Abstract
Background
The performance of various equations for estimated glomerular filtration rate (eGFR) in patients with diabetes remains controversial. We aimed to evaluate the performance of equations for eGFR in Chinese patients with diabetic nephropathy (DN).
Methods
This is a retrospective study included in 308 patients with type 2 diabetes and biopsy-proven DN who were followed up at least one year. eGFR was calculated using chronic kidney disease epidemiology (CKD-EPI) equations based on serum creatinine (eGFRCKD-EPI-Cr), cystatin C (eGFRCKD-EPI-CysC), and joint equations (eGFRCKD-EPI-Cr-CysC), respectively. End-stage kidney disease was defined by initiation of renal replacement therapy. The eGFR concordance between equations was assessed by Bland-Altman plots. Log-rank and multivariable logistic regression were employed to evaluate the performance of equations.
Results
Overall, the proportion of patients with eGFR < 60 mL/min/1.73m2 was 53%, 70%, and 61% by the equations of eGFRCKD-EPI-Cr, eGFRCKD-EPI-CysC, and eGFRCKD-EPI-Cr-CysC, respectively. Higher disconcordance was observed between equations when eGFR > 60 mL/min/1.73m2. Compared with eGFRCKD-EPI-Cr, 39% of patients were reclassified (reclassified group) from CKD 1-2 stages to CKD 3-5 stages by eGFRCKD-EPI-CysC and they presented significantly longer diabetic duration, heavier proteinuria, advanced pathological lesions, and poorer kidney outcomes. Multivariable logistic regression indicated cystatin C was independently associated with advanced glomerular classifications.
Conclusion
eGFR equations incorporating cystatin C are superior to eGFR based on creatinine alone for detecting kidney injury in the early stage. The independent association between cystatin C and glomerular classifications might contribute to it.
1. Introduction
The past few decades have witnessed a marked increasing prevalence of type 2 diabetes, especially in China, and the global prevalence of microvascular and macrovascular complications associated with diabetes increases dramatically [1, 2]. Diabetic nephropathy (DN) has become the leading cause of end-stage kidney disease (ESKD) worldwide [3, 4]. The utilization of renin-angiotensin-aldosterone system blockers and improvements in glycemic, blood pressure, and lipid control slow the progression of chronic kidney disease (CKD) to a degree [5]. Indeed, glomerular filtration rate (GFR) guides the clinical management of CKD and is an independent predictor of kidney injury, all-cause/cardiovascular mortality, and kidney failure [6]. Therefore, accurate estimation of GFR to identify CKD and predict kidney outcome is highlighted.
The Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the Evaluation and Management of CKD [7] recommends initial use of 2009 CKD-Epidemiology Collaboration equation based on serum creatinine (eGFRCKD-EPI-Cr) instead of the Modification of Diet in Renal Disease equation. They also suggest use of the 2012 CKD-EPI equations (eGFRCKD-EPI-CysC, eGFRCKD-EPI-Cr-CysC) to confirm kidney function when cystatin C has been measured, particularly for patients with eGFRCr of 45–59 mL/min/1.73 m2 who do not have markers of kidney damage. However, the performance of various equations in CKD cohorts remains controversial due to serum creatinine is influenced by age, muscle mass, sex, and race; cystatin C level is affected by ages, body mass index, diabetes, and inflammation. Patients with DN are recognized as a special community in CKD. Also, the implications and predictive potential of different equations in patients with DN have yet to be elucidated. The objective of the study was to compare the performance for detecting kidney injury of equations of eGFRCKD-EPI-Cr, eGFRCKD-EPI-CysC, and eGFRCKD-EPI-Cr-CysC in patients with DN in a single center in Southwest China.
2. Method
2.1. Study Population
This is a retrospective cohort study. From November 2003 to March 2018, a total of 308 Chinese patients with type 2 diabetes mellitus (T2DM) and biopsy-proven DN in West China Hospital of Sichuan University were recruited and followed up for at least one year by routine clinical visits. Patients with T2DM and proteinuria > 0.5 g/24 h or eGFR decline were indicated to receive kidney biopsy in our hospital. The diagnoses of T2DM and DN were based upon the criteria recommended by the American Diabetes Association (ADA) in 2018 [8] and the Renal Pathology Society in 2010 [9]. The ESKD was defined by initiation of renal replacement therapy (hemodialysis, peritoneal dialysis, or kidney transplantation). Patients who had malignances, nondiabetic renal disease (NDRD), and NDRD+DN were excluded from the study.
The protocol of study was approved by the ethics committee of West China Hospital of Sichuan University and conducted based on the principles of the Declaration of Helsinki; written informed consents were obtained at the time of biopsy from all the patients.
2.2. Clinical and Pathological Characteristics
Diabetic history, data including physical examinations (body mass index, blood pressure, and examination of diabetic retinopathy) and laboratory tests (HbA1c, 24-hour protein excretion, serum creatinine, serum cystatin C, serum lipid), were collected at the time of kidney biopsy from the hospital information system. Creatinine was measured using Jaffe's assay. Serum creatinine value was calibrated to isotope dilution mass spectrometry (IDMS). Serum cystatin C was measured using an automated particle-enhanced immunoturbidimetric method. Blood samples were collected after 12 hours of fasting in all the patients [10]. Pathological lesions were routinely assessed under light and electron microscopy by at least two nephropathologists according to criteria proposed by the Renal Pathology Society in 2010 [9].
2.3. Statistical Analysis
GFR was estimated using the equations [11] of eGFRCKD-EPI-Cr, eGFRCKD-EPI-CysC, and eGFRCKD-EPI-Cr-CysC, respectively. The CKD stages 1, 2, 3a, 3b, 4, and 5 were categorized by eGFR (≥90, 60-90, 45-60, 30-45, 15-30, ≤15 mL/min/1.73 m2) [12]. The term “reduced kidney function” indicated eGFR < 60 mL/min/1.73 m2. The bias (mean difference) between each two equations was assessed by Bland-Altman plots. The differences in variables were analyzed appropriately by Student's t-test, the Mann-Whitney test, or the chi-square test. Kidney outcomes were compared using the log-rank test and demonstrated by the Kaplan-Meier curves method. The association between variables was analyzed by multivariable logistic regression. All analyses were conducted using SPSS software 22.0 and GraphPad Prism 7.0, and a two-sided P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Baseline Demographic Clinical and Pathological Characteristics
A total of 308 patients with biopsy-proven DN were enrolled in the current study (Table 1). Among them, 69.5% of patients were male and the mean age was 51.4 ± 9.7 years old. The mean duration of diabetes was 97.8 ± 70.3 months. 44.8% and 86.0% of patients have diabetic retinopathy and hypertension, respectively. The mean serum creatinine and cystatin C were 130 ± 65 μmol/L and 1.61 ± 0.60 mg/L, respectively. The median (interquartile range) initial proteinuria was 4.3 (2.0-7.8) g/24 hours. The thyroid levels were in normal range. The mean GFR was higher when estimated using the CKD-EPI-Cr (62.6 ± 28.7 mL/min/1.73 m2) than using CKD-EPI-CysC (50.3 ± 23.3 mL/min/1.73 m2) and CKD-EPI-Cr-CysC (54.6 ± 24.9 mL/min/1.73 m2). 81.5% of patients received RAAS inhibitors, 72.1% of patients received insulin therapy, and 57.5% of patients received statins.
Table 1.
Baseline demographic, clinical, and pathological characteristics.
| Variables | Patients (n = 308) |
|---|---|
| Age (years) | 51.4 ± 9.7 |
| Gender (male) | 214 (69.5) |
| Duration of diabetes (months) | 97.8 ± 70.3 |
| Diabetic retinopathy (%) | 138 (44.8) |
| Body mass index (kg/m2) | 25.78 ± 3.78 |
| Hypertension (%) | 265 (86.0) |
| Systolic blood pressure (mmHg) | 145 ± 23 |
| Diastolic blood pressure (mmHg) | 86 ± 13 |
| HbA1C (%) | 7.3 (6.3-8.5) |
| Initial proteinuria (g/24 h) | 4.3 (2.0-7.8) |
| Serum creatinine (μmol/L) | 130 ± 65 |
| Cystatin C (mg/L) | 1.61 ± 0.60 |
| Triglyceride (mmol/L) | 2.19 ± 1.80 |
| Total cholesterol (mmol/L) | 5.22 ± 1.60 |
| Thyroid-stimulating hormone (mU/L) | 3.15 (1.96-5.60) (n = 155) |
| Free triiodothyronine 3 (pmol/L) | 3.92 (3.39-4.41) (n = 151) |
| Free triiodothyronine 4 (pmol/L) | 14.95 (13.17-16.61) (n = 154) |
| eGFRCKD-EPI-Cr | 62.6 ± 28.7 |
| eGFRCKD-EPI-CysC | 50.3 ± 23.3 |
| eGFRCKD-EPI-Cr-CysC | 54.6 ± 24.9 |
| RAAS inhibitors (%) | 251 (81.5) |
| Insulin therapy (%) | 222 (72.1) |
| Statins (%) | 177 (57.5) |
| Glomerular class | |
| I | 17 |
| IIa | 75 |
| IIb | 29 |
| III | 140 |
| IV | 47 |
| Interstitial fibrosis and tubular atrophy | |
| 0 | 10 |
| 1 | 138 |
| 2 | 127 |
| 3 | 33 |
| Interstitial inflammation | |
| 0 | 20 |
| 1 | 233 |
| 2 | 55 |
| Arteriolar hyalinosis | |
| 0 | 34 |
| 1 | 162 |
| 2 | 112 |
| Reach end-stage kidney disease | 131 |
| Median kidney survival time (months) | 20 (13-33) |
Data are presented as the mean ± standard and the median with range or counts and percentages.
All the patients in the study underwent kidney biopsy. Glomerular lesions were classified as follows. Seventeen patients had glomerular basement membrane thickening only and were classified as class I. 104 patients had mild or severe mesangial expansion, but without nodular sclerosis (Kimmelstiel-Wilson lesion), and were classified as class II. 140 patients who did not meet the criteria of class IV with at least one convincing Kimmelstiel-Wilson lesion were classified as class III. 47 patients with global glomerular sclerosis in ≥50% of glomeruli were classified as class IV. For the IFTA score, 10, 138, 127, and 33 patients were scored as 0, 1, 2, and 3, respectively. For interstitial inflammation, 20, 233, and 50 patients were scored as 0, 1, and 2, respectively. For arteriolar hyalinosis, 34, 162, and 112 patients were scored as 0, 1, and 2, respectively.
During a median follow-up period of 20 (13-33) months, a total of 131 (42.5%) patients reached ESKD.
3.2. CKD Stages Categorized Using Different Equations
Figure 1 shows the proportion of CKD stages categorized using the different equations. Overall, more patients tend to be categorized into advanced CKD stages by eGFRCKD-EPI-CysC and eGFRCKD-EPI-Cr-CysC compared with eGFRCKD-EPI-Cr. Approximately half of patients (47%) were included in CKD 1 and CKD 2 stages when using equation of eGFRCKD-EPI-Cr, but only 30% of patients in CKD 1 and 2 stages when using equation of eGFRCKD-EPI-CysC. On the contrary, 62 patients were categorized into CKD 3b stage and 39 patients were in CKD 4 stage when using eGFRCKD-EPI-Cr, but 89 and 58 patients were in CKD 3b and 4 stages, respectively, when using eGFRCKD-EPI-CysC.
Figure 1.
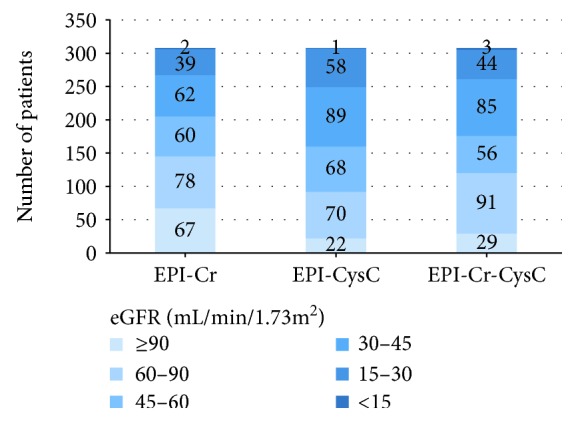
CKD stages categorized using different equations eGFR (mL/min/1.73m2). CKD stage 1: eGFR ≥ 90 mL/min/1.73m2; CKD stage 2: eGFR (60-90) mL/min/1.73m2; CKD stage 3a: eGFR (45-60) mL/min/1.73m2; CKD stage 3b: eGFR (30-45) mL/min/1.73m2; CKD stage 4: eGFR (15-30) mL/min/1.73m2; CKD stage 5: eGFR < 15 mL/min/1.73m2. More patients tend to be categorized into advanced CKD stages by eGFRCKD-EPI-CysC and eGFRCKD-EPI-Cr-CysC compared with eGFRCKD-EPI-Cr.
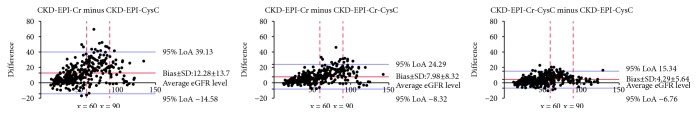
The Bland-Altman plot shown in Figure 2 revealed the bias between each two equations. Overall, high disconcordance was observed between eGFRCKD-EPI-Cr and eGFRCKD-EPI-CysC, especially when eGFR ≥ 60 mL/min/1.73 m2. The mean bias of eGFRCKD-EPI-Cr and eGFRCKD-EPI-CysC was 12.28 ± 13.7 mL/min/1.73 m2, and upper 95% limit of agreement was 39.13 mL/min/1.73 m2, which were beyond accepted limit. The mean bias of eGFRCKD-EPI-Cr and eGFRCKD-EPI-Cr-CysC was 7.98 ± 8.32 mL/min/1.73 m2, and the mean bias of eGFRCKD-EPI-Cr-CysC and eGFRCKD-EPI-CysC was 4.29 ± 5.64 mL/min/1.73 m2.
Figure 2.
Bland-Altman plots of the difference between eGFRs. The limits of agreement (LoA) are defined as the mean difference ± 1.96 SD of differences. The black line and the blue lines indicate mean difference and 95% LoA, respectively. The red dashed lines indicate when average eGFR are 60 and 90 mL/min/1.73 m2. High disconcordance was observed between eGFRCKD-EPI-Cr and eGFRCKD-EPI-CysC, especially when eGFR > 60 mL/min/1.73 m2. SD: standard deviation.
3.3. Reclassification to Reduced Kidney Function by eGFRCKD-EPI-CysC Equation
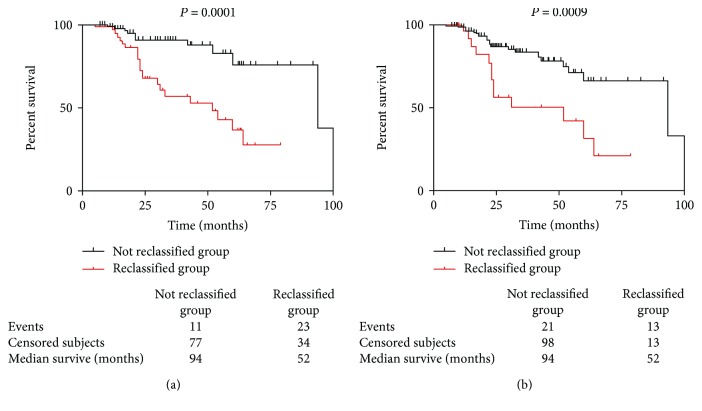
A total of 145 patients were with eGFR > 60 mL/min/1.73 m2 (CKD 1 and 2 stages) when using eGFRCKD-EPI-Cr equation; however, 57 of them were reclassified into CKD 3-5 stages when using eGFRCKD-EPI-CysC equation (Table 2). Compared with patients who were not reclassified (n = 88), the reclassified group presented significantly longer diabetic duration (106.8 ± 64.8 vs 83.4 ± 61.6 months), heavier proteinuria [4.2 (2.3-8.8) vs 2.4 (0.8-4.8) g/24 h, P = 0.001], higher level of cystatin C (1.45 ± 0.20 vs 1.02 ± 0.15 mg/L, P < 0.001), lower free triiodothyronine 3 [3.92 (3.46-4.35) vs 4.41 (3.92-5.10) pmol/L, P = 0.003], advanced glomerular classifications (P < 0.001), IFTA scores (P < 0.001), and interstitial inflammation (P < 0.001). In addition, during the follow-up, 11 (12.5%) patients reached the end point in the not reclassified group, while 21 (36.8%) patients reached the end point in the reclassified group; the kidney survival was significantly poorer by the log-rank test in the reclassified group (Figure 3(a)). Similarly, 26 patients were reclassified into CKD 3-5 stages using eGFRCKD-EPI-Cr-CysC equation from patients with eGFRCKD−EPI−Cr > 60 mL/min/1.73 m2. A significantly poorer kidney outcome was observed in patients who were reclassified by eGFRCKD-EPI-Cr-CysC during the follow-up period (Figure 3(b)).
Table 2.
Clinical and pathological characteristics of the not/reclassified group.
| Variables | Not reclassified group (n = 88) |
Reclassified group (n = 57) |
P value |
|---|---|---|---|
| eGFRCKD-EPI-Cr | 96.5 ± 17.0 | 76.1 ± 11.5 | <0.001 |
| eGFRCKD-EPI-CysC | 79.9 ± 17.1 | 49.3 ± 7.2 | <0.001 |
| Age (years) | 49.4 ± 10.8 | 51.3 ± 8.7 | NS |
| Gender (male) (%) | 62 (70.5) | 38 (66.7) | NS |
| Duration of diabetes (months) | 83.4 ± 61.6 | 106.8 ± 64.8 | 0.033 |
| Diabetic retinopathy (%) | 28 (32.6) | 22 (40.0) | NS |
| Body mass index (kg/m2) | 26.24 ± 3.35 | 25.67 ± 4.59 | NS |
| Systolic blood pressure (mmHg) | 138 ± 20 | 144 ± 22 | NS |
| Diastolic blood pressure (mmHg) | 86 ± 14 | 85 ± 13 | NS |
| HbA1C (%) | 7.8 (6.8-9.4) | 7.5 (6.3-10.2) | NS |
| Initial proteinuria (g/24 h) | 2.4 (0.8-4.8) | 4.2 (2.3-8.8) | 0.001 |
| Serum creatinine (μmol/L) | 74 ± 18 | 93 ± 19 | <0.001 |
| Cystatin C (mg/L) | 1.02 ± 0.15 | 1.45 ± 0.20 | <0.001 |
| Triglyceride (mmol/L) | 2.39 ± 2.17 | 2.03 ± 1.61 | NS |
| Total cholesterol (mmol/L) | 4.89 ± 1.36 | 5.36 ± 1.61 | NS |
| Thyroid-stimulating hormone (mU/L) | 2.93 (1.79-4.09) (n = 43) | 3.86 (2.61-6.58) (n = 29) | NS |
| Free triiodothyronine 3 (pmol/L) | 4.41 (3.92-5.10) (n = 42) | 3.92 (3.46-4.35) (n = 29) | 0.003 |
| Free triiodothyronine 4 (pmol/L) | 15.87 (14.65-17.26) (n = 42) | 14.54 (12.50-16.66) (n = 29) | NS |
|
| |||
| Pathological lesions | |||
| Glomerular classification | <0.001 | ||
| I | 14 | 1 | |
| IIa | 39 | 14 | |
| IIb | 6 | 7 | |
| III | 26 | 29 | |
| IV | 3 | 6 | |
| IFTA scores | <0.001 | ||
| 0 | 8 | 2 | |
| 1 | 62 | 27 | |
| 2 | 18 | 25 | |
| 3 | 0 | 3 | |
| Interstitial inflammation | <0.001 | ||
| 0 | 17 | 2 | |
| 1 | 70 | 45 | |
| 2 | 1 | 10 | |
| Arteriolar hyalinosis | NS | ||
| 0 | 18 | 10 | |
| 1 | 47 | 28 | |
| 2 | 23 | 19 | |
| Follow-up period (months) | 32 ± 22 | 30 ± 18 | NS |
| Progressed to ESKD (%) | 11 (12.5) | 21 (36.8) | <0.001 |
Patients in the reclassified group had significantly longer diabetic duration, heavier proteinuria, and advanced pathological lesions. Data are presented as the mean ± standard and the median with range or counts and percentages. IFTA: interstitial fibrosis and tubular atrophy; NS: not significant. A two-tailed P < 0.05 was considered statistically significant.
Figure 3.
Kidney survival of patients reclassified in advanced CKD stages (3-5) by equations based on cystatin C. Comparison of kidney survival between the not reclassified group and reclassified group by eGFRCKD-EPI-CysC (a) and eGFRCKD-EPI-Cr-CysC (b). The kidney survival was significantly poorer by the log-rank test in the reclassified groups.
3.4. The Association between Serum Creatinine and Cystatin C and Pathological Lesions
We then investigated the association between serum creatinine and cystatin C and pathological lesions. For glomerular classification, we defined class of III and IV as advanced lesion, and for tubular and interstitial lesions, we defined scores of 2 and 3 of IFTA as advanced lesions. We adjusted essential clinical variables including gender, age, blood pressure, diabetic duration, triglyceride, total cholesterol, and proteinuria for multivariable logistic regression. As shown in Table 3, only gender (odds ratio (OR) 0.349, 95% confidence interval (CI) 0.174-0.700, P = 0.003) and cystatin C (OR 3.771, 95% CI 1.140-12.472, P = 0.030) were independently associated with advanced glomerular lesions. Serum creatinine (OR 1.004, 95% CI 0.993-1.015, P > 0.05) was not independently associated with advanced glomerular lesions. However, total cholesterol (OR 1.249, 95% CI 1.009-1.545, P = 0.041) and serum creatinine (OR 1.012, 95% CI 1.002-1.023, P = 0.024) were independently associated with advanced tubular and interstitial injury.
Table 3.
Multivariable logistic regression of advanced glomerular classification and IFTA.
| Variables | Glomerular classification of III and IV | IFTA of 2 and 3 scores | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Gender (male) | 0.349 | 0.174-0.700 | 0.003 | 1.037 | 0.538-2.000 | NS |
| Age | 0.985 | 0.955-1.015 | NS | 0.985 | 0.956-1.015 | NS |
| Systolic blood pressure | 1.008 | 0.991-1.025 | NS | 1.004 | 0.988-1.020 | NS |
| Diastolic blood pressure | 0.995 | 0.966-1.025 | NS | 1.015 | 0.987-1.043 | NS |
| Diabetic duration | 1.003 | 0.999-1.008 | NS | 1.001 | 0.997-1.005 | NS |
| Triglyceride | 0.905 | 0.769-1.063 | NS | 0.941 | 0.803-1.104 | NS |
| Total cholesterol | 1.022 | 0.821-1.271 | NS | 1.249 | 1.009-1.545 | 0.041 |
| Proteinuria | 1.059 | 0.979-1.146 | NS | 1.018 | 0.949-1.092 | NS |
| Serum creatinine | 1.004 | 0.993-1.015 | NS | 1.012 | 1.002-1.023 | 0.024 |
| Cystatin C | 3.771 | 1.140-12.472 | 0.030 | 1.680 | 0.576-4.902 | NS |
Gender and cystatin C were independently associated with advanced glomerular classifications (III and IV stages); total cholesterol and serum creatinine were independently associated with higher IFTA scores. IFTA: interstitial fibrosis and tubular atrophy; CI: confidence interval; NS: not significant. A two-tailed P < 0.05 was considered statistically significant.
4. Discussion
Despite decades of research and heavy public health burden associated with DN, few new biomarkers have been applied to clinical practice in recent years [13]. Albuminuria and eGFR are still essential ones to monitor kidney function and guide management for patients with DN. However, the performance of GFR estimated by different equations is still under debate [14–16]. The current study showed the distribution of CKD stages categorized by different equations in patients with kidney-biopsy DN. 39% of patients with CKD 1 and 2 stages (by eGFRCKD-EPI-Cr) were reclassified into advanced CKD stages (by eGFRCKD-EPI-CysC) and they had longer diabetic duration, heavier proteinuria, advanced pathological lesions, and poorer prognosis. In addition, cystatin C, not creatinine, was independently associated with more severe glomerular classifications. Those findings suggest that equations incorporate cystatin C would improve the performance of detect glomerular lesions in the early stage in patients with DN.
Both serum creatinine and cystatin C are endogenous molecules. Serum creatinine is unstable and easily influenced by daily diet [10], secretion and reabsorption of tubular cells [17], and reduced muscle mass [18] which is common in patients with CKD [19]. Cystatin C is a low molecular basic protein, which is reabsorbed and catabolized by tubular cells completely. The serum concentrate is mainly affected by gender, obesity, diabetes, and hypertension [20, 21]. Criteria for selecting the optimal GFR estimating equation are accuracy, discrimination of kidney outcomes [22], and the population characteristic. Recently, substantial studies have evaluated the performance of different eGFR equations in the population of diabetes, CKD, or CKD with diabetes, but the conclusions remain largely controversial.
In the U.S. population of noninstitutionalized civilian, the prevalence of reduced kidney function was 6.5% when estimated using equations based on creatinine, compared with 8.7% when incorporated with cystatin C [23]. Similarly, a study enrolled 778 persons with diabetes detected the prevalence of reduced kidney function was 16.5% and 22.0% using eGFRCr and eGFRCysC, respectively. And patients with diabetes were more likely to be reclassified from preserved kidney function calculated by eGFRCr to reduced kidney function calculated by eGFRCysC [20]. However, a study included 199 diabetic patients demonstrated that both eGFRMDRD and eGFRCKD-EPI-Cr equations underestimated measured GFR (>90 mL/min/1.73 m2) [24]. Moreover, long-term GFR decline was proved to be largely underestimated in a cross-sectional and longitudinal analysis [21]. In the current study, a biopsy-proven DN cohort, 53% of patients were with reduced kidney function using eGFRCKD-EPI-Cr while 70% of which using eGFRCKD-EPI-CysC. Interestingly, patients who were reclassified by eGFRCKD-EPI-CysC have significantly heavier proteinuria, advanced pathological lesions, and faster progression of kidney disease than not reclassified patients, which suggested that eGFRCKD-EPI-CysC was more sensitive to detect kidney injury and predict kidney outcomes.
However, several studies questioned the improved performance of equations based on cystatin C. A latest study with 882 patients reported the misclassification was approximately 50% for creatinine-based equations and still 35% for cystatin C-based equations, and equations combined creatinine and cystatin C were not outperform equation only based on cystatin C [14]. In addition, eGFRCysC failed to improve the area under the curve for the diagnosis of reduced kidney function in patients with diabetes [25]. In the current study, we found that cystatin C, not the creatinine, was independently associated with advanced glomerular lesions. The contradictory can be explained by the characteristics of subjects partially. First, different equations were compared in CKD cohorts which include various primary or secondary kidney diseases. Cystatin C may be influenced by different disease status; even in CKD with diabetes cohort, the nondiabetic kidney disease may confound results [26, 27]. Second, subjects in previous studies were characteristic with obese (BMI 28-31 kg/m2) and older (>55 years old). Higher BMI is associated with increased fat mass which is a primary determinant of cystatin C generation [26]. And the accurate of cystatin C is decreased with age [28]. In the current study, the impact of age (51 years old) and BMI (25.78 kg/m2) on the performance of eGFRCysC is limited. Third, we aimed to explore the performance of equations to detect kidney injury, not the accuracy in estimating measured GFR.
These findings suggest that eGFRCysC is more sensitive to detect kidney injury in the early stage. Therefore, eGFRCysC should be considered, rather than eGFRCr alone, for clinical decision-making, especially when eGFRCr > 60 mL/min/1.73 m2.
There are several limitations of the current study that should be discussed. First, the sample size was limited due to that we only enrolled patients with biopsy-proven DN. Second, all the patients were ethnic Han in Southwest China; the performance of equations may be influenced by multiethnic setting such as muscle mass and meat intake. The results should be verified in more ethnic cohorts. Third, it was a retrospective cohort study; we did not have data of measured GFR to evaluate accuracy of different equations. Fourth, creatinine and cystatin C could be fluctuated; repeated measurements should be applied.
5. Conclusion
eGFR equations incorporating cystatin C is superior to eGFR based on creatine alone for detecting kidney injury in the early stage. The independent association between cystatin C and glomerular classifications might contribute to it.
Acknowledgments
This study was supported by Grant 81670662 from the National Natural Science Foundation of China. The Grant has supported our authors who did the work.
Data Availability
Original data can be provided if editors require.
Ethical Approval
The protocol of study was approved by the ethics committee of West China Hospital of Sichuan University and conducted based on the principles of the Declaration of Helsinki.
Consent
Written informed consents were obtained at the time of biopsy from all the patients.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Anders H. J., Huber T. B., Isermann B., Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nature Reviews Nephrology. 2018;14(6):361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M. C., Cooper M. E., Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nature Reviews Nephrology. 2016;12(2):73–81. doi: 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 3.Afkarian M., Zelnick L. R., Hall Y. N., et al. Clinical manifestations of kidney disease among us adults with diabetes, 1988-2014. JAMA. 2016;316(6):p. 602. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z.-H. Nephrology in China. Nature Reviews Nephrology. 2013;9(9):523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 5.Bakris G. L., Molitch M. Are all patients with type 1 diabetes destined for dialysis if they live long enough? Probably not. Diabetes Care. 2018;41(3):389–390. doi: 10.2337/dci17-0047. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K., Mahmoodi B. K., Woodward M., et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. Journal of the American Medical Association. 2012;307(18) doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney International Supplements. 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes 2018. Diabetes Care. 2018;41(Supplement 1):S13–S27. doi: 10.2337/dc18-s002. [DOI] [PubMed] [Google Scholar]
- 9.Tervaert T. W. C., Mooyaart A. L., Amann K., et al. Pathologic classification of diabetic nephropathy. Journal of the American Society of Nephrology. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 10.Nair S., O'Brien S. V., Hayden K., et al. Effect of a cooked meat meal on serum creatinine and estimated glomerular filtration rate in diabetes-related kidney disease. Diabetes Care. 2014;37(2):483–487. doi: 10.2337/dc13-1770. [DOI] [PubMed] [Google Scholar]
- 11.Levin A., Stevens P. E. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney International. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2) Supplement 1:S1–S266. [PubMed] [Google Scholar]
- 13.Doshi S. M., Friedman A. N. Diagnosis and management of type 2 diabetic kidney disease. Clinical Journal of the American Society of Nephrology. 2017;12(8):1366–1373. doi: 10.2215/CJN.11111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luis-Lima S., Escamilla-Cabrera B., Negrín-Mena N., et al. Chronic kidney disease staging with cystatin C or creatinine-based formulas: flipping the coin. Nephrology Dialysis Transplantation. 2018;34(2):287–294. doi: 10.1093/ndt/gfy086. [DOI] [PubMed] [Google Scholar]
- 15.Pottel H., Delanaye P., Schaeffner E., et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrology Dialysis Transplantation. 2017;32(3):497–507. doi: 10.1093/ndt/gfw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rule A. D., Kremers W. K. What is the correct approach for comparing GFR by different methods across levels of GFR? Clinical Journal of the American Society of Nephrology. 2016;11(9):1518–1521. doi: 10.2215/CJN.07530716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrone R. D., Madias N. E., Levey A. S. Serum creatinine as an index of renal function: new insights into old concepts. Clinical Chemistry. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 18.Oliveira E. A., Cheung W. W., Toma K. G., Mak R. H. Muscle wasting in chronic kidney disease. Pediatric Nephrology. 2018;33(5):789–798. doi: 10.1007/s00467-017-3684-6. [DOI] [PubMed] [Google Scholar]
- 19.Heymsfield S. B., Arteaga C., McManus C., Smith J., Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. The American Journal of Clinical Nutrition. 1983;37(3):478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 20.Tsai C.-W., Grams M. E., Inker L. A., Coresh J., Selvin E. Cystatin C– and creatinine-based estimated glomerular filtration rate, vascular disease, and mortality in persons with diabetes in the U.S. Diabetes Care. 2014;37(4):1002–1008. doi: 10.2337/dc13-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaspari F., Ruggenenti P., Porrini E., et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney International. 2013;84(1):164–173. doi: 10.1038/ki.2013.47. [DOI] [PubMed] [Google Scholar]
- 22.Rule A. D., Glassock R. J. GFR estimating equations: getting closer to the truth? Clinical Journal of the American Society of Nephrology. 2013;8(8):1414–1420. doi: 10.2215/CJN.01240213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grams M. E., Juraschek S. P., Selvin E., et al. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C−based estimates. American Journal of Kidney Diseases. 2013;62(2):253–260. doi: 10.1053/j.ajkd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacIsaac R. J., Ekinci E. I., Premaratne E., et al. The chronic kidney disease-epidemiology collaboration (CKD-EPI) equation does not improve the underestimation of glomerular filtration rate (GFR) in people with diabetes and preserved renal function. BMC Nephrology. 2015;16(1, article 198) doi: 10.1186/s12882-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliadis F., Didangelos T., Ntemka A., et al. Glomerular filtration rate estimation in patients with type 2 diabetes: creatinine- or cystatin C-based equations? Diabetologia. 2011;54(12):2987–2994. doi: 10.1007/s00125-011-2307-1. [DOI] [PubMed] [Google Scholar]
- 26.Lemoine S., Guebre-Egziabher F., Sens F., et al. Accuracy of GFR estimation in obese patients. Clinical Journal of the American Society of Nephrology. 2014;9(4):720–727. doi: 10.2215/CJN.03610413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi Y., Hu P., Xie Y., et al. Glomerular filtration rate measured by 99mTc‐DTPA renal dynamic imaging is significantly lower than that estimated by the CKD‐EPI equation in horseshoe kidney patients. Nephrology. 2016;21(6):499–505. doi: 10.1111/nep.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan L., Inker L. A., Rossert J., et al. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrology Dialysis Transplantation. 2014;29(6):1195–1203. doi: 10.1093/ndt/gft509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data can be provided if editors require.