/ijms19020417. [Zhou T, Prather ER, Garrison DE, et al. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle[J]. Int J Mol Sci, 2018, 19(2): E417.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol. 2017;233(1):R15–42. doi: 10.1530/JOE-16-0598. [Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources[J]. J Endocrinol, 2017, 233(1): R15-42.] [DOI] [PubMed] [Google Scholar]


