Abstract
Normal functioning of articulating tissues is required for many physiological processes occurring across length scales from the molecular to whole organism. Lubricating biopolymers are present natively on tissue surfaces at various sites of biological articulation, including eyelid, mouth, and synovial joints. The range of operating conditions at these disparate interfaces yields a variety of tribological mechanisms through which compressive and shear forces are dissipated to protect tissues from material wear and fatigue. This review focuses on recent advances in active agents and biomaterials for therapeutic augmentation of friction, lubrication, and wear in disease and injured states. Various small-molecule, biological, and gene delivery therapies are described, as are tribosupplementation with naturally-occurring and synthetic biolubricants and polymer reinforcements. While reintroduction of a diseased tissue’s native lubricant received significant attention in the past, recent discoveries and pre-clinical research are capitalizing on concurrent advances in the molecular sciences and bioengineering fields, with an understanding of the underlying tissue structure and physiology, to afford a desired, and potentially patient-specific, tissue mechanical response for restoration of normal function. Small and large molecule drugs targeting recently elucidated pathways as well as synthetic and hybrid natural/synthetic biomaterials for restoring a desired tissue mechanical response are being investigated for treatment of, for example, keratoconjunctivitis sicca, xeroderma, and osteoarthritis.
Keywords: Biotribology, Biolubrication, Biomechanics, Friction, Polymers, Biomaterials, Osteoarthritis
1. Introduction
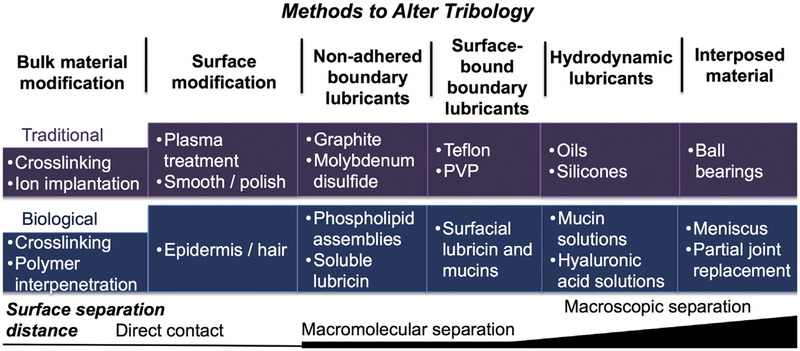
The function of many mechanical, electromechanical, and biological systems depends on appropriate lubrication at the articulating interface. In general, one is most familiar with friction and wear on metal surfaces in applications such as engines, turbines, and pistons [1,2]. Our deepening understanding of frictional behavior has given rise to various strategies for modulating friction. Today, strategies exist to alter tribology,1 [3] including application of lubricants such as graphite, introduction of rolling rather than sliding components, e.g., ball bearings, and the use of acoustic energy to dissipate friction. Further, the articulating substrates themselves may be modified, e.g., by plasma treating, smoothing, or hardening, or by conjugating lubricious polymers to the substrate, e.g., Teflon® used to coat cookware (Fig. 1).
Fig. 1.
Methods to alter tribology at articulating surfaces and representative examples of traditional and biological approaches and materials.
Friction, lubrication, and wear on biological surfaces is, in our opinion, more interesting and challenging to study given that the articulating substrates are living substrates of compositionally complex mixtures that may be stiff, viscoelastic, compact, or porous and possess smooth or rough surfaces. Numerous bodily tissue interfaces require efficient biolubrication1 for optimal physiological function, including diarthrodial joints [4–7] and mucosal membranes such as the eyelid, esophagus, intestine, and vagina (Fig. 1). When function becomes impaired due to injury, disease or old age, increased wear leads to reduced lubricity and tissue wear often associated with pain and restricted mobility. The tribology of biological systems, or biotribology, has been reviewed elsewhere [3,8–10] with particular emphases on diarthrodial joints [4–7], skin [11,12] endothelia [13], eye/contact lens interface [14], and tongue/mouth lubrication by saliva [15].
Normal functioning of articulating tissues is required for many physiological processes occurring across length scales from the molecular to the whole organism (Fig. 2). Whole-organism lubrication conserves energy [16] and enables evasion of predators [17] among swimming animals as well as prevents desiccation for amphibians [18]. Lubrication also plays critical roles during mammalian reproduction. For example, mammalian offspring (excluding egg-laying mammals) require whole-body lubrication during vaginal birth to reduce frictional stresses resulting from high cervical and vaginal pressures on the neonate [19]. Taken together, these examples highlight the levels of biotic organization over which biotribological principles govern significant biological functions.
Fig. 2.
Several exemplary tribological systems or interfaces classified by operating biotribological length scale and by level of biotic organization. Nearly all biotribological interfaces operate to some degree at the molecular and nanometer level.
Lubrication in humans is often mediated by aqueous solutions of macromolecules, macromolecule assemblies, and high-molecular weight biopolymers. Shear forces are transmitted to and dissipated by these naturally occurring biolubricants at the site of articulation. Of utmost importance are their chemical functionalities, structure, and conformation in their ability to provide extremely low, non-damage-inducing friction to their host surfaces. Mucins, the glycosylated bottle-brush-structured proteins found in saliva and mucus, bear hydrophilic hydroxyl, carboxyl, sulfate, and amino groups, and form lubricating gels through intermolecular complexation (Fig. 3a) [20,21]. Mucin-like glycoproteins function as effective boundary lubricants in low viscosity conditions [22], forming thin gel films [22,23] which repel interfacial contact (Fig. 3b). Mucins also lubricate under high viscosity conditions via hydrodynamic lubrication by forming thicker gel layers [24,25]. Assemblies of phospholipids are excellent boundary lubricants [26], due to several mechanisms including hydration lubrication [27–29] and roller-bearing-type lubrication (Fig. 3c) [30,31]. High molecular weight linear polysaccharides confer tissue lubrication even in the absence of conjugation to a protein core, exemplified by hyaluronic acid (HA) lubricating articular cartilage (Fig. 3d). Many examples of biological lubrication, including many of those described below, involve at least one of these classes of macromolecules; hydrophilic polysaccharide chains, whether in linear or branched form, or attached to a peptidic backbone, constitute the majority of lubricating macromolecules. This review begins with a discussion of the articulating tissues of interest, their relevance, and the diseases or injuries that decrease lubrication and increase wear, and then proceeds to discuss the therapeutic strategies which entail active agents (small molecules and biologics), polymer lubricants, and biomaterial-based reinforcement agents. Finally, we present a critical overview of the limitations of current systems to improve biolubrication and strengthen soft tissues, and evaluate the future outlook.
Fig. 3.
Schematic representations of common classes of biolubricant macromolecules with lubrication-conferring hydrophilic functional groups (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Tissues of interest and associated diseases
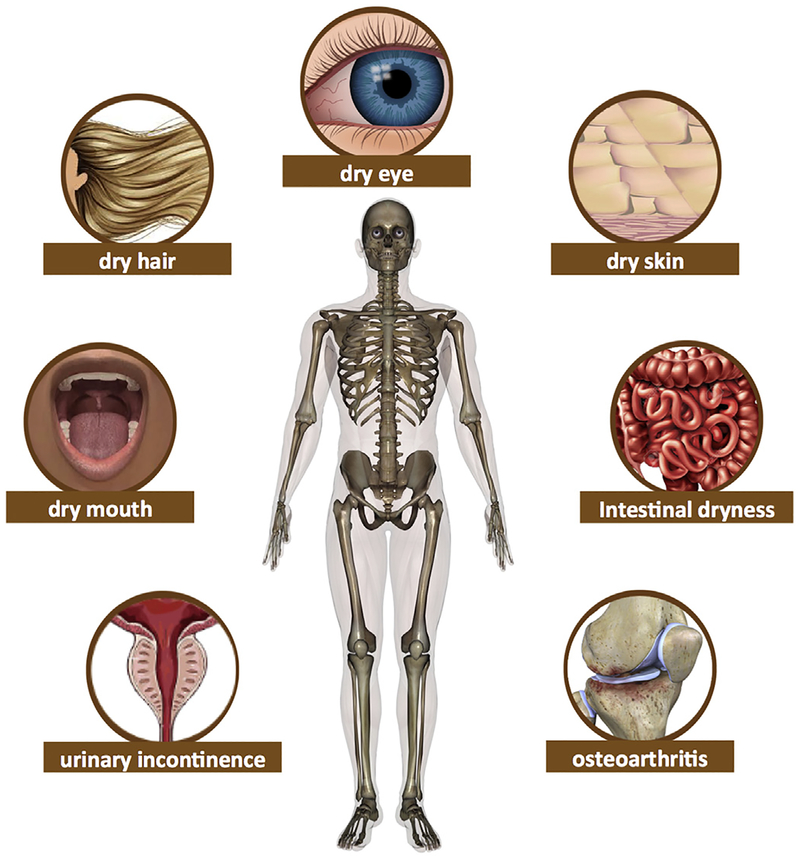
The following section highlights several key tissues and interfaces of biotribological importance in the human body (Fig. 4). Moreover, it describes the states of disease or injury associated with inadequate tissue lubrication or weakened structural integrity at each bodily site (Table 1).
Fig. 4.
Several key tissues and interfaces of biotribological importance in the human body and the disease associated with inadequate tissue lubrication or weakened structural integrity at each bodily site.
Table 1.
Key tissues and tissue interfaces of biological importance, and states of disease or injury associated with poor biotribological function.
| Bodily site | Tissue/Tissue interface | Native lubricant | Contributing macromolecules/molecules |
Common diseases and injuries |
|---|---|---|---|---|
| Eye (2.1)a | Eyelid—cornea | Tear film | Mucins, lipids | Keratoconjunctivitis sicca (dry eye) |
| Mouth (2.2) | Tongue—inner mouth | Saliva | Mucins | Xerostomia (dry mouth) |
| Gastrointestinal tract (2.3) |
Intestine—stool | Mucosal secretions, fats | Mucins, lipids | Intestinal dryness |
| Abdomen system (2.3) |
Abdominal organ—surrounding tissue, tendon—surrounding tissue | Mucosal secretions |
Mucins | Post-surgical (including peritendinous) adhesions |
| Reproductive system (2.4) |
Vagina—penis | Mucosal secretions |
Mucins | Vaginal dryness |
| Excretory system (2.5) |
Urethra—biomaterial, vascular lumen—biomaterial | - | − | Catheter-induced trauma, guidewire-induced trauma; urinary incontinence |
| Synovial joint (2.6) |
Articular cartilage—articular cartilage | Synovial fluid | HA, lubricin, phospholipids | Osteoarthritis |
| Skin & hair (2.7) | Dermis, Hair-hair | (Dry lubrication) | Atrophic cells and cellula debris; Keratin |
Xeroderma (dry skin); chronic wounds; Dry/ damaged hair |
Section heading within manuscript.
2.1. Eye
Tissue interface: eyelid—cornea. The human eye is a critical organ for vision and sense of sight. The cornea is the tissue through which light passes before striking photoreceptors in the rear of the eye. Corneal tissue is protected from damage and also hydrated by the outermost tissue of the eye, a non-permanent barrier, the eyelid. Given that the human eye blinks roughly 20,000 times per day [32,33], friction between the eyelid and cornea is largely mitigated by tear film. Tear film contains lubricating mucins and phospholipid assemblies, both of which are present in the fluid itself as well as adsorbed to the cornea and eyelid. The film also contains antimicrobial proteins and growth factors.
Disease: Keratoconjunctivitis sicca (dry eye). Various pathologies causing discomfort to the eye when blinking are colloquially termed dry eye. Insufficient lachrymation or xerophthalmia, clogged or poorly-functioning tear ducts, inflammation, or other pathologies may cause dry eye [34]. The most common treatment for dry eye involves over-the-counter eye drops known as artificial tears that directly administer lubricating macromolecules to the articulating ocular interface [35]. Artificial tears restore not only lubrication but also optimal tear film viscoelasticity and osmolality [36]. The many native and non-native polymers found in artificial tears, along with additional dry eye treatments, are described in Section 3.
2.2. Mouth
Tissue interface: Tongue—inner mouth. Frictional forces in the mouth, modulated by saliva’s lubrication, significantly affect the sensory experience of foods [23,37]. Moreover, the physical movements related to speech require low-friction sliding of the tongue against other soft and hard tissues in the mouth. Saliva contains an abundance of salivary mucins, which increase viscosity as well as form boundary-lubricating gels.
Disease: Xerostomia (dry mouth). Xerostomia is often associated with the effects of radiotherapy, salivary dysfunctions (reduced saliva production or altered saliva composition), or several medical conditions (Parkinson’s disease, hepatitis C, etc.) [38]. When insufficient lubrication is provided to the tongue-dinner mouth interface, several pathologies may result including poor speech, chewing, swallowing, altered perception of taste, as well as dental caries and periodontal diseases [39]. A leading treatment of dry mouth is the administration of lubricating sprays, gels, and rinses known as artificial saliva [40]. While these treatments temporarily mitigate the symptoms, other remedies aim to provide long-term therapeutic benefits are discussed later.
2.3. Abdominal system
Tissue interface: Abdominal organ—surrounding tissue, tendon—surrounding tissue. The exterior surfaces of nearly all abdominal and pelvic organs are coated with lubricating mucus, composed of mucin and surface adherent polysaccharides. Likewise, the exterior surfaces of tendons, ligaments, and muscles are lubricated by both viscous fluids as well as by boundary-lubricating polysaccharide-derived macromolecules. All of these intracorporeal organs and tissues articulate against and glide over one another during normal locomotion.
Disease: Post-surgical (including peritendinous) adhesions. Upon surgical or other disruption to abdominal organs or tendons (along with myriad other tissues in the body), the resulting scar tissue may disrupt the low-friction sliding of these tissues, resulting in fibrous adhesions. This phenomenon occurs as part of the natural healing process following incision, and also can form aberrantly in conditions such as adhesive capsulitis (frozen shoulder) [41]. Trauma following adhesion of the stomach, intestine, kidney, liver, and uterus to surrounding tissue causes severe organ system complications [42,43], and similar adhesion of tendons to surrounding tissue greatly impairs motor function and causes pain [44]. Thus, prevention of such adhesions is of critical importance. Numerous drug- and material-based strategies, in particular, a class of materials called adhesion barriers, along with alternate therapeutic techniques, exist to prevent post-surgical adhesions and have been thoroughly reviewed elsewhere [45–47].
2.4. Reproductive system
Tissue interface: Vagina—penis. Human reproduction classically involves insemination via vaginal-penile intercourse. The vaginal mucosal membrane secretes a mucin-rich fluid which serves as a lubricant to mitigate the frictional forces between the inner walls of the vagina and the male penis during intercourse, which involves, on average, approximately 100–500 thrusts [48]. Insufficient reduction of friction at this interface can cause microtrauma to the vaginal mucosa [49], discomfort for both partners, or vaginal or penile injuries [50–52].
Disease: Vaginal dryness. Vaginal dryness affects pre- and post-menopausal women due to a variety of underlying pathologies, and the most common symptom is insufficient production of vaginal mucosal secretions [53]. The resulting painful sexual intercourse that may occur is known as dyspareunia [54], and may significantly hinder sexual function. Personal lubricants deliver reduced friction during intercourse [55,56], and are typically water-, silicone-, or oil-based liquids [57]. They are also often used along with condoms, with the objective of providing improved tissue—biomaterial lubrication. Moreover, local or topical hormone therapies are used for augmenting mucosal secretions [58].
2.5. Excretory system
Tissue interface: Urethra—biomaterial, vascular lumen—biomaterial. Urinary catheters are used when the required systems for physiologically healthy urination are inoperative [59]. Frictional forces exerted upon insertion and removal of the device, as well as during daily life wherein gravity may exert downward forces, arise from kinetic or static friction of the catheter’s exterior against the urethral lumen. Likewise, vascular catheters passing through blood vessels exert frictional forces that are reduced by the polysaccharide-rich glycocalyx of the vascular lumen. Another medical device that must similarly pass through vasculature is a guidewire, and these frictional forces possess a similar potential for trauma.
Injury: Catheter-induced trauma, guidewire-induced trauma. Several hydrophilic, lubricious polymers (e.g., polyvinylpyrrolidone) have been implemented in catheter and guide-wire coatings to minimize shear forces transmitted to the lumen upon insertion and removal [60]. However, friction-related discomfort is experienced by many patients. Catheter and guide-wire coatings have been reviewed extensively elsewhere [61–64], and are non-exhaustively highlighted in the sections below.
Disease: Urinary incontinence. Aside from urinary incontinence arising from temporary pathophysiological changes, the muscles controlling urethral sphincters experience mechanical fatigue over decades of use and result in age-related urinary incontinence. Biomaterial treatments that address the fatigue-prone properties of aging sphincters to impart fatigue-resistance are under development.
Tissue interface: Intestine—stool. Food is lubricated by mucosal secretions and fats for its entire duration in the gastrointestinal tract; as soon as food enters the mouth it is lubricated by saliva and it continues to be lubricated by mucin-containing fluids throughout its passage through the esophagus, stomach, and intestines. In all compartments of the small and large intestine, ingested food articulates along the inner intestinal lumen and is lubricated by mucinous glycoproteins to dissipate shear forces that would otherwise damage the lumen. In addition, fats and oils present in ingested food serve to lubricate stool.
Disease: Intestinal dryness. Dehydration, unbalanced diet, stress, or some medications cause poor lubrication of stool passing through the gastrointestinal tract. Mucosal secretions in the lumen may also function poorly, thus withholding the mucin-containing fluid that is required to lubricate stool for pain-free articulation through the gastrointestinal tract. These disease states can cause constipation and abdominal pain. Laxatives (e.g., polyethylene glycol) are a class of ingestible treatments that aid in passage of stool, by lowering the stool-intestine friction.
2.6. Synovial joints
Tissue interface: Articular cartilage—articular cartilage. Articular cartilage is the hydrated soft tissue lining the ends of articulating bones in diarthrodial synovial joints. The tissue supports loads and provides a low friction interface between opposing bones. The native lubricant bathing articular cartilage is the synovial fluid, containing the linear polysaccharide hyaluronic acid (HA), allowing for maintenance of a viscous fluid film separating the cartilaginous surfaces upon sliding [65]. The glycoprotein lubricin provides hydrophilicity at the tissue surface to dissipate frictional forces [66]. Phospholipids at the articular cartilage surface also provide efficient boundary lubrication [67]. In addition to providing wear-resistance at the cartilage surface, structural macromolecules allow the applied load to be supported predominantly by the interstitial fluid phase of the tissue, and only minimally by the solid matrix of the cartilage. This phenomenon allows healthy tissue to endure decades of pressure without weakening in compressive strength or elasticity.
Disease: Osteoarthritis. Osteoarthritis is a chronic debilitating disease that affects weight bearing synovial joints. As osteoarthritis progresses, synovial fluid HA concentration and molecular weight decrease, thereby deteriorating the fluid’s rheological and friction-lowering properties [68]. Ultimately boundary mode lubrication prevails in advanced osteoarthritis, which is associated with increased friction opposed to hydrodynamic lubrication [69]. A clinical treatment known as viscosupplementation involves the intraarticular injection of viscous solutions of HA or lightly cross-linked HA intended to restore effective biolubrication to the synovial joint, and several preclinical cartilage-lubricating technologies are under development. Concomitant with the synovial fluid deterioration, cartilage permeability increases, which decreases interstitial fluid load support and increases fatigue as solid-solid contact becomes dominant [70,71]. Efforts to strengthen and reinforce fatigue-prone articular cartilage are described below.
2.7. Skin & hair
Tissue: Dermis. The outermost vascularized layer of the skin—the dermis—experiences many physical interactions throughout daily life, and is protected from frictional forces by the avascular outermost skin layer, or epidermis. Shear forces acting upon the skin are dissipated by a combination of sloughing of atrophic epidermal cells and barrier protection provided by dermal hair. Dermal and epidermal biolubrication is not dominated by fluid lubrication; instead, boundary-mode dry friction tends to operate under most daily conditions. Resisting damage occurring from excessive frictional forces is biologically important in order to maintain healthy mechanochemical transduction (i.e., sense of touch), integrin-mediated cell signaling, and generally keeping intracorporeal organs contained within the body [72].
Disease: Xeroderma (dry skin). During periods of relatively low humidity or in disease states affecting water balance throughout the body, dry skin may occur. Various lotions, creams, and other tissue protectants contain lubricating polymers which serve to maintain the tissues’ hydration and thus prevent frictional forces from damaging skin were it dehydrated [73].
Disease: Chronic wounds. Venous, diabetic, and pressure ulcers, along with other types of chronic wounds including chafing (e.g., diaper rash), are exacerbated by dermal frictional forces. In some instances, skin friction not only worsens but also initiates the wound. Typically, such wounds are covered with a barrier product, often either a solid substrate or a viscous gel, to allow frictional forces at the injured site to dissipate, to minimize pain, and to prevent infections [74].
Tissue interface: Hair—hair. Hair experiences frictional forces from wind and surrounding solid objects over the course of daily life. As in the case of skin friction, hair friction is typically mitigated through dry modes of lubrication. Hair fibers are made of the protein keratin, and boundary lubrication mechanisms of the proteinaceous fibers allow sliding with minimal damage to the macromolecular structure of the hair under optimally healthy conditions.
Disease: Dry/damaged hair. Hair-comprising keratin can exhibit several pathologies which give rise to dry hair [75]. In addition to inherent biological alterations, environmental conditions, affect hair fiber properties including the appearance of dryness [76]. Various shampoos and conditioners aim to restore lubricity to hair fibers by strengthening the fiber itself or by depositing boundary-lubricating macromolecules to the hair surface.
3. Therapeutic strategies
As a result of disease processes, injury, or many decades of gradual material wear and fatigue, tissue tribological and bulk structural properties worsen, causing sub-optimal or even painful outcomes. To meet the needs of patients with such conditions, active agents (small molecules, proteins, and genes section 3.1) are used to stimulate the body’s endogenous production of lubricants. Additionally, the replenishment of naturally-occurring biolubricants to the poorly lubricated interface is a widely utilized therapy to address the tissue’s malfunction (section 3.2). In addition to naturally-occurring lubricious macromolecules, synthetic polymer lubricants (often biomimetic in composition and structure) are used as therapeutic tissue tribosupplements (section 3.2). Beyond altering friction at the surface of tissues, reinforcement of tissue bulk improves wear- and fatigue-resistance (section 3.3). Alternate (non-drug and non-material) therapies for augmenting biolubrication also exist, providing further motivation for continued development of new non-conventional therapies (section 3.4).
3.1. Active agents
Both small and large molecule drugs, as well as gene therapies, are used to restore optimal biotribological tissue properties (Table 2).
Table 2.
Small molecules, proteins, and genes for treating common biotribological diseases and injuries.
| Common diseases and injuries | Small molecules (3.1.1)a | Proteins (3.1.2) | Genes (3.1.3) |
|---|---|---|---|
| Keratoconjunctivitis sicca (dry eye) | Cyclosporine [79], methylprednisolone [80], androgens [81] | Autologous human serum [102] | Interleukin-17 receptor gene [104] |
| Xerostomia (dry mouth) Intestinal dryness |
Pilocarpine and cevimeline [40] Senna [83], bisacodyl, bisoxatin, and sodium picosulfate [84], ricinoleic acid [85] |
Interferon alpha [103] | |
| Post-surgical (including peritendinous) adhesions |
Ibuprofen [86], corticosteroids (e.g. halcinonide) [88], heparin [89] | ||
| Vaginal dryness Osteoarthritis |
Estrogen [90], tibolone [93] Glucosamine [96], methylsulfonylmethane [95], chondroitin sulfate [96] |
Lubricin gene [105] |
Section heading within manuscript.
3.1.1. Small molecules
Keratoconjunctivitis sicca (dry eye) – Tear film hyperosmolarity, which is characteristic of dry eye, triggers the secretion of inflammatory cytokines that activate T lymphocytes [77]. This inflammatory environment gives rise to apoptosis of corneal and conjunctival cells, including the mucin-producing goblet cells responsible for tear film stability [78]. As a consequence of the self-perpetuating inflammatory cascade, agents such as cyclosporine are used to target specific inflammatory pathways in order to break the inflammatory cycle [79]. To relieve dry eye symptoms, additional common anti-inflammatory drugs such as the glucocorticoid methylprednisolone are used [80]. Recently, women have been identified to be at greater risk for dry eye, and, thus, androgens such as testosterone may be therapeutically administered to mitigate symptoms [81].
Xerostomia (dry mouth) – The decrease of salivary secretion is associated with a disturbance in the signal transduction process of the intracellular Ca2+ and aquaporin levels [82]. Mobilization of Ca2+ and aquaporin trafficking are both induced by muscarinic receptor stimulations. The small molecules pilocarpine (Salagen®) and cevimeline (Evoxac®), which are both muscarinic receptor agonists, promote a salivary secretion response in the treatment of dry mouth [40].
Intestinal dryness – The family of anthraquinone derivatives known as senna or senna glycosides (Senokot®) directly stimulate colonic smooth muscle and interfere with water and sodium reabsorption to increase intra-colonic fluid secretion which lubricates stool as it passes through the intestinal lumen [83]. Other small molecule stimulant laxatives include bisacodyl (Dulcolax®), bisoxatin, and sodium picosulfate [84]. Castor oil similarly has drug-like activity as a stimulant laxative, increasing intestinal secretions to aid in passage of stool via its active fatty acid, ricinoleic acid, binding to prostaglandin EP3 receptors in the intestine [85].
Post-surgical adhesions – Inflammatory response to surgery triggers the migration of inflammatory cells and fibroblasts to the site of injury, along with the formation of capillaries. Ibuprofen has long been used as a non-steroidal anti-inflammatory drug to prevent adhesion formation by inhibiting both cyclooxygenase COX-1 and COX-2 [86,87]. Locally administered ibuprofen prevents peritendinous adhesions following tendon repair in an in vivo chicken model following flexor digitorum profundus incision [86]. Corticosteroids, e.g., halcinonide (Halog Cream®), also show preclinical efficacy in reducing severity of intraperitoneal adhesions after cecum surgery [88]. Heparin, while a naturally-occurring polysaccharide, has drug-like activity in its anticoagulant and fibrinolysis-promoting activity [89].
Vaginal dryness – Hormone therapy, in particular, administration of estrogen, is widely used to mitigate symptoms of meno-pause including vaginal dryness [90], although estrogen’s use is more so directed towards increasing bone density and decreasing bone fractures in menopausal women [91,92]. Another steroid, tibolone (Livial®), whose metabolites have estrogenic, progestogenic and androgenic effects [92], clinically reduces vaginal dryness [93]. These steroids stimulate a multifaceted biological cascade of hormone levels in the body, one outcome of which is increased vaginal mucosal secretion.
Osteoarthritis – Monosaccharide glucosamine [94], methylsulfonylmethane (MSM) [95], and chondroitin sulfate [96] are taken as oral supplements with the intention of incorporating the structural component into diseased tissue to strengthen it and improve biotribological properties [96]. Among equine patients, glucosamine is often co-administered with HA (Polyglactin®) and clinically provides improvement in animal mobility [97]. Several other oral supplements with purported or possible drug-like action in treating osteoarthritis—so-called nutraceuticals—have been reviewed elsewhere, with variable outcomes [98,99]. Intraarticular HA viscosupplements were developed to function as non-biologically-active supplements, however more recent understanding indicates a secondary mechanism of action via binding synovial CD44 receptors to inhibit production of inflammatory mediators and thereby stimulate endogenous production of HA [100,101].
3.1.2. Proteins
Keratoconjunctivitis sicca (dry eye) – A recent clinical trial demonstrated that autologous human serum delivered via eye drops relieved dry eye symptoms [102]. This technique upregulates the production of the lubricating mucin MUC-1 in vitro. Moreover, serum proteins in combination with growth factors stimulate tear production as well as delivery of the essential tissue-protective proteins to the ocular surface.
Xerostomia (dry mouth) – Interferon a delivered by lozenge increased salivary secretion and decreased complaints related to dry mouth in a phase II clinical trial [103]. The placebo-controlled trial ruled out the effect of the lozenge alone on stimulating saliva production.
3.1.3. Gene therapies
Keratoconjunctivitis sicca (dry eye) – The delivery of interleukin-17 receptor via adenovirus-5 vector mitigates dry eye symptoms in a murine model; [104] efficacy has not yet been assessed in humans.
Osteoarthritis. The delivery of the gene encoding the glycoprotein lubricin to mice knee joints via adeno-associated viral vector protects against development of age-related as well as post-traumatic OA [105].
3.2. Biolubricants
For the purposes of this review, natural lubricants include those macromolecules and materials that are naturally-occurring lubricants in biological systems (either native to the target biological site of use, e.g., mucins for lubricating dry mouth, or non-native to the target site, e.g., a plant biopolymer used for treating an animal’s dry skin) (Table 3). Synthetic lubricants include macromolecules and materials that are synthesized not via naturally-occurring processes, but rather through advances in synthetic polymer chemistry and materials processing. Natural and synthetic polymers have related architectures and conformations (Table 4), which affect many of their physical properties including viscoelasticity, flexibility, and mode of lubrication.
Table 3.
Biolubricants for treating common biotribological diseases and injuries.
| Common diseases and injuries | Natural lubricants (3.2.1)a | Synthetic lubricants (3.2.2) |
|---|---|---|
| Keratoconjunctivitis sicca (dry eye) |
HA [106], HA-binding peptide [107,108], cellulose derivatives [36], dextran [36] |
PVA [153], PAA [223], PEG/polysorbate [224], PVP [156] |
| Xerostomia (dry mouth) | Mucins [111], cellulose derivatives, and xanthan gum [112] | PAA and PEG [112] |
| Intestinal dryness | Psyllium seed husk [113], castor oil [85] | Mineral oil [157], specifically liquid paraffin [158] |
| Post-surgical (including peritendinous) adhesions |
Icodextrin [114], ferric hyaluronate [225], HA solution [226], HA and carboxymethylcellulose barrier [115–117,227], regenerated cellulose [118] | Poly (tetrafluoroethylene) [160], PEG [161–163], PEG and carboxymethylcellulose [106], poly (g-glutamic acid) [165], pMPCb [166,167], PEG and poly (L-lactic acid) [86] |
| Vaginal dryness | Cellulose derivatives [57], pectin [119] | PEG [57], PAA and crosslinked PAA[119], silicones [228], mineral oil [170], PVP latex coating [171] |
| Catheter-induced trauma; guidewire- induced trauma |
Chitosan [120] | PVP [172,175,176,229], PEG [230], PVA [178], poly (dimethylacrylamide) [179], poly (sulfobetaine methacrylate) [183], pMPC [231] |
| Osteoarthritis | HA [121,122], HA-binding peptide [107], hydrophobically-modified HA [130,131], lubricin [134,135], phospholipid liposomes [137,138,232] and micelles[139], chitosan[141], HA/chitosan[142–144], alginate/chitosan[145], poly (g-glutamic acid) [78] |
HA mimetics [185], (Wathier two other manuscripts in prep) Lubricin mimetics [186–188], polyacrylamide [189–191], pMPCb; crosslinked PAA[233], linear [234] and crosslinked [195] PVP, cartilage substitutes of PVP and PVA [235,236], PVA and polyacrylamide [237], PVA and PAA [238], PEG and PVA [239], poly (3-sulfopropyl methacrylate)-grafted microparticles [196,197] |
| Xeroderma (dry skin); chronic wounds |
Carnauba wax [148], alginate [149,150] | Vaseline [198] |
| Dry/damaged hair | Quaternized cellulose derivatives (e.g. some polyquaterniums) [152] | Poly (diallyl dimethylammonium chloride) and other synthetic polyquaterniums [152], PEG-g-poly (L-lysine) [201] |
Section heading within manuscript.
pMPC, poly (2-methacryloyloxyethyl phosphorylcholine).
Table 4.
Natural and synthetic biolubricants’ microstructure related to their architecture and shape.
| Microstructure | Natural | Synthetic | |
|---|---|---|---|
 Linear |
HA | PVA | Polydimethylsiloxane |
| Cellulose | PEG | Dimethiconol | |
| Pectin | PAA | Polydimethylacrylamide | |
| Chitosan | PVP | Sodium (poly (7-oxanorbornene-2-carboxylate)) | |
| Alginate | PAM | Poly (diallyl dimethylammonium chloride) | |
| Poly (g-glutamic acid) | Poly (tetrafluoroethylene) | ||
| Carboxymethylcellulose | Poly (L-lactic acid) | ||
|
Brush |
Mucins | Poly (2-methacryloyloxyethyl phosphorylcholine) | |
| Xanthan gum | Poly (sulfobetaine methacrylate) | ||
| Icodextrin | PEG-g-poly (L-lysine) | ||
| Hydrophobic modified HA | |||
 Branched |
Dextran | Branched PEG | |
| PEG-containing star polymer | |||
| Polysorbate | |||
| Cyclomethicone | |||
 Crosslinked |
Ferric hyaluronate | Polycarbophil calcium pMPC crosslinked PAA crosslinked PVP poly (3-sulfopropyl methacrylate)-grafted microparticles |
|
3.2.1. Natural lubricants
Keratoconjunctivitis sicca (dry eye) – HA, present in native tear film, may be administered as a component of artificial tears to restore corneal and eyelid lubricity (e.g., Hylo-Vision®) [106]. Alternatively, a HA-binding peptide is covalently attached to a lens surface to concentrate HA on its surface in order to increase the amount of surface-bound HA and water molecules [107,108]. Cellulose derivatives are also commonly used for tear film recovery (e.g., Genteal® and Tears Naturale®) [36]. Hydroxypropyl cellulose ophthalmic inserts are another treatment available for moderate-to-severe dry eye syndrome [109]. The inserts act by stabilizing and thickening the tear film and prolonging tear film breakup time, as well as lubricating and protecting the eye. The treatments are indicated when the artificial tears therapy fails. Dextran is yet another polysaccharide used for this purpose (e.g., Visine Tears®), modulating friction, as well as viscosity [36]. Exogeneous mucins also lubricate and protect the cornea from trauma against hydrogel contact lenses [110].
Xerostomia (dry mouth) – A mucin-containing spray (Saliva Medac®) relieves dry mouth symptoms, particularly among older patients [111]. Cellulose derivatives and xanthan gum are contained in the Biotene®-brand series of oral sprays, gels, and oral rinses [112].
Intestinal dryness – A gel-like fraction of the fibrous content of psyllium seed husk relieves symptoms of constipation by lubricating stool on its passage through the gastrointestinal tract [113]. In addition to having drug-like effects, castor oil is a plant-derived lubricant that directly reduces friction of stool trans-locating through the gastrointestinal tract [85].
Post-surgical adhesions – Several gel lubricants and solid barrier lubricants derived from naturally-occurring biopolymers are commercially available for preventing post-surgical adhesions of pelvic (ovary, uterus), abdominal (intestine, stomach), and thoracic origin. Icodextrin (Adept®) prevents adhesions intraperitoneally following laparoscopic gynecological surgery [114]. A solid substrate made of HA and carboxymethylcellulose (Seprafilm®) is indicated for preventing adhesions following several surgical procedures including uterine myomectomy [115], intestinal resection [116], and general intraperitoneal surgeries [117]. A regenerated cellulose mesh (Interceed®) is among the most long-standing approved adhesion barriers and is indicated for prevention of adhesion following endometriosis repair and other gynecological surgery [118].
Vaginal dryness – Cellulose derivatives, including hydroxyethyl cellulose, are found in a variety of personal lubricants for decreasing friction and increasing a gliding sensation during sexual intercourse (K-Y Liquid® and Jelly®) [57]. Pectin is another polysaccharide derived from plant cell walls that is used in a vaginal moisturizer product (Summer’s Eve®) [119].
Catheter- and guidewire-induced trauma – While the majority of catheter-coating polymers are synthetic, chitosan, when derivatized with amphiphilic fatty acids to coat endovascular stents, reduces friction in vitro compared to non-coated stents [120].
Osteoarthritis – To treat osteoarthritis, HA is intraarticularly injected (known as viscosupplementation) to prevent cartilage wear in osteoarthritis [121,122]. While these agents have some biological activity (described earlier), the originally-intended mechanism of action of these viscous or gel-like aqueous medical device solutions is to restore viscosity and thus lubricity to the articulating surfaces of joints. Linear HA products (e.g. Supartz®, Orthovisc®, Euflexxa®) are generally metabolized more quickly, whereas crosslinked HA products (e.g. Synvisc®, Monovisc®, and Gel-One®) clear from the joint relatively more slowly, with intraarticular residence time half-lives of up to approximately 9 days (Table 5) [123]. However, several randomized controlled studies and meta-analyses have challenged the efficacy of this procedure [124–128]. Recently, the American Academy of Orthopedic Surgeons ceased to recommend viscosupplementation due to the lack of a clinically meaningful benefit for the majority of patients [127].
Table 5.
Intraarticular HA residence time studies.
| Author & year | Compound | Labeling method | Species | Half-life | Notes |
|---|---|---|---|---|---|
| Larsen 2012 [123] |
Hylan fluid (400 –800 kDa), hylan gel |
Fluid: 14C-acetate; gel: sulfonyl- bis-3H-ethyl crosslinks |
Lapine | Fluid 1.5 ± 0.2d, gel 8.8 ± 0.9d | Whole joint homogenized, SFa not discretely analyzed |
| Jackson 2006 [240] |
Hylan fluid, hylan gel | Fluid green fluorophore, gel red fluorophore | Caprine | (Fluid diminished rapidly, gel present to 28d) | Control: Dye alone eliminated within 24 h. |
| Lindenhayn 1997 [241] |
HA | 3H-HA | Lapine | 15.8 h (directb) and 17.5 ± 1.0h (indirectc) | Supports lymphatic clearance |
| Brown 1991 [242] |
HA (600 kDa) | 3H-HA | Lapine | 13.2 h (indirect) | Clearance was first-order; supports lymphatic clearance |
| Fraser 1993 [242] |
HA | 3H-HA | Ovine | 20.8 h (indirect) | Clearance was first-order |
| Antonas 1973 [243] |
HA | 3H-HA | Lapine | Not reported | Observed 3H2O in blood and tissue radiography |
| Page-Thomas 1987 [244] |
PGd (250 kDa) | 131I-Iodobeadse | Lapine | 12 h | |
| Edsman 2011 [245] |
Durolane or similar |
14C butanediol diglycidyl ether crosslinks |
Lapine | 32d (24–46d 95% CI) | Whole joint homogenized, SF not discretely analyzed |
| Lindqvist 2002 [246] |
Durolane or similar | 131I HA endgroup | Human | Reportedf: 1.5 h, 1.5d, and 4w; Effective: 2–3d | Whole joint gamma rays counted, SF not discretely analyzed |
SF, synovial fluid.
Direct measurement, 3H-HA signal measured in SF.
Indirect measurement, 3H2O signal measured in plasma.
PG, proteoglycan.
Iodobeads conjugated to PGs.
Half-lives reported as decay constants of tri-exponential curve fitted to clearance data; 1.5 h postulated to correspond to free linear HA, 1.5d to entrapped linear HA, 4w to crosslinked HA.
In addition to delivery via intraarticular injection, HA is administered orally as a supplement (Oralvisc®) and clinically relieves knee pain symptoms and improves function [129].
A technique to concentrate HA on cartilage surfaces is reported, wherein a heterobifunctional poly (ethylene glycol) (PEG) chain links an HA-binding peptide to the tissue surface (either through attachment to free amines or via a collagen-type-II-binding peptide); this technique reduces ex vivo cartilage friction and increases in vivo retention of HA in a rat model [107]. The PEG linker is believed not to serve a lubricating role in this study. Hydrophobically-modified HA is also investigated, prepared via several coupling mechanisms to attach butyl [130] and hexadecyl (FDA-approved Hymovis®) [131–133] functional groups to the HA backbone. Intra- and inter-chain hydrophobic interactions are intended to provide increased stability to associative HA conglomerates.
Another lubricious macromolecule, lubricin, is also directly injected into osteoarthritic joints. Such tribosupplementation demonstrates chondroprotective effects in rat [134,135] and mini-pig [136] models of osteoarthritis.
Phospholipid administration demonstrates clinical benefit in treating osteoarthritis. Both liposomes [137,138] and micelles (Flexiseq®) [139] relieved osteoarthritis-related knee pain in clinical studies. Co-administration of phospholipids with HA prevented cartilage damage in a rabbit model of osteoarthritis [140].
Not native to the human knee as a primary lubricating macromolecule, chitosan is generally safe upon intraarticular injection and is used in microbead form [141] (Arthrovisc®) as well as in combination with HA [142,143] (Chi2Knee®) for protection of osteoarthritic cartilage. The HA/chitosan combination product protects cartilage from degeneration in a rat meniscectomy model of osteoarthritis [144]. Chitosan formulated in combination with alginate as microgel particles also shows efficacy in preventing development of osteoarthritis in a rabbit model of osteoarthritis [145].
Another hydrophilic polymer, poly (γ-glutamic acid), is not natively found in the human body but is in a variety of medical and food applications [146]. Poly (γ-glutamic acid) is under development for intraarticular injection in treating osteoarthritis, although it is unknown whether its mechanism of action relates to lubrication, sequestration of inflammatory agents, or a combination of both phenomena [147].
Xeroderma (dry skin) – Carnauba wax, found in ChapStick®-brand lip protectants, is a plant-derived wax that both lubricates skin surfaces and also serves as a barrier to reduce skin dehydration by wind and dry air [148].
Chronic wounds. Chronic wounds such as ulcers are treated by a variety of naturally-occurring polymers. A calcium alginate-covered gauze reduces the amount of granulation tissue and causes less pain upon dressing change compared with petroleum jelly-covered gauze [149]. Alginate-based dressings are also effective in treating chronic skin ulcers [150].
Dry/damaged hair – Naturally-occurring biopolymers are often used in hair conditioners for their ability to reduce inter-fiber interaction and provide longevity to the hair without material degeneration. Cellulose derivatives for conditioner applications and general food, cosmetic, and pharmaceutical industries have been reviewed elsewhere [151], and examples include quaternary hydroxyethyl cellulose and hydroxypropyl cellulose [152].
3.2.2. Synthetic lubricants
Keratoconjunctivitis sicca (dry eye) – Hydrophilic polymers, such as PVA are used in artificial tears for lengthened duration of lubricant residence in the eye [153]. Additional hydrophilic polymers such as PAA or carbomer (Viscotears®) [154], PEG [155], and the PEG-containing star polymer polysorbate. Povidone is similarly used in lubricating drops (Soothe Hydration®) [156].
Xerostomia (dry mouth) – Polyacrylic acid (PAA) and PEG are also components in the Biotene®-brand series of oral sprays, gels, and oral rinses, along with naturally-occurring lubricants [112].
Intestinal dryness – Intestinal dryness may be treated via ingestion of mineral oil to lubricate the stool [157]. Specific forms of mineral oil used as lubricant laxatives include liquid paraffin and petroleum jelly or white petrolatum [158]. Orally administered PEG (Miralax®) is a synthetic polymer for treating constipation, although the mechanism of action is believed to be predominantly as an osmotic laxative and stool softener rather than as a lubricant per se [159].
Post-surgical adhesions – One of the earliest non-native bio-polymers to be commercially used as an adhesion barrier was poly (tetrafluoroethylene), or Teflon®. An expanded poly (tetrafluoroethylene) fabric (Gore-Tex® surgical membrane) is used to prevent adhesions during resections as well as during treatment of pelvic organ prolapse, and outperforms a regenerated cellulose barrier [160]. Sprayable solutions containing either linear PEG (SprayGel®) [161] or branched PEG (SprayShield®) [162] are currently CE-marked and are used for preventing adhesion following myomectomy [163] as well as ileostomy. Another PEG-containing barrier gel combines PEG with carboxymethylcellulose (Intercoat®) and prevents peritoneal adhesions following pelvic surgery [164]. Poly (γ-glutamic acid) is investigated for prevention of chest wall adhesions following thoracic incision in mice [165]. Poly (2-methacryloyloxyethyl phosphorylcholine) hydrogels, formed ex situ [166] or in situ [167], prevent peritendinous adhesions in healing rat Achilles tendons and chicken flexor digitorum profundus tendons. Poly (l-lactic acid)-PEG copolymers, electrospun into fibrous membranes, also prevent adhesions in the chicken flexor digitorum profundus tendon repair model [168].
Vaginal dryness – A variety of synthetic polymers are used in personal lubricants for reducing friction during sexual intercourse. PEG is used as a viscosity-modifying lubricant (Astroglide®) in aqueous solutions for use during intercourse, as are PAA (carbomer) and crosslinked PAA (polycarbophil) (Replens®) [119]. In contrast to aqueous personal lubricants, water-proof silicone personal lubricants have been developed. Silicone derivatives such as dimethicone, dimethiconol, and cyclomethicone are used in several approved products (e.g. Replens®, Astroglide®, K-Y Liquibeads®) [169]. A third class of personal lubricants includes oil-based lubricants. Mineral oil is the primary lubricating component in Johnson’s Baby Oil® [170]. Petroleum jelly or white petrolatum (Vaseline®) is another oil-based personal lubricant. In addition to lubricating tissue-tissue interfaces, personal lubricants are used to lubricate the interface between latex and vaginal mucosa. A lubricious photocured PVP-based latex coating increases latex hydro-philicity without significantly changing latex thickness or appearance and reduces latex friction [171]. The hydrophilic coating has not been evaluated for human use.
Catheter- and guidewire-induced trauma – PVP is among the most widely-utilized hydrophilic medical coating polymers [172,173], and when coated onto polyurethane stents increases lubricity, reduces bacterial adhesion, struvite, and hydroxyapatite encrustation via in vitro testing [174]. The SpeediCath® urinary catheter coated with PVP requires less withdrawal force than a non-coated catheter [175], and another demonstrates significantly improved user satisfaction in urethral as well as transanal (Navina®) applications [176]. A composite hydrogel catheter coating containing PEG and polyurethane (Aquavene®) shows similar resistance to encrustation in vitro [177]. Similarly, a PVA coating, applied to Foley catheters, exhibits markedly reduced friction coefficients in vitro compared to several conventional non-coated catheter materials [178]. Polydimethylacrylamide, grafted from poly (vinyl chloride) catheters, demonstrates a nearly twenty-fold reduction in frictional force against a silicone countersurface upon coating with polydimethylacrylamide [179]. A variety of zwitterionic polymers have been interpenetrated with or grafted to or from catheters due to their non-fouling and non-thrombogenic properties [180–182]. Linear poly (sulfobetaine methacrylate), grafted from the catheter surface (Teleflex®), reduces thrombosis in a thrombogenic canine model and decreases broad-spectrum microorganism adhesion in vitro [183]. Poly (2-methacryloyloxyethyl phosphorylcholine), coated on a polyurethane vascular catheter, reduces nano-scale friction using atomic force microscopy [184].
Osteoarthritis – For treatment of osteoarthritis, several synthetic injectable lubricant compositions have been recently investigated. HA mimetics are intended to confer lubrication via increased viscosity as well as potential boundary-lubricating effects. The linear polyanionic sodium (poly (7-oxanorbornene-2-carboxylate) protects ex vivo articular cartilage from wear while resisting enzymatic degradation by hyaluronidase and demonstrating cytocompatibility in vitro [185].
Lubricin mimetics, providing functionality similar to the boundary-lubricating glycoprotein lubricin, are reported. For example, Israelachvili et al. describe a functionalized PMMA backbone for mucin-like lubrication [186]. Another lubricin mimetic, synthesized from a chondroitin sulfate backbone forms supramacromolecular assemblies with HA [187]. A brush copolymer derived from a PAA core with grafted PEG side chains and a thiol end-group for cartilage binding adheres to ex vivo cartilage and reduces friction coefficients [188]. Degraded ex vivo cartilage treated with a brush copolymer composed of a polyglutamic acid backbone containing poly-2-methyl-2-oxazoline side chains for lubricity and benzaldehyde groups for binding to cartilage, via formation of a Schiff base, affords a low coefficient of friction similar to that observed in healthy cartilage. [189] To create a denser and more lubricious film on cartilage, the above brush polymer was modified to contain cyclic instead of linear poly-2-methyl-2-oxazoline side chains [190].
Several other classes of polymeric lubricants are not mimetic, per se, of native tissue lubricants; however, they are structurally and functionally inspired by biologically occurring hydrophilic macromolecules. Lightly crosslinked polyacrylamide networks demonstrate symptom-improving effects in goats, horses, and humans following intraarticular injection [191–194] such products are not currently approved for human use in the United States. Some evidence suggests the mechanism of polyacrylamide’s reduction of osteoarthritis symptoms involves lubrication of articular surfaces, while other evidence purports cushioning of loads and yet additional findings demonstrate integration of the polymer network into the joint synovial lining to provide proximal soft tissues with increased elasticity.
Similarly, lightly crosslinked PVP or crospovidone microgel particles afford minimal inflammatory reaction and a 30-day residence time upon injection into knee in a rabbit model [195]. Finally, silica nanoparticles grafted with the charged polymer sodium poly (3-sulfopropyl methacrylate) are recently reported to reduce friction in vitro; [196] animal studies have not to date been completed. The same polymer, grafted to poly (N-isopropylacrylamide) microgels, reduces polydimethylsiloxane-on-silicon friction via bench testing [197].
Xeroderma (dry skin) – Petroleum jelly or white petrolatum (Vaseline®) has found many medical uses over the past century since its original application for treating lacerations and burns. It is used as a moisture-preserver on the lips and hands to prevent chapping and indirectly functions as a lubricant to reduce the frictional force that wind exerts on the skin.
Chronic wounds – Petroleum jelly is further used as a lubricant to prevent chafing of skin with other contacting materials. For instance, it is used on the buttocks to prevent diaper rash or cyclist’s rash, on the nipples to prevent chafing during distance running, and on the crotch region of athletes. Petroleum jelly may also be used to protect and lubricate chronic diabetes-related and ulcerative wounds [198].
Dry/damaged hair – Various synthetic polymers are used in conditioners and shampoos to reduce frictional forces acting upon human hair fibers. Typical polymer lubricants include poly (diallyl dimethylammonium chloride) (polyquaternium-6) [152], and other linear polyelectrolytes designated in the polyquaternium family [199,200]. In fact, quaternary naturally occurring polymers including hydroxyethyl cellulose are designated as polyquaternium as well. A brush polymer having a PEG backbone with grafted poly (l-lysine) side chains adheres to human hair fibers and reduce friction in aqueous environments but not in dry environments using nanotribological assessment as determined by atomic force microscopy [201].
3.3. Reinforcing agents
An alternative approach to providing improved lubricating materials involves the strengthening or reinforcement of one or both articulating materials in order to prevent wear or fatigue (Table 6).
Table 6.
Reinforcing agents and other therapies for treating common biotribological diseases and injuries.
| Common diseases and injuries | Polymer reinforcements (3.3.1))a | Other therapies (3.3.2) |
|---|---|---|
| Xerostomia (dry mouth) | Gums/mints/candies/pastilles [213], electrostimulation [214] |
|
| Post-surgical (including peritendinous) adhesions |
Thermal preconditioning [217,218] | |
| Urinary incontinence Osteoarthritis | Biomimetic aggrecans [212] Glutaraldehyde [202], genipin [203,204], pyridinoline [206], biomimetic aggrecan [207], resurfacing with chondroitin sulfate/carboxymethylcellulose/HA and riboflavin [209], IPN [210,211] |
|
| Xeroderma (dry skin); chronic wounds |
Ultrasound, laser therapy, and electromagnetic stimulation [222] |
Section heading within manuscript.
3.3.1. Crosslinking and bulk reinforcement technologies
Osteoarthritis – Biotribological properties of cartilage have been augmented in vitro using crosslinking chemistry based on glutaraldehyde; [202] to address the relative toxicity of glutaraldehyde, an alternate crosslinking molecule, genipin, is recently described and strengthens tissue-engineered cartilage derived from an agarose scaffold [203] as well as from a cartilage-derived scaffold [204]. To the authors’ knowledge, this technique has not been applied in vivo to crosslink a patient’s tissue. Pyridinoline or hydroxylysylpyridinoline is a tri-(α-amino acid)-functional small molecule naturally occurring as a crosslinking moiety between type I collagen fibrils [205]. While it is minimally present in articular cartilage (predominantly natively type II collagen), application of pyridinoline crosslinking to tissue-engineered articular cartilage increases suture pull-out strength ex vivo and in vivo [206].
A hybrid synthetic/natural biomimetic aggrecan diffuses throughout bovine articular cartilage ex vivo; it has not to date demonstrated efficacy in improving mechanics of articular cartilage [207]. This brush polymer is also being investigated for strengthening intervertebral disc to treat degenerative disc disease; the biomimetic aggrecan resists enzymatic degradation and increases the compressive modulus of ex vivo intervertebral discs following injection into the nucleus propulsus [207,208]. Surfacial treatment of degraded ex vivo cartilage using chondroitin sulfate, carboxymethylcellulose, and HA strengthens and reduces compressive deformation during fatigue testing [209]. The reinforcing polymers covalently attached to the tissue surface via a free radical reaction using UV light and a riboflavin photoinitiator.
The friction-lowering properties of a cartilage-reinforcing biomaterial based on an interpenetrating polymer network strategy of entangling a synthetic hydrogel network within articular cartilage are recently reported [210]. The interpenetrating polymer network is synthesized in situ via diffusion of biomimetic monomers throughout cartilage tissue followed by green-light-activated photopolymerization. The interpenetrating network reduces cartilage friction coefficient under compressive equilibrium by up to 24% in the presence of IPN. The tissue-interpenetrating network also provides fatigue-resistance and significantly reduces tissue wear rate and worn volume following cyclic compression and articulation in ex vivo studies [211]. The treatment has not to date been evaluated in vivo.
Urinary incontinence – Numerous tissue fillers, approved and in development, allow for bulking of the proximal tissue surrounding the urethra for treating urinary incontinence. For example, a biomimetic, semi-synthetic/semi-natural aggrecan, synthesized from a PAA backbone with grafted chondroitin sulfate side chains, increases tissue volume and reduces stiffness when injected into ex vivo porcine urethral explants [212]. The main function of the device is as a filler, although the authors also suggest that the higher osmotic pressure of the filler will retain more water, and thus increase lubrication at the urethral tissue surface.
3.4. Other therapies
Xerostomia (dry mouth) – Gums, mints, candies, and pastilles, particularly those that are sugar-free and contain the sweetener xylitol, are often recommended for stimulation of saliva secretion [213]. An alternate method involves electrostimulation which, shown in a pilot clinical trial, increases saliva production and relieves dry mouth symptoms [214]. Similar to those used for dry mouth, lozenges or sucking candies can increase saliva production to coat and lubricate the throat and esophagus.224 However, all of these therapies are palliative, and researchers are focused on regenerative techniques for salivary glands. Thus far, several strategies are reported for the purpose of restoring salivary glands functions including: a) transplanting autologous salivary gland-derived epithelial stem/progenitor cells; [215] b) exploiting non epithelial cells and their bioactive lysates; and c) tissue engineering approaches using 3D biomaterials loaded with salivary gland cells [216].
Post-surgical adhesions – Thermal preconditioning has been shown preclinically to reduce inflammation and adhesion formation in a rabbit model of flexor tendon repair [217,218]. The preconditioning involved incubating the limb for 20 min at 41.5°C.
Chronic wounds – Hydrogel liquid bandages have been developed recently, with or without an antimicrobial agent, to prevent damage to wounds, skin or mucosal tissue resulting from applied pressure, friction and shear force [219]. Additionally, some modern dressings utilize silver ions and nanoparticles [220] for antibacterial action, while others contain activated charcoal for neutralizing the odor [221]. The role of the bandage is to provide a hydrated interface to the tissue and reduce wear. Physical therapies, such as ultrasound, laser therapy, and electrical/electromagnetic stimulation, have been clinically investigated in the treatment of pressure ulcers. While the number of clinical studies of these technologies are limited, and the data collected to date does not demonstrate overwhelming benefit provided from these treatments, they may prove beneficial pending additional robust trials and further developments [222].
4. Perspectives and future outlook
In conclusion, a variety of therapeutic strategies are clinically applied and preclinically investigated to improve outcomes for patients with suboptimal organ or tissue tribology. For a host of bodily tissues ranging in size (e.g., hair, ca. 100 μm and skin, ca. 1 m) and critical function (e.g., vision, digestion, reproduction, circulation, and ambulation), poor lubrication causes loss of structural integrity, wear, inflammation, and pain. Recently developed and developing active agents, biolubricants, polymer reinforcements, as well as non-drug/non-material approaches, effectively treat biomechanical deficiencies associated with the various diseases and injuries described herein. Biotribological principles are ubiquitous in nature, spanning wide ranges of physical length scales, levels of biological organization, and operating conditions (including, inter alia, normal stresses, shear stresses, coefficients of friction, articulation velocities, and lubricant viscosities). Significant advances in fundamental understanding of biological and biochemical processes give rise to engineering outcomes that can restore healthy levels of wear- and fatigue-resistance to diseased tissues.
For many diseases, it has proven necessary to take a multi-pronged approach and treat not only the tissue’s biological deficiency or only the biomechanical deficiency but rather both aspects congruously. One reason for these successes is the synergistic interaction of the drug and biomaterial, e.g., methylprednisolone’s anti-inflammatory effects coupled with hydroxypropyl cellulose’s lubrication, with one enabling the other to function more effectively. A need for multifaceted treatments becomes evident in diseases of biolubrication where the disease has progressed sufficiently such that tissue re-engineering is necessary. In fact, in these severe cases, a combination approach of drug and biomaterial often may not be sufficient to restore function, and a tri-pronged strategy including tissue engineering techniques such as gene/cellular hybrid therapies and natural or synthetic tissue grafting/implantation will be required for successful outcomes.
The biomaterial characteristics that are responsible for a successful clinical outcome are highly application dependent. Our approach to identifying the list of design requirements for a given practice is to discuss with the end user – the clinician, nurse, and/or surgeon – and we encourage all to do the same. Material safety is the top priority, given the caveat that a treatment for dry eye which is used topically and lasts for minutes has an in vivo contact exposure time and resulting in vivo biocompatibility response significantly different than a restorative treatment for osteoarthritic cartilage, where the biomaterial is in place for weeks to years. For instance, the defining characteristics of the polymers used for lubrication are hydrophilicity and a large molecular weight (or lightly crosslinked) to increase residence time. Natural bio-materials dominate the research field and commercial successes, likely due to known properties, availability, and prior use in regulatory approved devices. However, co-formulation with other natural materials or processing/manufacturing techniques may not provide the specific desired characteristics, and, thus, chemically modified natural materials and synthetic materials are investigated. These materials provide greater flexibility in controlling degradation rate and mechanism(s), in vivo resident time, adhesivity, osmolality, lubricity, rheological properties, modes and sites for one or more derivatizations, and the opportunity to introduce or one or more ligands for a cellular response or to attach one or more active agents (small molecule, protein, or nucleic acid) for a pharmacological effect. Finally a commercialized product does not mean, in our opinion, that all the design requirements have been satisfactorily met. Two product lines that showcase this point are viscosupplements for treating OA and barriers for prevention of peritoneal adhesions. In both cases, these biomaterial products are approved and used in the clinic – therefore a success by definition. However there is significant room for improvement as the clinical performance of these biomaterials is far from ideal with, for example, viscosupplements no longer being recommended by the society that uses them. We see such situations where the clinical need is large both in terms of patient numbers and performance enhancement as an opportunity to design and evaluate new biomaterials.
Several key areas remain clinically challenging to address. Osteoarthritis’s deterioration of articular cartilage and other synovial joint tissues remains an area of active investigation, as humans experience increasingly greater mechanical demands from their joints (athletics, work requirements) while living increasingly longer. Additionally, as surgeries are increasingly performed (due to improved surgical outcomes with minimally invasive techniques), there exists a growing demand for post-surgical adhesion therapies in sites of the body previously inaccessible with conventional surgery. Current therapies are generally single action or target only one symptom or condition of the disease or injury, and, thus, opportunities exist for the development of treatments, for example, that elicit both mechanical and biological outcomes – as discussed above. New biomaterial delivery techniques, such as targeted localization and in situ formulation, hold promise in future investigations of therapeutic biolubricants or reinforcing agents to address these emerging unmet needs. Finally, the continued advancement and clinical implementation of genetic and proteomic screening will lead to a detailed personalized understanding of the disease or injured site to guide treatment selection and subsequent management. Applied research at the interface of synthetic polymer chemistry, biomechanics, and biomedical engineering will provide biomaterial-based solutions for supplementing the human body’s biotribological sites of repeated applied stress as we live for increasing years with failure-prone tissues.
The purpose of this review is to stimulate critical discussions, highlight recent strategies, and provide further motivation for the design, development, and evaluation of active agents, biomaterials, and technologies to improve biolubrication and strengthen soft tissues in order to return them to a healthy or native-like performance state.
Acknowledgment
The authors thank Boston University, the National Science Foundation (BGC - Graduate Research Fellowship DGE-1247312) and the National Institutes of Health (AR066621) for support.
Funding sources
This material is supported in part by Boston University, the National Science Foundation (BGC - Graduate Research Fellowship DGE-1247312) and the National Institutes of Health (AR066621).
List of abbreviations
- FDA
Food and Drug Administration
- HA
Hyaluronic acid
- PAA
Poly(acrylic acid)
- PEG
Poly(ethylene glycol)
- PMMA
Polymethylmethacrylate
- PVP
Poly(vinyl pyrrolidone)
Footnotes
As defined by Merriam-Webster dictionary, tribology is a study that deals with the design, friction, wear, and lubrication of interacting surfaces in relative motion. It should be noted that the term biolubricant is alternately used in the literature to describe lubricants that derive from biological feedstock and are used for a variety of non-biological applications for the purposes of being more renewable, biodegradable, and environmentally friendly than conventional lubricants [24,25]. This class of materials is not included in the present review. Here, we use the term biolubricant to mean a macromolecule or material that reduces friction in a biological system.
Conflicts of interest
None.
References
- [1].Spikes H, The history and mechanisms of ZDDP, Tribol. Lett 17 (2004) 469–489. [Google Scholar]
- [2].Nicholls MA, Do T, Norton PR, Kasrai M, Bancroft GM, Review of the lubrication of metallic surfaces by zinc dialkyl-dithlophosphates, Tribol. Int 38 (2005) 15–39. [Google Scholar]
- [3].Dedinaite A, Biomimetic lubrication, Soft Matter 8 (2012) 273–284. [Google Scholar]
- [4].Mow VC, Ateshian GA, Spilker RL, Biomechanics of diarthrodial joints - a review of 20 years of progress, J. Biomech. Eng. ASME 115 (1993) 460–467. [DOI] [PubMed] [Google Scholar]
- [5].Klein J, Molecular mechanisms of synovial joint lubrication, Proc. Inst. Mech. Eng. Part J-Journal Eng. Tribol 220 (2006) 691–710. [Google Scholar]
- [6].Katta J, Jin Z, Ingham E, Fisher J, Biotribology of articular cartilage-A review of the recent advances, Med. Eng. Phys 30 (2008) 1349–1363. [DOI] [PubMed] [Google Scholar]
- [7].Neu CP, Komvopoulos K, Reddi AH, The interface of functional biotribology and regenerative medicine in synovial joints, Tissue Eng. B Rev 14 (2008) 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jin ZM, Stone M, Ingham E, Fisher J, Biotribology, Curr. Orthop 20 (2006) 32–40. [Google Scholar]
- [9].Yan Y, Neville A, Hesketh J, Dowson D, Real-time corrosion measurements to assess biotribocorrosion mechanisms with a hip simulator, Tribol. Int 63 (2013) 115–122. [Google Scholar]
- [10].Nosonovsky M, Bhushan B, Multiscale friction mechanisms and hierarchical surfaces in nano- and bio-tribology, Mater. Sci. Eng. R Rep 58 (2007) 62–193. [Google Scholar]
- [11].Derler S, Schrade U, Gerhardt L-C, Tribology of human skin and mechanical skin equivalents in contact with textiles, Wear 263 (2007) 1112–1116. [Google Scholar]
- [12].Derler S, Gerhardt LC, Tribology of skin: review and analysis of experimental results for the friction coefficient of human skin, Tribol. Lett 45 (2012) 1–27. [Google Scholar]
- [13].Dunn AC, Zaveri TD, Keselowsky BG, Sawyer WG, Macroscopic friction coefficient measurements on living endothelial cells, Tribol. Lett 27 (2007) 233–238. [Google Scholar]
- [14].Mann A, Tighe B, Contact lens interactions with the tear film, Exp. Eye Res 117 (2013) 88–98. [DOI] [PubMed] [Google Scholar]
- [15].Bongaerts JHH, Rossetti D, Stokes JR, The lubricating properties of human whole saliva, Tribol. Lett 27 (2007) 277–287. [Google Scholar]
- [16].Ehrenstein U, Eloy C, Skin friction on a moving wall and its implications for swimming animals, J. Fluid Mech 718 (2013) 321–346. [Google Scholar]
- [17].Mittal S, Pinky AK Mittal, Characterisation of glycoproteins in the secretory cells in the operculum of an Indian hill stream fish Garra lamta (Hamilton) (Cyprinidae, Cypriniformes), Fish Physiol. Biochem 26 (2002) 275–288. [Google Scholar]
- [18].Lillywhite HB, Licht P, Comparative study of integumentary mucous secretions in amphibians, Comp. Biochem. Physiol. A Physiol 51 (1975) 937–941. [DOI] [PubMed] [Google Scholar]
- [19].Kopas ML, A review of evidence-based practices for management of the second stage of labor, J. Midwifery Wom. Health 59 (2014) 264–276. [DOI] [PubMed] [Google Scholar]
- [20].Coles JM, Chang DP, Zauscher S, Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins, Curr. Opin. Colloid Interface Sci 15 (2010) 406–416. [Google Scholar]
- [21].Tabak LA, In defense of the oral cavity - structure, biosynthesis and function of salivary mucins, Annu. Rev. Physiol 57 (1995) 547–564. [DOI] [PubMed] [Google Scholar]
- [22].Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN, Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein, Biophys. J 92 (2007) 1693–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Veeregowda DH, van der Mei HC, de Vries J, Rutland MW, Valle-Delgado JJ, Sharma PK, Busscher HJ, Boundary lubrication by brushed salivary conditioning films and their degree of glycosylation, Clin. Oral Invest 16 (2012) 1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lai SK, Wang Y-Y, Cone R, Wirtz D, Hanes J, Altering mucus rheology to “solidify” human mucus at the nanoscale, PLoS One 4 (2009) e4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lai SK, Wang Y-Y, Hida K, Cone R, Hanes J, Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hills BA, Oligolamellar lubrication of joints by surface-active phospholipids,J. Rheumatol 16 (1989) 82–91. [PubMed] [Google Scholar]
- [27].Gaisinskaya A, Ma L, Silbert G, Sorkin R, Tairy O, Goldberg R, Kampf N, Klein J, Hydration lubrication: exploring a new paradigm, Faraday Discuss 156 (2012) 217–233. [DOI] [PubMed] [Google Scholar]
- [28].Jahn S, Klein J, Hydration lubrication: the macromolecular domain, Macromolecules 48 (2015) 5059–5075. [Google Scholar]
- [29].Ma L, Gaisinskaya-Kipnis A, Kampf N, Klein J, Origins of hydration lubrication, Nat. Commun 6 (2015) 6060. [DOI] [PubMed] [Google Scholar]
- [30].Sorkin R, Kampf N, Dror Y, Shimoni E, Klein J, Origins of extreme boundary lubrication by phosphatidylcholine liposomes, Biomaterials 34 (2013) 5465–5475. [DOI] [PubMed] [Google Scholar]
- [31].St Dennis JE, Jin K, John VT, Pesika NS, Carbon microspheres as ball bearings in aqueous-based lubrication, ACS Appl. Mater. Interfaces 3 (2011) 2215–2218. [DOI] [PubMed] [Google Scholar]
- [32].Doughty MJ, Further assessment of gender- and blink pattern-related differences in the spontaneous eyeblink activity in primary gaze in young adult humans, Optom. Vis. Sci 79 (2002) 439–447. [DOI] [PubMed] [Google Scholar]
- [33].Sforza C, Rango M, Galante D, Bresolin N, Ferrario VF, Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion, Ophthalmic Physiol. Optic 28 (2008) 345–353. [DOI] [PubMed] [Google Scholar]
- [34].Pflugfelder SC, Solomon A, Stern ME, The diagnosis and management of dry eye - a twenty-five-year review, Cornea 19 (2000) 644–649. [DOI] [PubMed] [Google Scholar]
- [35].Calonge M, The treatment of dry eye, Surv. Ophthalmol 45 (2001) S227–S239. [DOI] [PubMed] [Google Scholar]
- [36].Doughty MJ, Glavin S, Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review, Ophthalmic Physiol. Optic 29 (2009) 573–583. [DOI] [PubMed] [Google Scholar]
- [37].Veeregowda DH, Busscher HJ, Vissink A, Jager DJ, Sharma PK, van der Mei HC, Role of structure and glycosylation of adsorbed protein films in biolubrication, PLoS One 7 (2012), e42600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tsibouklis J, Middleton AM, Patel N, Pratten J, Toward mucoadhesive hydrogel formulations for the management of xerostomia: the physico-chemical, biological, and pharmacological considerations, J. Biomed. Mater. Res 101 (2013) 3327–3338. [DOI] [PubMed] [Google Scholar]
- [39].Ozdemir T, Fowler EW, Hao Y, Ravikrishnan A, Harrington DA, Witt RL, Farach-Carson MC, Pradhan-Bhatt S, Jia XQ, Biomaterials-based strategies for salivary gland tissue regeneration, Biomater. Sci 4 (2016) 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Porter SR, Scully C, Hegarty AA, An update of the etiology and management of xerostomia, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 97 (2004) 28–46. [DOI] [PubMed] [Google Scholar]
- [41].Bunker TD, Anthony PP, The pathology of frozen shoulder - a Dupuytren-like disease, J. Bone Jt. Surg. British 77 (1995) 677–683. [PubMed] [Google Scholar]
- [42].Ouaissi M, Gaujoux S, Veyrie N, Deneve E, Brigand C, Castel B, Duron JJ, Rault A, Slim K, Nocca D, Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature, J. Visc. Surg 149 (2012) E104–E114. [DOI] [PubMed] [Google Scholar]
- [43].Pados G, Venetis CA, Almaloglou K, Tarlatzis BC, Prevention of intraperitoneal adhesions in gynaecological surgery: theory and evidence, Reprod. Biomed. Online 21 (2010) 290–303. [DOI] [PubMed] [Google Scholar]
- [44].Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N, Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br. Med. Bull 90 (2009) 85–109. [DOI] [PubMed] [Google Scholar]
- [45].Ahmad G, Duffy JMN, Farquhar C, Vail A, Vandekerckhove P, Watson A, Wiseman D, Barrier agents for adhesion prevention after gynaecological surgery, Cochrane Database Syst. Rev 4 (2008), CD000475. [DOI] [PubMed] [Google Scholar]
- [46].Kumar S, Wong PF, Leaper DJ, Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after nongynaecological abdominal surgery, Cochrane Database Syst. Rev 1 (2009), CD005080. [DOI] [PubMed] [Google Scholar]
- [47].Wu W, Cheng RY, das Neves J, Tanga JC, Xiao JY, Ni Q, Liu XN, Pan GQ, Li DC, Cui WG, Sarmento B, Advances in biomaterials for preventing tissue adhesion, J. Contr. Release 261 (2017) 318–336. [DOI] [PubMed] [Google Scholar]
- [48].Hardy SB, Whitten PL, Patterning of sexual activity, in: Primate Soc, Chicago University Press, Chicago, 1987, pp. 370–384. [Google Scholar]
- [49].Norvell MK, Benrubi GI, Thompson RJ, Investigation of microtrauma after sexual intercourse, J. Reprod. Med 29 (1984) 269–271. [PubMed] [Google Scholar]
- [50].Randolph ME, Pinkerton SD, Bogart LM, Cecil H, Abramson PR, Sexual pleasure and condom use, Arch. Sex. Behav 36 (2007) 844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Higgins JA, Hoffman S, Graham CA, Sanders SA, Relationships between condoms, hormonal methods, and sexual pleasure and satisfaction: an exploratory analysis from the Women’s well-being and sexuality study, Sex. Health 5 (2008) 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fennell J, “And Isn’t that the point?”: pleasure and contraceptive decisions, Contraception 89 (2014) 264–270. [DOI] [PubMed] [Google Scholar]
- [53].Nelson HD, Menopause, Lancet 371 (2008) 760–770. [DOI] [PubMed] [Google Scholar]
- [54].Sutton KS, Boyer SC, Goldfinger C, Ezer P, Pukall CF, To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia, J. Sex. Med 9 (2012) 240–250. [DOI] [PubMed] [Google Scholar]
- [55].Herbenick D, Reece M, Hensel D, Sanders S, Jozkowski K, Fortenberry JD, Association of lubricant use with Women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study, J. Sex. Med 8 (2011) 202–212. [DOI] [PubMed] [Google Scholar]
- [56].Herbenick D, Reece M, Schick V, Sanders SA, Fortenberry JD, Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of american adults, J. Sex. Med 11 (2014) 642–652. [DOI] [PubMed] [Google Scholar]
- [57].Dezzutti CS, Brown ER, Moncla B, Russo J, Cost M, Wang L, Uranker K, Ayudhya R, Pryke K, Pickett J, LeBlanc MA, Rohan LC, Is wetter Better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity, PLoS One 7 (2012), e48328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sturdee DW, Panay N, Int menopause soc writing, g., recommendations for the management of postmenopausal vaginal atrophy, Climacteric 13 (2010) 509–522. [DOI] [PubMed] [Google Scholar]
- [59].Schumm K, Lam TBL, Types of urethral catheters for management of short-term voiding problems in hospitalised adults, Cochrane Database Syst. Rev 2 (2008), CD004013. [DOI] [PubMed] [Google Scholar]
- [60].Hedlund H, Hjelmas K, Jonsson O, Klarskov P, Talja M, Hydrophilic versus non-coated catheters for intermittent catheterization, Scand. J. Urol. Nephrol 35 (2001) 49–53. [DOI] [PubMed] [Google Scholar]
- [61].Wyman P, Hydrophilic coatings for biomedical applications in and ex vivo, in: Coatings Biomed. Appl, Woodhead Publishing Limited, Oxford, 2012, pp. 3–42. [Google Scholar]
- [62].Lorenzoni R, Ferraresi R, Manzi M, Roffi M, Guidewires for lower extremity artery angioplasty: a review, Eurointervention 11 (2015) 799–807. [DOI] [PubMed] [Google Scholar]
- [63].Lawrence EL, Turner IG, Materials for urinary catheters: a review of their history and development in the UK, Med. Eng. Phys 27 (2005) 443–453. [DOI] [PubMed] [Google Scholar]
- [64].Beiko DT, Knudsen BE, Watterson JD, Cadieux PA, Reid G, Denstedt JD, Urinary tract biomaterials J Urol. 171 (2004) 2438–2444. [DOI] [PubMed] [Google Scholar]
- [65].Mow VC, Huiskes R, Basic Orthopedic Biomechanics and Mechanobiology, third ed, Lippincott Williams & Wilkins, Philadelphia, 2005. [Google Scholar]
- [66].Swann DA, Silver FH, Slayter HS, Stafford W, Shore E, The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids, Biochem. J 225 (1985) 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sarma AV, Powell GL, LaBerge M, Phospholipid composition of articular cartilage boundary lubricant, J. Orthop. Res 19 (2001) 671–676. [DOI] [PubMed] [Google Scholar]
- [68].Mathieu P, Conrozier T, Vignon E, Rozand Y, Rinaudo M, Rheologic behavior of osteoarthritic synovial fluid after addition of hyaluronic acid: a pilot study, Clin. Orthop. Relat. Res 467 (2009) 3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Crockett R, Boundary lubrication in natural articular joints, Tribol. Lett 35 (2009) 77–84. [Google Scholar]
- [70].Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF, Osteoarthritis: new insights. Part 1: the disease and its risk factors, Ann. Intern. Med 133 (2000) 635–646. [DOI] [PubMed] [Google Scholar]
- [71].Maroudas AI, Balance between swelling pressure and collagen tension in normal and degenerate cartilage, Nature 260 (1976) 808–809. [DOI] [PubMed] [Google Scholar]
- [72].Silver FH, Siperko LM, Seehra GP, Mechanobiology of force transduction in dermal tissue, Skin Res. Technol 9 (2003) 3–23. [DOI] [PubMed] [Google Scholar]
- [73].Loden M, Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders, Am. J. Clin. Dermatol 4 (2003) 771–788. [DOI] [PubMed] [Google Scholar]
- [74].Thomas DR, Prevention and treatment of pressure ulcers, J. Am. Med. Dir. Assoc 7 (2006) 46–59. [DOI] [PubMed] [Google Scholar]
- [75].Moll R, Divo M, Langbein L, The human keratins: biology and pathology, Histochem. Cell Biol 129 (2008) 705–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wortmann FJ, Stapels M, Chandra L, Humidity-dependent bending recovery and relaxation of human hair, J. Appl. Polym. Sci 113 (2009) 3336–3344. [Google Scholar]
- [77].Stevenson W, Chauhan SK, Dana R, Dry eye disease an immune-mediated ocular surface disorder, Arch. Ophthalmol 130 (2012) 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M, Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting, Ocul. Surf 11 (2013) 246–258. [DOI] [PubMed] [Google Scholar]
- [79].Sall K, Stevenson OD, Mundorf TK, Reis BL, Cs APSG, Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease, Ophthalmology 107 (2000) 631–639. [DOI] [PubMed] [Google Scholar]
- [80].Marsh P, Pflugfelder SC, Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome, Ophthalmology 106 (1999) 811–816. [DOI] [PubMed] [Google Scholar]
- [81].Sullivan DA, Wickham LA, Rocha EM, Krenzer KL, Sullivan BD, Steagall R, Cermak JM, Dana MR, Ullman MD, Sato EH, Gao JP, Rocha FJ, Ono M, Silveira LA, Lambert RW, Kelleher RS, Tolls DB, Toda I, Androgens and dry eye in Sjogren’s syndrome, Neuroendocr. Immune Basis Rheum. Dis 876 (1999) 312–324. [DOI] [PubMed] [Google Scholar]
- [82].Li JC, Ha YM, Ku NY, Choi SY, Lee SJ, Oh SB, Kim JS, Lee JH, Lee EB, Song YW, Park K, Inhibitory effects of autoantibodies on the muscarinic receptors in Sjogren’s syndrome, Lab. Invest 84 (2004) 1430–1438. [DOI] [PubMed] [Google Scholar]
- [83].Sondheimer JM, Gervaise EP, Lubricant versus laxative in the treatment of chronic functional constipation of children - a comparative study, J. Pediatr. Gastroenterol. Nutr 1 (1982) 223–226. [DOI] [PubMed] [Google Scholar]
- [84].Jauch R, Hankwitz R, Beschke K, Pelzer H, Bis-(para-hydroxyphenyl)-pyridyl-2-methane - common laxative principle of bisacodyl and sodium picosulfate, Arzneimittel-Forschung/Drug Res. 25 (1975) 1796–1800. [PubMed] [Google Scholar]
- [85].Tunaru S, Althoff TF, Nusing RM, Diener M, Offermanns S, Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 9179–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liu S, Hu CM, Li FF, Li XJ, Cui WG, Fan CY, Prevention of peritendinous adhesions with electrospun ibuprofen-loaded poly(l-lactic acid)-polyethylene glycol fibrous membranes, Tissue Eng. 19 (2013) 529–537. [DOI] [PubMed] [Google Scholar]
- [87].Lee JH, Go AK, Oh SH, Lee KE, Yuk SH, Tissue anti-adhesion potential of ibuprofen-loaded PLLA-PEG diblock copolymer films, Biomaterials 26 (2005) 671–678. [DOI] [PubMed] [Google Scholar]
- [88].Zhang YD, Yao W, Wu CX, Chi QM, Zhang JY, Li M, Topical application of halcinonide cream reduces the severity and incidence of intraperitoneal adhesions in a rat model, Am. J. Surg 184 (2002) 74–77. [DOI] [PubMed] [Google Scholar]
- [89].Diamond MP, Linsky CB, Cunningham T, Kamp L, Pines E, Decherney AH, Dizerega GS, Adhesion reformation - reduction by the use of Interceed (TC7) plus heparin, J. Gynecol. Surg 7 (1991) 1–6. [DOI] [PubMed] [Google Scholar]
- [90].Suckling J, Lethaby A, Kennedy R, Local oestrogen for vaginal atrophy in postmenopausal women, Cochrane Database Syst. Rev 8 (2006), CD001500. [DOI] [PubMed] [Google Scholar]
- [91].Johnson MS, Klein JD, Loveland-Cherry C, Moyer VA, Ockene JK, Petitti DB, Siu AL, Teutsch SM, Yawn BP, Force USPST, Hormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the US preventive services task force, Ann. Intern. Med 142 (2005) 855–860. [PubMed] [Google Scholar]
- [92].Fogelman I, Oestrogen, the prevention of bone loss and osteoporosis, Br. J. Rheumatol 30 (1991) 276–281. [DOI] [PubMed] [Google Scholar]
- [93].Hammar M, Christau S, Nathorst-Boos J, Rud T, Garre K, A double-blind, randomised trial comparing the effects of tibolone and continuous combined hormone replacement therapy in postmenopausal women with menopausal symptoms, Br. J. Obstet. Gynaecol 105 (1998) 904–911. [DOI] [PubMed] [Google Scholar]
- [94].Gregory PJ, Sperry M, Wilson AF, Dietary supplements for osteoarthritis, Am. Fam. Physician 77 (2008) 177–184. [PubMed] [Google Scholar]
- [95].Kim LS, Axelrod LJ, Howard P, Buratovich N, Waters RF, Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial, Osteoarthr. Cartil 14 (2006) 286–294. [DOI] [PubMed] [Google Scholar]
- [96].Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, Bradley JD, Bingham CO, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR, Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ, Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis, N. Engl. J. Med 354 (2006) 795–808. [DOI] [PubMed] [Google Scholar]
- [97].Frisbie DD, McIlwraith CW, Kawcak CE, Werpy NM, Evaluation of intraarticular hyaluronan, sodium chondroitin sulfate and N-acetyl-D-glucosamine combination versus saline (0.9% NaCl) for osteoarthritis using an equine model, Vet. J 197 (2013) 824–829. [DOI] [PubMed] [Google Scholar]
- [98].Ameye LG, Chee WS, Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence, Arthritis Res. Ther 8 (2006) R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Fajardo M, Di Cesare PE, Disease-modifying therapies for osteoarthritis -current status, Drugs Aging 22 (2005) 141–161. [DOI] [PubMed] [Google Scholar]
- [100].Wang C-T, Lin Y-T, Chiang B-L, Lin Y-H, Hou S-M, High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis, Osteoarthr. Cartil 14 (2006) 1237–1247. [DOI] [PubMed] [Google Scholar]
- [101].Altman RD, Vinod D, Jun T, Review of the mechanism of action for supartz fx in knee osteoarthritis, Cartilage2 9 (2018) 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, Shimmura S, Treatment of dry eye by autologous serum application in Sjogren’s syndrome, Br. J. Ophthalmol 83 (1999) 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ship JA, Fox PC, Michalek JE, Cummins MJ, Richards AB, Grp IFNPS, Treatment of primary Sjogren’s syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: a phase II clinical trial, J. Interf. Cytokine Res 19 (1999) 943–951. [DOI] [PubMed] [Google Scholar]
- [104].Nguyen CQ, Yin HE, Lee BH, Chiorini JA, Peck AB, IL17: potential therapeutic target in Sjogren’s syndrome using adenovirus-mediated gene transfer, Lab. Invest 91 (2011) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ruan MZC, Erez A, Guse K, Dawson B, Bertin T, Chen Y, Jiang M-M, Yustein J, Gannon F, Lee BHL, Proteoglycan 4 expression protects against the development of osteoarthritis, Sci. Transl. Med 5 (2013) 176ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yokoi N, Komuro A, Nishida K, Kinoshita S, Effectiveness of hyaluronan on corneal epithelial barrier function in dry eye, Br. J. Ophthalmol 81 (1997) 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Singh A, Corvelli M, Unterman SA, Wepasnick KA, McDonnell P, Elisseeff JH, Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid, Nat. Mater 13 (2014) 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Singh A, Li P, Beachley V, McDonnell P, Elisseeff JH, A hyaluronic acid-binding contact lens with enhanced water retention, Contact Lens Anterior Eye 38 (2015) 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nguyen T, Latkany R, Review of hydroxypropyl cellulose ophthalmic inserts for treatment of dry eye, Clin. Ophthalmol 5 (2011) 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Winkeljann B, Boettcher K, Balzer BN, Lieleg O, Mucin coatings prevent tissue damage at the cornea-contact lens interface, Adv. Mater. Interf 4 (2017). [Google Scholar]
- [111].Momm F, Guttenberger R, Treatment of xerostomia following radiotherapy: does age matter? Support. Care Canc 10 (2002) 505–508. [DOI] [PubMed] [Google Scholar]
- [112].Epstein JB, Emerton S, Le ND, Stevenson-Moore P, A double-blind crossover trial of Oral Balance gel and Biotene (R) toothpaste versus placebo in patients with xerostomia following radiation therapy, Oral Oncol. 35 (1999) 132–137. [DOI] [PubMed] [Google Scholar]
- [113].Marlett JA, Kajs TM, Fischer MH, An unfermented gel component of psyllium seed husk promotes laxation as a lubricant in humans, Am. J. Clin. Nutr 72 (2000) 784–789. [DOI] [PubMed] [Google Scholar]
- [114].Brown CB, Luciano AA, Martin D, Peers E, Scrimgeour A, Dizerega GS, Grp A, Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study, Fertil. Steril 88 (2007) 1413–1426. [DOI] [PubMed] [Google Scholar]
- [115].Diamond MP, Bieber E, Coddington C, Franklin R, Grunert G, Gunn D, Lotze E, Rowe G, Grainger D, Tjaden B, Holtz G, Patton G, Johns DA, Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study, Fertil. Steril 66 (1996) 904–910. [PubMed] [Google Scholar]
- [116].Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG, Corman M, Beart RW, Wexner SD, Becker JM, Monson JRT, Kaufman HS, Beck DE, Bailey HR, Ludwig KA, Stamos MJ, Darzi A,Bleday R, Dorazio R, Madoff RD, Smith LE, Gearhart S, Lillemoe K, Gohl J, Reduction in adhesive small-bowel obstruction by Seprafilm (R) stop adhesion barrier after intestinal resection, Dis. Colon Rectum 49 (2006) 1–11. [DOI] [PubMed] [Google Scholar]
- [117].Vrijland WW, Tseng LNL, Eijkman HJM, Hop WC, Jakimowicz JJ, Leguit P, Stassen LPS, Swank DJ, Haverlag R, Bonjer J, Jeekel H, Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane - a randomized clinical trial, Ann. Surg 235 (2002) 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sekiba K, Use of Interceed (TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery, Obstet. Gynecol 79 (1992) 518–522. [PubMed] [Google Scholar]
- [119].Caswell M, Kane M, Comparison of the moisturization efficacy of two vaginal moisturizers: Pectin versus polycarbophil technologies, J. Cosmet. Sci 53 (2002) 81–87. [PubMed] [Google Scholar]
- [120].Niemczyk A, El Fray M, Franklin SE, Friction behaviour of hydrophilic lubricious coatings for medical device applications, Tribol. Int 89 (2015) 54–61. [Google Scholar]
- [121].Balazs EA, Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results, Surg. Technol. Int 12 (2004) 278–289. [PubMed] [Google Scholar]
- [122].Hunter DJ, Viscosupplementation for osteoarthritis of the knee, N. Engl. J. Med 372 (2015) 1040–1047. [DOI] [PubMed] [Google Scholar]
- [123].Larsen NE, Dursema HD, Pollak CT, Skrabut EM, Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models, J. Biomed. Mater. Res. B Appl. Biomater 100B (2012) 457–462. [DOI] [PubMed] [Google Scholar]
- [124].Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH, Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements, Tribol. Int 31 (1998) 17–33. [Google Scholar]
- [125].Mattei L, Di Puccio F, Piccigallo B, Ciulli E, Lubrication and wear modelling of artificial hip joints: a review, Tribol. Int 44 (2011) 532–549. [Google Scholar]
- [126].Liang H, Shi B, Fairchild A, Cale T, Applications of plasma coatings in artificial joints: an overview, Vacuum 73 (2004) 317–326. [Google Scholar]
- [127].Jevsevar DS, Treatment of osteoarthritis of the knee: evidence-based guideline, second ed. J. Am. Acad. Orthop. Surg 21 (2013) 571–576. [DOI] [PubMed] [Google Scholar]
- [128].Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P, American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee, Arthritis Care Res. 64 (2012) 465–474. [DOI] [PubMed] [Google Scholar]
- [129].Nelson FR, Zvirbulis RA, Zonca B, Li KW, Turner SM, Pasierb M, Wilton P, Martinez-Puig D, Wu W, The effects of an oral preparation containing hyaluronic acid (Oralvisc(A (R))) on obese knee osteoarthritis patients determined by pain, function, bradykinin, leptin, inflammatory cytokines, and heavy water analyses, Rheumatol. Int 35 (2015) 43–52. [DOI] [PubMed] [Google Scholar]
- [130].Su H, Wang X, Du M, Song Y, Zheng Q, Boundary lubricating properties of hydrophobically modified polyacrylamide, RSC Adv. 6 (2016) 5695–5702. [Google Scholar]
- [131].Smith MM, Russell AK, Schiavinato A, Little CB, A hexadecylamide derivative of hyaluronan (HYMOVIS (R)) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan, J. Inflamm. London 10 (2013) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Galesso D, Finelli I, Paradossi G, Renier D, Viscoelastic properties and elastic recovery of HYADD(R) 4 hydrogel compared to crosslinked HA-based commercial viscosupplements, Osteoarthr. Cartil 20 (2012) S292. [Google Scholar]
- [133].Benazzo F, Perticarini L, Padolino A, Castelli A, Gifuni P, Lovato M, Manzini C, Giordan N, A multi-centre, open label, long-term follow-up study to evaluate the benefits of a new viscoelastic hydrogel (Hymovis®) in the treatment of knee osteoarthritis, Eur. Rev. Med. Pharmacol. Sci 20 (2016) 959–968. [PubMed] [Google Scholar]
- [134].Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, Blanchet T, Gleghorn JP, Bonassar LJ, Bendele AM, Morris EA, Glasson SS, Prevention of cartilage degeneration in a rat model of osteo-arthritis by intraarticular treatment with recombinant lubricin, Arthritis Rheum. 60 (2009) 840–847. [DOI] [PubMed] [Google Scholar]
- [135].Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, Teeple E, Waller KA, Elsaid KA, Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection, Arthritis Rheum. 62 (2010) 2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Waller KA, Chin KE, Jay GD, Zhang LX, Teeple E, McAllister S, Badger GJ, Schmidt TA, Fleming BC, Intra-articular recombinant human proteoglycan 4 mitigates cartilage damage after destabilization of the medial meniscus in the yucatan minipig, Am. J. Sports Med 45 (2017) 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sivan S, Schroeder A, Verberne G, Merkher Y, Diminsky D, Priev A, Maroudas A, Halperin G, Nitzan D, Etsion I, Barenholz Y, Liposomes act as effective biolubricants for friction reduction in human synovial joints, Langmuir 26 (2010) 1107–1116. [DOI] [PubMed] [Google Scholar]
- [138].Verberne G, Schroeder A, Halperin G, Barenholz Y, Etsion I, Liposomes as potential biolubricant additives for wear reduction in human synovial joints, Wear 268 (2010) 1037–1042. [DOI] [PubMed] [Google Scholar]
- [139].Conaghan PG, Bijlsma JW, Kneer W, Wise E, Kvien TK, Rother M, Drug-free gel containing ultra-deformable phospholipid vesicles (TDT 064) as topical therapy for the treatment of pain associated with osteoarthritis: a review of clinical efficacy and safety, Curr. Med. Res. Opin 30 (2014) 599–611. [DOI] [PubMed] [Google Scholar]
- [140].Kawano T, Miura H, Mawatari T, Moro-Oka T, Nakanishi Y, Higaki H,Iwamoto Y, Mechanical effects of the intraarticular administration of high molecular weight hyaluronic acid plus phospholipid on synovial joint lubrication and prevention of articular cartilage degeneration in experimental osteoarthritis, Arthritis Rheum. 48 (2003) 1923–1929. [DOI] [PubMed] [Google Scholar]
- [141].Henrotin Y, Oprenyeszk F, Comblain F, Dubuc JE, Boileau C, Chausson M, Lecler R, Rocasalbas G, Douette P, Gautier S, Novel chitosan hydrogels for the treatment of osteoarthritis: mechanical support, lubrication and prevention of cartilage degradation in a rabbit model of osteoarthritis, Arthritis Rheum. 67 (2015) 1150. [Google Scholar]
- [142].Kaderli S, Viguier E, Watrelot-Virieux D, Roger T, Gurny R, Scapozza L,Moller M, Boulocher C, Jordan O, Efficacy study of two novel hyaluronic acid-based formulations for viscosupplementation therapy in an early osteoarthrosic rabbit model, Eur. J. Pharm. Biopharm 96 (2015) 388–395. [DOI] [PubMed] [Google Scholar]
- [143].Kaderli S, Boulocher C, Pillet E, Watrelot-Virieux D, Rougemont AL, Roger T, Viguier E, Gurny R, Scapozza L, Jordan O, A novel biocompatible hyaluronic acid-chitosan hybrid hydrogel for osteoarthrosis therapy, Int. J. Pharm 483 (2015) 158–168. [DOI] [PubMed] [Google Scholar]
- [144].Patchornik S, Ram E, Ben Shalom N, Nevo Z, Robinson D, Chitosan-hyaluronate hybrid gel intraarticular injection delays osteoarthritis progression and reduces pain in a rat meniscectomy model as compared to saline and hyaluronate treatment, Adv. Orthop 2012 (2012), 979152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Oprenyeszk F, Chausson M, Maquet V, Dubuc JE, Henrotin Y, Protective effect of a new biomaterial against the development of experimental osteoarthritis lesions in rabbit: a pilot study evaluating the intra-articular injection of alginate-chitosan beads dispersed in an hydrogel, Osteoarthr. Cartil 21 (2013) 1099–1107. [DOI] [PubMed] [Google Scholar]
- [146].Ogunleye A, Bhat A, Irorere VU, Hill D, Williams C, Radecka I, Poly-gamma-glutamic acid: production, properties and applications, Microbiology-Sgm 161 (2015) 1–17. [DOI] [PubMed] [Google Scholar]
- [147].Prescott A, Stock LR, Shen V, Poly-gamma-glutamic acid as a novel material for viscosupplementation and treating OA of the knee: a pilot study, in: 3rd Int. Conf. Exhib. Orthop. & Rheumatol. Omi. Gr. Conf. San Fr. CA vol. 3, 2014,p. 36. [Google Scholar]
- [148].Shellhammer TH, Rumsey TR, Krochta JM, Viscoelastic properties of edible lipids, J. Food Eng 33 (1997) 305–320. [Google Scholar]
- [149].Lalau JD, Bresson R, Charpentier P, Coliche V, Erlher S, Van GH, Magalon G, Martini J, Moreau Y, Pradines S, Rigal F, Wemeau JL, Richard JL, Efficacy and tolerance of calcium alginate versus vaseline gauze dressings in the treatment of diabetic foot lesions, Diabetes Metab. 28 (2002) 223–229. [PubMed] [Google Scholar]
- [150].Huang GB, Sun TQ, Zhang L, Wu QH, Zhang KY, Tian QF, Huo R, Combined application of alginate dressing and human granulocyte-macrophage colony stimulating factor promotes healing in refractory chronic skin ulcers, Exp. Ther. Med 7 (2014) 1772–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kumar V, Banker GS, Chemically-modified cellulosic polymers, Drug Dev. Ind. Pharm 19 (1993) 1–31. [Google Scholar]
- [152].Guzman E, Ortega F, Baghdadli N, Luengo GS, Rubio RG, Effect of the molecular structure on the adsorption of conditioning polyelectrolytes on solid substrates, Colloid. Surface. Physicochem. Eng. Aspect 375 (2011) 209–218. [Google Scholar]
- [153].McDonald CC, Kaye SB, Figueiredo FC, Macintosh G, Lockett C, A randomised, crossover, multicentre study to compare the performance of0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome, Eye 16 (2002) 601–607. [DOI] [PubMed] [Google Scholar]
- [154].Sullivan LJ, McCurrach F, Lee S, Taylor HR, Rolando M,MarechalCourtois C, CreuzotGarcher C, Karabatsas C, Hoh MB, Faschinger C, Laroche L, Efficacy and safety of 0.3% carbomer gel compared to placebo in patients with moderate-to-severe dry eye syndrome, Ophthalmology 104 (1997) 1402–1408. [DOI] [PubMed] [Google Scholar]
- [155].Tung CI, Kottaiyan R, Koh S, Wang Q, Yoon G, Zavislan JM, Davio SR, Aquavella JV, Noninvasive, objective, multimodal tear dynamics evaluation of 5 over-the-counter tear drops in a randomized controlled trial, Cornea 31 (2012) 108–114. [DOI] [PubMed] [Google Scholar]
- [156].Wegener AR, Meyer LM, Schonfeld CL, Effect of viscous agents on corneal density in dry eye disease, J. Ocul. Pharmacol. Therapeut 31 (2015) 504–508. [DOI] [PubMed] [Google Scholar]
- [157].Borowitz SM, Cox DJ, Kovatchev B, Ritterband LM, Sheen J, Sutphen J, Treatment of childhood constipation by primary care physicians: efficacy and predictors of outcome, Pediatrics 115 (2005) 873–877. [DOI] [PubMed] [Google Scholar]
- [158].Candy B, Jones L, Goodman ML, Drake R, Tookman A, Laxatives or methylnaltrexone for the management of constipation in palliative care patients, Cochrane Database Syst. Rev (2011), CD003448. [DOI] [PubMed] [Google Scholar]
- [159].DiPalma JA, DeRidder PH, Orlando RC, Kolts BE, Cleveland MV, A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative, Am. J. Gastroenterol 95 (2000) 446–450. [DOI] [PubMed] [Google Scholar]
- [160].Haney AF, Hesla J, Hurst BS, Kettel LM, Murphy AA, Rock JA, Rowe G, Schlaff WD, Expanded polytetrafluoroethylene (GORE-TEX surgical membrane) is superior to oxidized regenerated cellulose (INTERCEED TC7+) in preventing adhesions, Fertil. Steril 63 (1995) 1021–1026. [PubMed] [Google Scholar]
- [161].Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR, A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients undergoing myomectomy, Fertil. Steril 82 (2004) 398–404. [DOI] [PubMed] [Google Scholar]
- [162].Banasiewicz T, Horbacka K, Karon J, Malinger S, Antos F, Rudzki S, Kala Z, Stojcev Z, Kossi J, Krokowicz P, Preliminary study with SprayShield (TM) adhesion barrier system in the prevention of abdominal adhesions, Video-surgery Other Miniinvasive Tech 8 (2013) 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Tchartchian G, Hackethal A, Herrmann A, Bojahr B, Wallwiener C, Ohlinger R, Ebert AD, De Wilde RL, Evaluation of SprayShield (TM) Adhesion Barrier in a single center: randomized controlled study in 15 women undergoing reconstructive surgery after laparoscopic myomectomy, Arch. Gynecol. Obstet 290 (2014) 697–704. [DOI] [PubMed] [Google Scholar]
- [164].Sardo AD, Spinelli M, Bramante S, Scognamiglio M, Greco E, Guida M, Cela V, Nappi C, Efficacy of a polyethylene oxide-sodium carboxymethyl-cellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery, J. Minim. Invasive Gynecol 18 (2011) 462–469. [DOI] [PubMed] [Google Scholar]
- [165].Izumi Y, Takahashi Y, Kohno M, Nomori H, Cross-Linked poly(gamma-glutamic acid) attenuates pleural and chest wall adhesions in a mouse thoracotomy model, Eur. Surg. Res 48 (2012) 93–98. [DOI] [PubMed] [Google Scholar]
- [166].Ishiyama N, Moro T, Ohe T, Miura T, Ishihara K, Konno T, Ohyama T, Kimura M, Kyomoto M, Saito T, Nakamura K, Kawaguchi H, Reduction of peritendinous adhesions by hydrogel containing biocompatible phospholipid polymer MPC for tendon repair, J. Bone Jt. Surg. Am 93A (2011) 142–149. [DOI] [PubMed] [Google Scholar]
- [167].Ishiyama N, Moro T, Ishihara K, Ohe T, Miura T, Konno T, Ohyama T, Kimura M, Kyomoto M, Nakamura K, Kawaguchi H, The prevention of peritendinous adhesions by a phospholipid polymer hydrogel formed in situ by spontaneous intermolecular interactions, Biomaterials 31 (2010) 4009–4016. [DOI] [PubMed] [Google Scholar]
- [168].Liu S, Hu CM, Li FF, Li XJ, Cui WG, Fan CY, Prevention of peritendinous adhesions with electrospun ibuprofen-loaded poly(l-lactic acid)-polyethylene glycol fibrous membranes, Tissue Eng. 19 (2013) 529–537. [DOI] [PubMed] [Google Scholar]
- [169].O’Lenick AJ, Silicones - basic chemistry and selected applications, J. Surfactants Deterg. 3 (2000) 229–236. [Google Scholar]
- [170].Steiner M, Piedrahita C, Glover L, Joanis C, Spruyt A, Foldesy R, The impacts of lubricants on latex condoms during vaginal intercourse, Int. J. STD AIDS 5 (1994) 29–36. [DOI] [PubMed] [Google Scholar]
- [171].Chin SL, Xiao RQ, Cooper BG, Varongchayakul N, Buch K, Kim D, Grinstaff MW, Macromolecular photoinitiators enhance the hydrophilicity and lubricity of natural rubber, J. Appl. Polym. Sci 133 (2016) 43930. [Google Scholar]
- [172].Nagaoka S, Akashi R, Low-friction hydrophilic surface for medical devices, Biomaterials 11 (1990) 419–424. [DOI] [PubMed] [Google Scholar]
- [173].Lowe S, O’Brien-Simpson NM, Connal LA, Antibiofouling polymer interfaces: poly(ethylene glycol) and other promising candidates, Polym. Chem 6 (2015) 198–212. [Google Scholar]
- [174].Tunney MM, Gorman SP, Evaluation of a poly(vinyl pyrollidone)-coated biomaterial for urological use, Biomaterials 23 (2002) 4601–4608. [DOI] [PubMed] [Google Scholar]
- [175].Stensballe J, Looms D, Nielsen PN, Tvede V, Hydrophilic-coated catheters for intermittent catheterisation reduce urethral microtrauma: a prospective, randomised, participant-blinded, crossover study of three different types of catheters, Eur. Urol 48 (2005) 978–983. [DOI] [PubMed] [Google Scholar]
- [176].Vapnek JM, Maynard FM, Kim J, A prospective randomized trial of the lofric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization, J. Urol 169 (2003) 994–998. [DOI] [PubMed] [Google Scholar]
- [177].Gorman SP, Tunney MM, Keane PF, Van Bladel K, Bley B, Characterization and assessment of a novel poly(ethylene oxide)/polyurethane composite hydrogel (Aquavene (R)) as a ureteral stent biomaterial, J. Biomed. Mater. Res 39 (1998) 642–649. [DOI] [PubMed] [Google Scholar]
- [178].Graiver D, Durall RL, Okada T, Surface-morphology and friction coefficient of various types of Foley catheter, Biomaterials 14 (1993) 465–469. [DOI] [PubMed] [Google Scholar]
- [179].Uyama Y, Tadokoro H, Ikada Y, Low-frictional catheter materials by photoinduced graft-polymerization, Biomaterials 12 (1991) 71–75. [DOI] [PubMed] [Google Scholar]
- [180].Goda T, Matsuno R, Konno T, Takai M, Ishihara K, Protein adsorption resistance and oxygen permeability of chemically crosslinked phospholipid polymer hydrogel for ophthalmologic biomaterials, J. Biomed. Mater. Res. B Appl. Biomater 89B (2009) 184–190. [DOI] [PubMed] [Google Scholar]
- [181].Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N, Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res 39 (1998) 323–330. [DOI] [PubMed] [Google Scholar]
- [182].Ishihara K, Aragaki R, Ueda T, Watenabe A, Nakabayashi N, Reduced thrombogenicity of polymers having phospholipid polar groups, J. Biomed. Mater. Res 24 (1990) 1069–1077. [DOI] [PubMed] [Google Scholar]
- [183].Smith RS, Zhang Z, Bouchard M, Li J, Lapp HS, Brotske GR, Lucchino DL, Weaver D, Roth LA, Coury A, Biggerstaff J, Sukavaneshvar S, Langer R,Loose C, Vascular catheters with a nonleaching poly-sulfobetaine surface modification reduce thrombus formation and microbial attachment, Sci. Transl. Med 4 (2012), 153ra132. [DOI] [PubMed] [Google Scholar]
- [184].Ho SP, Nakabayashi N, Iwasaki Y, Boland T, LaBerge M, Frictional properties of poly(MPC-co-BMA) phospholipid polymer for catheter applications, Biomaterials 24 (2003) 5121–5129. [DOI] [PubMed] [Google Scholar]
- [185].Wathier M, Lakin BA, Bansal PN, Stoddart SS, Snyder BD, Grinstaff MW, A large-molecular-weight polyanion, synthesized via ring-opening metathesis polymerization, as a lubricant for human articular cartilage, J. Am. Chem. Soc 135 (2013) 4930–4933. [DOI] [PubMed] [Google Scholar]
- [186].Banquy X, Burdynska J, Lee DW, Matyjaszewski K, Israelachvili J, Bio-inspired bottle-brush polymer exhibits low friction and amontons-like behavior, J. Am. Chem. Soc 136 (2014) 6199–6202. [DOI] [PubMed] [Google Scholar]
- [187].Lawrence A, Xu X, Bible MD, Calve S, Neu CP, Panitch A, Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface, Biomaterials 73 (2015) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Samaroo KJ, Tan M, Putnam D, Bonassar LJ, Binding and lubrication of biomimetic boundary lubricants on articular cartilage, J. Orthop. Res 35 (2017) 548–557. [DOI] [PubMed] [Google Scholar]
- [189].Morgese G, Cavalli E, Müller M, Zenobi-Wong M, Benetti EM, Nano-assemblies of tissue-reactive,polyoxazoline graft-copolymers restore thelubrication properties of degraded cartilage, ACS Nano 11 (2017) 2794–2804. [DOI] [PubMed] [Google Scholar]
- [190].Morgese G, Cavalli E, Rosenboom J-G, Zenobi-Wong M, Benetti EM, Cyclic polymer grafts that lubricate and protect damaged cartilage, Angew. Chem. Int. Ed 57 (2018) 1621–1626. [DOI] [PubMed] [Google Scholar]
- [191].Zar VV, Zargorodniy NV, Martinov DV, Effectiveness and safety of injectable endoprosthetics of synovial fluid by cross-linked polymer noltrex for treatment OA knee, Eur. J. Musculoskelet. Dis 1 (2012) 23–32. [Google Scholar]
- [192].Tnibar A, Schougaard H, Camitz L, Rasmussen J, Koene M, Jahn W, Markussen B, An international multi-centre prospective study on the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow-up, Acta Vet. Scand 57 (2015) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Tnibar A, Persson AB, Nielsen H, Svalastoga E, Westrup U, McEvoy F, Knudsen J, Thomsen PD, Berg LC, Jacobsen S, Christensen LH, Evaluation of a polyacrylamide hydrogel in the treatment of induced osteoarthritis in a goat model: a randomized controlled pilot study, Osteoarthr. Cartil 22 (2014). S477–S477. [Google Scholar]
- [194].Christensen LH, Camitz L, Illigen KE, Hansen M, Sarvaa R, Conaghan PG, The effects of polyacrylamide hydrogel in normal and osteoarthritic animal joints, Osteoarthr. Cartil 24 (2016) S449–S450. [DOI] [PubMed] [Google Scholar]
- [195].Pielka S, Zywicka B, Rosiak J, Henke A, Ulanski P, Paluch D, Szymonowicz M, Solski L, Staniszewska-Kus J, Tissue reaction after injection of polyvinylpyrrolidone (PVP) preparation into knee joint, Exp. Polim. Med 34 (2004) 3–8. [PubMed] [Google Scholar]
- [196].Liu GQ, Cai MR, Zhou F, Liu WM, Charged polymer brushes-grafted hollow silica nanoparticles as a novel promising material for simultaneous joint lubrication and treatment, J. Phys. Chem. B 118 (2014) 4920–4931. [DOI] [PubMed] [Google Scholar]
- [197].Liu GQ, Liu ZL, Li N, Wang XL, Zhou F, Liu WM, Hairy polyelectrolyte brushes-grafted thermosensitive microgels as artificial synovial fluid for simultaneous biomimetic lubrication and arthritis treatment, ACS Appl. Mater. Interfaces 6 (2014) 20452–20463. [DOI] [PubMed] [Google Scholar]
- [198].Tormo Maicas V, Rochina IJ, Martinez Martinez C, Marin Fontana MJ, Sauvadet A, Bohbot S, Hydrocolloids and lubricating materials in the treatment of acute and chronic wounds, Rev. Enferm 26 (2003) 15–20. [PubMed] [Google Scholar]
- [199].Wu WJ, Alkema J, Shay GD, Basset DR, Quantitative methods for evaluating optical and frictional properties of cationic polymers, J. Cosmet. Sci 52 (2001) 51–65. [PubMed] [Google Scholar]
- [200].Obermeier B, Langguth P, Frey H, Partially quarternized amino functional poly(methacrylate) terpolymers: versatile drug permeability modifiers, Biomacromolecules 12 (2011) 425–431. [DOI] [PubMed] [Google Scholar]
- [201].Lee S, Zurcher S, Dorcier A, Luengo GS, Spencer ND, Adsorption and lubricating properties of poly(l-lysine)-graft-poly(ethylene glycol) on human-hair surfaces, ACS Appl. Mater. Interfaces 1 (2009) 1938–1945. [DOI] [PubMed] [Google Scholar]
- [202].Oungoulian SR, Hehir KE, Zhu KC, Willis CE, Marinescu AG, Merali N, Ahmad CS, Hung CT, Ateshian GA, Effect of glutaraldehyde fixation on the frictional response of immature bovine articular cartilage explants, J. Biomech 47 (2014) 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lima EG, Tan AR, Tai T, Marra KG, DeFail A, Ateshian GA, Hung CT, Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement, J. Biomed. Mater. Res 91A (2009) 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Cheng NC, Estes BT, Young TH, Guilak F, Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondro-genesis, Tissue Eng. 19 (2013) 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Knott L, Bailey AJ, Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance, Bone 22 (1998) 181–187. [DOI] [PubMed] [Google Scholar]
- [206].DuRaine GD, Arzi B, Lee JK, Lee CA, Responte DJ, Hu JC, Athanasiou KA, Biomechanical evaluation of suture-holding properties of native and tissue-engineered articular cartilage, Biomechanics Model. Mechanobiol 14 (2015) 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Phillips E, Lefchak M, Mulcahey M, Prudnikova K, Marcolongo MI, Cartilage molecular engineering using biomimetic aggrecan shows infiltration and distribution of biomimetic aggrecan throughout the cartilage extracellular matrix, in: 10th World Biomater. Congr. Montr. Canada, 2016, 02684. [Google Scholar]
- [208].Madende D, Prudnikova K, Lightfoot S, Vresilovic E, Marcolongo M, New biomimetic aggrecan for treatment of intervertebral disc degeneration, in: 2012 38th Annu. Northeast Bioeng. Conf, 2012, pp. 213–214. [Google Scholar]
- [209].Grenier S, Donnelly PE, Gittens J, Torzilli PA, Resurfacing damaged articular cartilage to restore compressive properties, J. Biomech 48 (2015) 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Cooper BG, Lawson TB, Snyder BD, Grinstaff MW, Reinforcement of articular cartilage with a tissue-interpenetrating polymer network reduces friction and modulates interstitial fluid load support, Osteoarthr. Cartil 25 (2017) 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Cooper BG, Stewart RC, Burstein D, Snyder BD, Grinstaff MW, A tissue-penetrating double network restores the mechanical properties of degenerated articular cartilage, Angew. Chem. Int. Ed 55 (2016) 4226–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kriete A, Parellada J, Prudnikova K, Marcolongo M, Novel biomimetic aggrecan for treatment of urinary incontinence, 2014. 40th Annu. Northeast Bioeng. Conf. (n.d.). [Google Scholar]
- [213].Hooper P, Tincello DG, Richmond DH, The use of salivary stimulant pastilles to improve compliance in women taking oxybutynin hydrochloride for detrusor instability: a pilot study, Br. J. Urol 80 (1997) 414–416. [DOI] [PubMed] [Google Scholar]
- [214].Talal N, Quinn JH, Daniels TE, The clinical effects of electrostimulation on salivary function of Sjograns-syndrom patients - a placebo controlled study, Rheumatol. Int 12 (1992) 43–45. [DOI] [PubMed] [Google Scholar]
- [215].Shubin AD, Felong TJ, Schutrum BE, Joe DSL, Ovitt CE, Benoit DSW, Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics, Acta Biomater. 50 (2017) 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sciences M, Arbor A, Campus N, Arbor A, Surgery M, Surgery M, Translational and Clinical Concise Review: Salivary Gland Regeneration: Therapeutic Approaches from Stem Cells to Tissue Organoids, 2017, pp. 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Healy C, Mulhall KJ, Patrick DF, Kay EW, Bouchier-Hayes D, The effect of thermal preconditioning of the limb on flexor tendon healing, J. Hand Surg. Br. Eur 32E (2007) 289–295. [DOI] [PubMed] [Google Scholar]
- [218].Mulhall KJ, McLaughlin R, Kay E, Kiely P, Bouchier-Hayes D, Murray P, Thermal preconditioning prevents peritendinous adhesions and inflammation, Clin. Orthop. Relat. Res (2002) 258–266. [DOI] [PubMed] [Google Scholar]
- [219].Salamone JC, Salamone AB, Swindle-Reilly K, Leung KX-C, McMahon RE, Grand challenge in Biomaterials-wound healing, Regen. Biomater 3 (2016) 127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Khampieng T, Wongkittithavorn S, Chaiarwut S, Ekabutr P, Pavasant P, Supaphol P, Silver nanoparticles-based hydrogel: characterization of material parameters for pressure ulcer dressing applications, J. Drug Deliv. Sci. Technol 44 (2018) 91–100. [Google Scholar]
- [221].Jaul E, Assessment and management of pressure ulcers in the elderly, Drugs Aging 27 (2010) 311–325. [DOI] [PubMed] [Google Scholar]
- [222].Manesh AO, Flemming K, Cullum NA, Ravaghi H, Electromagnetic therapy for treating pressure ulcers, Cochrane Database Syst. Rev 19 (2006), CD002930. [DOI] [PubMed] [Google Scholar]
- [223].Sullivan LJ, McCurrach F, Lee S, Taylor HR, Rolando M, MarechalCourtois C, CreuzotGarcher C, Karabatsas C, Hoh MB,Faschinger C, Laroche L, Efficacy and safety of 0.3% carbomer gel compared to placebo in patients with moderate-to-severe dry eye syndrome, Ophthalmology 104 (1997) 1402–1408. [DOI] [PubMed] [Google Scholar]
- [224].Tung CI, Kottaiyan R, Koh S, Wang Q, Yoon G, Zavislan JM, Davio SR, V Aquavella J, Noninvasive, Objective, multimodal tear dynamics evaluation of 5 over-the-counter tear drops in a randomized controlled trial, Cornea 31 (2012) 108–114. [DOI] [PubMed] [Google Scholar]
- [225].Johns DB, Keyport GM, Hoehler F, diZerega GS, Intergel Adhesion Prevention S, Reduction of postsurgical adhesions with Intergel (R) adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery, Fertil. Steril 76 (2001) 595–604. [DOI] [PubMed] [Google Scholar]
- [226].Diamond MP, Sepracoat G, Adhesion Study, Reduction of de novo post-surgical adhesions by intraoperative precoating with Sepracoat* (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study, Fertil. Steril 69 (1998) 1067–1074. [DOI] [PubMed] [Google Scholar]
- [227].Fossum GT, Silverberg KM, Miller CE, Diamond MP, Holmdahl L, Gynecologic use of Sepraspray Adhesion Barrier for reduction of adhesion development after laparoscopic myomectomy: a pilot study, Fertil. Steril 96 (2011) 487–491. [DOI] [PubMed] [Google Scholar]
- [228].O’Lenick AJ, Silicones - basic chemistry and selected applications, J. Surfactants Deterg 3 (2000) 229–236. [Google Scholar]
- [229].Tunney MM, Gorman SP, Evaluation of a poly(vinyl pyrollidone)-coated biomaterial for urological use, Biomaterials 23 (2002) 4601–4608. [DOI] [PubMed] [Google Scholar]
- [230].Gorman SP, Tunney MM, Keane PF, Van Bladel K, Bley B, Characterization and assessment of a novel poly(ethylene oxide)/polyurethane composite hydrogel (Aquavene (R)) as a ureteral stent biomaterial, J. Biomed. Mater. Res 39 (1998) 642–649. [DOI] [PubMed] [Google Scholar]
- [231].Ho SP, Nakabayashi N, Iwasaki Y, Boland T, LaBerge M, Frictional properties of poly(MPC-co-BMA) phospholipid polymer for catheter applications, Biomaterials 24 (2003) 5121–5129. [DOI] [PubMed] [Google Scholar]
- [232].Zheng R, Zhan J, Wang X, Kaplan D, Pesika N, John VT, Lubrication properties of phospholipid liposome coated silk microspheres, Part. Part. Syst. Char 30 (2013) 133–137. [Google Scholar]
- [233].Rosser JM, Carbomer viscosupplementation in the equine middle carpal joint, J. Vet. Sci. Technol 5 (2014) 158. [Google Scholar]
- [234].Guo Y, Hao ZX, Wan C, Tribological characteristics of polyvinylpyrrolidone (PVP) as a lubrication additive for artificial knee joint, Tribol. Int 93 (2016) 214–219. [Google Scholar]
- [235].Ma RY, Xiong DS, Miao F, Zhang JF, Peng Y, Friction properties of novel PVP/PVA blend hydrogels as artificial cartilage, J. Biomed. Mater. Res 93A (2010) 1016–1019. [DOI] [PubMed] [Google Scholar]
- [236].Katta JK, Marcolongo M, Lowman A, Mansmann KA, Friction and wear behavior of poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for articular cartilage replacement, J. Biomed. Mater. Res 83A (2007) 471–479. [DOI] [PubMed] [Google Scholar]
- [237].Bodugoz-Senturk H, Macias CE, Kung JH, Muratoglu OK, Poly(vinyl alcohol)-acrylamide hydrogels as load-bearing cartilage substitute, Bio-materials 30 (2009) 589–596. [DOI] [PubMed] [Google Scholar]
- [238].Choi J, Kung HJ, Macias CE, Muratoglu OK, Highly lubricious poly(vinyl alcohol)-poly(acrylic acid) hydrogels, J. Biomed. Mater. Res. B Appl. Biomater 100B (2012) 524–532. [DOI] [PubMed] [Google Scholar]
- [239].Bodugoz-Senturk H, Choi J, Oral E, Kung JH, Macias CE, Braithwaite G, Muratoglu OK, The effect of polyethylene glycol on the stability of pores in polyvinyl alcohol hydrogels during annealing, Biomaterials 29 (2008) 141–149. [DOI] [PubMed] [Google Scholar]
- [240].Jackson DW, Simon TM, Intra-articular distribution and residence time of Hylan A and B: a study in the goat knee, Osteoarthr. Cartil 14 (2006) 1248–1257. [DOI] [PubMed] [Google Scholar]
- [241].Lindenhayn K, Heilmann HH, Niederhausen T, Walther HU, Pohlenz K, Elimination of tritium-labelled hyaluronic acid from normal and osteoarthritic rabbit knee joints, Eur. J. Clin. Chem. Clin. Biochem 35 (1997) 355–363. [DOI] [PubMed] [Google Scholar]
- [242].Brown TJ, Laurent UBG, Fraser JRE, Turnover of hyaluronan in synovial joints - elimination of labeled hyaluronan from the knee-joint of the rabbit, Exp. Physiol 76 (1991) 125–134. [DOI] [PubMed] [Google Scholar]
- [243].Antonas KN, Muirden KD, Fraser JRE, Distribution of biologically labeled radioactive hyaluronic acid injected into joints, Ann. Rheum. Dis 32 (1973) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Pagethomas DP, Bard D, King B, Dingle JT, Clearance of proteoglycan from joint cavities, Ann. Rheum. Dis 46 (1987) 934–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Edsman K, Hjelm R, Larkner H, Nord LI, Karlsson A, Wiebensjo A, Hoglund AU, Kenne AH, Nasstrom J, Intra-articular duration of durolane (TM) after single injection into the rabbit knee, Cartilage 2 (2011) 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Lindqvist U, Tolmachev V, Kairemo K, Astrom G, Jonsson E, Lundqvist H, Elimination of stabilised hyaluronan from the knee joint in healthy men, Clin. Pharmacokinet 41 (2002) 603–613. [DOI] [PubMed] [Google Scholar]