Abstract
目的
体外探讨鼠李糖乳杆菌上清液(LGG-CM)能否通过抑制NF-κB通路来阻断大肠杆菌K1(E.coli K1)株引起的细菌性脑膜炎。
方法
用人脑微血管内皮细胞(HBMEC)构建体外血脑屏障模型;采用免疫印迹研究LGG-CM能否抑制E.coli K1激活NF-κB通路;通过侵袭实验和中性粒细胞迁移实验,研究LGG-CM能否抑制细菌侵袭和中性粒细胞迁移;通过免疫印迹研究黏附分子CD44和紧密连接蛋白ZO-1的表达;免疫荧光检测ZO-1蛋白的细胞内分布;用Transwell建立体外血脑屏障模型,通过跨细胞内皮电阻(TEER)值和细菌迁移实验评价LGG-CM对细胞屏障完整性的保护作用。
结果
免疫印迹结果表明LGG-CM能抑制E.coli K1激活NF-κB通路,藉此抑制E.coli K1的侵袭和中性粒细胞迁移。同时,LGG-CM可抑制E.coli K1上调CD44蛋白和下调紧密连接蛋白ZO-1。此外,LGG-CM能够明显减缓TEER值的降低和抑制E.coli K1穿越体外血脑屏障。
结论
体外实验表明,LGG-CM能够通过抑制NF-κB通路激活、阻断E.coli K1侵袭和中性粒细胞迁移及维护血脑屏障完整性来预防E.coli K1引起的细菌性脑炎。
Keywords: 鼠李糖乳杆菌, 上清液, 脑膜炎, 人脑血管内皮细胞, E.coli K1, NF-κB
Abstract
Objective
To investigate whether Lactobacillus rhamnosus GG conditioned medium (LGG-CM) has preventive effect against E. coli K1-induced neuropathogenicity in vitro by inhibiting nuclear factor-κB (NF-κB) signaling pathway.
Methods
An in vitro blood-brain barrier (BBB) model was constructed using human brain microvascular endothelial cells (HBMECs). The effect of LGG-CM on E. coli-actived NF-κB signaling pathway was assayed using Western blotting. Invasion assay and polymorphonuclear leukocyte (PMN) transmigration assay were performed to explore whether LGG-CM could inhibit E. coli invasion and PMN transmigration across the BBB in vitro. The expressions of ZO-1 and CD44 were detected using Western blotting and immunofluorescence. The changes of trans-epithelial electric resistance (TEER) and bacterial translocation were determined to evaluate the BBB permeability.
Results
Pre-treament with LGG-CM inhibited E. coli-activated NF-κB signaling pathway in HBMECs and decreased the invasion of E. coli K1 and transmigration of PMN. Western blotting showed that LGG-CM could alleviate E. coli-induced up-regulation of CD44 and down-regulation of ZO-1 expressions in HBMECs. In addition, pre-treatment with LGG-CM alleviated E. coli K1-induced reduction of TEER and suppressed bacterial translocation across the BBB in vitro.
Conclusion
LGG-CM can block E. coli-induced activation of NF-κB signaling pathway and thereby prevents E. coli K1-induced neuropathogenicity by decreasing E. coli K1 invasion rates and PMN transmigration.
Keywords: Lactobacillus rhamnosus GG, conditioned medium, meningitis, human brain microvascular endothelial cells, E. coli K1, nuclear factor-κB
大肠杆菌E.coli K1是引起新生儿菌血症和脑膜炎最常见的革兰阴性菌[1]。最近的研究表明E.coli K1在侵袭血脑屏障并引发脑膜炎的过程中有着典型的三联症特征,即NF-κB信号通路激活、细菌侵袭脑微血管内皮细胞(BMEC)以及中性粒细胞迁移穿越血脑屏障[2-3]。E.coli K1引起的三联炎症是一个动态过程:首先,E.coli K1毒力蛋白IbeA结合脑微血管内皮细胞的波形蛋白,激活NF-κB信号通路。其次,激活的NF-κB信号通路可改变BMEC的脂筏分布及骨架蛋白表达,引发E.coli K1侵袭并穿越血脑屏障,进入脑内。最后,E.coli K1引起脑内炎症因子和趋化因子释放,募集中性粒细胞迁移和穿越血脑屏障进入炎症灶,进一步加重炎症反应[4-6]。由此可见,NF-κB信号通路的激活在E.coli K1脑膜炎的发病中起着至关重要的作用,且前期实验中我们发现NF-κB信号通路抑制剂可以抑制细菌性脑膜炎三联征的发生[2]。
NF-κB作为一种信号转导途径的枢纽,在许多生理、病理过程发挥了重要作用[7-8]。抑制NF-κB通路激活不仅可调节某些生理活动,同时也可预防和治疗一些相关疾病。尽管目前已经发现许多种类的NF-κB抑制剂,但是大部分抑制剂都存在许多不足,因此,目前为止只有少数抑制剂应用于临床[9]。开发新的NF-κB信号途径抑制剂已成为药物研发的热点和趋势。目前研究表明鼠李糖乳杆菌的培养上清液(LGG-CM)有着类似于LGG菌体本身的益生作用,对其益生作用机制的研究也逐渐成为了热点。本课题组前期研究发现LGG-CM能抑制肠上皮细胞NF-κB信号通路激活,但是对于其能否抑制基于NF-κB通路的E.coli K1三联炎症却未有报道。因此,本研究探讨了LGG-CM能否预防E.coli K1三联炎症并初步研究了其相关机制。
1. 材料和方法
1.1. 材料
1.1.1. 细胞、菌株及用到的试剂
HBMEC和大肠杆菌E.coli K1由南方医科大学微生物学系保存,鼠李糖乳杆菌(LGG)由美国南加州大学洛杉矶儿童医院黄胜和教授惠赠。乳杆菌培养基(De Man, Rogosa, andSharpe broth, MRS)购于广东环凯微生物科技有限公司,脑心浸液培养基(brain heart infusion, BHI)购于美国BD。胎牛血清购于德国PAN生物科技公司,RPMI1640基础培养基购于赛默飞生物科技有限公司。兔抗ZO-1、p65和CD44抗体均购于英国Abcam。0.22 μm滤器和截留量为30 000的超滤蛋白浓缩管购于美国Millipore。其它细胞培养器材,如Transwell小室、24孔和6孔细胞培养板均购于美国Corning。人外周血中性粒细胞分离液Polymorphprep购于挪威Axis-Shield。
1.2. 方法
1.2.1. LGG-CM的制备
LGG接种于MRS培养基中,置于37 ℃培养24 h。将菌液离心取上清,用0.22 μm的滤膜过滤除菌。将上清液装入浓缩管中,在4 ℃条件下4000 r/min离心40 min。因为研究报道的LGG上清活性成分主要集中于蛋白相对分子质量≥30 000部分,我们的预实验结果也表明相对于 < 30 000组分,≥30 000组分确实能明显抑制E.coli K1侵袭HBMEC。因此,在本研究中,所有的实验均使用≥30 000组分。
1.2.2. 体外细胞侵袭实验
HBMEC接种于24孔板中培养,待细胞汇合后,弃去培养液,用PBS清洗3次,将LGG-CM与无抗生素培养基按不同体积混合,配置成如下几个实验组:0.5%(5:1000)、1%(10:1000)、2%(20:1000),每组设3个复孔。用上清处理1 h后,向每孔中加入1×107菌落形成单位(CFU)的E.coli K1,使感染复数(MOI)达到100:1。37 ℃,5% CO2培养箱中共同孵育1.5 h。用PBS清洗细胞3次,再加入含有100 μg/mL庆大霉素的培养基,37 ℃,5% CO2培养箱中孵育1 h,以杀死细胞外细菌。再用PBS清洗3次,加入100 μL的0.5% tritonx-100。置于培养箱中处理8 min,立即加入100 μL蒸馏水终止裂解。反复吹打后,吸出细胞裂解液,梯度稀释后涂板计数菌落数。侵袭率=胞内存活细菌数/加入细菌数×100%,重复3次取平均值,以单独感染E.coli K1组为100%,计算相对侵袭率。
1.2.3. PMN细胞迁移实验
取人外周血约10 mL,按Polymorphprep中性粒细胞分离液说明书分离中性粒细胞(PMN)。将HBMEC接种于Transwell小室上层,待细胞汇合后继续生长4 d用于后续实验。设置对照组、E.coli K1组和2% LGG-CM+E.coli K1 3组,每组分别加PBS或2% LGG-CM预处理1 h。然后向下室中加入2×105 CFU的E.coli K1处理2 h。再向上室中加入1×106个PMN处理4 h,最后取Transwell下层培养液用血球计数板计数迁移至下层的PMN数量。
1.2.4. 免疫印迹检测各组ZO-1,CD44和p65蛋白的表达
细胞分组如1.2.3,将细胞培养于6孔板中。细菌处理完毕后,按试剂盒说明书,裂解并提取细胞核蛋白及胞质蛋白。取10 μL变性后的蛋白进行蛋白电泳。截取相应相对分子质量的蛋白胶,将蛋白转至PVDF膜。然后用5%脱脂奶粉封闭1 h,分别加入兔抗ZO-1、CD44、p65及β-actin抗体,4 ℃孵育过夜。辣根过氧化物酶标记的二抗室温孵育1 h,TBST洗3次,上机观察各组蛋白表达量。
1.2.5. 跨内皮细胞电阻检测
将HBMEC消化和接种至Transwell上室,下室中加入1 mL培养液,待细胞汇合后继续生长4 d。用跨内皮细胞电阻(TEER)评价体外血脑屏障的完整性,实验分组和处理情况同1.2.1,加入1×106 CFU的E.coli K1于Transwell上室感染细胞。在感染后的0、1、2、3、4、5 h用EVOM2电阻仪检测TEER值,方法严格按照说明书。记录各个时间段的电阻值,作出变化曲线。在第5小时取Transwell下层培养液涂板,计算下层细菌数量。
1.2.6. 免疫荧光技术检测各组细胞间ZO-1蛋白的形态及分布
实验分组同1.2.3,处理完后,加入4%多聚甲醛固定10 min,弃去液体,预冷的PBST清洗3次,加入200 μL封闭液(含5% BSA的PBST)室温孵育2 h,弃去封闭液加入100 μL兔抗ZO-1抗体稀释液,4 ℃孵育过夜。然后用预冷的PBST清洗3次,加入Alexa Fluor488包被的二抗稀释液,室温避光孵育1 h,弃去二抗,PBST清洗3次。最后加入10 μg/mL的细胞核荧光染料DAPI孵育10 min,用荧光显微镜观察ZO-1蛋白的分布。
1.2.7. 统计学处理
用SPSS 20.0软件对所有数据进行单因素方差分析,统计数据皆采用均数±标准差表示,以P < 0.05认为差异据有统计学意义。
2. 结果
2.1. LGG-CM能够抑制E.coli K1激活NF-κB通路
细胞经受相关外界因素刺激后,可激活NF-κB通路,活化的p65可从细胞质进入细胞核启动相关基因表达。因此,细胞核中的p65蛋白表达增加表明NF-κB通路处于激活状态。免疫印迹实验结果显示(图 1A,B):经E.coli K1处理后,细胞核中的p65含量明显增加,表明E.coli K1可激活HBMEC的NF-κB通路。LGGCM预处理能够明显下调E.coli K1引起的细胞核中p65蛋白上升(**P < 0.01)。这些结果表明LGG的培养上清液可抑制E.coli K1激活NF-κB信号通路。
1.
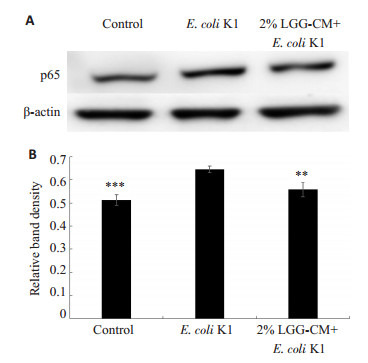
LGG-CM对E.coli K1激活NF-κB通路的影响
Effects of LGG-CM on E. coli-induced activation of NF-κB pathway in HBMECs. **P < 0.01, ***P=0.000 vs E.coli K1.
2.2. LGG-CM可抑制E.coli K1侵袭HBMEC
NF-κB通路激活可介导后续的E.coli K1侵袭和PMN迁移[2],因此,我们后续研究了LGG-CM是否可抑制E.coli K1侵袭HBMEC。实验结果显示(图 2),随着LGG-CM的浓度增高,抑制E.coli K1侵袭的能力也增强(*P < 0.05,**P < 0.01,***P=0.000),其抑制效果呈剂量依赖性。
2.
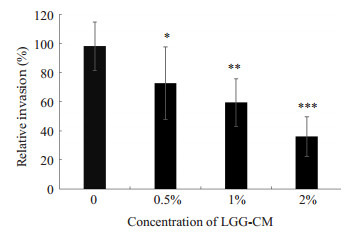
LGG-CM对E. coli K1侵袭HBMEC的影响
Effects of LGG-CM on invasion of E. coli K1 to HBMEC. *P < 0.05, **P < 0.01, ***P=0.000 vs control group.
2.3. LGG-CM抑制PMN迁移及下调CD44的表达
PMN迁移是引发细菌性脑膜炎的重要因素之一,黏附分子CD44在PMN迁移的过程中发挥了重要作用。实验中我们分别检测了LGG-CM对PMN迁移及HBMEC中CD44表达的影响。PMN细胞迁移实验结果如图 3:对照组及LGG-CM处理组PMN相对迁移率明显低于E.coli K1组(*P < 0.05,***P=0.000)。免疫印迹结果显示(图 4A,B):对照组及LGG-CM处理组HBMEC的CD44表达量明显低于E.coli K1组(*P < 0.05,**P < 0.01)。
3.
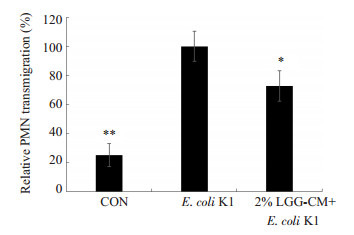
LGG-CM对E. coli K1诱导PMN迁移的影响
Effects of LGG-CM on E. coli-induced PMN transmigration. *P < 0.05, **P < 0.01 vs E. coli K1.
4.
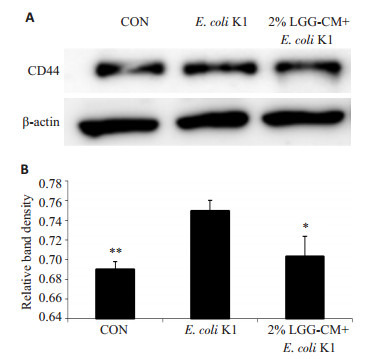
LGG-CM对HBMEC的CD44蛋白表达的影响
Effects of LGG-CM on expression of CD44 in HBMECs. A: Western blot analysis; B: Quantitative analysis of relative expression of CD44. *P < 0.05, **P < 0.01 vs E. coli K1.
2.4. LGG-CM可抑制E.coli K1破坏体外血脑屏障的完整性
实验中将HBMEC接种于Transwell上室模拟体外血脑屏障模型,通过检测TEER值变化和Transwell下层细菌数以验证血脑屏障的完整性和通透性。实验结果如图 5所示:LGG-CM能够明显减缓E.coli K1引起的TEER值下降(**P < 0.01,***P=0.000),且具有剂量依赖性。取第5 h下层液体涂板计数细菌(图 6),当LGGCM浓度为2%时,下层细菌CFU明显降低(*P < 0.05)。
5.
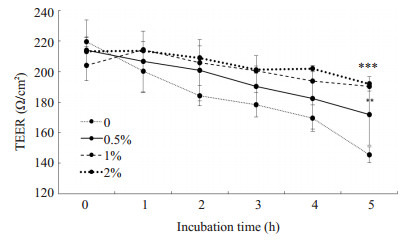
LGG-CM对血脑屏障TEER值的影响
Effects of LGG-CM on TEER value in HBMECs. **P < 0.01, ***P=0.000 vs control group.
6.
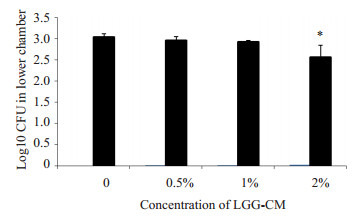
LGG-CM对Transwell下室细菌CFU数量的影响
Effects of LGG-CM on bacterial translocation. *P < 0.05 vs control group.
2.5. LGG-CM可抑制E.coli K1破坏血脑屏障的紧密连接
紧密连接是维持血脑屏障完整性和通透性的重要结构,紧密连接蛋白ZO-1是其重要组成蛋白之一。通过检测ZO-1的表达和分布情况可研究LGG-CM是否能够抑制E.coli K1对血脑屏障的破坏。实验中通过免疫印迹及免疫荧光技术观察紧密连接蛋白ZO-1的表达和分布,结果表明如(图 7A,B):感染E.coli K1能显著降低ZO-1的表达,而用LGG-CM预处理能够抑制E.coli K1下调ZO-1蛋白表达(**P < 0.05)。免疫荧光结果显示(图 7C):E.coli K1感染能够明显破坏ZO-1结构,细胞间ZO-1分布松散且断裂明显,而LGG-CM能显著减轻E.coli K1对ZO-1的破坏作用。
7.
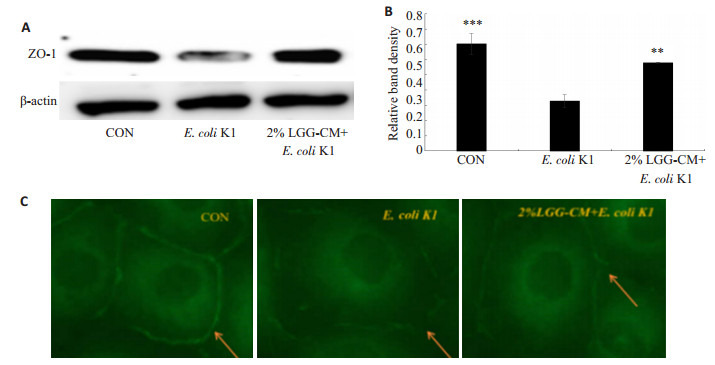
LGG-CM对ZO-1蛋白表达的影响
Effects of LGG-CM on expression of ZO-1 in HBMECs. A: Western blot analysis; B: Quantitative analysis of relative ZO-1 expression (**P < 0.01, ***P=0.000 vs control); C: Immunofluorescence detection of ZO-1 (Original magnification: ×400).
3. 讨论
LGG是目前研究最为广泛的益生菌之一,已被证明能有效的预防和治疗某些疾病,如肠道感染、腹泻、全身系统性炎症及过敏性疾病等[10-12]。最近的研究发现LGG还能促进新生小鼠肠道发育、预防大鼠肠道癌症及炎症[13-14]。然而,作为一种活的细菌,益生菌的安全性一直受到质疑,有报道称在一些免疫缺陷病人及儿童中使用益生菌会引起菌血症[15-16],且美国FDA明令禁止对免疫功能不全的未成年儿童使用活的菌株。益生菌上清液作为一种益生菌的代谢产物,其相对于益生菌来说更加安全。有研究表明LGG-CM能降低致病菌和氧化应激引起的肠道损伤[17-18];通过对小鼠灌服LGG-CM可以降低过敏反应[19]及增强单核巨噬细胞的免疫功能[20]。许多研究还揭示了益生菌或其产物的肠外益生特性,如通过肠-脑轴影响血脑屏障的形成、脑的运作和调节机体生理活动甚至宿主的行为[21-23]。然而目前研究发现的脑益生作用都是由益生菌或其代谢产物直接作用于肠道,通过肠-脑轴传递引发。而在本研究中,我们发现LGG-CM可直接作用于脑微血管内皮细胞,抑制E.coli K1对其NF-κB信号通路的激活,藉此阻断炎症反应的发生。同时我们还发现LGG-CM能够调节血脑屏障的紧密连接蛋白ZO-1表达,抑制E.coli K1对血脑屏障的损坏。这些结果初步表明LGG-CM可用于预防和治疗E.coli K1引起的脑膜炎。
NF-κB通路的激活是细菌性脑膜炎发生的重要特征之一,其在介导E.coli K1侵袭HBMEC及PMN迁移穿过血脑屏障中发挥了重要作用[2]。p65蛋白是组成NF-κB通路系统的五种蛋白之一,当NF-kB通路系统被激活时,细胞质中的p65蛋白会进入细胞核中,调节相应的基因表达[23]。实验发现LGG-CM能够阻断E.coli K1引发的p65核转运,从而抑制NF-kB通路系统的激活。黏附和侵袭是大肠杆菌穿越血脑屏障进入脑内的首要条件[25]。本实验中我们通过用不同浓度的上清处理细胞,发现LGG-CM能明显降低E.coli K1侵袭HBMEC且具有浓度依赖性。PMN募集是一把双刃剑,尽管它能有效的清除外来入侵细菌,但同时也会引起炎症介质的释放[26],引发和加重炎症反应。CD44作为一种黏附分子,在介导炎症细胞聚集上具有重要作用[27]。实验发现LGG-CM能够下调CD44蛋白的表达及穿过血脑屏障PMN的数量。
检测跨内皮细胞电阻值和紧密连接蛋白表达是评价细胞屏障完整性的经典方法[28-29]且已有报道发现益生菌可以维持肠道屏障的完整性[30-31]。本研究发现随着LGG-CM浓度升高,TEER值下降幅度减缓且Transwell下层细菌数量也显著下降,表明LGG-CM能抑制E.coli K1破坏血脑屏障,以维持血脑屏障的完整性。紧密连接蛋白是一种与细胞间紧密连接性相关的蛋白,其破坏及重排都会增大细胞间通透性[32],其中ZO-1蛋白是一种典型的与内皮细胞紧密连接相关的蛋白[33],其表达量的多少与细胞间紧密连接性呈正相关。实验中我们发现相对于致病菌组,LGG-CM预处理组可以抑制E.coli K1降解ZO-1蛋白,TEER值、下层细菌数量及紧密连接蛋白的检测都证明了LGG-CM能够维持细胞屏障的完整性。
综上所述,本研究发现LGG-CM能够抑制基于NF-κB通路激活的细菌性脑膜炎三联征,表明LGG-CM有望成为预防和治疗细菌性脑膜炎新药物的潜力。但是,LGG-CM成分复杂,到底是哪种活性成分起作用目前还不清楚。已有学者从LGG-CM中分离出P70和P45两种蛋白,发现它们在保护肠道方面具有较好的作用,这对于我们进一步的研究具有重要的指导意义。总之,本研究初步证明LGG-CM能够通过抑制细菌性脑膜炎三联征和维持血脑屏障完整性来预防细菌性脑膜炎的发生。同时,本研究也表明LGG-CM有望成为新的NF-κB通路抑制剂,为该抑制剂的发掘和探究提供了新的研究思路和方向。
Biography
曾庆,硕士,E-mail: 870686982@qq.com
Funding Statement
国家自然科学基金(81370740);南方医科大学公卫学院院长基金(GW201601)
Supported by National Natural Science Foundation of China (81370740)
Contributor Information
曾 庆 (Qing ZENG), Email: 870686982@qq.com.
曹 虹 (Hong CAO), Email: gzhcao@smu.edu.cn.
References
- 1.Huang SH, Stins MF, Kim KS. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2000;2(10):1237–44. doi: 10.1016/S1286-4579(00)01277-6. [Huang SH, Stins MF, Kim KS. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis [J]. Microbes Infect, 2000, 2(10): 1237-44.] [DOI] [PubMed] [Google Scholar]
- 2.Chi F, Bo T, Wu CH, et al. Vimentin and PSF act in concert to regulate IbeA + E. coli K1 induced activation and nuclear translocation of NF-κB in human brain endothelial cells. PLoS One. 2012;7(4):e35862. doi: 10.1371/journal.pone.0035862. [Chi F, Bo T, Wu CH, et al. Vimentin and PSF act in concert to regulate IbeA + E. coli K1 induced activation and nuclear translocation of NF-κB in human brain endothelial cells[J]. PLoS One, 2012, 7(4): e35862.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang SF, Peng L, Gai ZT, et al. Pathogenic triad in bacterial meningitis: pathogen invasion, NF-kappa B activation, and leukocyte transmigration that occur at the blood-brain barrier. http://journal.frontiersin.org/article/10.3389/fmicb.2016.00148/pdf. Front Microbiol. 2016;7(5):47. doi: 10.3389/fmicb.2016.00148. [Wang SF, Peng L, Gai ZT, et al. Pathogenic triad in bacterial meningitis: pathogen invasion, NF-kappa B activation, and leukocyte transmigration that occur at the blood-brain barrier[J]. Front Microbiol, 2016, 7(5): 47.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics. 2000;105(2):316–9. doi: 10.1542/peds.105.2.316. [Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis[J]. Pediatrics, 2000, 105(2): 316-9.] [DOI] [PubMed] [Google Scholar]
- 5.Tuerlinckx D, Bodart E, Garrino MG, et al. Clinical data and cerebrospinal fluid findings in Lyme meningitis versus aseptic meningitis. https://www.researchgate.net/publication/7858618_Clinical_data_and_cerebrospinal_fluid_findings_in_Lyme_meningitis_versus_aseptic_meningitis. Eur J Pediatr. 2003;162(3):150–3. doi: 10.1007/s00431-002-1119-1. [Tuerlinckx D, Bodart E, Garrino MG, et al. Clinical data and cerebrospinal fluid findings in Lyme meningitis versus aseptic meningitis[J]. Eur J Pediatr, 2003, 162(3): 150-3.] [DOI] [PubMed] [Google Scholar]
- 6.Ponka A, Ojala K, Teppo AM, et al. Differential diagnosis of aseptic and bacterial meningitis on the basis of cerebrospinal fluid findings. http://cn.bing.com/academic/profile?id=84e7dcc4b1da83e785d2a8db2df09f40&encoded=0&v=paper_preview&mkt=zh-cn. Duodecim. 1983;99(7):470–6. [Ponka A, Ojala K, Teppo AM, et al. Differential diagnosis of aseptic and bacterial meningitis on the basis of cerebrospinal fluid findings [J]. Duodecim, 1983, 99(7): 470-6.] [PubMed] [Google Scholar]
- 7.Jakkampudi A, Jangala R, Reddy BR, et al. NF-κB in acute pancaeatitis: Mechanisms and therapeutic potential. Pancreatology. 2016;16(4):477–88. doi: 10.1016/j.pan.2016.05.001. [Jakkampudi A, Jangala R, Reddy BR, et al. NF-κB in acute pancaeatitis: Mechanisms and therapeutic potential[J]. Pancreatology, 2016, 16(4): 477-88.] [DOI] [PubMed] [Google Scholar]
- 8.Collins PE, Mitxitorena I, Carmody RJ. The ubiquitination of NF-κB subunits in the control of transcription. Cells. 2016;5(2):23. doi: 10.3390/cells5020023. [Collins PE, Mitxitorena I, Carmody RJ. The ubiquitination of NF-κB subunits in the control of transcription[J]. Cells, 2016, 5(2): 23.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.康文渊, 田菲菲. NF-κB信号通路及抑制剂研究进展[EB/OL].北京:中国科技论文在线.
- 10.Hojsak I, Snovak N, Abdovic S, et al. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin Nutrit. 2010;29(3):312–6. doi: 10.1016/j.clnu.2009.09.008. [Hojsak I, Snovak N, Abdovic S, et al. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial[J]. Clin Nutrit, 2010, 29(3): 312-6.] [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Chatterjee M, Ganguly S, et al. Effect of lactobacillus rhamnosus GG in persistent diarrhea in Indian children -A randomized controlled trial. J Clin Gastroenterol. 2007;41(8):756–60. doi: 10.1097/01.mcg.0000248009.47526.ea. [Basu S, Chatterjee M, Ganguly S, et al. Effect of lactobacillus rhamnosus GG in persistent diarrhea in Indian children -A randomized controlled trial[J]. J Clin Gastroenterol, 2007, 41(8): 756-60.] [DOI] [PubMed] [Google Scholar]
- 12.Zhang LY, Li N, Des Robert C, et al. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr. 2006;42(5):545–52. doi: 10.1097/01.mpg.0000221905.68781.4a. [Zhang LY, Li N, Des Robert C, et al. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model[J]. J Pediatr Gastroenterol Nutr, 2006, 42(5): 545-52.] [DOI] [PubMed] [Google Scholar]
- 13.Yan F, Liu L, Cao H, et al. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. http://cn.bing.com/academic/profile?id=4c3fd93b18ffb3e2832e35f33b33cac0&encoded=0&v=paper_preview&mkt=zh-cn. Mucosal Immunol. 2016;20(7):43. doi: 10.1038/mi.2016.43. [Yan F, Liu L, Cao H, et al. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood[J]. Mucosal Immunol, 2016, 20(7): 43.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamallat Y, Meyiah A, Kuugbee ED, et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. https://www.researchgate.net/publication/305498474_Lactobacillus_rhamnosus_induced_epithelial_cell_apoptosis_ameliorates_inflammation_and_prevents_colon_cancer_development_in_an_animal_model. Biomed Pharmacother. 2016;83(7):536–41. doi: 10.1016/j.biopha.2016.07.001. [Gamallat Y, Meyiah A, Kuugbee ED, et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model[J]. Biomed Pharmacother, 2016, 83(7): 536-41.] [DOI] [PubMed] [Google Scholar]
- 15.Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy. https://www.researchgate.net/profile/Michael_Cannon2/publication/8101012_Lactobacillus_sepsis_associated_with_probiotic_therapy/links/568c4aea08ae153299b65f6d.pdf?origin=publication_list. Pediatrics. 2005;115(1):178–81. doi: 10.1542/peds.2004-2137. [Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy[J]. Pediatrics, 2005, 115(1): 178-81.] [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases:a modern perspective on a traditional therapy. Clin Infect Dis. 2001;32(11):1567–76. doi: 10.1086/320518. [Alvarez-Olmos MI. Oberhelman RA. Probiotic agents and infectious diseases:a modern perspective on a traditional therapy[J]. Clin Infect Dis, 2001, 32(11): 1567-76.] [DOI] [PubMed] [Google Scholar]
- 17.Yan F, Cao H, Cover TL, et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132(2):562–75. doi: 10.1053/j.gastro.2006.11.022. [Yan F, Cao H, Cover TL, et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth[J]. Gastroenterology, 2007, 132(2): 562-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Y, Drabik KA, Waypa TS, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. http://d.scholar.cnki.net/detail/SJPD2059_U/SJPD12102300039232. Am J Physiol Cell Physiol. 2006;290(4):C1018–30. doi: 10.1152/ajpcell.00131.2005. [Tao Y, Drabik KA, Waypa TS, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells[J]. Am J Physiol Cell Physiol, 2006, 290(4): C1018-30.] [DOI] [PubMed] [Google Scholar]
- 19.Harb H, Van Tol E, Heine H, et al. Neonatal supplementation of processed supernatant from Lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in Life. Clinic Exper Allergy. 2013;43(3):353–64. doi: 10.1111/cea.2013.43.issue-3. [Harb H, Van Tol E, Heine H, et al. Neonatal supplementation of processed supernatant from Lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in Life[J]. Clinic Exper Allergy, 2013, 43(3): 353-64.] [DOI] [PubMed] [Google Scholar]
- 20.Fong FL, Kirjavainen PV, El-Nezami H. Immunomodulation of lactobacillus rhamnosus GG (LGG)-derived soluble factors on antigen-presenting cells of healthy blood donors. Sci Rep. 2016;6(4):22845. doi: 10.1038/srep22845. [Fong FL, Kirjavainen PV, El-Nezami H. Immunomodulation of lactobacillus rhamnosus GG (LGG)-derived soluble factors on antigen-presenting cells of healthy blood donors[J]. Sci Rep, 2016, 6(4): 22845.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quigley EM, Shanahan F. The future of probiotics for disorders of the Brain-Gut Axis. https://www.researchgate.net/publication/263711186_The_Future_of_Probiotics_for_Disorders_of_the_Brain-Gut_Axis?_sg=0Mcp2FdpzK0Glt7NO0YgI_yK8CGO1ZZS9hVdF1uPdkl66Tzw4bEf8cTAdE3HE4qUQRxOTxONocUYCNwowJKL-Q. Adv Exp Med Biol. 2014;817(9):417–32. doi: 10.1007/978-1-4939-0897-4_19. [Quigley EM, Shanahan F. The future of probiotics for disorders of the Brain-Gut Axis[J]. Adv Exp Med Biol, 2014, 817(9): 417-32.] [DOI] [PubMed] [Google Scholar]
- 22.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice[J]. Sci Transl Med, 2014, 6(263): 263ra158.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umbrello G, Esposito S. Microbiota and neurologic diseases: potential effects of probiotics. J Transl Med. 2016;14(1):298. doi: 10.1186/s12967-016-1058-7. [Umbrello G, Esposito S. Microbiota and neurologic diseases: potential effects of probiotics[J]. J Transl Med, 2016, 14(1): 298.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saegusa M, Hashimura M, Kuwata T, et al. Crosstalk between NF-kappa B/p65 and beta-catenin/TCF4/p300 signalling pathway through alterations in GSK-3 beta expression during transdifferntiation of endometrial carcinoma cells. J Pathol. 2007;213(1):35–45. doi: 10.1002/path.v213:1. [Saegusa M, Hashimura M, Kuwata T, et al. Crosstalk between NF-kappa B/p65 and beta-catenin/TCF4/p300 signalling pathway through alterations in GSK-3 beta expression during transdifferntiation of endometrial carcinoma cells[J]. J Pathol, 2007, 213 (1): 35-45.] [DOI] [PubMed] [Google Scholar]
- 25.Burns JL, Griffith A, Barry JJ, et al. Transcytosis of gastrointestinal epithelial cells by Escherichia coli K1. Pediatr Res. 2001;49(1):30–7. doi: 10.1203/00006450-200101000-00010. [Burns JL, Griffith A, Barry JJ, et al. Transcytosis of gastrointestinal epithelial cells by Escherichia coli K1[J]. Pediatr Res, 2001, 49(1): 30-7.] [DOI] [PubMed] [Google Scholar]
- 26.Echchannaoui H, Frei K, Letiembre M, et al. CD14 deficiency leads to increased MIP-2 production, CXCR2 expression, neutrophil transmigration, and early death in pneumococcal infection. J Leukoc Biol. 2005;78(3):705–15. doi: 10.1189/jlb.0205063. [Echchannaoui H, Frei K, Letiembre M, et al. CD14 deficiency leads to increased MIP-2 production, CXCR2 expression, neutrophil transmigration, and early death in pneumococcal infection[J]. J Leukoc Biol, 2005, 78(3): 705-15.] [DOI] [PubMed] [Google Scholar]
- 27.He XL, Shi XL, Puthiyakunnon S, et al. CD44-mediated monocyte transmigration across Cryptococcus neoformans-infected brain microvascular endothelial cells is enhanced by HIV-1 gp41-I90 ectodomain. J Biomed Sci. 2016;23(1):1. doi: 10.1186/s12929-015-0217-0. [He XL, Shi XL, Puthiyakunnon S, et al. CD44-mediated monocyte transmigration across Cryptococcus neoformans-infected brain microvascular endothelial cells is enhanced by HIV-1 gp41-I90 ectodomain[J]. J Biomed Sci, 2016, 23(1): 1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinton P, Nougayrede JP, Del RJ, et al. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol Appl Pharmacol. 2009;237(1):41–8. doi: 10.1016/j.taap.2009.03.003. [Pinton P, Nougayrede JP, Del RJ, et al. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression[J]. Toxicol Appl Pharmacol, 2009, 237 (1): 41-8.] [DOI] [PubMed] [Google Scholar]
- 29.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84(3):282–91. doi: 10.1038/labinvest.3700050. [Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease[J]. Lab Invest, 2004, 84(3): 282-91.] [DOI] [PubMed] [Google Scholar]
- 30.Mao XB, Gu CS, Hu HY, et al. Dietary lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PLoS One. 2016;11(1):e0146312. doi: 10.1371/journal.pone.0146312. [Mao XB, Gu CS, Hu HY, et al. Dietary lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus[J]. PLoS One, 2016, 11(1): e0146312.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh CY, Osaka T, Moriyama E, et al. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. 2015;3(3):e12327. doi: 10.14814/phy2.12327. [Hsieh CY, Osaka T, Moriyama E, et al. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum[J]. Physiol Rep, 2015, 3(3): e12327.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SW, Kim WJ, Jun HO, et al. Angiopoietin-1 reduces vascular endothelial growth factor-induced brain endothelial permeability via upregulation of ZO-2. https://www.researchgate.net/publication/23792444_Angiopoietin-1_reduces_vascular_endothelial_growth_factor-induced_brain_endothelial_permeability_via_upregulation_of_ZO-2. Int J Mol Med. 2009;23(2):279–84. [Lee SW, Kim WJ, Jun HO, et al. Angiopoietin-1 reduces vascular endothelial growth factor-induced brain endothelial permeability via upregulation of ZO-2[J]. Int J Mol Med, 2009, 23(2): 279-84.] [PubMed] [Google Scholar]
- 33.Furuse M. Molecular basis of the core structure of tight junctions. https://www.researchgate.net/publication/41562819_Molecular_Basis_of_the_Core_Structure_of_Tight_Junctions. Cold Spring Harb Perspect Biol. 2010;2(1):a002907. doi: 10.1101/cshperspect.a002907. [Furuse M. Molecular basis of the core structure of tight junctions[J]. Cold Spring Harb Perspect Biol, 2010, 2(1): a002907.] [DOI] [PMC free article] [PubMed] [Google Scholar]


