Abstract
目的
研究苯丁酸钠对前列腺癌多烯紫杉醇耐药细胞株的增值抑制与凋亡诱导作用,并探讨其作用机制。
方法
构建多烯紫杉醇耐药前列腺癌PC3细胞株(PC3/DTX),采用0、1、2、4 mmol/L的苯丁酸钠处理PC3/DTX,MTT检测细胞增殖、细胞活力以及抑制率,计算不同浓度苯丁酸钠处理后各组多烯紫杉醇对PC3/DTX细胞的半致死浓度(IC50);流式细胞仪检测不同处理组细胞凋亡水平;Western blot检测p21、Cyclin D1与Survivin的表达水平。
结果
0、1、2、4 mmol/L的苯丁酸钠处理PC3/DTX细胞48 h后,细胞存活率分别是(99.85±2.69)%,(84.68±3.87)%,(68.65±4.54)%和(43.54±5.69)%(P < 0.05);抑制率分别是(10.69±3.65)%,(5.78±4.58)2%,(54.68±3.98)%和(69.84±6.54)%(P < 0.05);多烯紫杉醇对各处理组细胞的IC50分别为135.98±2.69、109.65±3.87、87.65±3.84、64.62±2.98 nmol/L(P < 0.05);细胞的凋亡率分别为(7.20±0.80)%,(10.20±0.90)%,(19.80±2.10)%和(27.40±2.50)%(P < 0.05);Western blot显示苯丁酸钠促进p21蛋白的表达,抑制Cyclin D1与Survivin蛋白的表达。
结论
苯丁酸钠可以增加前列腺癌耐药细胞株对多烯紫杉醇的药敏性,并影响前列腺癌耐药细胞株p21、Cyclin D1与Survivin蛋白的表达量。
Keywords: 前列腺癌, 化疗耐药, 苯丁酸钠, 多烯紫杉醇
Abstract
Objective
To investigate the effect of sodium phenylbutyrate (SPB) in modulating docetaxel resistance in human prostate cancer cells in vitro.
Methods
A PC3/docetaxel-resistant human prostate cancer cell line PC3/DTX was induced and examined for proliferation, viability, and cell inhibition rate in the presence of SPB. The concentration of concentration of docetaxel required to kill 50% of PC3/DTX cells incubated with 0, 1, 2, and 4 mmol/L SPB was determined using MTT assay. Cell apoptosis rate was analyzed with flow cytometry and the cellular expressions of p21, cyclin D1 and survivin proteins were detected using Western blotting.
Results
Treatment of PC3/DTX cells with 0, 1, 2, and 4 mmol/L of SPB for 48 h resulted in cell viabilities of (99.85±2.69)%, (84.68±3.87)%, (68.65±4.54)% and (43.54±5.69)%, and cell inhibition rates of (10.69±3.65)%, (25.78±4.58)%, (54.68±3.98)% and (69.84±6.54)%, respectively (P < 0.05). The concentration of docetaxel required to kill 50% of PC3/DTX cells cultured in the presence of with 0, 1, 2, and 4 mmol/L SPB was 135.98±2.69, 109.65±3.87, 87.65±3.84 and 64.62±2.98 nmol/L, respectively (P < 0.05), and the cell apoptosis rates were (7.2±0.8)%, (10.2±0.9)%, (19.8±2.1)% and (27.4±2.5)%, respectively. SPB treatment promoted the protein expression of p21 and suppressed the expressions of cyclin D1 and survivin in PC3/DTX cells.
Conclusion
SPB can affect the expressions of p21, cyclin D1, and survivin in PC3/DTX cells and increase the sensitivity to the drug-resistant cells to docetaxel.
Keywords: prostate cancer, drug resistance, sodium phenylbutyrate, docetaxel
近年来前列腺癌在我国的发病率显著上升,2008年起成为泌尿系统中发病率最高的肿瘤。失去手术机会的前列腺癌往往在内分泌治疗、中位缓解18~24个月后,逐渐对激素产生非依赖而发展为去势抵抗前列腺癌(CRPC)[1]。以多烯紫杉醇(DTX)为代表的标准化疗方案虽然可以控制CRPC患者的疼痛、提高生活质量,但由于化疗中易于产生耐药性,其中位生存时间亦不过延长18个月。苯丁酸钠(SPB)是一种组蛋白去乙酰化酶抑制剂(HDIs),能通过阻滞细胞周期、诱导分化和凋亡等机制抑制肿瘤细胞生长,具有广谱抗肿瘤的特性,近年来成为肿瘤辅助治疗手段的研究热点[2-6],但其对化疗耐药性CRPC期前列腺癌的作用国内外鲜见报告。本研究通过建立前列腺癌细胞(PC3)多烯紫杉醇耐药细胞株PC3/DTX,研究SPB对PC3/DTX细胞株增殖、凋亡的作用,进一步探讨SPB对P21、Cyclin D1及Survivin基因表达的影响及三者之间的关系,为化疗耐药性CRPC的临床治疗提供新思路及理论依据。
1. 材料和方法
1.1. 材料和试剂
苯丁酸钠(上海远慕生物有限公司),多烯紫杉醇(上海北诺生物科技有限公司),PC3细胞株购自中国科学院典型培养物保藏委员会细胞库,F-12培养基、胎牛血清(美国Gibico),DAB显色试剂盒(福州迈新生物技术有限公司),p21抗体、Cyclin D1抗体、Survivin抗体、GAPDH抗体(美国Abcam),MTT试剂盒(武汉博士德生物工程有限公司),Annexin V-FITC/PI凋亡检测试剂盒(美国Sigma)、流式细胞分析仪(美国BD),全自动酶标仪(ELISA Reader, Japan)。
1.2. 方法
1.2.1. 耐药细胞株建立
前列腺癌细胞株PC3细胞在含有10%的胎牛血清的F-12培养基中培养,并置于37 ℃、5% CO2饱和湿度的培养箱中培养备用。根据Guo等[7]建立的方法构建多烯紫杉醇耐药PC3细胞株PC3/ DTX。耐药倍数=IC50(耐药细胞株PC3/DTX)/IC50(野生型细胞株PC3)。
1.2.2. MTT法检测细胞活性
将5×104的PC3/DTX细胞悬液分别等量接种于96孔板,每孔15 μL,置入CO2培养箱中,待细胞完全贴壁后,分组,每个处理组设4个复孔,分别用0、1、2、4 mmol/L的SPB进行处理。持续培养72 h后每孔加5 mg/mL MTT 20 μL,置于孵箱内继续孵育4 h,小心吸弃培养液,每孔加DMSO 150 μL,振荡混匀10 min,酶联免疫检测仪540 nm波长比色,比色时以空白孔调零。记录每组细胞的吸光度A值,取其平均值计算细胞存活率和抑制率。细胞存活率=(实验组A值/对照组A值)×100%;细胞抑制率=(1-实验组A值/对照组A值)×100%。
1.2.3. 流式细胞仪检测细胞凋亡
用0、1、2、4 mmol/L的SPB处理PC3/DTX细胞48 h后,以0.25%胰酶消化,使附壁的细胞分离脱壁,将细胞悬液收入5 mL试管中,1500 r/min离心5 min,以PBS液漂洗两次后以100目筛网过滤,1500 r/min再离心5 min。按照Annexin VFITC/PI试剂盒进行染色,实验方法依照试剂盒说明书进行。室温下避光反应15 min,加入300 μL Bingding Buffer,上机检测。
1.2.4. Western blot检测
用0、1、2、4 mmol/L的SPB处理PC3/DTX细胞48 h后,收集细胞,采用SDS裂解液提取总蛋白,BCA蛋白定量试剂盒对总蛋白进行定量。上样相同质量的总蛋白,12%的SDS-聚丙烯酰胺凝胶电泳分离蛋白,电泳结束后,将蛋白电转至PVDF膜,含5%脱脂奶粉的TBST封闭PVDF膜,4 ℃过夜。封闭完成后,以TBST溶液按照比例稀释p21(1:1000)、Cyclin D1(1:800)、Survivin(1:1200)和GAPDH(1:1500)抗体,室温孵育2 h。取出PVDF膜,室温下以TBST液在平缓摇动条件下漂洗3次以上,每次各5 min。以TBST溶液按照1:1000的比例稀释过氧化物酶标记的二抗,室温下孵育1 h。暗房胶片曝光检测目的蛋白表达水平。
1.3. 统计学处理
利用SPSS 13.0统计软件对实验结果进行分析,数据以均数±标准差表示,各组之间采用单因素方差分析或写成One way ANOVA进行比较,以P < 0.05存在差异有统计学意义。
2. 结果
2.1. 成功构建耐药细胞株
取对数生长期的细胞接种于96孔板,将多烯紫杉醇按照3倍的浓度梯度分别加人各组细胞中,最低浓度为12.36 pM,并设置空白对照组。培养72 h后,实验测得野生型细胞株PC3对DTX的IC50为12.36 nmol/L,耐药株PC3/DTX对DTX的IC50为136.84 nmol/L,PC3/ DTX耐药细胞株的耐药倍数为11.07,说明PC3/DTX细胞株耐药性强,符合实验设计的要求。
2.2. 苯丁酸钠抑制PC3/DTX细胞增殖实验
采用0、1、2、4 mmol/L的SPB处理PC3/DTX细胞48 h后,采用MTT法检测细胞增殖能力,实验结果发现SPB能够显著抑制PC3/DTX细胞的增殖(图 1A,P < 0.05)。0、1、2、4 mmol/L的SPB处理PC3/DTX细胞48 h后,细胞存活率分别是99.85±2.69%,84.68±3.87%,68.65±4.54%和(43.54±5.69)%(图 1B,P < 0.05)。不同浓度SPB处理PC3/DTX细胞48 h后,多烯紫杉醇对PC3/DTX细胞的抑制率分别是(10.69±3.65)%,(25.78±4.58)%,(54.68±3.98)%和(69.84±6.54)%(图 1C,P < 0.05)。不同浓度SPB处理PC3/DTX细胞后,多烯紫杉醇对各处理组细胞的IC50分别为135.98±2.69、109.65±3.87、87.65±3.84、64.62±2.98 nmol/L(图 1D,P < 0.05)。实验结果表明,SPB能够显著抑制PC3/DTX细胞的增殖,并增加PC3/DTX细胞对多烯紫杉醇的药敏性。
1.
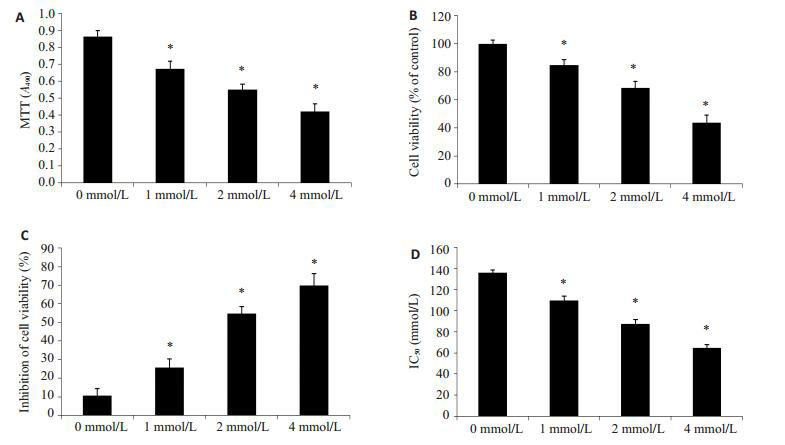
苯丁酸钠抑制PC3/DTX细胞增殖
Sodium phenylbutyrate inhibits PC3/DTX cell proliferation. A: MTT assay for detecting cell proliferation; B: MTT assay for detecting cell viability; C: Cell inhibition rate analyzed using MTT assay after culture with SPB; D: Concentration required to kill 50% of PC3/DTX cells after cultured with different concentration of SPB. Different letters means significantly difference between different groups, *P < 0.05.
2.3. 苯丁酸钠促进PC3/DTX细胞凋亡实验
采用不同浓度(0、1、2、4 mmol/L)SPB处理PC3/ DTX细胞48 h,流式细胞仪检测细胞凋亡水平。不同浓度SPB处理PC3/DTX细胞后,不同处理组细胞的凋亡率分别为(7.2±0.8)%,(10.2±0.9)%,(19.8±2.1)%和(27.4±2.5)%(图 2,P < 0.05)。实验结果表明,SPB能够促进PC3/DTX细胞凋亡。
2.
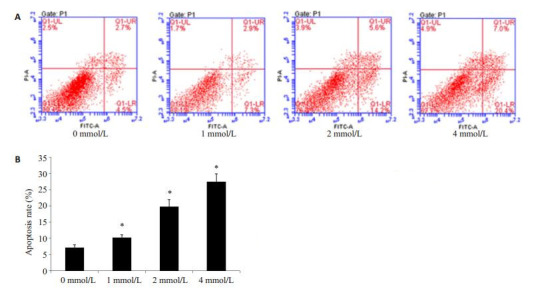
苯丁酸钠促进PC3/DTX细胞凋亡
Sodium phenylbutyrate promotes PC3/DTX cell apoptosis. A: Flow cytometry with Annexin V-FITC/PI staining; B: Cell apoptosis rate in different groups. All experiments were performed for 3 times. Different letters means significantly difference between different groups, *P < 0.05.
2.4. 苯丁酸钠对p21、Cyclin D1、Survivin蛋白表达的影响
采用0、1、2、4 mmol/L的SPB处理PC3/DTX细胞48 h后,采用Western blot检测p21、Cyclin D1、Survivin蛋白的表达水平,实验结果发现,SPB处理后能够促进p21蛋白的表达,并随着浓度的增大,表达量增加;SPB处理后能够抑制Cyclin D1和Survivin蛋白的表达,随着浓度的增加,表达量降低(图 3)。
3.
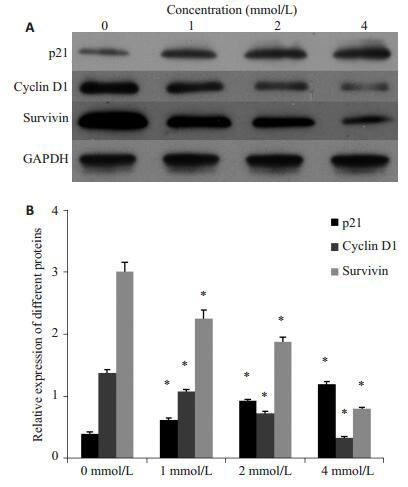
苯丁酸钠对p21、Cyclin D1、Survivin蛋白表达的影响
Western blotting (A) for detecting p21, cyclin D1, and survivin protein expressions and their quantitative analysis (B) in PC3/DTX cells treated with sodium phenylbutyrate. All experiments were performed for 3 times. Different letters means significantly difference between different groups, *P < 0.05.
3. 讨论
已知HDIs通过抑制组蛋白去乙酰化酶活性、增强组蛋白乙酰转移酶活性,致使组蛋白高度乙酰化,进而通过调节特异性基因的转录,抑制肿瘤细胞的增殖,诱导细胞分化或凋亡[8-9],包括前列腺癌[10-12]在内的数个人类恶性肿瘤的进展,都与HDAC活性的改变有关。SPB作为一种广谱HDIs,能够抑制某些肿瘤细胞的增殖、促进凋亡,在体内外对多种血液病及实体肿瘤细胞均有抑制生长和诱导分化作用,一定程度上可以抑制肿瘤细胞侵袭和复发[13-16]。
SPB对前列腺癌作用的相关研究报道不多,Lenka等[17]的研究发现丁酸钠可以降低体外培养的前列腺癌LNCap和C4-2细胞的活力、减弱其粘附力、促进细胞自培养瓶壁脱落、抑制雄激素受体基因的表达但对前列腺特异性抗原基因表达的影响微弱;目前缺乏SPB对CRPC期化疗耐药性前列腺癌细胞效应及其可能机制的报道。增殖和凋亡失衡是肿瘤发生的重要原因,本研究通过构建多烯紫杉醇耐药PC3细胞株PC3/DTX,以梯度浓度的SPB作用于PC3/DTX,探讨SPB是否能够改善PC3/DTX耐药细胞株对化疗药物多烯紫杉醇的药敏性。实验结果表明,在4 mmol/L浓度范围内,SPB能够显著抑制PC3/DTX细胞的增殖,并增加PC3/ DTX细胞对多烯紫杉醇的药敏性,其效应随SPB浓度增加而增强。
由于前列腺癌确诊时患者一般处于高龄、前列腺癌高危家族中拟表型的出现和遗传物质的复杂性,很难确认出前列腺癌的易感基因,目前倾向于前列腺癌的易感受多基因调控。p21、Cyclin D1和Survivin是目前认识得较为透彻的3个细胞活性相关基因。
P21 [18]蛋白是CKI家族中的重要一员,是细胞周期内具有双重特征的通用抑制物,具有广泛抑制激酶活性的细胞周期抑制蛋白,P21基因表达异常与前列腺癌预后不良相关[19];Cyclin D1 [20]是原癌基因,在肿瘤细胞G1/ S转换中起促进作用;Survivin [21]是凋亡蛋白抑制剂家族中的成员,具有促进细胞增殖的作用,是迄今发现的作用最强的凋亡抑制因子,在细胞中的表达中有严格的细胞周期依赖性。Survivin和cyclin D1的异常高表达与前列腺癌的发生、发展以及预后有密切关系,survivin在不同的前列腺癌细胞株均有表达,在前列腺肿瘤的发生、发展中扮演着重要的角色,其在前列腺肿瘤的高水平表达与肿瘤的分期、分级有关。Survivin的选择性分布特点使其成为肿瘤基因治疗的理想靶点,针对Survivin和cyclin D1的基因治疗具有良好的靶向性、特异性和安全性,可以促进体外培养的PC3细胞凋亡并减缓前列腺癌实体瘤的生长速度[22-24]。
我们的研究发现,经SPB处理后,PC3/DTX细胞Cyclin D1和Survivin蛋白的表达量随着SPB浓度的增加而降低,p21蛋白的表达量随着浓度的增大而增加。研究结果提示SPB通过在某种程度上调控细胞周期和细胞凋亡、增加前列腺癌耐药细胞株对多烯紫杉醇药敏性,其中p21、Cyclin D1和Survivin蛋白可能发挥了相关的重要生理功能。本研究探讨了SPB对PC3/DTX细胞株p21、Cyclin D1与Survivin的表达水平的影响,SPB是靶向作用于相关基因、还是通过上游基因的逐级调控,需要进一步研究。事实上,目前已证实PC3细胞中,p21和Cyclin D1的表达依赖上游基因的调控[25-27]。
化疗耐药的机制复杂,不仅仅由单个因素参与其中。在此过程中,SPB可以增加前列腺癌耐药细胞株对多烯紫杉醇的药敏性,并能调节p21、Cyclin D1与Survivin蛋白的表达。但是,SPB调节p21、Cyclin D1与Survivin表达以及增加前列腺癌耐药细胞株药敏性的具体分子生物学机制有待进一步研究阐明。
Funding Statement
广东省科技计划项目(2013B022000066)
References
- 1.Zhu Y, Yang XQ, Han CT, et al. Pathological features of localized prostate cancer in China: a contemporary analysis of radical prostatectomy specimens. PLoS One. 2015;10(3):e0121076–80. doi: 10.1371/journal.pone.0121076. [Zhu Y, Yang XQ, Han CT, et al. Pathological features of localized prostate cancer in China: a contemporary analysis of radical prostatectomy specimens[J]. PLoS One, 2015, 10(3): e0121076-80.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusaczuk M, Bartoszewicz M, Cechowska-pasko M. Phenylbutyric acid: simple structure-multiple effects. Curr Pharm Desin. 2015;21(16):2147–66. doi: 10.2174/1381612821666150105160059. [Kusaczuk M, Bartoszewicz M, Cechowska-pasko M. Phenylbutyric acid: simple structure-multiple effects[J]. Curr Pharm Desin, 2015, 21(16): 2147-66.] [DOI] [PubMed] [Google Scholar]
- 3.Fadeev P, Kharisov I, Kovan'ko G, et al. Study of antitumor activity of Sodium phenylbutyrate, histon deacetylase inhibitor, on ehrlich carcinoma model. Bull Exp Biol Med. 2015;159(5):652–4. doi: 10.1007/s10517-015-3039-x. [Fadeev P, Kharisov I, Kovan'ko G, et al. Study of antitumor activity of Sodium phenylbutyrate, histon deacetylase inhibitor, on ehrlich carcinoma model[J]. Bull Exp Biol Med, 2015, 159(5): 652-4.] [DOI] [PubMed] [Google Scholar]
- 4.Kusaczuk M, Krętowski R, Bartoszewicz M, et al. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol. 2016;37(1):931–42. doi: 10.1007/s13277-015-3781-8. [Kusaczuk M, Krętowski R, Bartoszewicz M, et al. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line[J]. Tumour Biol, 2016, 37(1): 931-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iannitti T, Palmieri B. Clinical and experimental applications of Sodium phenylbutyrate. Drugs R D. 2011;11(3):227–49. doi: 10.2165/11591280-000000000-00000. [Iannitti T, Palmieri B. Clinical and experimental applications of Sodium phenylbutyrate[J]. Drugs R D, 2011, 11(3): 227-49.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.孙 素霞, 李 文军, 陈 思强, et al. 丁酸钠对人结肠癌细胞株HCT-116细胞凋亡及基质相互作用分子和Orail蛋白活性的影响. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=201202189. 南方医科大学学报. 2012;32(2):189–92. [孙素霞, 李文军, 陈思强, 等.丁酸钠对人结肠癌细胞株HCT-116细胞凋亡及基质相互作用分子和Orail蛋白活性的影响[J].南方医科大学学报, 2012, 32(2): 189-92.] [PubMed] [Google Scholar]
- 7.Guo B, Villeneuve DJ, Hembruff L, et al. Cross-resistance studies of isogenic drug-resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel May be strongly compromised by prior doxorubicin exposure. Breast Cancer Res Treat. 2004;85(1):31–51. doi: 10.1023/B:BREA.0000021046.29834.12. [Guo B, Villeneuve DJ, Hembruff L, et al. Cross-resistance studies of isogenic drug-resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel May be strongly compromised by prior doxorubicin exposure[J]. Breast Cancer Res Treat, 2004, 85(1): 31-51.] [DOI] [PubMed] [Google Scholar]
- 8.Ma N, Luo Y, Wang Y, et al. Selective histone deacetylase inhibitors with anticancer activity. https://www.researchgate.net/publication/281057768_Selective_Histone_Deacetylase_Inhibitors_with_Anticancer_Activity. Curr Top Med Chem. 2016;16(4):415–26. doi: 10.2174/1568026615666150813145629. [Ma N, Luo Y, Wang Y, et al. Selective histone deacetylase inhibitors with anticancer activity[J]. Curr Top Med Chem, 2016, 16(4): 415-26.] [DOI] [PubMed] [Google Scholar]
- 9.Mottamal M, Zheng S, Huang TL, et al. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20(3):3898–941. doi: 10.3390/molecules20033898. [Mottamal M, Zheng S, Huang TL, et al. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents [J]. Molecules, 2015, 20(3): 3898-941.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrazzo A, Fiorito J, Zappalà L, et al. Antiproliferative activity of phenylbutyrate ester of haloperidol metabolite Ⅱ[(±)-MRJF4] in prostate cancer cells. Eur J Med Chem. 2011;46(1):433–8. doi: 10.1016/j.ejmech.2010.10.012. [Marrazzo A, Fiorito J, Zappalà L, et al. Antiproliferative activity of phenylbutyrate ester of haloperidol metabolite Ⅱ[(±)-MRJF4] in prostate cancer cells[J]. Eur J Med Chem, 2011, 46(1): 433-8.] [DOI] [PubMed] [Google Scholar]
- 11.Di Giacomo V, Di Valerio V, Rapino M, et al. MRJF4, a novel histone deacetylase inhibitor, induces p21 mediated autophagy in PC3 prostate cancer cells. https://www.researchgate.net/publication/280033184_MRJF4_a_novel_histone_deacetylase_inhibitor_induces_p21_mediated_autophagy_in_PC3_prostate_cancer_cells. Cell Mol Biol (Noisy-le-grand) 2015;61(3):17–23. [Di Giacomo V, Di Valerio V, Rapino M, et al. MRJF4, a novel histone deacetylase inhibitor, induces p21 mediated autophagy in PC3 prostate cancer cells[J]. Cell Mol Biol (Noisy-le-grand), 2015, 61(3): 17-23.] [PubMed] [Google Scholar]
- 12.Weaver EM, Zamora FJ, Puplampu-Dove YA, et al. Regulation of T-type Calcium Channel expression by Sodium butyrate in prostate cancer cells. Eur J Pharmacol. 2015;749(9):20–31. doi: 10.1016/j.ejphar.2014.12.021. [Weaver EM, Zamora FJ, Puplampu-Dove YA, et al. Regulation of T-type Calcium Channel expression by Sodium butyrate in prostate cancer cells[J]. Eur J Pharmacol, 2015, 749(9): 20-31.] [DOI] [PubMed] [Google Scholar]
- 13.Khan A, Ahmad R, Srivastava N. Effect of methyl butyrate aroma on the survival and viability of human breast cancer cells in vitro. J Egypt Natl Canc Inst. 2016;28(2):81–8. doi: 10.1016/j.jnci.2016.02.005. [Khan A, Ahmad R, Srivastava N. Effect of methyl butyrate aroma on the survival and viability of human breast cancer cells in vitro[J]. J Egypt Natl Canc Inst, 2016, 28(2): 81-8.] [DOI] [PubMed] [Google Scholar]
- 14.Tailor D, Hahm R, Kale K, et al. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion. 2014;16(5):55–64. doi: 10.1016/j.mito.2013.10.004. [Tailor D, Hahm R, Kale K, et al. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells[J]. Mitochondrion, 2014, 16(5): 55-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takuo M, Shingo Y. Apoptosis of bladder cancer by sodium butyrate and cisplatin. J Infect Chemother. 2012;18(3):288–95. doi: 10.1007/s10156-011-0322-2. [Takuo M, Shingo Y. Apoptosis of bladder cancer by sodium butyrate and cisplatin[J]. J Infect Chemother, 2012, 18(3): 288-95.] [DOI] [PubMed] [Google Scholar]
- 16.Zuo L, Lu M, Zhou Q, et al. Butyrate suppresses proliferation and migration of RKO colon cancer cells though regulating endocan expression by MAPK signaling pathway. https://www.researchgate.net/publication/259702159_Butyrate_suppresses_proliferation_and_migration_of_RKO_colon_cancer_cells_though_regulating_endocan_expression_by_MAPK_signaling_pathway. Food Chem Toxicol. 2013;62(7):892–900. [Zuo L, Lu M, Zhou Q, et al. Butyrate suppresses proliferation and migration of RKO colon cancer cells though regulating endocan expression by MAPK signaling pathway[J]. Food Chem Toxicol, 2013, 62(7): 892-900.] [PubMed] [Google Scholar]
- 17.Paskova L, Smesny TK, Fialova B, et al. Different effect of Sodium butyrate on cancer and normal prostate cells. Toxicol In Vitro. 2013;27(5):1489–95. doi: 10.1016/j.tiv.2013.03.002. [Paskova L, Smesny TK, Fialova B, et al. Different effect of Sodium butyrate on cancer and normal prostate cells[J]. Toxicol In Vitro, 2013, 27(5): 1489-95.] [DOI] [PubMed] [Google Scholar]
- 18.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. http://www.pubfacts.com/fulltext/27156098/Multiple-functions-of-p21-in-cell-cycle-apoptosis-and-transcriptional-regulation-after-DNA-damage. DNA Repair (Amst) 2016;42(3):63–71. doi: 10.1016/j.dnarep.2016.04.008. [Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage [J]. DNA Repair (Amst), 2016, 42(3): 63-71.] [DOI] [PubMed] [Google Scholar]
- 19.Xie HJ, Li C, Dang Q, et al. Infiltrating mast cells increase prostate cancer chemotherapy and radiotherapy resistances via modulation of p38/p53/p21 and ATM signals. https://www.researchgate.net/publication/285541949_Infiltrating_mast_cells_increase_prostate_cancer_chemotherapy_and_radiotherapy_resistances_via_modulation_of_p38p53p21_and_ATM_signals/fulltext/568e98a608aead3f42f059a6/285541949_Infiltrating_mast_cells_increase_prostate_cancer_chemotherapy_and_radiotherapy_resistances_via_modulation_of_p38p53p21_and_ATM_signals.pdf?origin=publication_detail. Oncotarget. 2016;7(2):1341–53. doi: 10.18632/oncotarget.6372. [Xie HJ, Li C, Dang Q, et al. Infiltrating mast cells increase prostate cancer chemotherapy and radiotherapy resistances via modulation of p38/p53/p21 and ATM signals[J]. Oncotarget, 2016, 7(2): 1341-53.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jirawatnotai S, Sittithumcharee G. Paradoxical roles of cyclin D1 in DNA stability. https://www.researchgate.net/profile/Siwanon_Jirawatnotai/publication/301759172_Paradoxical_roles_of_cyclin_D1_in_DNA_stability/links/572b0fed08ae057b0a08d067.pdf?origin=publication_detail. DNA Repair (Amst) 2016;42(1):56–62. doi: 10.1016/j.dnarep.2016.04.011. [Jirawatnotai S, Sittithumcharee G. Paradoxical roles of cyclin D1 in DNA stability[J]. DNA Repair (Amst), 2016, 42(1): 56-62.] [DOI] [PubMed] [Google Scholar]
- 21.Altieri DC. Survivin-The inconvenient IAP. seminars in cell & developmental. Biology (Basel) 2015;39(3):91–6. doi: 10.1016/j.semcdb.2014.12.007. [Altieri DC. Survivin-The inconvenient IAP. seminars in cell & developmental[J]. Biology (Basel), 2015, 39(3): 91-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalieri F, Beretta L, Cui JW, et al. Redox-Sensitive PEGPolypeptide nanoporous particles for survivin silencing in prostate cancer cells. Biomacromolecules. 2015;16(7):2168–78. doi: 10.1021/acs.biomac.5b00562. [Cavalieri F, Beretta L, Cui JW, et al. Redox-Sensitive PEGPolypeptide nanoporous particles for survivin silencing in prostate cancer cells[J]. Biomacromolecules, 2015, 16(7): 2168-78.] [DOI] [PubMed] [Google Scholar]
- 23.Casimiro MC, Di Sante G, Ju X, et al. Cyclin D1 promotes Androgen-Dependent DNA damage repair in prostate cancer cells. Cancer Res. 2016;76(2):329–38. doi: 10.1158/0008-5472.CAN-15-0999. [Casimiro MC, Di Sante G, Ju X, et al. Cyclin D1 promotes Androgen-Dependent DNA damage repair in prostate cancer cells [J]. Cancer Res, 2016, 76(2): 329-38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta V, Garg M, Chaudhry M, et al. Role of cyclin D1 immunoreactivity and AgNOR staining in the evaluation of benign and malignant lesions of the prostate. Prostate Int. 2014;2(2):90–6. doi: 10.12954/PI.14041. [Gupta V, Garg M, Chaudhry M, et al. Role of cyclin D1 immunoreactivity and AgNOR staining in the evaluation of benign and malignant lesions of the prostate[J]. Prostate Int, 2014, 2(2): 90-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JE, Kim JH. Valproic acid inhibits the invasion of PC3 prostate cancer cells by upregulating the metastasis suppressor protein NDRG1. Genet Mol Biol. 2015;38(4):527–33. doi: 10.1590/S1415-475738420150028. [Lee JE, Kim JH. Valproic acid inhibits the invasion of PC3 prostate cancer cells by upregulating the metastasis suppressor protein NDRG1[J]. Genet Mol Biol, 2015, 38(4): 527-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik WK, Kim S, Lim IK. Protein methylation and interaction with the antiproliferative gene, BTG2(/TIS21/Pc3) Yonsei Med J. 2014;55(2):292–303. doi: 10.3349/ymj.2014.55.2.292. [Paik WK, Kim S, Lim IK. Protein methylation and interaction with the antiproliferative gene, BTG2(/TIS21/Pc3)[J]. Yonsei Med J, 2014, 55(2): 292-303.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi OR, Ryu MS, Lim IK. Shifting p53-induced senescence to cell death by TIS21(/BTG2/Pc3) gene through posttranslational modification of p53 protein. Cell Signal. 2016;28(9):1172–85. doi: 10.1016/j.cellsig.2016.05.014. [Choi OR, Ryu MS, Lim IK. Shifting p53-induced senescence to cell death by TIS21(/BTG2/Pc3) gene through posttranslational modification of p53 protein[J]. Cell Signal, 2016, 28(9): 1172-85.] [DOI] [PubMed] [Google Scholar]


