Abstract
目的
探讨氧化低密度脂蛋白(ox-LDL)对CD8+记忆性T细胞亚群分化的影响及其机制。
方法
将雌性10周龄未发生糖尿病NOD小鼠随机分为2组(n=5):ox-LDL组及对照组。断颈处死各组小鼠磁珠分离脾脏CD8+T细胞体外培养(IL-2),ox-LDL组加入ox-LDL 30 μg/mL体外刺激24 h,ox-LDL未处理组作为对照组。24 h后分离细胞。体外实验利用流式细胞仪检测各组以下指标:胞内CD8+IFN-γ+确定胰岛β细胞特异性杀伤性CD8+T细胞的比例;细胞表面分子CD8、CD44、CD62L确定CD8+记忆性T细胞的不同亚群分化;T细胞相关转录因子TCF-1及STAT-3的活化确定关键性转录因子的作用。体内实验中,将各组CD8+记忆性T细胞通过尾静脉注射的方法移植给未发病NOD小鼠,同时设立未移植的模型对照组,每5 d检测血糖1次,监测小鼠的血糖改变及生存率。
结果
相比对照组,ox-LDL(30 μg/mL)作用24 h后,胰岛β细胞特异性杀伤性CD8+T细胞活化减少([ 0.7±0.03)% vs(2.7±0.14)%,P < 0.01],记忆性T细胞中的效应记忆性T细胞形成数目减少([ 10.3±0.71)% vs(30.3±1.36)%,P < 0.01],干细胞样记忆性T细胞增加([ 72.3±3.8)% vs(55.1±2.61)%,P < 0.05]。T细胞转录因子TCF-1及p-STAT-3活化的也受到抑制[TCF-1:(14.5±0.82)% vs(34.2±1.23)%,磷酸化STAT-3:(3.3±0.12)% vs(22.1±1.1)%,P < 0.01]。T细胞移植给未发病NOD小鼠后,ox-LDL处理组小鼠血糖升高明显低于对照组(P < 0.05),且生存率提高(P < 0.05)。
结论
ox-LDL干扰记忆性CD8+T细胞核转录因子TCF-1活化及STAT-3的磷酸化,抑制长效杀伤作用的效应性和记忆性CD8+T细胞形成,诱导干细胞样记忆性CD8+T细胞形成,从而阻止胰岛β细胞自身免疫损伤及Ⅱ型糖尿病的发生
Keywords: 氧化低密度脂蛋白, 记忆性CD8+T细胞, 自身免疫性糖尿病
Abstract
Objective
To investigate effect of oxidized low-density lipoprotein (ox-LDL) on memory CD8+T cell subpopulation differentiation in mice with autoimmune diabetes.
Methods
Cultured splenic CD8+T cells from pre-diabetic NOD mice isolated with magnetic beads were treated with 30 μg/mL ox-LDL and 10 U/mL interleukin-2 (IL-2) for 24 h and the control cells were treated with IL-2 only. Flow cytometry was used to determine the percentage of splenic CD8+IFN-γ+T cells, expressions of CD8, CD44 and CD62L on the T cells, and the activation of T cell factor-1 (TCF-1) and STAT-3. The CD127+ memory T cells were purified and transplanted into the pre-diabetic NOD mice via the tail vein, and the blood glucose was recorded weekly and survival time of the mice was monitored.
Results
Treatment with ox-LDL significantly reduced islet β cell-specific cytotoxic CD8+T cells as compared with the control group [(0.7±0.03)% vs (2.7±0.14)%, P < 0.01]. The percentage of effector memory CD8+T cells (Tem) in the total memory CD8+T cells was reduced [(10.3±0.71)% vs (30.3±1.36)%, P < 0.01] and that of stem cell-like memory T cells was significantly increased[(72.3 ± 3.8)% vs (55.1 ± 2.61)%, P < 0.05] following ox-LDL treatment, which also resulted in significantly decreased activation of TCF-1 [(14.5 ± 0.82)% vs (34.2 ± 1.23)%, P < 0.01] and pSTAT-3[(3.3±0.12)% vs (22.1±1.1)%, P < 0.01]. Transplantation of ox-LDL-treated memory T cells in pre-diabetic NOD mice obviously inhibited the increase of blood glucose and prolonged the survival time of the mice (P < 0.05).
Conclusion
Ox-LDL decreases the activation of transcriptional factors TCF-1 and phosphorylation of STAT-3, inhibits the formation of effector memory CD8+T cells with long-term cytotoxicity, but promote the generation of stem cell-like memory CD8+Tcells, which result in suppression of islet β cell-specific effector cytotoxic CD8+T cell differentiation to lessen autoimmune injury to the islet β cells
Keywords: oxidized low-density lipoprotein, memory CD8+T cell subtypes, autoimmune diabetes
抗原刺激初次入侵机体,初始性CD8+T细胞活化增殖分化,95%形成效应性CD8+杀伤性T细胞(CTL),发挥短期杀伤效应后自身凋亡,其余5%变成记忆性CD8+T细胞(Tm)长期存在,相同抗原再次入侵机体时,Tm迅速分化增殖,发挥高效杀伤的再次应答作用[1]。因此,记忆性CD8+T细胞分化在自身免疫病、感染、肿瘤及疫苗的等多个领域都具有极重要的理论意义及应用价值[2]。氧化低密度脂蛋白(ox-LDL)作为机体重要代谢因素,对免疫系统的研究主要集中于其介导巨噬细胞或树突状细胞转化为泡沫细胞[3-4],以及刺激机体产生ox-LDL特异性杀伤性CD8+T细胞[5]。然而,ox-LDL对抗原特异性记忆性CD8+T细胞亚群的影响,国内外均无报道。针对Tm对多种疾病的重要作用以及ox-LDL影响记忆性T细胞相关研究的欠缺,本研究拟在前期工作的基础上,探索ox-LDL是否干扰Tm的亚群形成与分化,从而影响Tm对特异性抗原的应答,有助于从代谢水平理解CD8+记忆性T细胞为基础的再次免疫应答,ox-LDL的水平是否能影响再次免疫应答,从而影响免疫系统功能等,因此,本研究具有重要的理论意义与应用价值。
Ⅰ型糖尿病是一种杀伤性CD8+T淋巴细胞介导的,以胰岛β细胞特异性免疫损伤为主要特征的器官特异性自身免疫性疾病,而记忆性CD8+T细胞持续形成杀伤性T细胞,记忆性T细胞分化影响了胰岛β细胞损伤及后续的糖尿病进展[6-7]。因此,自身免疫性糖尿病NOD小鼠模型是一个研究记忆性CD8+细胞的理想模型。本研究拟利用自身免疫性糖尿病小鼠脾脏的CD8+ T细胞,观察ox-LDL对CD8+Tm细胞分化的影响,以及胰岛β细胞特异性CD8+CTL形成并探讨其机制,从而确定ox-LDL对抗原特异性Tm的影响。
1. 材料和方法
1.1. 主要材料
抗小鼠CD8,CD44,CD62L,IFN-γ,CD127,TCF-1,STAT-3的荧光抗体及胞内细胞染色破膜液、固定液及Golgi液(Biolegend);RPMI 1640培养基及胎牛血清(Gibco);细胞因子IL-2(Peprotech);CD8+T免疫分选磁珠(Stemcell);小鼠胰岛β细胞NIT-1及自身免疫性糖尿病NOD小鼠均由华中科技大学同济医学院王从义教授赠送。
1.2. 方法
1.2.1. 实验分组
清洁级健康级雌性10周龄未发生糖尿病NOD小鼠,体质量22~26 g,采用随机数字表法将其分为2组(n=5):ox-LDL组(实验组)及ox-LDL未处理组(对照组)。对待动物遵循动物伦理学标准。无菌条件断颈处死小鼠并获取脾脏细胞制备单细胞悬液,利用磁珠分离脾脏CD8+T细胞,加入RPMI 1640,10%胎牛血清及IL-2(10 U/mL)进行体外培养,37 ℃培养24 h后,实验组加入ox-LDL 30 μg/mL体外刺激24 h,ox-LDL未处理组仅加入IL-2(10 U/mL),不加入ox-LDL处理。4 ℃ 1500 r/min,5 min离心去除上清,细胞备用。
1.2.2. LDL的制备与氧化修饰
采用1次性密度超速离心法制法[8]。室温低速1500 g离心10 min分离血清,加入溴化钾调密度至1.30 g/mL,4 ℃ 50 000 r/min离心5 h收集淡黄色中间层即为LDL。采用铜诱导法氧化LDL并鉴定。
1.2.3. 流式细胞术表面标志染色
按抗体说明书,106细胞加入2 μg相应表面分子抗体CD8,CD44,CD62L,CD127进行染色,4 ℃染色30 min,PBS洗3次,流式细胞仪检测。
1.2.4. 胞内转录因子流术细胞术检测
完成表面分子CD8染色后的细胞,利用转录因子固定液2 mL重悬细胞并室温固定30 min,再加入1 mL破膜液,室温离心1500 r/min,5 min,洗涤2次。分别加入转录因子抗体TCF-1,STAT-3室温染色30 min,2 mL破膜液洗涤2次,上机检测。
1.2.5. 胞内细胞因子流术细胞术染色
取小鼠2×106脾细胞,加入Golgi终止液3 μL,1×106胰岛β细胞37 ℃共培养刺激2 h,PBS洗2次,表面分子CD8染色,利用固定液2 mL重悬细胞并室温固定30 min,加入1 mL破膜液,室温离心1500 r/min,5 min,洗涤2次。加入抗IFN-γ抗体室温4度染色30 min,2 mL破膜液洗涤2次,上机检测。
1.2.6. 记忆性T细胞移植未发病NOD小鼠
CD127+ CD8+分选各组脾细胞的记忆性CD8+T细胞,按106细胞/只通过尾静脉输注10周龄未发病的NOD小鼠中,同时以不移植任何细胞的模型对照组进行参照,每隔1周检测外周血血糖,并监测生存期。
1.3. 统计学处理
应用SPSS 13.0统计软件进行处理,计量数据以均数±标准差表示,检测结果比较采用单因素方差分析,多重比较用LSD法,P < 0.05表示差异具有统计学意义。
2. 结果
2.1. ox-LDL抑制自身胰岛β细胞抗原特异性CD8+T细胞活化
未发病NOD小鼠的脾脏中胰岛β细胞反应性CD8+胞内IFN-γ水平代表胰岛β细胞特异性杀伤性T细胞的强度(图 1)。对照组中,脾细胞中(2.70±0.14)%胰岛β细胞特异性杀伤性CD8+T细胞。ox-LDL作用,仅有(0.7± 0.03)%胰岛β细胞特异性杀伤性CD8+T细胞。对照组与实验组差异具有统计学意义(P < 0.01)。
1.
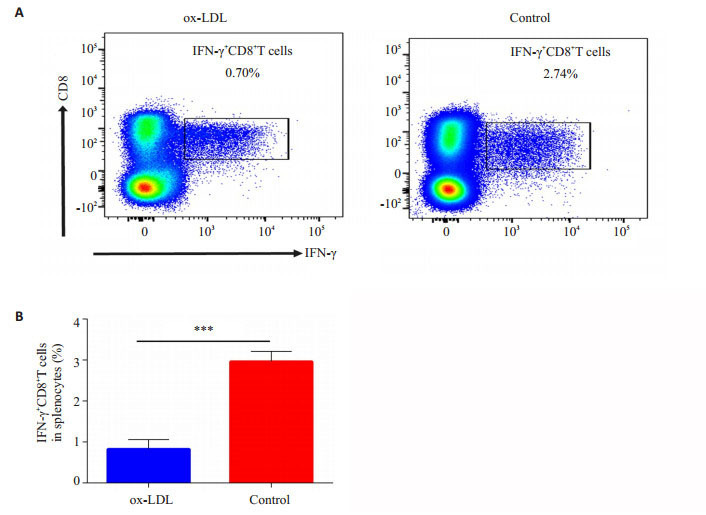
流式细胞仪检测脾细胞经过胰岛β细胞体外刺激后CD8+IFN-γ+T细胞比例
Flow cytometric analysis of formation of CD8+IFN-γ+T cells in the spleen with islet β cell stimulation in vitro. A: Representative plots of splenic CD8+IFN-γ+T cells in experimental group and control group; B: Statistical analysis of splenic CD8+IFN-γ+T cells between experimental group and control group. ***P < 0.01.
2.2. ox-LDL抑制胰岛β细胞特异性记忆性CD8+Tem细胞的分化
小鼠记忆性CD8+T细胞分为干细胞样记忆性T细胞(Tscm, CD44-CD62L+),中央记忆性T细胞(Tcm, CD44+ CD62L+),效应记忆性T细胞(Tem, CD44+CD62L-)。通过对脾脏CD8+T细胞进行CD44、CD62L染色,确定记忆性CD8+ T细胞分类(图 2)。对照组中,Tem为(30.3 ± 1.36)%,Tcm为(8.7 ± 0.58)%,Tscm为(55.1 ± 2.61)%。ox-LDL作用下Tem为(10.3±0.71)%,Tcm为(13.4±0.62)%,Tscm为(72.3±3.8)%。ox-LDL显著降低了效应记忆性CD8+T细胞的形成(P < 0.01),使记忆性T细胞保持在更为原始的Tscm的状态(P < 0.01),从而降低了效应性杀伤性CD8+T细胞的形成。
2.
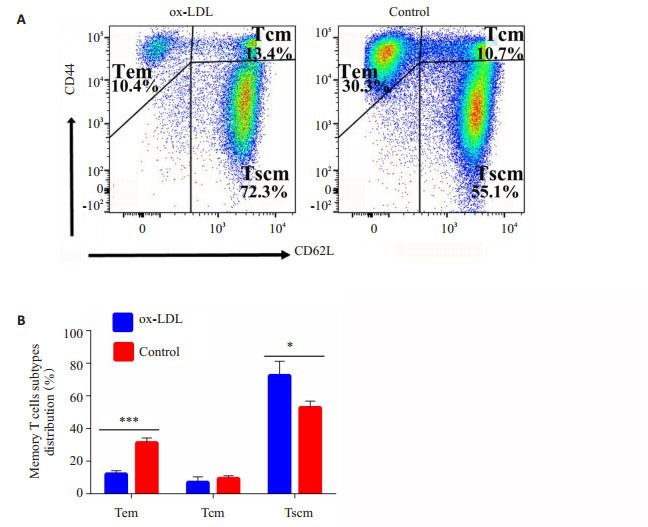
流式细胞术检测脾细胞中记忆性CD8+T细胞亚群分布
Distribution of memory CD8+T cells subtypes in spleen detected with flow cytometry. A: Representative plots of distribution of splenic CD8+Tm in experimental group and control group; B: Statistical analysis of distribution of splenic CD8+Tm between experimental group and control group. *P < 0.05, ***P < 0.01.
2.3. ox-LDL通过影响CD8+T细胞核转录因子TCF-1的活化及STAT-3磷酸化影响记忆性T细胞分化
记忆性T细胞亚群分化受到多个T细胞转录因子的影响。本研究利用特异性抗体检测与记忆性T细胞分化密切相关的转录因子TCF-1(图 3A)及磷酸化STAT-3(p-STAT-3)的水平(图 3B)。结果显示,对照组中,TCF-1阳性CD8+细胞为(34.1 ± 1.23)%,磷酸化STAT-3阳性CD8+细胞为(22.1±1.1)%。经过ox-LDL作用后,TCF-1阳性CD8+细胞为(14.5±0.82)%,磷酸化STAT-3阳性CD8+细胞为(3.3±0.12)%,两组差异均有统计学意义(P < 0.01)。
3.
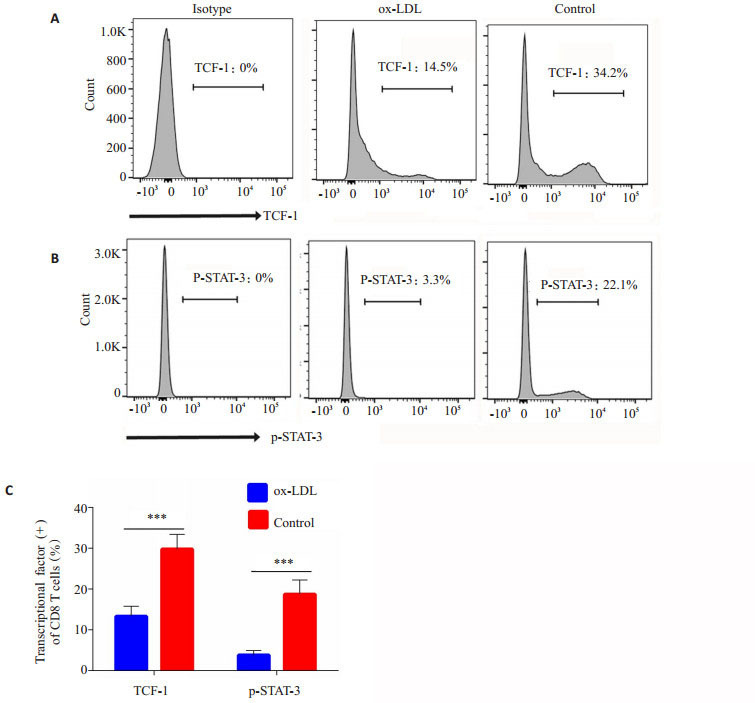
流式细胞术检测CD8+T细胞胞内转录因子TCF-1及p-STAT-3的活化
Activation of intracellular transcriptional factors TCF-1 and p-STAT-3 detected with flow cytometry. A: Representative plots of TCF-1 activation of splenic CD8+T cells in experimental group and control group; B: Representive plots of STAT-3 phosphorylation of splenic CD8+T cells in experimental group and control group; C: The statistical analysis of the activation of transcriptional factors TCF-1 and p-STAT3 in splenic CD8+T cells in the groups. ***P < 0.01.
2.4. ox-LDL抑制胰岛β细胞特异性CD8+T细胞过继转移介导的自身免疫型糖尿病
CD127+CD8+分选各组脾细胞中的记忆性T细胞,按106细胞/只通过尾静脉输注未发病的NOD小鼠,同时设立未移植的小鼠为模型对照组,记录血糖与小鼠生存期(图 4)。结果显示,ox-LDL未处理组与模型对照组小鼠血糖升高均快于ox-LDL处理组,生存期均短于ox-LDL处理组(P < 0.05)。
4.
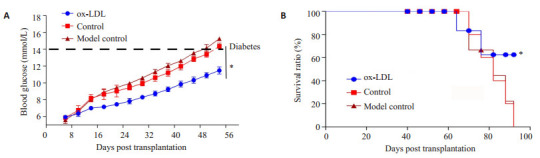
NOD小鼠血糖及生存期监测
Changes of blood glucose and survival time of NOD mice in the 3 groups. *P < 0.05.
3. 讨论
ox-LDL现有研究集中于对天然免疫应答的巨噬细胞,树突状细胞的影响[9],及特异性免疫应答中ox-LDL特异的效应性T细胞的形成[10]。然而,关于ox-LDL直接影响记忆性CD8+T细胞亚群分化的研究仅有急性冠脉综合症病人外周血记忆性CD8+T细胞总数减少[11]。本研究证实,ox-LDL直接影响脾脏记忆性CD8+T细胞亚群的分化,抑制具有长效杀伤能力的Tem CD8+T的分化,减少胰岛β细胞特异性杀伤性CD+T细胞的形成,将记忆性T细胞保持在较为原始的Tscm亚群。记忆性T细胞是指对特异性抗原有具有记忆能力,寿命较长的T淋巴细胞[12-13]。Tm细胞抗原活化阈值低[14],对共刺激信号依赖性也较弱[15],少量抗原再次刺激即可迅速活化,分化成效应性T细胞,快速高效清除抗原。记忆性T细胞根据分化度、功能及表型分为不同的亚群:Tscm(鼠:CD44-CD62L+,人:CD45RO-CCR7+CD62L+CD127+ CD95+CD122+),Tcm(鼠:CD44+CD62L+,人:CD45RO+ CD27+CD57-CCR +),Tem(鼠:CD44+CD62L-,人:CD45RO-CD27+CD57+CCR7-)[16]。Tscm位于分化早期,杀伤能力最弱,分化抗凋亡能力最强,最接近于初始性T细胞。Tem位于分化终止期,杀伤能力最强,抗凋亡能力最弱,最接近于杀伤性T细胞,是发挥持续杀伤作用的主要细胞。而Tcm介于两者之间。抗原再次刺激时,CD8+Tem大量分化为效应性杀伤性T细胞,发挥杀伤作用[17-18]。然而也有新理论提出初始性T细胞先形成Tscm,依次分化为Tcm、Tem,最终转化为CTL[19]。本研究报道ox-LDL体外直接调节了Tem及Tscm的分化,发现代谢因素影响记忆性CD8+T细胞应答新的线索。体内实验中,移植经过ox-LDL处理的记忆性CD8+T细胞给未发病的NOD小鼠,也发现血糖控制较好,生存率明显提高,证实了ox-LDL抑制长效杀伤作用的Tem,从而胰岛β细胞免疫损伤减少。
记忆性T细胞亚群的分化受到多种转录因子可以调控[20]。本研究发现ox-LDL作用后,记忆性T细胞胞内转录因子TCF-1的活化以及STAT-3的磷酸化均受到明显的抑制。TCF-1是重要的T细胞分化调节转录因子,受上游分子wnt/β-catenin的调控,TCF-1缺陷导致T细胞抗凋亡分子Bcl-2的缺陷,IL-15受体功能障碍,记忆性T细胞的生存明显受抑[21],同时导致Tem转化CTL的关键性转录因子Eomes失活,无法形成具有IFN-γ和颗粒酶等杀伤分子的CTL [22]。STAT-3通路在记忆性T细胞的形成与维持中起着重要的作用,研究证实,STAT-3活化缺陷小鼠CD8+T细胞表面IL-7受体形成障碍,而IL-7是记忆性T细胞形成的极为重要的细胞因子,因此STAT-3缺陷小鼠无法有效形成病毒特异性的Tcm与Tem [23]。STAT-3缺陷的高IgE血症病人外周血中Tcm及Tem数目也明显少于正常人[24]。ox-LDL可能通过与TLR4结合发挥功能,T细胞表面的TLR4受体在受到不同配体刺激时,会形成不同类别的记忆性CD8+T细胞,产生不同的细胞因子。LPS通过TLR4-MyD88-NF-κB诱导CD8+Tm合成IFN-γ,转变成Tem及CTL介导杀伤[25]。而Hsp70通过TLR4-TOLLIP活化SOCS-1抑制NF-κB,合成IL-10及PD-1表达,介导T细胞耐受[26-27]。作为TLR4配体之一的ox-LDL,可能也通过类似通路影响记忆性T细胞的分化。然而,现有文献中多证实在巨噬细胞及平滑肌细胞中ox-LDL-TLR4-NF-κB发挥作用[28-29],与本实验结果不一致,有待深入研究记忆性T细胞与巨噬细胞对ox-LDL-TLR4通路活化的差异。
综上所述,本研究利用自身免疫性糖尿病小鼠模型,报道了ox-LDL直接影响记忆性CD8+T细胞亚群的分化,抑制具有长效杀伤功能的记忆性效应性T细胞及快速杀伤功能效应性杀伤性CTL,诱导了具有较原始的具有强大分化能力的Tscm,并有效降低自身免疫性糖尿病小鼠的发病率,延长其生存期。初步证明了ox-LDL的作用是通过抑制记忆性T细胞核转录因子TCF-1的活化及STAT-3磷酸化实现的。本研究为ox-LDL影响CD8+记忆性T细胞的形成与分化提供了新的线索,为高脂条件下免疫记忆的形成及相关的疫苗研究提供了初步的理论基础。
Biography
郑华, 副主任医师, E-mail: gzhzmd@126.com
Funding Statement
广东省自然科学基金(2015A030313305); 南方医院院长基金(2013B008)
Contributor Information
郑 华 (Hua ZHENG), Email: gzhzmd@126.com.
吴 砂 (Sha WU), Email: shawu99@outlook.com.
References
- 1.Mckinney EF, Smith KG. T-cell exhaustion: understanding the interface of chronic viral and autoinflammatory diseases. Immunol Cell Biol. 2016;94(10):935–42. doi: 10.1038/icb.2016.81. [Mckinney EF, Smith KG. T-cell exhaustion: understanding the interface of chronic viral and autoinflammatory diseases[J]. Immunol Cell Biol, 2016, 94(10): 935-42.] [DOI] [PubMed] [Google Scholar]
- 2.Nus M, Mallat Z. Immune-mediated mechanisms of atherosclerosis and implications for the clinic. Expert Rev Clin Immunol. 2016;12(11):1217–37. doi: 10.1080/1744666X.2016.1195686. [Nus M, Mallat Z. Immune-mediated mechanisms of atherosclerosis and implications for the clinic[J]. Expert Rev Clin Immunol, 2016, 12(11): 1217-37.] [DOI] [PubMed] [Google Scholar]
- 3.Foks AC, Engelbertsen D, Kuperwaser F, et al. Blockade of Tim-1 and Tim-4 enhances atherosclerosis in Low-Density lipoprotein Receptor-Deficient mice. Arterioscler Thromb Vasc Biol. 2016;36(3):456–65. doi: 10.1161/ATVBAHA.115.306860. [Foks AC, Engelbertsen D, Kuperwaser F, et al. Blockade of Tim-1 and Tim-4 enhances atherosclerosis in Low-Density lipoprotein Receptor-Deficient mice[J]. Arterioscler Thromb Vasc Biol, 2016, 36(3): 456-65.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christ A, Bekkering S, Latz E, et al. Long-term activation of the innate immune system in atherosclerosis. Semin Immunol. 2016;28(4):384–93. doi: 10.1016/j.smim.2016.04.004. [Christ A, Bekkering S, Latz E, et al. Long-term activation of the innate immune system in atherosclerosis[J]. Semin Immunol, 2016, 28(4): 384-93.] [DOI] [PubMed] [Google Scholar]
- 5.Mueller SN, Gebhardt T, Carbone FR, et al. Memory T cell subsets, migration patterns, and tissue residence. http://www.annualreviews.org/doi/abs/10.1146/annurev-immunol-032712-095954?journalCode=immunol. Annu Rev Immunol. 2013;31(9):137–61. doi: 10.1146/annurev-immunol-032712-095954. [Mueller SN, Gebhardt T, Carbone FR, et al. Memory T cell subsets, migration patterns, and tissue residence[J]. Annu Rev Immunol, 2013, 31(9): 137-61.] [DOI] [PubMed] [Google Scholar]
- 6.Chee J, Ko HJ, Skowera A, et al. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol. 2014;192(2):572–80. doi: 10.4049/jimmunol.1302100. [Chee J, Ko HJ, Skowera A, et al. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes[J]. J Immunol, 2014, 192(2): 572-80.] [DOI] [PubMed] [Google Scholar]
- 7.Delgado E, Perez BM, Suarez AB, et al. Modulation of autoimmune T-Cell memory by stem cell educator therapy: phase 1/2 clinical trial. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4703710/ EBio Medicine. 2015;2(12):2024–36. doi: 10.1016/j.ebiom.2015.11.003. [Delgado E, Perez BM, Suarez AB, et al. Modulation of autoimmune T-Cell memory by stem cell educator therapy: phase 1/2 clinical trial [J]. EBio Medicine, 2015, 2(12): 2024-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.马 立勤, 郑 华, 吴 赛珠. 不同类型冠心病患者C反应蛋白与氧化型低密度脂蛋白自身抗体水平的比较. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2007071068. 南方医科大学学报. 2007;27(7):1068–70. [马立勤, 郑华, 吴赛珠.不同类型冠心病患者C反应蛋白与氧化型低密度脂蛋白自身抗体水平的比较[J].南方医科大学学报, 2007, 27 (7): 1068-70.] [PubMed] [Google Scholar]
- 9.Kiss M, Czimmerer Z, Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J Allergy Clin Immunol. 2013;132(2):264–86. doi: 10.1016/j.jaci.2013.05.044. [Kiss M, Czimmerer Z, Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology[J]. J Allergy Clin Immunol, 2013, 132(2): 264-86.] [DOI] [PubMed] [Google Scholar]
- 10.Houben T, Brandsma E, Walenbergh SM, et al. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochim Biophys Acta. 2017;1862(4):416–29. doi: 10.1016/j.bbalip.2016.07.008. [Houben T, Brandsma E, Walenbergh SM, et al. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis[J]. Biochim Biophys Acta, 2017, 1862(4): 416-29.] [DOI] [PubMed] [Google Scholar]
- 11.Zidar DA, Mudd JC, Juchnowski S, et al. Altered maturation status and possible immune exhaustion of CD8 T lymphocytes in the peripheral blood of patients presenting with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 2016;36(2):389–97. doi: 10.1161/ATVBAHA.115.306112. [Zidar DA, Mudd JC, Juchnowski S, et al. Altered maturation status and possible immune exhaustion of CD8 T lymphocytes in the peripheral blood of patients presenting with acute coronary syndromes [J]. Arterioscler Thromb Vasc Biol, 2016, 36(2): 389-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerritsen B, Pandit A. The memory of a killer T cell: models of CD8(+) T cell differentiation. Immunol Cell Biol. 2016;94(3):236–41. doi: 10.1038/icb.2015.118. [Gerritsen B, Pandit A. The memory of a killer T cell: models of CD8(+) T cell differentiation[J]. Immunol Cell Biol, 2016, 94(3): 236-41.] [DOI] [PubMed] [Google Scholar]
- 13.Cui G, Staron MM, Gray SM, et al. IL-7-Induced glycerol transport and TAG synthesis promotes memory CD8+T cell longevity. Cell. 2015;161(4):750–61. doi: 10.1016/j.cell.2015.03.021. [Cui G, Staron MM, Gray SM, et al. IL-7-Induced glycerol transport and TAG synthesis promotes memory CD8+T cell longevity[J]. Cell, 2015, 161(4): 750-61.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MT, Kurup SP, Starbeck GR, et al. Manipulating memory CD8 T cell numbers by timed enhancement of IL-2 signals. J Immunol. 2016;197(5):1754–61. doi: 10.4049/jimmunol.1600641. [Kim MT, Kurup SP, Starbeck GR, et al. Manipulating memory CD8 T cell numbers by timed enhancement of IL-2 signals[J]. J Immunol, 2016, 197(5): 1754-61.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagoya Y, Nakatsugawa M, Ochi T, et al. Transient stimulation expands superior antitumor T cells for adoptive therapy. http://www.ncbi.nlm.nih.gov/pubmed/28138559. JCI Insight. 2017;2(2):e89580–4. doi: 10.1172/jci.insight.89580. [Kagoya Y, Nakatsugawa M, Ochi T, et al. Transient stimulation expands superior antitumor T cells for adoptive therapy[J]. JCI Insight, 2017, 2(2): e89580-4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall HD, Kaech SM. Generating CD8 T cell heterogeneity: attack of the clones. Immunity. 2013;39(2):203–5. doi: 10.1016/j.immuni.2013.08.008. [Marshall HD, Kaech SM. Generating CD8 T cell heterogeneity: attack of the clones [J]. Immunity, 2013, 39(2): 203-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Speiser DE, Lichterfeld M, et al. T memory stem cells in health and disease. Nat Med. 2017;23(1):18–27. doi: 10.1038/nm.4241. [Gattinoni L, Speiser DE, Lichterfeld M, et al. T memory stem cells in health and disease[J]. Nat Med, 2017, 23(1): 18-27.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelsamed HA, Moustaki A, Fan Y, et al. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med. 2017;214(6):1593–606. doi: 10.1084/jem.20161760. [Abdelsamed HA, Moustaki A, Fan Y, et al. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis [J]. J Exp Med, 2017, 214(6): 1593-606.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn JK, Gorry PR. Stem memory T cells (TSCM)-their role in cancer and HIV immunotherapies. Clinical Translat Immunol. 2014;3(7):e20–5. doi: 10.1038/cti.2014.16. [Flynn JK, Gorry PR. Stem memory T cells (TSCM)-their role in cancer and HIV immunotherapies[J]. Clinical Translat Immunol, 2014, 3(7): e20-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Best JA, Blair DA, Knell J, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14(4):404–12. doi: 10.1038/ni.2536. [Best JA, Blair DA, Knell J, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation[J]. Nat Immunol, 2013, 14(4): 404-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolz JC, Harty JT. IL-15 regulates memory CD8+T cell O-glycan synthesis and affects trafficking. J Clin Invest. 2014;124(3):1013–26. doi: 10.1172/JCI72039. [Nolz JC, Harty JT. IL-15 regulates memory CD8+T cell O-glycan synthesis and affects trafficking[J]. J Clin Invest, 2014, 124(3): 1013-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Yu S, Zhao DM, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–40. doi: 10.1016/j.immuni.2010.08.002. [Zhou X, Yu S, Zhao DM, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1[J]. Immunity, 2010, 33(2): 229-40.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui W, Liu Y, Weinstein JS, et al. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+T cells. Immunity. 2011;35(5):792–805. doi: 10.1016/j.immuni.2011.09.017. [Cui W, Liu Y, Weinstein JS, et al. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+T cells[J]. Immunity, 2011, 35(5): 792-805.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–18. doi: 10.1016/j.immuni.2011.09.016. [Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory[J]. Immunity, 2011, 35(5): 806-18.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh AK, Sinha D, Mukherjee S, et al. LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8(+) T cells. Cytokine. 2015;73(1):44–52. doi: 10.1016/j.cyto.2015.01.018. [Ghosh AK, Sinha D, Mukherjee S, et al. LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8(+) T cells[J]. Cytokine, 2015, 73(1): 44-52.] [DOI] [PubMed] [Google Scholar]
- 26.Biswas R, Mukherjee S, Sinha D, et al. Culture-differentiated CD8(+) T cells acquire innate memory-like traits and respond to a pathogen-associated molecule. Immunol Cell Biol. 2014;92(4):368–76. doi: 10.1038/icb.2013.94. [Biswas R, Mukherjee S, Sinha D, et al. Culture-differentiated CD8(+) T cells acquire innate memory-like traits and respond to a pathogen-associated molecule[J]. Immunol Cell Biol, 2014, 92(4): 368-76.] [DOI] [PubMed] [Google Scholar]
- 27.Kim MT, Harty JT. Impact of inflammatory cytokines on effector and memory CD8+T cells. http://www.ncbi.nlm.nih.gov/pubmed/24995011. Front Immunol. 2014;5(5):295–8. doi: 10.3389/fimmu.2014.00295. [Kim MT, Harty JT. Impact of inflammatory cytokines on effector and memory CD8+T cells [J]. Front Immunol, 2014, 5(5): 295-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janeesh PA, Sasikala V, Dhanya CR, et al. Robinin modulates TLR/ NF-κB signaling pathway in oxidized LDL induced human peripheral blood mononuclear cells. Int Immunopharmacol. 2014;18(1):191–7. doi: 10.1016/j.intimp.2013.11.023. [Janeesh PA, Sasikala V, Dhanya CR, et al. Robinin modulates TLR/ NF-κB signaling pathway in oxidized LDL induced human peripheral blood mononuclear cells[J]. Int Immunopharmacol, 2014, 18(1): 191-7.] [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Zhang L, Chen C, et al. The critical role of ABCG1 and PPARγ/LXRα signaling in TLR4 mediates inflammatory responses and lipid accumulation in vascular smooth muscle cells. Cell Tissue Res. 2017;368(1):145–57. doi: 10.1007/s00441-016-2518-3. [Cao X, Zhang L, Chen C, et al. The critical role of ABCG1 and PPARγ/LXRα signaling in TLR4 mediates inflammatory responses and lipid accumulation in vascular smooth muscle cells[J]. Cell Tissue Res, 2017, 368(1): 145-57] [DOI] [PubMed] [Google Scholar]


