Abstract
目的
探讨血清检测磷酯酰肌醇蛋白聚糖-3(GPC3)对原发性肝细胞癌(PHC)诊断及疗效评估的临床意义。
方法
采用双抗夹心ELISA法, 对60例PHC, 60例转移性肝癌, 50例肝硬化, 50例慢性病毒性肝炎, 20例肝囊肿, 20例脂肪肝, 20例肝血管瘤, 20例药物性肝炎及40例健康人血清中GPC3进行定量检测。同时采用化学发光法检测PHC患者血清中甲胎蛋白水平, 并对治疗前后PHC患者血清中GPC3及甲胎蛋白水平进行比较分析。
结果
PHC组血清GPC3水平显著高于其他肝病组及健康对照组, 差异有统计学意义(P < 0.05)。转移性肝癌、肝硬化及病毒性肝炎组血清GPC3水平显著高于其他良性肝病组和健康对照组, 差异有统计学意义(P < 0.05)。PHC组血清GPC3水平与甲胎蛋白水平及肝功能状况无关。GPC3与甲胎蛋白诊断PHC的阳性率分别为65%和56.7%, 两者联合检测可将PHC的诊断率提高至85%。在23例治疗有效的PHC患者中, 15例患者血清中GPC3水平较治疗前呈现稳定的下降趋势, 而仅有10例患者血清中甲胎蛋白呈现与疗效相似的下降趋势。
结论
GPC3与甲胎蛋白联合检测有望提高PHC的早期诊断率, 同时血清GPC3的不同阈值或可在PHC及其他各类肝脏疾病的鉴别诊断过程中发挥作用。GPC3具有较甲胎蛋白更优的特异性及灵敏度, 或可成为判断PHC治疗疗效的一种特异性血清学标志
Keywords: 原发性肝细胞肝癌, 肿瘤标志物, 磷酯酰肌醇蛋白聚糖-3, 甲胎蛋白
Abstract
Objective
To explore the clinical value of detecting serum glypican-3 in the diagnosis and therapeutic effect evaluation of primary hepatocellular carcinoma (PHC).
Methods
Using sandwich ELISA, we detected serum glypican-3 levels in 60 patients with PHC, 60 with metastatic liver cancer, 50 with liver cirrhosis, 50 with chronic viral hepatitis, 20 with hepatic cyst, 20 with fatty liver, 20 with hepatic hemangioma and 20 with drug-induced hepatitis as well as in 40 healthy subjects (control). We also analyzed the changes in serum levels of glypican-3 and alpha fetoprotein (AFP) in PHC patients after treatment.
Results
PHC patients had significantly higher serum levels of glypican-3 than patients with other liver diseases and the control subjects (P < 0.05). The levels of serum glypican-3 were significantly higher in patients with metastatic liver cancer, liver cirrhosis and viral hepatitis than in those with other benign liver diseases and the control subjects (P < 0.05). Glypican-3 level was not associated with AFP level or liver function in PHC patients, in whom the positivity rates for glypican-3 and AFP were 65% and 56.7%, respectively. The detection rate of PHC increased to 85% by a combined detection of AFP and glypican-3. In the 23 PHC patients who responded positively to treatments, serum glypican-3 level showed a steady decline compared with that in 15 patients before treatment, while serum AFP level showed a similar decrease only in 10 patients.
Conclusion
Combined detection of glypican-3 and AFP is expected to improve the early diagnosis rate of PHC. The different thresholds of serum glypican-3 may play a role in the differential diagnosis of PHC and other various liver diseases. Glypican-3 may serve as a better marker than AFP with a high specificity and sensitivity for evaluating the therapeutic effect in PHC patients
Keywords: primary hepatocellular carcinoma, tumor markers, glypican-3, alpha fetoprotein
原发性肝细胞肝癌(PHC)是世界高发的恶性肿瘤之一,其发病率呈逐年上升趋势,且早期诊断率及五年生存率极低[1-2]。影像技术如超声、CT及磁共振的快速发展为PHC的早期诊断提供了可能。肿瘤标志物的出现,如甲胎蛋白(AFP)、AFP异质体-L3和高尔基体蛋白73也使得PHC的诊断率较前大大提高[3-4]。AFP是目前广泛用于PHC筛查及诊断的肿瘤标志物,但因血清AFP水平易受到肝炎及其他肝病的影响[5],10%~20%早期PHC患者血清AFP为正常水平,导致AFP在PHC筛查方面,以及对于肝脏内一些异常增生的小结节及其他良性肝细胞损害的诊断具有一定的局限性[6-7]。因此寻找敏感性及特异性更高的肿瘤标志物一直是研究的热点。
磷酯酰肌醇蛋白聚糖3(GPC3)是近年来发现的一种新的最有希望成为PHC特异性肿瘤标志物的蛋白。国内外多数研究认为GPC3在诊断PHC的敏感性及特异性方面可能更有优势[8-9],但该结论仍受到一些研究者的质疑[10-11]。一项荟萃分析显示PHC患者血清GPC3水平显著高于健康人群,但尚无法确定GPC3在鉴别诊断PHC和肝硬化中的临床价值[12]。对于血清中GPC3的表达水平在PHC鉴别诊断方面的价值,目前研究很少,证据很不充分。本研究通过检测各种肝病患者血清中GPC3水平,旨在进一步探讨GPC3对诊断和鉴别诊断PHC,以及在预后评估方面的临床价值,为GPC3在临床上的广泛应用提供理论依据。
1. 资料和方法
1.1. 一般资料
选择2006年9月~2014年3月间我院消化科及肿瘤科收治的不同肝脏疾病患者共300例作为研究对象。其中PHC60例,转移性肝癌60例,肝硬化50例,慢性病毒性肝炎50例,肝囊肿20例,脂肪肝20例,肝血管瘤20例,药物性肝炎20例。男性共187例,女性共113例,年龄范围26~80岁,平均50.2±11.7岁。另收集40例健康体检者作为研究对照,其中男性22例,女性18例,年龄范围27~77岁,平均51.5±12.4岁。PHC诊断根据2011年原发性肝癌诊疗规范[13]。PHC的分期分级根据UICC TNM标准。PHC患者的纳入标准:(1)以病理学诊断初次确诊的PHC患者;(2)随访时间超过1年;(3)第1次采血前未行治疗,包括姑息治疗;(4)临床资料完整。排除标准:(1)缺少病理学诊断;(2)病危或合并其他肿瘤;(3)资料不全等影响疗效判断者。慢性病毒性肝炎的诊断根据2000年病毒性肝炎防治方案[14]。其他肝病均结合B超、CT及检验结果以确诊。
1.2. 方法
1.2.1. 血清标本的采集和保存
抽取患者及健康体检者清晨空腹全血3 mL,血清分离后于-80 ℃保存,分批次集中检测血清中GPC3和AFP水平。
1.2.2. 血清GPC3蛋白浓度测定
取酶标板,设空白孔,将试剂盒中的GPC3标准品倍比稀释至5个浓度依次加入各孔;同时用稀释液将样本血清按照1:200稀释后加入孔中,100 µL/孔37 ℃温育30 min。弃去液体后,用洗涤液先过洗1遍,后洗涤5次,每次洗涤轻微振摇酶标板30 s后弃去洗液。每孔加入100 µL酶联检测抗体37 ℃温育30 min。弃去板内液体,相同方法洗涤5次。加入每孔依次加入50 µL底物A及B,显色。避光显色3 min,加入50 µL终止液终止后用酶标仪检测各孔于波长450 nm的光密度值(A)。制定标准曲线,计算样本中GPC3的浓度。
1.2.3. 血清AFP浓度检测
采用化学发光法定量检测血清AFP水平,按卫生部《原发性肝癌诊疗规范》(2011年版),AFP≥200 ng/mL判定为阳性[13]。
1.2.4. 肝脏生化及凝血指标测定
谷草转氨酶(AST)、谷丙转氨酶(ALT)、总胆红素(TBIL)、白蛋白(ALB)均采用全自动生化分析仪测定;血浆凝血酶原时间(PT)采用比浊法测定。
1.3. 统计学分析
采用SPSS 19.0软件进行统计学分析。呈正态分布的计量资料以均数±标准差表示。非正态分布计数资料的以中位数表示。计量资料两组间均数比较采用t检验,多组间均数比较采用单因素方差分析。计数资料比较采用χ2检验。P < 0.05具有统计学差异。
2. 结果
2.1. 各种肝脏疾病患者血清中GPC3蛋白浓度的差异
利用检测试剂盒中的GPC3标准品,倍比稀释后测得的吸光度值建立标准曲线(图 1)以判断样本中的GPC3浓度。结果判定值GPC3蛋白 > 5 ng/mL为阳性,并将此浓度确定为1:200倍稀释后血清样本中GPC3阳性值下线。各组GPC3检测结果(表 1)如下:PHC(39/60,65%);转移性肝癌(7/60,11.7%);肝硬化(3/50,6%);慢性病毒性肝炎(4/50,8%);肝囊肿(1/20,5%);脂肪肝、肝血管瘤、药物性肝炎及健康对照组血清中GPC3均为阴性。GPC3在各组间的差异具有统计学意义(P=0.00),其中PHC患者血清中GPC3蛋白水平明显高于其他各种肝脏疾病及健康人血清中的GPC3蛋白水平,差异有统计学意义(P < 0.05)。统计数据显示转移性肝癌、肝硬化及慢性病毒性肝炎患者血清中GPC3水平与其它良性肝病及健康对照组相比,差异显著(P < 0.05,表 1)。
1.
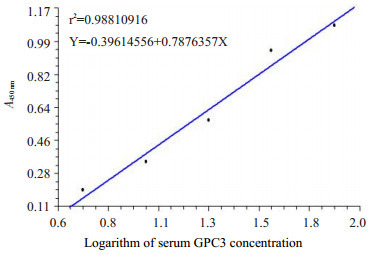
ELISA检测血清中GPC3蛋白
Quantification of GPC3 protein in the sera using ELISA. Standard curve to quantify the GPC3 protein was derived based on the absorbance data of serially diluted GPC3 standard sample.
1.
各种肝脏疾病患者及健康人血清中GPC3水平
Serum levels of GPC3 in patients with different liver diseases and healthy controls
| Disease | n | Gender | Virus-positive donor | GPC3 (ng/mL) | GPC3 positive rate | |||
| Male | Female | HBV | HCV | |||||
| Compared with the other groups, *P < 0.05; Compared with the other groups, ΔP < 0.05. | ||||||||
| PHC | 60 | 42 | 18 | 42 | 3 | 21.67±19.36* | 39/60 (65%) | |
| Metastatic liver cancer | 60 | 35 | 25 | 5 | 0 | 5.15±4.45Δ | 7/60 (11.7%) | |
| Liver cirrhosis | 50 | 36 | 14 | 32 | 4 | 4.27±3.36Δ | 3/50 (6%) | |
| Chronic viral hepatitis | 50 | 31 | 19 | 40 | 10 | 3.96±3.60Δ | 4/50 (8%) | |
| Hepatic cyst | 20 | 13 | 7 | 4 | 0 | 2.55±1.22 | 1/20 (5%) | |
| Adiposis hepatica | 20 | 12 | 8 | 0 | 0 | 2.54±1.22 | 0/20 (0%) | |
| Hepatic hemangioma | 20 | 10 | 10 | 0 | 0 | 2.55±1.15 | 0/20 (0%) | |
| Drug-induced hepatitis | 20 | 10 | 0 | 0 | 0 | 1.92±1.10 | 0/20 (0%) | |
| Healthy control | 40 | 22 | 18 | 0 | 0 | 1.68±0.94 | 0/40 (0%) | |
2.2. PHC患者血清中GPC3升高与AFP无关
ELISA结果显示(表 2),60例PHC患者中AFP及GPC3两种指标检测的阳性率分别为39/60(65%);34/ 60(56.7%)。而两种指标联合检测可使诊断率显著提高至51/60(85%)。另外GPC3(+),AFP(-)的PHC患者中有5例分期为Ib期,因此GPC3作为一种新型的肿瘤标志物联合多种检测指标不仅使PHC的诊断率显著提高,并有望提高PHC的早期诊断率。
2.
PHC患者血清中GPC3和AFP及二项联合检测的阳性率比较
Comparison of positivity rates of serum GPC3 and AFP and binomial test in PHC patients
| Protein | Positive number | Negative number | Positive rate |
| GPC3 | 39 | 21 | 65% |
| AFP | 34 | 26 | 56.70% |
| GPC3+AFP | 51 | 9 | 85% |
2.3. 血清中GPC3浓度与肝细胞受损情况及肝功状态无关
用AST和ALT来做为评价肝细胞受损情况的指标;用TBIL、ALB、PT及有无腹水来评价肝脏功能情况。将60份PHC血清标本分为GPC3(+)组及GPC3(-)组,通过各种评价指标比较两组在肝细胞受损情况及肝功能状态方面的差异。卡方检验的结果显示(表 3),6种评价指标在两组间均无显著差异(P > 0.05)。表明PHC患者血清中GPC3蛋白浓度与肝细胞损伤情况及肝功能状态无关,肝炎活动以及多种良性肝脏疾病造成的肝细胞损伤不会影响血清中GPC3蛋白浓度。进一步说明了GPC3蛋白在患者血清中浓度的变化直接与PHC相关。
3.
PHC患者血清GPC3水平与肝细胞及肝功状态无关
Correlation of serum GPC3 levels with hepatocyte injury and liver function in 60 patients with PHC
| Variables | GPC3 (+) N=39 | GPC3 (-) N=21 | χ2 | P |
| Aspartate transaminase (AST); Alanine aminotransferase (ALT); Total bilirubin(TBIL); Albumin(ALB); Prothrombin time(PT). | ||||
| AST | 28/39 (71.8%) | 14/21 (66.7%) | 0.171 | 0.771 |
| ALT | 27/39 (69.2%) | 12/21 (57.1%) | 0.877 | 0.402 |
| TBIL | 26/39 (66.7%) | 10/21 (47.6%) | 2.063 | 0.176 |
| ALB | 19/39 (48.7%) | 11/21 (52.4%) | 0.073 | 1.000 |
| PT | 4/39 (10.3%) | 3/21 (14.3%) | 0.215 | 0.687 |
| Ascites | 9/39 (23.1%) | 4/21 (19.0%) | 0.131 | 1.000 |
2.4. 治疗前后PHC患者血清中GPC3及AFP的动态变化
60例确诊为PHC患者中,有47例接受了规范治疗,包括手术、肝动脉栓塞化疗术、射频消融术、靶向治疗和/或光子刀治疗后,随后对治疗后的患者进行连续追踪的影像学评价及临床分析。其中23例患者,肿瘤的大小减小及数目明显减少且患者的一般状况及肝功能状况改善,判断临床治疗有效。我们对这些患者治疗前后血清样本中GPC3和AFP的浓度进行跟踪检测。23例PHC患者治疗后血清检测结果显示,15例患者在治疗前后血清中GPC3蛋白浓度呈现稳定的下降趋势,而AFP仅在10例患者中呈现明显的下降趋势。表明血清中AFP变化在治疗有效的PHC患者中变化尚不稳定。与AFP相比,GPC3作为PHC肿瘤标志物具有更高的敏感性,提示GPC3不仅可用于PHC的早期诊断,可能还可做为一种判断疗效及预后的敏感性指标。
3. 讨论
GPC3是一种细胞膜糖蛋白,相对分子质量约为60 000,属硫酸肝素蛋白多糖家族。目前共发现6名该家族成员,包括GPC1-5和GPC6 [15-16]。GPC3在妊娠期主要由胎肝合成,出生后除少数组织有较少表达外,其余正常组织均无明显表达[17]。其与PHC、肝母细胞瘤、大肠癌、子宫内膜癌、黑素瘤、肺鳞癌、卵巢透明细胞癌等多种肿瘤的发生发展关系密切[18-19]。GPC3通过与各种配体和受体(包括细胞粘附分子,基质成分,生长因子,酶及其抑制剂)的相互作用参与多种细胞生理和病理过程,同时也可能参与抑制和调节大多数中胚层组织和器官的生长,具体机制目前尚不清楚[20]。有研究者报道GPC3可能通过调节Wnt信号通路在PHC中发挥作用[21]。细胞表面的GPC3还可作为肝素结合生长因子的存储位点,通过ERK和/或AKT信号通路影响肝癌细胞的侵袭性生长[22]。近期的一项研究显示PHC中过表达的GPC3可通过活化ERK促进上皮间质转化[23]。表明GPC3是PHC发生发展过程中的关键因素之一。因此本研究对GPC3在PHC诊断中的临床应用价值进行了探讨。
GPC3的氨基端(N端)可做为一种可溶性GPC3片段被分泌至细胞外,其在血清中的特异性较高[24]。通过ELISA方法检测PHC及其他肝脏疾病中可溶性GPC3的水平为PHC的血清学诊断开辟了新的途径[8, 25-26]。仍有一些研究者并不同意上述观点[10, 27-28]。Yasuda等[27]的研究并没有发现PHC患者及慢性肝病患者血清中GPC3的表达差异,慢性肝病患者血清中的GPC3似乎更高,但他们发现GPC3在PHC患者组织中高表达,表明GPC3是由PHC组织产生的,同时提出这种不同的结果可能是由于检测方式和过程不同所致。为证实GPC3是否具有更优的特异性及灵敏度,可在PHC的诊断及鉴别诊断中发挥作用。我们利用商品化的检测试剂盒,对PHC、转移性肝癌、肝硬化、慢性病毒性肝炎、肝囊肿、脂肪肝、肝血管瘤、药物性肝炎及健康人进行了较大范围的诊断性检测。在本实验室建立的标准体系中,GPC3在5~80 ng/mL的浓度范围呈现较好的线性趋势,GPC3在PHC、转移性肝癌、肝硬化、慢性病毒性肝炎患者中的阳性率分别为:65%、11.7%、6%及8%;20例肝囊肿患者中有1例表现为血清GPC3水平升高,脂肪肝、肝血管瘤、药物性肝炎及健康对照组血清中GPC3均为阴性。PHC血清中GPC3蛋白水平显著高于其他肝脏疾病及健康患者。该结果与国内外文献报道相似[10, 29]。此外有报道称肿瘤组织中GPC3表达有助于鉴别PHC及胆管细胞癌和转移性腺癌[30]。值得注意的是我们的研究中也发现GPC3水平在PHC及转移性肝癌组中具有显著差异,而转移性肝癌、肝硬化及慢性病毒性肝炎患者血清GPC3水平明显高于良性肝脏疾病及健康对照。提示血清GPC3的不同阈值或可在PHC及其他各类肝脏疾病的鉴别诊断过程中发挥作用。
做为影像学诊断的补充,AFP是目前临床上最常用和最有价值的血清学诊断指标。虽AFP诊断HCC具有较高的特异性但其灵敏度约40%~65% [8]。据文献统计仅依赖AFP来诊断PHC,漏诊率约40%。因此在PHC的筛查中有必要采用两种特异性肿瘤标志物联合检测的方式以提高疾病诊断率降低漏诊率[31]。本项研究在验证PHC患者血清GPC3水平与其他各类肝病患者中具有显著差异的基础上,对比分析了GPC3及AFP两种肿瘤标志物在PHC患者血清中的检出情况。60例PHC患者,GPC3及AFP诊断的阳性率分别为65%和56.7%。其中GPC3(+)患者中有11例患者血清中AFP(-);AFP(+)患者中有7例患者血清中GPC3(-),表明PHC患者血清中GPC3水平与AFP无关。GPC3和AFP两项指标联合检测可使PHC诊断阳性率显著提高至85%,与相关文献报道相似[32]。虽从总体而言,GPC3在PHC中的增幅并不如AFP显著,但两种检测指标在诊断上有很大的互补作用。两种血清肿瘤标记物联合检测有助于提高PHC的诊断价值。另外让人兴奋的是我们的研究中发现GPC3(+)、AFP(-)PHC患者中有5例为Ib期肿瘤,结合之前研究者发现GPC3在伴有门脉癌栓或肝外转移的早期小肝癌患者中显著升高[33-34],提示GPC3可能在PHC的早期诊断方面可能具有一定的临床价值,而GPC3联合其他血清学指标检测有望进一步提高PHC的早期诊断率。Nguyen等[35]的研究结果发现GPC3在低分化的PHC患者中敏感性更高,与Arg-1联合检测几乎可检出所有的低分化PHC。另外,本文还证实了GPC3在血清中浓度的升高与肝细胞的受损情况及肝功能状况无关。说明病毒性肝炎活动状态或其他良性肝脏疾病造成的肝细胞损伤并不会引起血清GPC3升高。而血清GPC3在转移性肝脏恶性肿瘤中未出现线束升高,进一步表明血清GPC3具有较好的特异性,其表达水平的变化可能直接与PHC相关。
基于上述研究基础,我们通过对23例临床治疗有效的PHC患者的追踪随访,治疗前后分别检测GPC3及AFP的动态变化。血清中GPC3蛋白水平呈现了比AFP更稳定的下降趋势,表明GPC3具有较AFP更高的灵敏度,有希望成为预后评估的指标。Ofuji等[36]也报道了血清GPC3水平可预测Ⅰ期PHC术后的复发风险。综上,我们认为GPC3作为一种新型的肿瘤胚胎抗原,在PHC的诊断及鉴别诊断中显示出了一定的优势,具备成为肿瘤诊断标志物的可能;同时GPC3有助于对PHC患者进行较好的疗效判断及预后分析,具有很好的临床应用价值。目前GPC3过表达可作为肿瘤标志物和预后评估已在肿瘤的临床诊治中初显其应用价值,抗GPC3抗体已进入肝癌治疗的临床实验阶段[37]。因此,深入地研究GPC3在多种恶性肿瘤中的功能及致瘤机制必将推动肿瘤的诊断及以GPC3为靶点的抗体、疫苗研究的不断深入,肿瘤免疫治疗将成为继肿瘤手术、放疗和化疗之后又一有效治疗方法。
Biography
王媛媛, 博士, 主治医师, E-mail: doc_wang@126.com
Funding Statement
国家消化系统疾病临床医学研究中心科技支撑项目(2015BAI13B07); 广州市科技计划项目(2014Y2-00088)
Contributor Information
王 媛媛 (Yuanyuan WANG), Email: doc_wang@126.com.
肖 冰 (Bing XIAO), Email: fjxb@163.com.
References
- 1.Blechacz B, Mishra L. Hepatocellular carcinoma biology. http://www.ncbi.nlm.nih.gov/pubmed/22941010. Recent Results Cancer Res. 2013;190(7):1–20. doi: 10.1007/978-3-642-16037-0_1. [Blechacz B, Mishra L. Hepatocellular carcinoma biology[J]. Recent Results Cancer Res, 2013, 190(7): 1-20.] [DOI] [PubMed] [Google Scholar]
- 2.付 艳, 邢 卉春. 原发性肝癌的流行状况及危险因素分析. http://www.cnki.com.cn/Article/CJFDTOTAL-GZBZ201402039.htm. 中国肝脏病杂志. 2014;25(2):87–90. [付艳, 邢卉春.原发性肝癌的流行状况及危险因素分析[J].中国肝脏病杂志, 2014, 25(2): 87-90.] [Google Scholar]
- 3.Wang NY, Wang C, Li W, et al. Prognostic value of serum AFP, AFP-L3, and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation. Asian Pac J Cancer Prev. 2014;15(4):1539–44. doi: 10.7314/APJCP.2014.15.4.1539. [Wang NY, Wang C, Li W, et al. Prognostic value of serum AFP, AFP-L3, and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation[J]. Asian Pac J Cancer Prev, 2014, 15(4): 1539-44.] [DOI] [PubMed] [Google Scholar]
- 4.Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, et al. Hepatocellular carcinoma: a comprehensive review of biomarkers, clinical aspects, and therapy. http://www.ncbi.nlm.nih.gov/pubmed/28545181. Asian Pac J Cancer Prev. 2017;18(4):863–72. doi: 10.22034/APJCP.2017.18.4.863. [Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, et al. Hepatocellular carcinoma: a comprehensive review of biomarkers, clinical aspects, and therapy[J]. Asian Pac J Cancer Prev, 2017, 18 (4): 863-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43(3):434–41. doi: 10.1016/j.jhep.2005.03.019. [Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial [J]. J Hepatol, 2005, 43(3): 434-41.] [DOI] [PubMed] [Google Scholar]
- 6.European Association For The Study Of The Liver1; European Organisation For Research And Treatment of Cancer. EASL-EORTC clinical practice guidelines: management ofhepatocellular carcinoma. https://www.mendeley.com/research-papers/easl-eortc-clinical-practice-guidelines-management-hepatocellular-carcinoma-european-organisation-re/ Eur J Cancer. 2012;56(4):908–43. doi: 10.1016/j.jhep.2011.12.001. [European Association For The Study Of The Liver1; European Organisation For Research And Treatment of Cancer. EASL-EORTC clinical practice guidelines: management ofhepatocellular carcinoma[J]. Eur J Cancer, 2012, 56(4): 908-43.] [DOI] [PubMed] [Google Scholar]
- 7.吴 黎黎, 邵 璇璇, 张 曼, et al. 血清肿瘤标志物的联合检测在原发性肝癌中的诊断价值. http://www.cnki.com.cn/Article/CJFDTOTAL-JYYL201506014.htm. 检验医学与临床. 2015;24(6):754–5. [吴黎黎, 邵璇璇, 张曼, 等.血清肿瘤标志物的联合检测在原发性肝癌中的诊断价值[J].检验医学与临床, 2015, 24(6): 754-5.] [Google Scholar]
- 8.Lee HJ, Yeon JE, Suh SJ, et al. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver. 2014;8(2):177–85. doi: 10.5009/gnl.2014.8.2.177. [Lee HJ, Yeon JE, Suh SJ, et al. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma[J]. Gut Liver, 2014, 8(2): 177-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Shang W, Yu X, et al. Glypican-3: a promising biomarker for hepatocellular carcinoma diagnosis and treatment[J]. Med Res Rev, 2017, 16. doi: <a href="http://dx.doi.org/10.1002/med.21455.[Epubaheadofprint]." target="_blank">10.1002/med.21455.[Epubaheadofprint].</a>http://www.ncbi.nlm.nih.gov/pubmed/28621802
- 10.Wang YC, Yang HY, Xu HF, et al. Golgi protein 73, not Glypican-3, May be a tumor marker complementary to alpha-Fetoprotein for hepatocellular carcinoma diagnosis. J Gastroenterol Hepatol. 2014;29(3):597–602. doi: 10.1111/jgh.12461. [Wang YC, Yang HY, Xu HF, et al. Golgi protein 73, not Glypican-3, May be a tumor marker complementary to alpha-Fetoprotein for hepatocellular carcinoma diagnosis[J]. J Gastroenterol Hepatol, 2014, 29(3): 597-602.] [DOI] [PubMed] [Google Scholar]
- 11.Jeon Y, Jang ES, Choi YS, et al. Glypican-3 level assessed by the enzyme-linked immunosorbent assay is inferior to alpha-fetoprotein level for hepatocellular carcinoma diagnosis. Clin Mol Hepatol. 2016;22(3):359–65. doi: 10.3350/cmh.2016.0033. [Jeon Y, Jang ES, Choi YS, et al. Glypican-3 level assessed by the enzyme-linked immunosorbent assay is inferior to alpha-fetoprotein level for hepatocellular carcinoma diagnosis[J]. Clin Mol Hepatol, 2016, 22(3): 359-65.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SL, Fang X, Huang ZZ, et al. Can serum glypican-3 be a biomarker for effective diagnosis of hepatocellular carcinoma? A meta-analysis of the literature[J]. Dis Markers, 2014: 127831. Epub 2014 Oct 14.http://www.ncbi.nlm.nih.gov/pubmed/25378766
- 13.中华人民共和国卫生部(卫办医政发[2011]121号) 原发性肝癌诊疗规范. 临床肿瘤学杂志. 2011;16(10):929–46. doi: 10.3969/j.issn.1009-0460.2011.10.017. [中华人民共和国卫生部(卫办医政发[2011]121号).原发性肝癌诊疗规范[J].临床肿瘤学杂志, 2011, 16(10): 929-46.] [DOI] [Google Scholar]
- 14.中华医学会传染病与 寄生虫病学分会, 肝病学分会.病毒性肝炎防治方案. http://www.cnki.com.cn/Article/CJFDTOTAL-ZUAN200004041.htm. 中华肝脏病杂志. 2000;18(6):324–9. [中华医学会传染病与.寄生虫病学分会, 肝病学分会.病毒性肝炎防治方案[J].中华肝脏病杂志, 2000, 18(6): 324-9.] [Google Scholar]
- 15.Ho M, Kim H. Glypican-3: a new target for Cancer immunotherapy. Eur J Cancer. 2011;47(3):333–8. doi: 10.1016/j.ejca.2010.10.024. [Ho M, Kim H. Glypican-3: a new target for Cancer immunotherapy [J]. Eur J Cancer, 2011, 47(3): 333-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Cat B, David G. Developmental roles of the glypicans. Semin Cell Dev Biol. 2001;12(2):117–25. doi: 10.1006/scdb.2000.0240. [De Cat B, David G. Developmental roles of the glypicans[J]. Semin Cell Dev Biol, 2001, 12(2): 117-25.] [DOI] [PubMed] [Google Scholar]
- 17.Liu JW, Zuo XL, Wang S. Diagnosis accuracy of serum Glypican-3 level in patients with hepatocellular carcinoma and liver cirrhosis: a meta-analysis. http://www.ncbi.nlm.nih.gov/pubmed/26502856. Eur Rev Med Pharmacol Sci. 2015;19(19):3655–73. [Liu JW, Zuo XL, Wang S. Diagnosis accuracy of serum Glypican-3 level in patients with hepatocellular carcinoma and liver cirrhosis: a meta-analysis[J]. Eur Rev Med Pharmacol Sci, 2015, 19(19): 3655-73.] [PubMed] [Google Scholar]
- 18.Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle. 2008;7(18):2787–90. doi: 10.4161/cc.7.18.6672. [Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer [J]. Cell Cycle, 2008, 7(18): 2787-90.] [DOI] [PubMed] [Google Scholar]
- 19.Zhou S, O'gorman MR, Yang F, et al. Glypican 3 as a serum marker for hepatoblastoma. http://www.ncbi.nlm.nih.gov/pubmed/28378832. Sci Rep. 2017;5(7):45932. doi: 10.1038/srep45932. [Zhou S, O'gorman MR, Yang F, et al. Glypican 3 as a serum marker for hepatoblastoma[J]. Sci Rep. 2017, 5(7): 45932.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Z, Chen C, Long H, et al. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis. http://www.ncbi.nlm.nih.gov/pubmed/23338845. Mol Med Rep. 2013;7(3):969–74. doi: 10.3892/mmr.2013.1279. [Pan Z, Chen C, Long H, et al. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis [J]. Mol Med Rep, 2013, 7(3): 969-74.] [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Wang SK, Zhang K, et al. Expression of glypican 3 enriches hepatocellular carcinoma development-related genes and associates with carcinogenesis in cirrhotic livers. Carcinogenesis. 2015;36(2):232–42. doi: 10.1093/carcin/bgu245. [Liu X, Wang SK, Zhang K, et al. Expression of glypican 3 enriches hepatocellular carcinoma development-related genes and associates with carcinogenesis in cirrhotic livers[J]. Carcinogenesis, 2015, 36 (2): 232-42.] [DOI] [PubMed] [Google Scholar]
- 22.Gao W, Kim H, Ho M. Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-Mediated migration and motility of hepatocellular carcinoma cells. PLoS One. 2015;10(9):e0137664. doi: 10.1371/journal.pone.0137664. [Gao W, Kim H, Ho M. Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-Mediated migration and motility of hepatocellular carcinoma cells[J]. PLoS One, 2015, 10(9): e0137664.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Liu H, Weng H, et al. Glypican-3 promotes epithelialmesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. http://www.ncbi.nlm.nih.gov/pubmed/25572615. Int J Oncol. 2015;46(3):1275–85. doi: 10.3892/ijo.2015.2827. [Wu Y, Liu H, Weng H, et al. Glypican-3 promotes epithelialmesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway[J]. Int J Oncol, 2015, 46(3): 1275-85.] [DOI] [PubMed] [Google Scholar]
- 24.Hippo Y, Watanabe K, Watanabe A, et al. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64(7):2418–23. doi: 10.1158/0008-5472.CAN-03-2191. [Hippo Y, Watanabe K, Watanabe A, et al. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma[J]. Cancer Res, 2004, 64(7): 2418-23] [DOI] [PubMed] [Google Scholar]
- 25.Montalbano M, Georgiadis J, Masterson AL, et al. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma (Review) http://www.ncbi.nlm.nih.gov/pubmed/28098909. Oncol Rep. 2017;37(3):1291–300. doi: 10.3892/or.2017.5387. [Montalbano M, Georgiadis J, Masterson AL, et al. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma (Review) [J]. Oncol Rep, 2017, 37(3): 1291-300.] [DOI] [PubMed] [Google Scholar]
- 26.Waidely E, Al-Yuobi AR, Bashammakh AS, et al. Serum protein biomarkers relevant to hepatocellular carcinoma and their detection. Analyst. 2016;141(1):36–44. doi: 10.1039/C5AN01884F. [Waidely E, Al-Yuobi AR, Bashammakh AS, et al. Serum protein biomarkers relevant to hepatocellular carcinoma and their detection [J]. Analyst, 2016, 141(1): 36-44.] [DOI] [PubMed] [Google Scholar]
- 27.Yasuda E, Kumada T, Toyoda H, et al. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma. Hepatol Res. 2010;40(5):477–85. doi: 10.1111/(ISSN)1872-034X. [Yasuda E, Kumada T, Toyoda H, et al. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma[J]. Hepatol Res, 2010, 40(5): 477-85.] [DOI] [PubMed] [Google Scholar]
- 28.Nault JC, Guyot E, Laguillier C, et al. Serum proteoglycans as prognostic biomarkers of hepatocellular carcinoma in patients with alcoholic cirrhosis. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1343–52. doi: 10.1158/1055-9965.EPI-13-0179. [Nault JC, Guyot E, Laguillier C, et al. Serum proteoglycans as prognostic biomarkers of hepatocellular carcinoma in patients with alcoholic cirrhosis[J]. Cancer Epidemiol Biomarkers Prev, 2013, 22 (8): 1343-52.] [DOI] [PubMed] [Google Scholar]
- 29.Yu JP, Xu XG, Ma RJ, et al. Development of a clinical chemiluminescent immunoassay for serum GPC3 and simultaneous measurements alone with AFP and CK19 in diagnosis of hepatocellular carcinoma. J Clin Lab Anal. 2015;29(2):85–93. doi: 10.1002/jcla.21733. [Yu JP, Xu XG, Ma RJ, et al. Development of a clinical chemiluminescent immunoassay for serum GPC3 and simultaneous measurements alone with AFP and CK19 in diagnosis of hepatocellular carcinoma[J]. J Clin Lab Anal, 2015, 29(2): 85-93.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geramizadeh B, Seirfar N. Diagnostic value of arginase-1 and glypican-3 in differential diagnosis of hepatocellular carcinoma, cholangiocarcinoma and metastatic carcinoma of liver. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539736/ Hepat Mon. 2015;15(7):e30336. doi: 10.5812/hepatmon30336v2. [Geramizadeh B, Seirfar N. Diagnostic value of arginase-1 and glypican-3 in differential diagnosis of hepatocellular carcinoma, cholangiocarcinoma and metastatic carcinoma of liver[J]. Hepat Mon, 2015, 15(7): e30336.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo Y, Kimura O, Shimosegawa T. Significant biomarkers for the management of hepatocellular carcinoma. Clin J Gastroenterol. 2015;8(3):109–15. doi: 10.1007/s12328-015-0568-9. [Kondo Y, Kimura O, Shimosegawa T. Significant biomarkers for the management of hepatocellular carcinoma [J]. Clin J Gastroenterol, 2015, 8(3): 109-15.] [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16(35):4410–5. doi: 10.3748/wjg.v16.i35.4410. [Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma[J]. World J Gastroenterol, 2010, 16(35): 4410-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen IP, Ariizumi S, Nakano M, et al. Positive glypican-3 expression in early hepatocellular carcinoma predicts recurrence after hepatectomy. J Gastroenterol. 2014;49(1):117–25. doi: 10.1007/s00535-013-0793-2. [Chen IP, Ariizumi S, Nakano M, et al. Positive glypican-3 expression in early hepatocellular carcinoma predicts recurrence after hepatectomy[J]. J Gastroenterol, 2014, 49(1): 117-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao M, Yao DF, Bian YZ, et al. Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12(2):171–9. doi: 10.1016/S1499-3872(13)60028-4. [Yao M, Yao DF, Bian YZ, et al. Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma[J]. Hepatobiliary Pancreat Dis Int, 2013, 12(2): 171-9.] [DOI] [PubMed] [Google Scholar]
- 35.Nguyen T, Phillips D, Jain D, et al. Comparison of 5 immunohistochemical markers of hepatocellular differentiation for the diagnosis of hepatocellular carcinoma. Arch Pathol Lab Med. 2015;139(8):1028–34. doi: 10.5858/arpa.2014-0479-OA. [Nguyen T, Phillips D, Jain D, et al. Comparison of 5 immunohistochemical markers of hepatocellular differentiation for the diagnosis of hepatocellular carcinoma[J]. Arch Pathol Lab Med, 2015, 139 (8): 1028-34.] [DOI] [PubMed] [Google Scholar]
- 36.Ofuji K, Saito K, Suzuki S, et al. Perioperative plasma glypican-3 level May enable prediction of the risk of recurrence after surgery in patients with stage Ⅰ hepatocellular carcinoma. http://www.ncbi.nlm.nih.gov/pubmed/28035063. Oncotarget. 2017;8(23):37835–44. doi: 10.18632/oncotarget.14271. [Ofuji K, Saito K, Suzuki S, et al. Perioperative plasma glypican-3 level May enable prediction of the risk of recurrence after surgery in patients with stage Ⅰ hepatocellular carcinoma[J]. Oncotarget, 2017, 8(23): 37835-44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawada Y, Yoshikawa T, Ofuji K, et al. Phase Ⅱ study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5):e1129483. doi: 10.1080/2162402X.2015.1129483. [Sawada Y, Yoshikawa T, Ofuji K, et al. Phase Ⅱ study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients[J]. Oncoimmunology, 2016, 5(5): e1129483] [DOI] [PMC free article] [PubMed] [Google Scholar]


