Abstract
Purpose of Review:
The treatment of debilitating pain and loss of function secondary to lumbar stenosis is in high demand with the aging patient population. Options, including epidural steroid injections (ESIs) and medication therapy, are limited and it is unclear if they provide any functional improvements. In this prospective study, we evaluate functional outcomes in older adults with symptomatic lumbar stenosis treated with ESIs compared to those managed with medications by introducing the Short Physical Performance Battery (SPPB). Our study was IRB-approved and included sixteen patients, 68 to 83 years old, with symptomatic back and radicular leg pain secondary to lumbar stenosis. Patients could elect to undergo a lumbar ESI (n = 11) or be treated via medication management (n = 5). Numeric pain score, SPPB score, and adverse events were measured and compared at baseline and a 1-month follow-up visit.
Recent Findings:
Statistically significant improvements were observed from baseline compared to the 1-month follow-up for total SPPB score in the injection group. Similar improvements in the injection group were observed for pain scores and the SPPB sub-components such as the 4-meter walk test, chair stand time and balance score. Comparatively, no statistically significant improvements were observed in the medication group.
Summary:
Lumbar ESIs improved objective physical capacity parameters and pain scores in elderly patients with symptomatic lumbar stenosis compared to medication management. In addition, the SPPB is an easy-to-use tool to measure changes in physical function in older adults and could easily be integrated into an outpatient pain clinic.
Keywords: Short Physical Performance Battery (SPPB), Epidural steroid injections, Lumbar stenosis and radicular pain, Elderly
Older adults are the fastest-growing patient population and suffer disproportionately from chronic pain conditions. Lower back pain related to degenerative changes in the spine is the most common source of pain with a prevalence of >30% in the elderly [1]. Degenerative changes in the spine (lumbar stenosis) frequently affect facet joints and cause narrowing of the spinal canal and lateral recess/neuroforamen and present clinically with debilitating lower back pain and pain with walking (neurogenic claudication) and radicular leg pain, placing these patients at risk for losing their function and independence [25].
Medical management with prescription opioids, neuropathic pain medications, and anti-inflammatory agents is limited, especially in the elderly, related to medication side effects. Spinal surgery is an option but is associated with numerous perioperative risks in older adults [3–9].
Regarding injection therapy, findings have been inconsistent. Still, a general consensus is that an epidural steroid injection (ESI) is a reasonable, non-surgical treatment option [5–16]. The goal of an ESI is to suppress pain and the inflammatory response, thereby increasing function to the point where a patient can engage in functional rehabilitation [5,8,9,12–15]. Lumbar ESI is widely used but rigorous outcome data are limited, especially data evaluating objective evidence of functional outcomes. Most ESI studies use metrics that include improvements in pain scores and/or questionnaires [5,8,9,12–16]. Friedly et al. [15], often considered the gold standard study for lumbar spinal stenosis and ESI, compared patients (n = 400) receiving glucocorticoids plus lidocaine versus lidocaine alone. Both groups demonstrated reduced pain intensity based on the Roland-Morris Disability Questionnaire after 3 and 6 weeks compared to baseline; however, no significant group differences were observed. The Roland-Morris Disability Questionnaire is a validated measure of disability and patient-reported function but does not include any objective or observable measures that can be measured by investigators to gauge functional changes.
There are few combined functional outcomes associated with pain control measures in older adults. Guralnik et al. [17] developed the Short Physical Performance Battery (SPPB), which comprises the Chair Rise, Balance, and Walk/Gait speed tests. A summary score is calculated on a 0 to 12 scale, with lower scores indicating a greater level of disability and higher scores indicating more normal functional levels. These authors longitudinally followed 5,000 older adults for 6 years and used the SPPB scores to assess mortality. A 10-fold increase in mortality was observed in patients that had a SPPB score of 12 (12.3 deaths per 100 person-years) compared to a SPPB score of 0 (1.3 deaths per 100 person years). Additionally, a SPPB score of 12 was associated with 19.6 nursing home admissions per 100 person-years versus 0.7 nursing home admissions per 100 person-years if the SPPB score was 0.
The objective of our pilot study, therefore, was to determine the feasibility of employing the SPPB in a busy pain clinic and to test if functional outcomes differences could be detected by the SPPB in patients with symptomatic lumbar stenosis causing lower back and leg pain who receive ESI versus medication management.
METHODS
The present investigation was a prospective, Institutional Review Board-approved, observational study in adults > 65 years old. Informed written consent was obtained for every patient. The outcome measures were also approved by the University of Texas Medical Branch Institutional Review Board (Protocol 12–160). Table 1 shows inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
MRI, magnetic resonance imaging; ADL, activities of daily living
Sixteen patients, 68 to 83 years old, with symptomatic back and/or leg pain secondary to lumbar stenosis causing lower back pain and radicular leg pain were enrolled over 9 months. Patients could self-select to undergo an interlaminar lumbar ESI (10 mg of dexamethasone) at a surgery center under fluoroscopic guidance and sterile conditions or to enroll in medication management with gabapentin and tramadol, the doses of which were titrated on an individual basis according to side effects and dose response. Typically, tramadol was prescribed at 50 mg as needed every 6 hours and gabapentin was titrated to 600 mg every 8 hours. Patients were reimbursed for their time and participation in the study. The following metrics were tested at baseline and at a 1-month follow-up period: numeric pain score, total SPPB score (and subcomponents), adverse events, and complications.
Outcome Measures
The primary outcome measures were the SPPB score and self-reported pain scores, which were evaluated at baseline and at 1 month for both groups [the injection (lumber ESI) and medication management]. Percentage of improvement pre- and post-intervention was calculated for each component of the SPPB and for the numeric pain score.
The SPPB includes three objective tests of lower body function: 1) a timed 4-meter walk at a normal pace using the best of two times; 2) five timed, repeated chair stands measuring the time required to perform five rises from a chair to an upright position as fast as possible with the arms kept across the chest; and 3) three individual tests of standing balance, which include a side-by-side stand, a semi-tandem stand, and a tandem stand, with the maximum score awarded for successfully standing for 10 seconds in each individual test. There is a maximum score of 4 in each category, and summing the three individual test items creates the SPPB summary score. There is a potential range from 0 to 12, with higher scores indicating better lower body function.
Statistical Analysis
In the present investigation, measures were summarized by means ± standard deviations for scale measures and percentages for categorical measures. Fisher’s exact tests and Mann-Whitney U tests were used to assess baseline differences in age, gender, race, and body mass index. Mann-Whitney U tests were also used to assess differences in outcomes at baseline. Wilcoxon signed rank tests (paired tests) were used to assess differences pre- to post-intervention (i.e., baseline to 1-month follow-up) in SPPB total, 4-meter walk test, chair stand, balance, and pain in the injection and medication management groups.
Eleven patients chose lumbar ESI (injection group) versus five patients who continued with optimized conservative management (medication management group). P < 0.05 was considered statistically significant. At 80% power (α = 0.05), our study had the power to detect differences of 2 or 3.2 points (SD 2) in SPPB total scores and 1.5 or 2.5 points (SD 1.5) in pain scores with the Wilcoxon signed rank test for the injection (n = 11) and medication groups (n = 5), respectively. All analyses were performed in JMP 12.0 (SAS, Cary, NC).
RESULTS
Table 2 reports baseline patient characteristics for the injection and medication management groups; there were no statistically significant group differences in baseline patient characteristics. At baseline, pain scores (P = 0.03) were greater in the injection group versus the medication management group (Table 3).
Table 2.
Baseline characteristics for injection and medication management groups.
| Measure | Injection, n = 11 | Medication management, n = 5 | P* |
|---|---|---|---|
| Age, mean years ± SD | 75.0 ± 5.0 | 76.6 ± 4.7 | 0.46 |
| Gender, % | 0.24 | ||
| Male | 81.8% | 40.0% | |
| Female | 18.2% | 60.0% | |
| Race, % | 0.31 | ||
| White | 54.6% | 20.0% | |
| Non-White | 45.5% | 80.0% | |
| BMI, mean ± SD | 32.9 ± 10.0 | 25.0 ± 4.3 | 0.16 |
P values from Fisher’s exact test for categorical measures and Mann-Whitney U test for scale measures. BMI, body mass index.
Table 3.
Mean differences in baseline scores between injection and medication management groups.
| Measure | Injection, n = 11 | Medication management, n = 5 | P* |
|---|---|---|---|
| Mean ± SD | |||
| SBBP total score | |||
| Baseline | 4.1 ± 2.1 | 4.2 ± 2.7 | 0.95 |
| SPPB 4-m walk test (sec) | |||
| Baseline | 9.1 ± 3.1 | 9.2 ± 4.7 | 0.24 |
| SBBP chair stand time (sec) | |||
| Baseline | 30.3 ± 13.2 | 19.5 ± 10.5 | 0.53 |
| SPPB balance score | |||
| Baseline | 2.3 ± 1.5 | 2.4 ± 1.5 | 0.50 |
| Pain score | |||
| Baseline | 7.9 ± 2.0 | 5.4 ± 1.1 | 0.03 |
P values from Mann-Whitney U tests. SPPB, Short Physical Performance Battery.
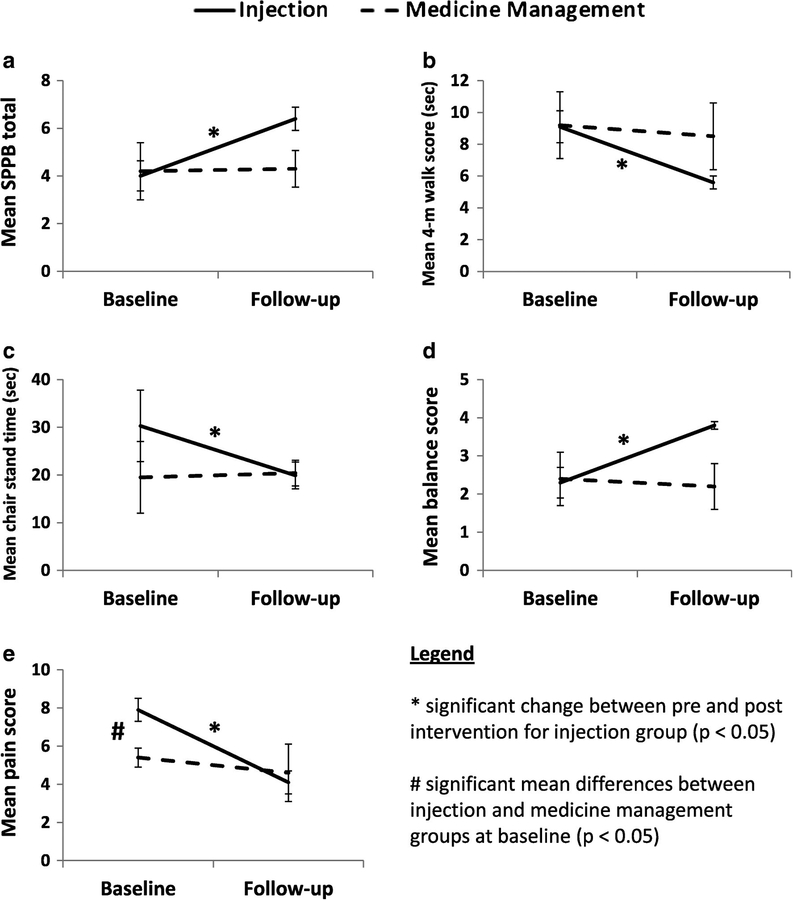
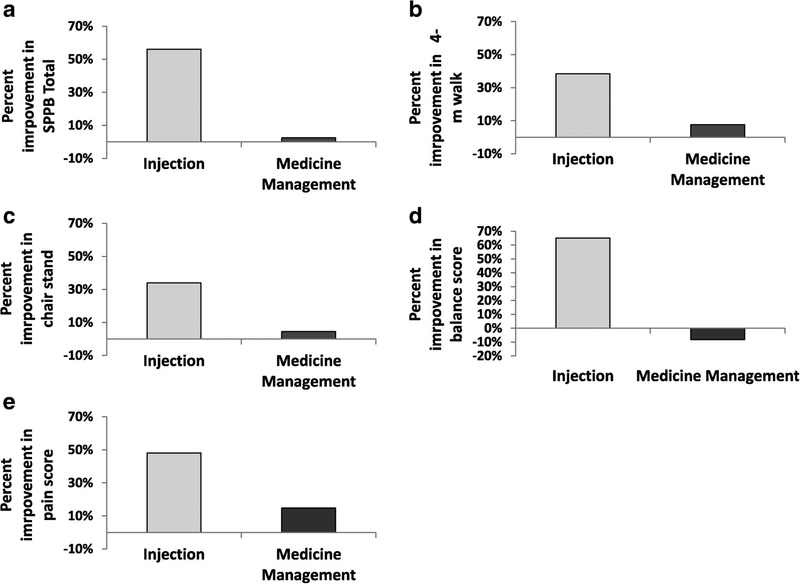
There were statistically significant improvements from baseline to 1-month follow-up in the injection group for total SPPB scores (P = 0.001). There were similar improvements in the individual functional measures: 4-meter walk test (P = 0.001), chair stand time (P = 0.047), balance (P = 0.008), and pain scores (P = 0.001; Fig. 1, A–E). Comparatively, no statistically significant improvements (P range = 0.13–1.0) were observed for those in the medication management group in any functional measure (Fig. 1, A–E). These differences translated into consistently higher percent (%) improvement from baseline to 1-month follow-up in the injection group (34%–65.2%) compared to the medication management group (−8.3% to 14.8%) across all outcomes (Fig. 2, A–E). No complications were observed.
Fig 1.
Injection versus medical management group differences in mean change between pre- and post-intervention in (A) Short Physical Performance Battery (SPPB) total scores. (B) 4-meter walk test time (C), chair stand time (D), balance scores, and (E) pain scores for injection and medication management groups. Error bars represent standard error. An asterisk (*) indicates significant (P < 0.05, Wilcoxon signed rank tests) group differences between pre- and post-intervention within either injection or medication management group. Pound sign (#) indicates significant (P < 0.05, Mann-Whitney U test, Table 3) baseline differences injection or medication management group.
Fig 2.
Percent change between pre- and post-intervention in (A) Short Physical Performance Battery (SPPB) total scores. (B) 4-meter walk test time (C), chair stand time (D), balance scores, and (E) pain scores for injection and medication management groups.
DISCUSSION
Improvement in subjective levels of pain is the most commonly used outcome measure in studies on effects of treatment for lumbar stenosis-related pain, especially in outpatient pain clinics. Improvement in functional capacity is less frequently used to gauge a patient’s response to intervention, possibly because it is viewed as a complex and difficult concept to measure. Yet, functional outcomes are a primary criteria that patients and insurance rely upon when assessing treatment options [18]. Tomkins-Lane et al. [18] have described “capacity” as the ability to perform a given task in a controlled environment that can be measured through any type of functional measure (e.g., walking test, sit and reach, timed up and go). The present investigation revealed improvements in functional capacity using the SPPB after lumbar ESI in patients whose disease process limited their ability to engage in lower body functioning. Additionally, it provides additional evidence to the current body of literature regarding how ESI can improve functional capacity and is one of a few studies to incorporate objective measures of functional capacity rather than relying on subjective questionnaires to measure functional improvements.
The SPPB was originally developed to predict disability and mortality in older patients as it relates to lower body function. Guralnik and Simonsick et al. [17] first described the test in 1994, showing a correlation between low SPPB scores and increased risk of death and nursing home admissions, which has been corroborated in the subsequent literature [19–25]. However, the SPPB has not been used to assess pain and pain treatment in older patients.
Strengths and Limitations
In reviewing our findings, several limitations should be considered, including that the present investigation has a small sample size (n = 16), especially in the medication management group (n = 5), which could limit the scope of our analyses. Additionally, our sample was not randomized and there were statistically significant differences between treatment groups in baseline measurements for pain scores, with an initially higher score in the group of patients who selected ESI that could have potentially have skewed results. This study did not have the power to stratify results by baseline pain scores. However, baseline values in other functional measures were similar. More specifically, the SPPB demonstrated improvement in the injection group but not in the medication management group. These results support the notion that an ESI provides superior efficacy than medication management in this population. Despite the noted limitations, this study is one of only a few that has used objective measures of function to gauge changes using ESI in patients suffering from lower back and radicular leg pain and therefore could be used as preliminary data for a larger study in the future. The SPPB was easily administered in a busy pain clinic without any difficulties or complications.
CONCLUSIONS
In our study of 16 patients, lumbar ESI improved objective physical capacity parameters and pain scores in elderly patients with symptomatic lumbar spinal stenosis compared to medication management. Although this study had the limitations of a small sample size and self-selection, these clinically relevant results can add to the present literature through the use of objective functional parameter testing to measure improvements after ESI. The SPPB is an easy-to-use tool to measure changes in physical function in older adults and could easily be integrated into the outpatient pain clinic setting. Even a 1-point change in an SPPB score and its subsets has been shown to be clinically meaningful and to correlate with decreased mortality and disability [17,19–25]. The present investigation found that the patients in the injection group had a 2.4-point improvement from baseline to 1-month follow-up. Although ESI does not provide a permanent cure for the degenerative process of lumbar stenosis, as no present treatment has demonstrated, it has the potential to provide long-term improvements in symptoms and can be viewed as a tool to decrease pain and increase lower body function to assist patients in more easily engaging in rehabilitation and ideally in decreasing fall risk, disability, and mortality. Based on this initial pilot study, larger randomized controlled trials are warranted.
Acknowledgments
The authors wish to disclose and thank the sponsor of the study. The study was conducted by Dr. Rene Przkora and was sponsored by the National Institutes of Health-funded University of Texas Medical Branch (UTMB) Clinical and Translational Science Award (grant no. UL1TR001439) and by the UTMB Claude D. Pepper Older Americans Independence Center (grant no. P30 AG024832 to E.V.) Research Career Development Project (project no. RCD4 to R.P.). The sponsorship was limited to supplies and expenses. It had no influence or interference after the protocol was designed. Role of Sponsor: The financial sponsor of this work had no role in the design and conduct of the study or the collection, management, analysis, or interpretation of the data. The sponsor also did not have a role in the preparation or review of the manuscript or the decision to submit.
Footnotes
Conflict of interest: Each author certifies that he or she, or a member of his or her immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript.
REFERENCES
- 1.Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J. 2009;9:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: A systematic review of randomized controlled trials. Spine. 2011; 36:E1335–1351. [DOI] [PubMed] [Google Scholar]
- 3.Issack PS, Cunningham ME, Pumberger M, Hughes AP, Cammisa FP. Degenerative lumbar spinal stenosis: Evaluation and management. J Am Acad Orthop Surg. 2012;20:527–535. [DOI] [PubMed] [Google Scholar]
- 4.Borenstein D. Mechanical low back pain: a rheumatologist’s view. Nat Rev Rheumatol. 2013;9:643–653. [DOI] [PubMed] [Google Scholar]
- 5.Kreiner SD, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC, Reitman CA; North American Spine Society. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013;13:734–743. [DOI] [PubMed] [Google Scholar]
- 6.Pathophysiology Kobayashi S., diagnosis and treatment of intermittent claudication in patients with lumbar canal stenosis. World J Orthop. 2014;5:134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H; SPORT Investigators. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costandi S, Chopko B, Mekhail M, Dews T, Mekhail N. Lumbar spinal stenosis: Therapeutic options review. Pain Pract. 2015;15:68–81. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: A comprehensive, evidence-based review. Reg Anesth Pain Med. 2013;38:175–200. [DOI] [PubMed] [Google Scholar]
- 10.Amundsen T, Weber H, Lilleas F. Lumbar spinal stenosis. Conservative or surgical management: A 10-year prospective trial. Spine. 2000;25:1424–1436. [DOI] [PubMed] [Google Scholar]
- 11.Chad MD, David A. Lumbar spinal stenosis. Neurol Clin. 2007;25:407–418. [DOI] [PubMed] [Google Scholar]
- 12.Manchikanti L, Kaye AD, Manchikanti K, Boswell M, Pampati V, Hirsch J. Efficacy of epidural injections in the treatment of lumbar central canal spinal stenosis: A systematic review. Anesth Pain Med. 2015;5:e23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen SP, Hanling S, Bicket MC, White RL, Veizi E, Kurihara C, Zhao Z, Hayek S, Guthmiller KB, Griffith SR, Gordin V, White MA, Vorobeychik Y, Pasquina PF. Epidural steroid injections compared with gabapentin for lumbosacral radicular pain: Multicenter randomized double-blind comparative efficacy study. BMJ. 2015;350:h1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delport EG, Cucuzzella AR, Marley JK, Pruitt CM, Fisher JR. Treatment of lumbar spinal stenosis with epidural steroid injections: a retrospective outcome study. Arch Phys Med Rehabil. 2004;85:479–484. [DOI] [PubMed] [Google Scholar]
- 15.Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Sullivan SD, Bauer Z, Bresnahan BW, Avins AL, Nedeljkovic SS, Nerenz DR, Standaert C, Kessler L, Akuthota V, Annaswamy T, Chen A, Diehn F, Firtch W, Gerges FJ, Gilligan C, Goldberg H, Kennedy DJ, Mandel S, Tyburski M, Sanders W, Sibell D, Smuck M, Wasan A, Won L, Jarvik JG. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014;371:11–21. [DOI] [PubMed] [Google Scholar]
- 16.Ammendolia C, Stuber K, Tomkins-Lane CC. What interventions improve walking ability in neurogenic claudication with lumbar spinal stenosis? A systematic review. Eur Spine J. 2014; 23:1282–1301. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 18.Tomkins-Lane CC, Haig AJ. A review of activity monitors as a new technology for objectifying function in lumbar spinal stenosis. J Back Musculoskelet Rehabil. 2012;25:177–185. [DOI] [PubMed] [Google Scholar]
- 19.Arnau A, Espaulella J, Méndez T, Serrarols M, Canudas J, Formiga F, Ferrer M. Lower limb function and 10-year survival in population aged 75 years and older. Fam Pract. 2016;33:10–16. [DOI] [PubMed] [Google Scholar]
- 20.Fisher S, Ottenbacher KJ, Goodwin JS, Graham JE, Ostir GV. Short Physical Performance Battery in hospitalized older adults. Aging Clin Exp Res. 2009;21:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corsonello A, Lattanzio F, Pedone C, Garasto S, Laino I, Bustacchini S, Pranno L, Mazzei B, Passarino G, Incalzi RA; Pharmacosurveillance In The Elderly Care Pvc Study Investigators. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012;15:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pamoukdjian F, Paillaud E, Zelek L, Laurent M, Lévy V, Landre T, Sebbane G. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J Geriatr Oncol. 2015;6:484–496. [DOI] [PubMed] [Google Scholar]
- 24.Cosgrove JL, Bertolet M, Chase SL, Cosgrove GK. Epidural steroid injections in the treatment of lumbar spinal stenosis: Efficacy and predictability of successful response. Am J Phys Med Rehabil. 2011;90:1050–1055. [DOI] [PubMed] [Google Scholar]
- 25.Tomkins-Lane CC, Conway J, Hepler C, Haig AJ. Changes in objectively measured physical activity (performance) after epidural steroid injection for lumbar spinal stenosis. Arch Phys Med Rehabil. 2012;93:2008–2014. [DOI] [PubMed] [Google Scholar]