Abstract
Malaria is one of the most rampant diseases today not only in Uganda but also throughout Africa. Hence, it needs very close attention as it can be severe, causing many deaths, especially due to the rising prevalence of pathogenic resistance to current antimalarial drugs. The majority of the Ugandan population relies on traditional herbal medicines for various health issues. Thus, herein, we review various plant resources used to treat malaria across communities in Uganda so as to provide comprehensive and valuable ethnobotanical data about these plants. Approximately 182 plant species from 63 different plant families are used for malaria treatment across several communities in Uganda, of which 112 plant species have been investigated for antimalarial activities and 96% of the plant species showing positive results. Some plants showed very strong antimalarial activities and could be investigated further for the identification and validation of potentially therapeutic antimalarial compounds. There is no record of an investigation of antimalarial activity for approximately 39% of the plant species used for malaria treatment, yet these plants could be potential sources for potent antimalarial remedies. Thus, the review provides guidance for areas of further research on potential plant resources that could be sources of compounds with therapeutic properties for the treatment of malaria. Some of the plants were investigated for antimalarial activities, and their efficacy, toxicity, and safety aspects still need to be studied.
1. Introduction
Malaria, a dangerous and life-threatening disease caused by Plasmodium parasites is spread to humans through bites of infected female Anopheles mosquitoes [1]. It is one of the most widespread diseases today not only in Uganda but also throughout Africa. Hence, careful monitoring of malaria is required as the disease can be severe and can cause many deaths, especially due to the increasing prevalence of resistance to current antimalarial drugs. Among the five parasitic species that cause malaria to humans, Plasmodium falciparum and Plasmodium vivax are the deadliest [2, 3]. P. falciparum and P. vivax being the most prevalent malaria parasites in sub-Saharan Africa and regions of the Americas, respectively, were responsible for about 99.7% and 74.1% of malaria cases in 2017 [4]. In Southeast Asia, Plasmodium knowlesi is the most common cause of malaria, accounting for up to 70% of malaria cases, although it has been known to infect Old-World monkeys more [5]. Two other species of Plasmodium, Plasmodium malariae and Plasmodium ovale, generally cause mild fevers. Approximately 216 million malaria cases were registered in 2016, with a death toll of up to 445,000 [1]. According to the World Health Organization [6], the incidence of malaria in Uganda, at 47.8%, was the highest worldwide in 2005. According to Njoroge and Bussman [7], malaria is responsible for one to two million deaths annually in Africa. Typical symptoms of malaria include high fever, fatigue, headache, muscle ache, nausea, abdominal discomfort, and profuse sweating. However, in extreme cases and cases of prolonged illness without treatment, brain tissue injury, pulmonary edema, kidney failure, severe anemia, yellow discoloration of the skin, and low blood sugar may be noted (Figure 1) [1, 2]. In Uganda, malaria is one of the major causes of illness and death [7]. Statistically, it accounts for 46% of children's sicknesses, almost 40% of outpatient visits to hospitals and clinics, 25% of hospital admissions, 14% of inpatient deaths, and approximately 23% of infant mortalities [7].
Figure 1.
Illustration of some common symptoms of malaria.
In different parts of the world, the use of herbs and herbal extracts in the management and treatment of malaria is very common since herbs are cheap and readily available besides being effective. In fact, the use of herbal medicine for treatment worldwide is on the rise. Over 80% of the Ugandan population relies directly on herbal plants for their health care primarily [8]. A great majority of the population uses traditional herbal medicines because of their confirmed therapeutic value [8]. The increase in preference for herbal remedies coupled with resistance exhibited by pathogenic strains, including Plasmodium species, to the modern drugs available is the driving force behind researchers' interest in herbal plants for possible alternatives for more effective antimalarial drugs [9, 10].
This review was aimed at providing comprehensive ethnobotanical information about various plant resources with antimalarial properties that are primarily used to manage and treat malaria across communities in Uganda, based on which further evaluation of these plants such as those of their efficacy and safety for the treatment of malaria may be based.
2. Methods and Materials
In the review, the data search processes employed by Komakech et al. [11] were modified to gather information on herbal plants for malaria treatment in Uganda from peer-reviewed articles in English published in scientific journals and other verifiable databases, with a focus on plant species and families, plant parts used, antimalarial activities of the extracts from herbal plants, and mechanisms of action of novel antimalarial phytochemicals and derivatives. Electronic literature databases such as PubMed, Medline, Scopus, SciFinder, Google Scholar, and Science Direct were carefully searched for suitable information. The following words were used as key search terms: (“Herbal medicine in Uganda” OR “Herbs in Uganda” OR “Traditional remedies in Uganda” OR “Natural remedies in Uganda” OR “Anti-malarial herbs in Uganda” OR “Anti-malarial plants in Uganda” OR “Ugandan herbs” OR “Ugandan ethno-medicine” OR “Ugandan phyto-medicine”), AND (“anti-plasmodial activities” OR “anti-malarial activities” OR “anti-plasmodial effects” OR “anti-malarial effects” OR “malaria treatment” OR “malaria management”) OR (“Malaria in Uganda” AND “prevalence” OR “occurrence” OR “distribution” OR “herbal treatment” OR “herbal remedies” OR “phyto-medicine” OR “phyto remedy” OR “plant parts used for treatment”) OR (Phytochemicals for malaria treatment OR Artemisinins OR Quinine OR Noble anti-malarial compounds OR Plant derived anti-malarial compounds AND mechanisms of action OR modes of action) OR (“Malaria herbal medicine in Uganda” OR “Herbal medicine in Uganda” OR “Herbal malaria remedy in Uganda” OR “Natural malaria medicine in Uganda” OR “Traditional malaria herbal medicine” OR “Malaria herbal recipe” AND “dosage” OR “dose” OR “dose given” OR “mode of administration” OR “means of traditional extraction” OR “traditional extraction” OR “Toxicity” OR “Safety and toxicity” OR “Policy framework” OR “other ethno-pharmacological uses” OR “other ethno-pharmacological utilizations” OR “other ethno-medicinal uses”). The information gathered was verified separately for its reliability; any discrepancies discovered were resolved by discussions between the authors. Thereafter, these data were summarized and analyzed, and comparisons were made to draw conclusions.
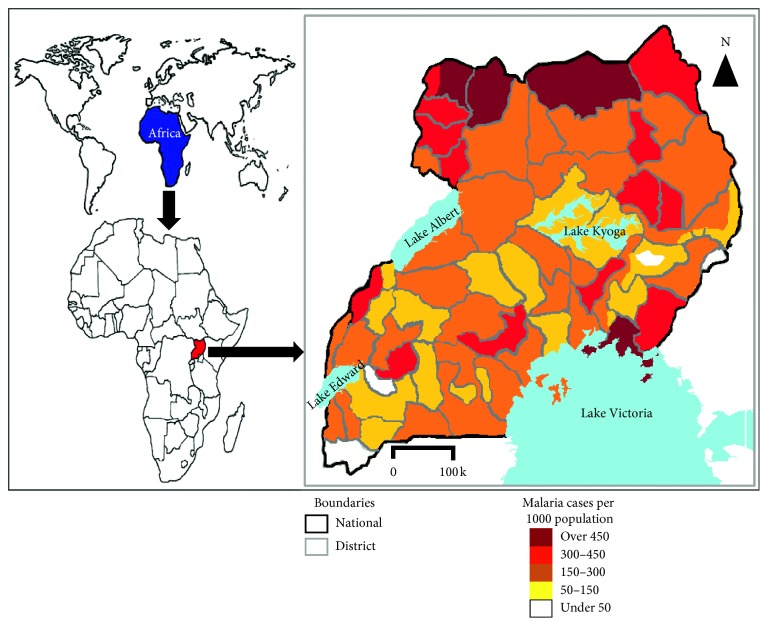
3. Prevalence of Malaria
Malaria in Uganda is highly endemic because the climate is favorable for its consistently stable and year-round transmission in about 99% of the country, with the country's entire population being at risk for contraction [12]. The most vulnerable groups of people at great risk for malaria are expectant mothers and young children under the age of 5 years [12]. The malarial parasite, P. falciparum, is most commonly the cause of malaria throughout Uganda, accounting for over 90% of malaria cases. However, Betson et al. [13] have warned of the potential for the emergence of infections due to P. malariae and P. ovale spp. as well, since there is much focus on countering P. falciparum infections. In 2016, Larocca et al. [14] indicated that Uganda was one of the leading countries in the world with malaria incidence rate as high as 478 cases per 1,000 population per year. Specifically, overall registered death cases caused by malaria in children were between 70,000 and 100,000 annually in Uganda [14]. Tremendous effort has been made to control malaria in Uganda by the government-headed Uganda Malaria Reduction Strategic Plan and Mass Action Against Malaria. These efforts have greatly reduced the malaria burden and incidence from 272 cases per 1000 population in 2016/17 to 191 cases per 1000 population in 2017/18 [12]. Although there has been a general reduction in the incidence of malaria, studies indicate that malaria prevalence along lakes, for example, Lake Victoria, and in remote areas of the country (villages) as well as areas closer to forests are much higher, with over 450 malaria cases per 1000 population (Figure 2) [12, 13, 15]. Communities around lakeshores in Uganda have always had high prevalence of malaria among children and especially the young ones despite routine treatments [12, 16]. Through the government initiative to control malaria, the prevalence in some districts remained as low as 4.3% in 2018 [12]. Malaria control strategies including indoor residual spraying along with house to house distribution of mosquito nets treated with insecticides resulted in a remarkable reduction in malaria burdens in many parts of the country [17]. Raouf et al. [18] observed that significant reductions in the levels of malaria in Uganda cannot be sustained if the current control measures are terminated.
Figure 2.
Malaria prevalence in Uganda (modified from [12]).
4. Mechanisms of Actions of Novel Phytochemicals in Malaria Treatment
Herbal plants are extremely rich in phytochemicals that are highly efficacious in the treatment of malaria, such as sesquiterpenes and sesquiterpene lactones, fluoroquinolones, chalcones, flavanones, phenolics, quinones, coumarins, and alkaloids (Table 1) [35, 36]. The herbal plants that are used as prophylactic measures to prevent malaria as well contain some of these compounds (Table 2). From these groups of compounds, active metabolites including quinine and artemisinin have been derived and the most successful antimalarial drugs to date have been obtained. Artemisinins from Artemisia annua a plant belonging to the family Asteraceae have actually been an integral part of the fight against malaria, with artemisinin-based combination therapy contributing enormously to modern day treatments [36]. They have been effective against all strains of P. falciparum including multi-drug-resistant ones [36, 37].
Table 1.
Herbs used in the treatment of malaria in Uganda.
| Plant family | Scientific name | Local name | Part used | Growth form | Mode of preparation | Dose and mode of administration for malaria | Status of antimalarial/antiplasmodial activity investigation | Other ailments treated | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | Justicia betonica L. | Nalongo/quinine | Leaves/whole plant | Herb | Decoction | About 120 ml every 8 hours for a week | Investigated | Diabetes, yellow fever, diarrhea | [10, 19] |
| Justicia anselliana (Nees) T. Anderson | Kwiniini omuganda | Leaves/twig | Herb | Decoction | Orally taken, dose not specified | No record | [20] | ||
| Monechma subsessile C. B. Clarke | Erazi | Leaves | Decoction | Orally taken, dose not specified | No record | Abdominal pain | [19] | ||
| Thunbergia alata Sims | Kasaamusaamu/ntudde buleku | Leaves/whole plant | Climber | Decoction | About 120 ml every 8 hours for a week | No record | False teeth | [8, 10] | |
| Alliaceae | Allium cepa L. | Katungulu | Bulb | Herb | No record | [21] | |||
| Aloeaceae | Aloe dawei A. Berger (wild/cultivated) | Kigagi | Leaves | Herb | Decoction | A glassful once a day for 7 days | Investigated | Candida | [10] |
| Aloe kedongensis (wild) | Kigagi | Leaves | Herb | Decoction | Orally taken, dose not specified | Investigated | [19, 22] | ||
| Aloe volkensii (cultivated) | Kigagi | Leaves | Herb | Decoction/infusion | Orally taken, dose not specified | No record | [19] | ||
| Aloe ferox Mill | Kigagi | Leaves | Herb | Decoction | Orally taken, dose not specified | Investigated | Wounds, digestive disorders, rheumatic arthritis | [18, 19] | |
| Aloe lateritia (wild) | Kigagi | Leaves/root | Herb | Decoction | Orally taken, dose not specified | No record | [19] | ||
| Amaranthaceae | Amaranthus hybridus L. | Bbuga | Leaves | Herb | Decoction | Half a glass every 24 hours for 7 days | No record | [10] | |
| Anacardiaceae | Mangifera indica L. | Muyembe gwakona | Leaves/bark | Tree | Decoction | 4 and 3 teaspoons after every 8 hours for adults and children, respectively, for a week | Investigated | Diarrhea, dysentery, body pain, venereal diseases, cough, syphilis | [10, 23] |
| Rhus natalensis Bernh. Ex Krauss | Omesheshe | Leaves | Shrub | Decoction | Orally taken, dose not specified | Investigated | [24] | ||
| Rhus vulgaris Meikle | Kakwasokwaso/tebudda | Leaves | Shrub | Decoction | Half a glass every 8 hours for 7 days | No record | Skin rush, erectile dysfunction | [10] | |
| Apiaceae | Heteromorpha trifoliata Eckl. & Zeyh. | Omumemena | Leaves/roots | Herb | Decoction | Orally taken, dose not specified | No record | [19] | |
| Centella asiatica (L.) Urb. | Kabo Kabakyala/mbutamu | Leaves/whole plant | Herb | Decoction | 4 teaspoons thrice a day for 4 days | Investigated | [10] | ||
| Apocynaceae | Alstonia boonei De Wild. | Mubajangalabi | Bark | Tree | Decoction | Orally taken, dose not specified | Investigated | [8] | |
| Carissa edulis (Forssk.) Vahl | Muyunza, ekamuriei | Roots | Herb | Decoction | Orally taken, dose not specified | Investigated | Epilepsy, fever, cough, syphilis, measles, dysentery | [21, 23] | |
| Carissa spinarum Lodd. ex A. DC. | Omuyonza | Roots | Decoction | Orally taken, dose not specified | Investigated | [19] | |||
| Catharanthus roseus G. Don | Sekagya | Leaves | Herb | Decoction | About 120 ml every 8 hours for a week | Investigated | [10] | ||
| Araceae | Culcasia faleifolia Engl. | Ntangawuzi yomukibira | Roots | Herb | Decoction | About 120 ml once a day for a week | No record | [10] | |
| Aristolochiaceae | Aristolochia elegans Mast. | Musuja welaba/nakasero | Seeds/sap | Vine | Steeped in water and drunk | A glassful once a day | Investigated | Abdominal pain, East coast fever | [8, 19] |
| Aristolochia tomentosa Sims. | Kankapu | Stem | Climber | Infusion | Oral, dose not specified | No record | Wounds, skin diseases, snake bites | [23] | |
| Asclepiadaceae | Gomphocarpus physocarpus E. Mey. | Kafumbo | Leaves | Herb | Decoction | Half a glass daily for a week | No record | [10] | |
| Asphodelaceae | Aloe vera (L.) Burm. f. | Kigagi/alovera | Leaves | Herb | Decoction | 1 teaspoon and 1 tablespoon 3 times a day for children and adults, respectively, for a week | Investigated | Stomach ache | [8, 25] |
| Asteraceae | Ageratum conyzoides L. | Namirembe | Whole plant/leaves | Herb | Decoction | A glassful thrice a day for 7 days | Investigated | Worms, weakness in pregnancy | [8, 10] |
| Artemisia annua L. | Sweet anne | Leaves | Herb | Decoction | Oral, dose not specified | Investigated | Fever | [19] | |
| Artemisia afra Jacq. ex Willd | Pasile | Leaves | Herb | Infusion | Oral, dose not specified | Investigated | Fever | [10] | |
| Aspilia africana (Pers.) C. D. Adams | Makayi, ekarwe | Whole plant/leaves/roots | Herb | Decoction | 8 teaspoons 3 times a day for a week | Investigated | Abdominal aches, measles, diarrhea, wounds, induction of appetite | [10, 19] | |
| Baccharoides adoensis (Sch. Bip. ex Walp.) H. Rob. | Okellokello | Leaves | Shrub | Decoction | 1 teaspoon and 1 tablespoon 3 times a day for children and adults, respectively, for a week; bath-leaves squeezed and added to bathing water | Investigated | Flu, skin rush, ear infections | [25, 26] | |
| Bidens grantii Sherff | Ehongwa | Leaves, flower | Herb | Decoction | Oral, dose not specified | No record | Pregnancy disorders, prehepatic jaundice | [19] | |
| Bidens pilosa L. | Sere/labika | Whole plant/leaves | Herb | Decoction/fresh leaf extract | 4 teaspoons thrice a day for 4 days | Investigated | Diarrhea, wounds | [10, 23] | |
| Bothriocline longipes N. E. Br. | Ekyogayanja | Leaves | Decoction | Oral, dose not specified | Investigated | Fever, ague, paludism | [19, 24] | ||
| Conyza bonariensis (L.) | Ndasha | Leaves | Decoction | Oral, dose not specified | No record | Stomach ache, body pain, anemia, respiratory problems | [19] | ||
| Conyza floribunda H. B. K. | Kafumbe | Leaves | Herb | Decoction | About 120 ml once a day for a week | No record | Headache | [10] | |
| Conyza sumatrensis (Retz.) E. H. Walker | Kati kati | Leaves | Herb | No record | Wounds, sore throat, ringworms | [21, 27] | |||
| Crassocephalum vitellinum | Kitonto | Leaves | Herb | Honey added to decoction | 2 teaspoons thrice a day for 7 days | Investigated | [10, 19] | ||
| Emilia javanica (Burm. F.) C. B. Rob. | Nakate | Whole plant | Herb | Decoction | Half a glass once a day for a week | No record | [10] | ||
| Guizotia scabra Chiov. | Ekiterankuba | Leaves | Decoction | Oral, dose not specified | Investigated | Stomach ache, HIV/AIDS opportunistic infections | [19] | ||
| Gynura scandens O. Hoffm. | Ekizimya-muriro | Leaves | Decoction | Oral, dose not specified | No record | Febrile convulsions | [19] | ||
| Melanthera scandens (Schumach. & Thonn.) Roberty | Makaayi | Leaves | Herb | Decoction | Oral, dose not specified | Investigated | Stomach ache, body odour, yellow fever | [8] | |
| Pluchea ovalis DC. | Omuneera | Leaves | Decoction | Oral, dose not specified | No record | [19] | |||
| Microglossa pyrifolia (Lam.)O. Ktze | Kafugankande | Whole plant/leaves/roots | Herb | Decoction | Half a glass thrice a day for a week | Investigated | Cough, abdominal disorders, chest pain | [10, 19, 28] | |
| Schkuhria pinnata (Lam.) | Apunait | Leaves | Herb | Infusion | 1 teaspoon and 1 tablespoon 3 times a day for children and adults, respectively, for a week | Investigated | Wounds, skin diseases, diabetes, ear infections, wounds | [23, 25] | |
| Sigesbeckia orientalis L. | Kyaryaho | Roots | Decoction | Oral, dose not specified | No record | Wounds, stomach ache | [19] | ||
| Solanecio mannii (Hook. f.) C. Jeffrey | Omusununu | Leaves | Decoction | Oral, dose not specified | Investigated | Fever, indigestion | [19] | ||
| Sonchus oleraceus L. | Entahutara | Leaves | Decoction | Oral, dose not specified | No record | Stomach ache, scars, anemia, diarrhea | [8, 19] | ||
| Tagetes minuta L. | Kawunyira | Whole plant/leaves | Herb | Decoction | Half a glass thrice a day for a week | Investigated | Flu, headache, convulsions | [10] | |
| Tithonia diversifolia A. Gray | Kimyula | Leaves | Herb | Decoction | Half a glass thrice a day for a week | Investigated | Diabetes, abdominal pain | [10, 19, 25] | |
| Vernonia adoensis Sch. Bip. ex Walp. | Nyakajuma | Leaves/flowers | Decoction | Oral, dose not specified | Investigated | Diarrhea, dizziness | [19] | ||
| Vernonia amygdalina Delile | Mululuza/labwori | Whole plant/roots | Shrub | Decoction | Half a glass 2 times a day for 5 days | Investigated | Headache, stomach ache, burns, baths | [8, 10, 19, 20] | |
| Vernonia cinerea (L.) Less. | Kayayana | Bark | Tree | Decoction | Half a glass thrice a day for a week | Investigated | Fever, vomiting, inflammation | [10] | |
| Vernonia lasiopus O. Hoffm. | Kaluluza kasajja | Roots/leaves | Shrub | Fresh leaf extract/root decoction | 2 teaspoons thrice a day for 7 days | Investigated | Abdominal pain, cough, migraine headache, delayed delivery | [8, 10, 19, 20] | |
| Bignoniaceae | Markhamia lutea (Benth.) K. Schum. | Musambya/muzanganda | Roots | Tree | Decoction | A glassful once a day for 7 days | Investigated | Cough, diarrhea | [8, 10, 19] |
| Spathodea campanulata Buch. -Harm. ex DC. | Kifabakazi | Bark | Tree | Decoction | Half a glass 3 times a day for 5 days | Investigated | Increased vaginal fluid, skin infection, infertility, hernia | [8, 10] | |
| Caesalpiniaceae | Cassia didymobotrya Fres. | Mukyula | Leaves | Shrub | Decoction | About 120 ml every 8 hours for a week | Investigated | [10] | |
| Chamaecrista nigricans Greene | Epeduru lo didi | Leaves | Herb | Infusion | Oral, dose not specified | No record | Labour induction, hypertension, retained placenta | [23] | |
| Erythrophleum pyrifolia | Omurama | Leaves/roots | Investigated | [24] | |||||
| Senna spectabilis (DC.) H. S. Irwin & Barneby | Gasiya | Leaves | Tree | Decoction | Half a glass twice a day for 5 days | Investigated | [10] | ||
| Caesalpinioideae | Cassia hirsuta | Kasagalansansi | Roots | Herb | Infusion | Investigated | Stomach pains | [23] | |
| Canelliaceae | Warbugia ugandensis Sprague | Omukuzanume | Bark/leaves | Tree | Decoction/powder swallowed with banana | Half a glass once a day for a week | Investigated | Toothache, flu, skin diseases, asthma, stomach ache, body and muscle pain | [10, 20, 27] |
| Caricaceae | Carica papaya L. | Paapali essajja | Leaves | Tree | Decoction | Half a glass twice a day for 3 days | Investigated | Snake bite, sterility, cough, cancer, body pain, induces labour | [10, 19, 23, 25] |
| Celastraceae | Maytenus senegalensis | Echomai | Roots | Tree | Decoction | Oral, dose not specified | Investigated | Toothache, skin diseases, chest pain, wound, fever | [23] |
| Chenopodiaceae | Chenopodium ambrosioides L. | Kawuna wuna | Leaves | Investigated | Headache, epilepsy | [21] | |||
| Chenopodium opulifolium Koch & Ziz | Namuvu | Leaves | No record | Oral wounds, skin rush, toothache | [8, 21] | ||||
| Combretaceae | Combretum molle G. Don | Ndagi | Bark | Tree | Decoction | Half a glass once a day for 3 days | Investigated | Cough, | [10, 21] |
| Crassulaceae | Kalanchoë densiflora Rolfe | Kisanasana | Leaves | Herb | No record | [21] | |||
| Cucurbitaceae | Cucurbita maxima Lam. | Kasuunsa | Leaves | Herb | Decoction | Half a glass once a day for 7 days | Investigated | Abdominal pain | [10, 25, 27] |
| Momordica foetida Schumach. | Orwihura | Leaves | Decoction | Oral, dose not specified | Investigated | Vomiting, baths, cough, flue, worms | [19, 26, 28] | ||
| Dracaenaceae | Dracaena steudneri Engl. | Kajjolyenjovu | Leaves | Herb | Decoction | Half a glass thrice a day for a week | No record | Scars, cough, syphilis, kidney stones, snake bites | [8, 10] |
| Ebenaceae | Euclea latideus Staff | Emusi | Roots | Shrub | Decoction | Oral, dose not specified | Investigated | Ringworms, swollen legs | [23] |
| Euphorbiaceae | Alchornea cordifolia (Schumach.) Mull. Arg. | Luzibaziba | Leaves | Herb | Decoction | Half a glass once a day for 7 days | Investigated | Shaking body | [8, 10] |
| Bridelia micrantha Baill. | Katazamiti | Bark | Tree | Decoction | Half a glass thrice a day for a week | Investigated | [10] | ||
| Clutia abyssinica Jaub. & Spach | Omubarama | Leaves | Decoction | Oral, dose not specified | Investigated | Fever, diarrhea | [19] | ||
| Croton macrostachyus Olive. | Ookota | Roots/bark | Tree | Decoction | Oral, dose not specified | Investigated | Tuberculosis, stomach ache, cough, fever, asthma | [23] | |
| Fluegea virosa (Roxb. ExWillb.)Voigt | Lukandwa/mukandula | Leaves | Shrub | Decoction | Half a glass 3 times a day for a week | Investigated | Miscarriage, chest pains, infertility in women | [8, 10, 21, 23] | |
| Jatropha curcas L. | Kirowa | Leaves | Shrub | Investigated | Tooth decay, headache, weakness in pregnancy | [21] | |||
| Macaranga schweinfurthii Pax | Kyeganza | Bark | Tree | Decoction | Half a glass 3 times a day for a 5 days | No record | [10] | ||
| Phyllanthus (pseudo) niruri Mull. Arg. | Nakitembe | Leaves | Shrub | Decoction | Half a glass 3 times a day for a 7 days | Investigated | [10] | ||
| Shirakiopsis elliptica (Hochst.) H.–J. Esser | Musasa | Back | Tree | Decoction | Oral, dose not specified | No record | [20] | ||
| Tetrorchidium didymostemon (Baill.) Pax & K. Hoffm. | Ekiziranfu | Bark | Decoction | Used as enema | No record | Jaundice, measles, gastrointestinal disorders, enema | [8, 19] | ||
| Fabaceae | Arachis hypogea (NC) | Ebinyobwa | Leaves | Fresh extract | Oral, dose not specified | No record | [19] | ||
| Cajanus cajan (L.) Druse | Entondaigwa | Leaves | Shrub | Fresh extract | 100 ml once a day for a week | Investigated | Diarrhea, body pain | [27] | |
| Crotalaria agatiflora Schweinf. | Kijjebejebbe | Whole shoot | Shrub | Fresh extract | Daily bath | No record | High blood pressure | [10] | |
| Crotalaria ochroleuca G. Don | Alayo | Leaves | Herb | Fresh extract | 1 teaspoon and 1 tablespoon 3 times a day for children and adults, respectively, for a week | No record | Stomach ache | [28] | |
| Entada abyssinica Steud. ex A. Rich. | Mwolola | Leaves | Tree | Decoction | Investigated | Oral wounds, body weakness, wounds, skin infections | [8, 20, 26] | ||
| Entada africana Guill. & Perr. | Mwolola | Bark | Tree | Decoction | 4 and 3 teaspoons after every 8 hours for adults and children, respectively, for a week | Investigated | [10] | ||
| Erythrina abyssinica Lam. | Girikiti/lacoro | Bark | Tree | Decoction | Half a glass 3 times a day for a 5 days | Investigated | Fever, leprosy, burns, tuberculosis, toothache, syphilis | [10, 23] | |
| Erythrina excelsa Bak. | Bajjangala | Bark | Tree | Decoction | Half a glass 3 times a day for a week | No record | Wounds, candida | [10] | |
| Indigofera arrecta Hochst. Ex A. Rich | Omushoroza | Roots/bark | No record | Abdominal pain | [19] | ||||
| Indigofera congesta Baker | Namasumi | Twig | Herb | Infusion | Oral, dose not specified | No record | [8, 20] | ||
| Indigofera emerginella Steud. ex A. Rich | Omunyazabashumba | Leaves/roots | Shrub | Decoction | Oral, dose not specified | Investigated | Cough | [19] | |
| Macrotyloma axillare Verdc. | Akihabukuru | Leaves | No record | Impotence, dizziness | [19] | ||||
| Pseudarthria hookeri Wight & Arn | Omukongorani/kikakala | Leaves/whole plant | Herb | Decoction | One teaspoon thrice a day for 4 days | No record | Fever | [19, 20, 25, 29] | |
| Rhynchosia viscosa DC | Omutegansi | Flower | No record | Labour induction | [19] | ||||
| Senna absus (L.) Roxb. | Mucuula | Shrub | Leaves | Fresh extract | Oral, dose not specified | No record | Prolonged embryo in uterus | [8] | |
| Senna didymobotrya (Fresen.) H. S. Irwin & Barneby | Omugabagaba/kivumuzi | Herb | Leaves, twig | Decoction | Oral, dose not specified | Investigated | Change of sex of child | [8, 19, 20, 29] | |
| Senna siamea (Lam.) H. S. Irwin & Barneby | Garcia | Roots | Tree | Fresh extract | A cupful (500 ml) once a day for 3 days | Investigated | Abdominal pain, sore throat | [25, 27] | |
| Tamarindus indica L. | Cwaa/nkoge | Bark | Tree | Decoction | Oral, dose not specified | Investigated | Convulsions, fever | [8, 21] | |
| Flacourtiaceae | Ocoba spinosa Forssk | Ekalepulepu | Roots | Herb | Decoction | Oral, dose not specified | No record | Syphilis, skin problems, wounds, headache, impotence, stomach ache | [23] |
| Trimeria bakeri Gilg. | Omwatanshare | Leaves | Shrub | Decoction | Oral, dose not specified | Investigated | [24] | ||
| Hypericaceae | Harungana madagascariensis Lam. | Mukaabiransiko/mulirira | Bark | Tree | Decoction | 2 tablespoons thrice a day for 3 days | Investigated | Yellow fever | [8, 10] |
| Labiatae | Hyptis pectinata Poir. | Bongoloza | Whole plant | Herb | Decoction | Oral, dose not specified | No record | [20, 29] | |
| Lamiaceae | Aeolanthus repens Oliv. | Ntulagi | Leaves | Herb | Decoction | Quarter a glass thrice a day for 3 days | No record | [10] | |
| Ajuga remota Benth. | Kitinwa | Leaves | Herb | Decoction | Half a glass once a day for a week | Investigated | Stomach ache | [10] | |
| Clerodendrum myricoides R. Br. | Kikonge | Leaves | Shrub | Decoction | Half a glass daily for a week | Investigated | Syphilis, intestinal problems, induction of labour | [10, 28] | |
| Clerodendrum rotundifolium Oliv. | Kisekeseke | Roots/leaves | Shrub | Fresh leaf extract/root decoction | Half a glass daily for a 5 days | Investigated | Diabetes | [10] | |
| Hoslundia opposita Vahl. | Kamunye | Leaves | Herb | Decoction | Half a glass 3 times a day for a week; bath | Investigated | Ulcers | [8, 10, 25] | |
| Leonotis nepetifolia Schimp. exBenth | Kifumufumu | Whole plant | Herb | Decoction | A glassful thrice a day for 3 days | Investigated | Headache | [10, 21] | |
| Ocimum basilicum | Emopim | Leaves | Herb | Infusion | Half a glass 3 times a day for a week | Investigated | Fever, eye cataract | [23, 27] | |
| Ocimum gratissimum Willd. | Mujaaja | Leaves | Herb | Decoction | Half a glass 3 times a day for 5 days | Investigated | Wounds, ear infections, chest pain | [10, 21] | |
| Ocimum lamiifolium Hochst. | Omwenyi | Leaves | Decoction | Half a glass 3 times a day for a week | Investigated | Abdominal pain | [19] | ||
| Plectranthus barbatus | Ebiriri omutano | Whole plant/leaves, roots/stem | Herb | Infusion | Oral, dose not specified | Investigated | Fever, heart disease, snake bite | [10, 23] | |
| Plectranthus caninus Roth | Kibwankulata | Leaves | Herb | Decoction | 4 and 2 teaspoons thrice a day for adults and children, respectively, for a week | No record | [10] | ||
| Plectranthus cf. forskohlii | Ekizera | Leaves | Decoction | Oral, dose not specified | No record | [19] | |||
| Rosmarinus officinalis L. | Rosemary | Leaves | Herb | Decoction | Half a glass twice a day for 5 days | Investigated | Chest pain | [10] | |
| Tetradenia riparia (Hochst.) Codd | Kyewamala | Leaves | Herb | Decoction | One teaspoon twice a day for a week | Investigated | [10] | ||
| Lauranceae | Persea americana Mill. | Ovakedo | Leaves | Tree | Decoction | Oral, dose not specified | Investigated | Fungal and bacterial infection, high blood pressure, intestinal worms and parasites | [23] |
| Loranthaceae | Tapinanthus constrictiflorus (Engl.) Danser | Enzirugaze | Leaves | Herb | Decoction | A glass daily for 7 days | No record | [10] | |
| Malvaceae | Hibiscus surattensis L. | Nantayitwako musota | Leaves | Shrub | Decoction | Half a glass thrice a day for 7 days | No record | High blood pressure | [10] |
| Meliaceae | Azadirachta indica A. Juss. | Neem | Leaves | Tree | Decoction | About 120 ml once a day for 7 days | Investigated | Dental decay/ache, yellow fever, cough, skin diseases, diabetes, nausea | [10, 19, 23, 25] |
| Carapa grandiflora Sprague | Omukeete | Leaves/bark | Tree | Decoction | Half a glass twice a day for 7 days | No record | [10] | ||
| Melia azedarach | Elira | Leaves | Tree | Decoction | Oral, dose not specified | Investigated | Fever, skin disease, itching wounds, parasitic worms | [23] | |
| Menispermaceae | Cissampelos mucronata A. Rich. | Kavawala | Leaves/whole plant | Herb | Decoction | Half a glass twice a day for 5 days | Investigated | [10] | |
| Mimosaceae | Acacia hockii De willd | Ekisim | Roots | Tree | Decoction | Oral, dose not specified | No record | Diarrhea, syphilis, dysentery | [23, 30] |
| Acacia nilotica | Investigated | [31] | |||||||
| Acacia sieberiana | Etiriri | Roots | Tree | Decoction | Oral, dose not specified | No record | Dysentery, epilepsy, cough | [21, 23] | |
| Albizia coriaria Welw. | Lugavu | Bark | Tree | Decoction | 1 and 3 teaspoons thrice a day for children and adults, respectively, for a week. | Investigated | Skin diseases, diarrhea | [10] | |
| Albizia grandibracteata Taube | Nongo | Bark | Tree | Decoction | Half a glass once a day for a week | Investigated | Yellow fever, anemia, fungal infections of scalp | [8, 10, 32] | |
| Albizia zygia (DC.) Macbr. | Mulongo | Bark | Tree | Investigated | [21] | ||||
| Newtonia buchananii (Baker) Gilb. & Perr. | Mpewere | Bark | Tree | Dried, powdered, added to boiling water | Half a glass once a day for a week | No record | [10] | ||
| Moraceae | Antiaris toxicaria Lesch. | Kirundu | Bark | Tree | Decoction | Half a glass once a day for a week | Investigated | Weakness in pregnancy, headache | [8, 10] |
| Ficus natalensis Hochst | Tree | Investigated | Gonorrhea | [8, 33] | |||||
| Ficus saussureana DC. | Muwo | Bark | Tree | Decoction | Half a glass thrice a day for 7 days | No record | [10] | ||
| Milicia excels (Welw.) C. C. Berg. | Mivule | Bark | Tree | Decoction | Half a glass thrice a day for 7 days | Investigated | Burns, fresh cuts, skin rush | [8, 10] | |
| Moringaceae | Moringa oleifera Lam | Moringa | Leaves/roots | Tree | Decoction/chewed raw | A glassful thrice a day for 7 days; a handful of fresh leaves chewed 3 times for 4 days | Investigated | Joint pains | [21, 25] |
| Musaceae | Musa paradisiaca (NC) | Kabalagala | Leaves | Herb | Decoction | Oral, dose not specified | Investigated | Jaundice, prolonged embryo in uterus | [19] |
| Myricaceae | Myrica kandtiana Engl. (NC) | Omujeeje | Leaves | Decoction | Oral, dose not specified | No record | Vomiting, diarrhea | [19] | |
| Myristicaceae | Pycnanthus angolensis (Welw.)Warb. | Lunaba | Leaves | Tree | Decoction | Half a glass a day | Investigated | [10] | |
| Myrsinaceae | Maesa lanceolata Forssk. | Kiwondowondo | Leaves | Shrub | Decoction | Half a glass thrice a day for 7 days | Investigated | Febrile convulsions | [10, 19, 24] |
| Myrtaceae | Eucalyptus grandis Maiden. | Kalitunsi | Leaves | Tree | Decoction | Half a glass a day | No record | Cough | [8, 10] |
| Psidium guajava L. | Mupeera | Leaves | Tree | Decoction | Half a glass thrice a day for a week | Investigated | Bloody diarrhea, typhoid, wounds, cough | [10, 23] | |
| Syzygium cordatum Hochst. | Mugeege | Bark | Tree | Decoction | Oral, dose not specified | Investigated | Dry cough, skin rush, wounds | [8, 10, 20, 29] | |
| Syzygium cumini (L.) Skeels | Jambula | Leaves | Tree | Decoction | Half a glass thrice a day for a week | Investigated | Cough | [32] | |
| Syzygium guineense (Willd.) DC. | Kalunginsanvu | Bark | Tree | Decoction | Oral, dose not specified | Investigated | [20] | ||
| Papillionaceae | Butyrospermuum paradoxum | Ekunguri | Roots | Tree | Decoction | Oral, dose not specified | No record | Labour pains, headaches | [23] |
| Ormocarpum trachycarpum | Ederut | Roots | Shrub | Decoction | Oral, dose not specified | No record | Pneumonia, snake bite | [23] | |
| Passifloraceae | Passiflora edulis Sims | Akatunda | Leaves | Herb | Fresh extract | Oral, dose not specified | No record | Diarrhea, cough | [19] |
| Pittosporaceae | Pittosporum brachcalya | Not defined | Not defined | Shrub | No record | [34] | |||
| Pittosporum mannii Hook. f. Subsp. ripicola (J. Leon)Cuf. | Mubajjankon | Leaves | Shrub | Infusion/decoction | Half a glass a day for a week | No record | [10] | ||
| Poaceae | Cymbopogon citratus Stapf. | Kisubi | Leaves | Grass | Decoction | 120 ml every after 8 hours for a week | Investigated | Dental caries, influenza, cough, cancer, indigestion, fever | [10, 19, 23] |
| Digitaria scalarum Chiov. | Lumbugu | Leaves | Grass | Decoction | 120 ml every after 8 hours for a week | No record | [10] | ||
| Imperata cylindrical (L.) Beauv. var. africana (Anderss.) C. E. Hubbard | Lusenke | Roots | Grass | Dried, powdered, added boiling water/decoction | 120 ml once a day for a week | No record | Abdominal pain | [10] | |
| Zea mays L. | Luyange lwakasoli | Flowers/husks | Cereal grass | Decoction | 120 ml every after 8 hours for a week | Investigated | Boosts immunity | [10] | |
| Polygalaceae | Securidaca longipedunculata Fresen. | Eliloi | Roots | Shrub | Decoction | Oral, dose not specified | Investigated | Skin diseases, measles, cough, hernia, diarrhea | [23] |
| Maesopsis eminii Engl. | Musizi | Bark | Tree | Decoction | Half a glass thrice a day for a week | No record | [10] | ||
| Portulacaceae | Talinum portulacifolium (Forssk.) Asch. ex Schweinf. | Mpozia | Leaves | Herb | Oral, dose not specified | No record | [21] | ||
| Rosaceae | Prunus africana (Hook. f.) Kalkman | Ntaseesa or Ngwabuzito | Bark | Tree | Decoction | 2 and 3 teaspoons thrice a day for children and adults, respectively, for a week | Investigated | Fainting, cancer | [8, 10] |
| Rubus steudneri schweinf. | Nkenene | Leaves | Herb | Decoction | Half a glass once a day for a week | No record | [10] | ||
| Rubiaceae | Coffea canephora Froehner | Mwanyi | Leaves | Shrub | Decoction | Oral, dose not specified | No record | [21] | |
| Hallea rubrostipulata (K. Schum.) J.-F. Leroy | Muziku | Bark | Tree | Decoction | Oral, dose not specified | Investigated | [20] | ||
| Pentas longiflora Oliv. | Ishagara | Leaves | Decoction | Oral, dose not specified | Investigated | Fever | [19] | ||
| Vangueria apiculata K. Schum. | Matugunda | Bark | Shrub | Decoction | 2 and 3 teaspoons thrice a day for children and adults, respectively, for a week | No record | [10] | ||
| Rutaceae | Citrus reticulata | Omuqugwa | Roots | Tree | Decoction | Oral, dose not specified | Investigated | Weight loss induction, cancer, skin diseases | [23] |
| Citrus sinensis | Omucungwa/cungwa | Roots | Tree | Decoction | Oral, dose not specified | Investigated | Vomiting, cough, diabetes | [21, 23, 25] | |
| Teclea nobilis Delile | Omuzo | Aerial parts | Decoction | Oral, dose not specified | Investigated | Body cleanser | [32] | ||
| Toddalia asiatica Baill. | Kawule | Roots | Climber | Decoction | Half a glass thrice a day for a week | Investigated | Cough, abdominal pain | [10, 19, 24] | |
| Zanthoxylum chalybeum Engl. | Ntale ya ddungu | Roots | Tree | Decoction | Oral, dose not specified | Investigated | Body swellings, stomach ache, cough, fever, chest pain | [10, 23, 28] | |
| Zanthoxyllum leprieurii Guill. & Perr. | Mutatembwa/munyenye | Bark | Tree | Decoction drunk | Half a glass thrice a day for a week | No record | [10] | ||
| Salicaceae | Trimeria grandifolia ssp. tropica (Hochst.) Warb. | Omwatanshare | Leaves | Decoction | Oral, dose not specified | Investigated | [19] | ||
| Sapindaceae | Blighia unijugata Baker | Nkuzanyana | Bark | Tree | Decoction drunk | Half a glass twice a day for a week | Investigated | Wounds, vomiting, skin diseases, fibroids, cervical cancer | [8, 10] |
| Sapotaceae | Manilkara obovata (Sabine & G. Don) | Nkunya | Bark | Tree | Decoction | Oral, dose not specified | No record | [20] | |
| Scrophulariaceae | Sopubia ramosa (Hochst.) Hochst. | Kakulunkanyi | Whole plant | Herb | Decoction | Oral, dose not specified | No record | [20] | |
| Simaroubaceae | Harrisonia abyssinica Olive. | Ekeroi | Roots/leaves | Shrub | Decoction | Oral, dose not specified | Investigated | Fever, wounds, syphilis, snake bite, abdominal pain | [23] |
| Solanaceae | Datura stramonium Thunb. | Amadudu | Leaves | Herb | Decoction drunk | Half a glass thrice a day for a week | No record | Ulcers, stomach ache, chest pain | [10] |
| Physalis peruviana L. | Ntuntunu | Leaves | Herb | Decoction drunk | Half a glass 3 times a day for a week | No record | Vomiting, febrile convulsions, fainting | [8, 10, 19] | |
| Solanum nigrum L. | Nsugga | Leaves | Herb | Decoction drunk | Half a glass 3 times a day for a week | Investigated | Ear infection, headache, epilepsy, STI, diarrhea | [8, 10] | |
| Tiliaceae | Trumfetta rhomboidea Jacq. | Musombankoko | Roots | Shrub | Decoction drunk | Half a glass once a day for a week | No record | [10] | |
| Ulmaceae | Celtis africana L. | Akasisa | Leaves | Tree | Decoction drunk | Half a glass a day for a week | Investigated | [10] | |
| Umbelliferae | Steganotania araliacea Hoeshst | Ematule | Roots/leaves | Tree | Decoction | Oral, dose not specified | No record | Measles, body swelling | [23] |
| Verbenaceae | Lantana camara | Kanpanga | Leaves | Shrub | Decoction | Oral, dose not specified | Investigated | Wounds, measles, tuberculosis, pneumonia, snake bite, chest pain | [23] |
| Lantana trifolia L. | Omuhukye | Leaves | Decoction | Orally taken, dose not specified | Investigated | Yellow fever, ringworms, muscle pain, prolapsed rectum | [8, 19] | ||
| Zingiberaceae | Curcuma longa L. | Binjali | Rhizome | Herb | Fresh extract | 30 ml thrice a day for 3 days | Investigated | [28] |
Table 2.
Some herbs used in malaria prevention amongst communities in Uganda.
| Plant family | Plant species | Local name | Plant form | Mode of use to prevent malaria | Reference(s) |
|---|---|---|---|---|---|
| Cleomaceae | Cleome gynandra L. | Akeyo | Herb | Leaves are cooked and eaten as a prophylactic measure | [25] |
| Cucurbitaceae | Cucurbita maxima Duchesne | Acuga | Scrambler | Leaves cooked and pasted with groundnut then eaten | [25] |
| Euphorbiaceae | Manihot esculenta Crantz | Gwana | Herb | Tuber peelings are dried then burnt in house so that smoke repels mosquitoes | [25] |
| Fabaceae | Crotalaria ochroleuca G. Don | Alayo | Herb | Leaves are cooked and eaten as a prophylactic measure | [25] |
| Lamiaceae | Ocimum forsskaolii Benth. | Yat cola | Herb | Leaves dried and burnt so that smoke chases away mosquitoes; bath infusion to repel mosquito | [25] |
| Rosmarinus officinalis L. | Rosemary | Herb | Leaves are cooked and eaten as a prophylactic measure; planted around the house to repel mosquitoes | [10] | |
| Malvaceae | Gossypium hirsutum L. | Pama | Shrub | Cotton lint is dried and burnt so that smoke keeps away mosquitoes | [25] |
| Musaceae | Musa sp. | Labolo kwon | Shrub | Fruit peeling are dried and burnt in the house to produce smoke that keeps away mosquitoes | [25] |
| Myrtaceae | Eucalptus grandis Maiden. | Kalitunsi | Tree | Leave and branches are burnt to repel mosquitoes | [25] |
| Poaceae | Cymbopogon citratus Stapf. | Kisubi | Grass | Planted around the house to repel mosquitoes; taken in tea as a prophylactic measure | [19, 23] |
| Solanaceae | Solanum americanum Mill. | Ocuga | Herb | Leaves are cooked and eaten as a prophylactic measure | [25] |
The mechanism of action of artemisinin is widely debated but the most accepted theory is that of activation of the molecule by heme, which enables it to produce free radicals that then destroy the proteins needed for parasite survival [36]. The presence of an uncommon chemical peroxide linkage bridge in artemisinin, a sesquiterpene lactone, is the most probable reason for its antimalarial effects. Cleavage of the peroxide linkage bridge in the presence of iron (II) ions (from heme) forms very reactive free radicals that undergo rapid rearrangement to form more stable carbon-centered radicals, which chemically modify the parasite and inhibit various processes within the parasite molecules, resulting in its death [36]. Artemisinin acts on primarily the trophozoite parasitic phase and prevents disease progression. It kills circulating ring-stage parasites, thus increasing the therapeutic response [37]. Mok et al. [38] suggested that artemisinin is linked to the upregulation of unfolded protein response pathways, which leads to decreased parasitic growth and development. Shandilya et al. [39] suggested that artemisinin is activated by iron, which then functionally inhibits PfATP6, a calcium pump, by terminating phosphorylation, nucleotide binding, and actuator domains, eventually leading to a functional loss of PfATP6 of the Plasmodium parasite and its death. A study by Mbengue et al. [40] indicated that artemisinin strongly inhibits phosphoinositide-3-kinase (PfPI3K), an enzyme important in cellular activities including growth, multiplication, differentiation, and survival in P. falciparum.
Cinchona tree bark, from which quinine was isolated, has been used to treat malaria since 1632 [41]. The World Health Organization listed quinine as one of the important medicines needed in a health system [42]. It is however only used to treat malaria caused by chloroquine-resistant strain of P. falciparum in the absence of artemisinins [43]. A popular hypothesis about the mechanism of action of quinine is based on chloroquine, another quinoline drug which is closely linked to quinine and has been comprehensively studied. Quinine inhibits the pathway of biocrystallization of hemozoin, resulting in the accumulation of the free cytotoxic heme which eventually kills the parasite [44].
Most of the plants used in the treatment of malaria in Uganda contain alkaloids greatly implicated in antiplasmodial activity (Table 3). A number of alkaloids target apicoplast, an organelle in the Plasmodium parasite, while others such as benzylisoquinoline alkaloids in Cissampelos mucronata, a plant belonging to the family Menispermaceae inhibits protein synthesis in the parasite [99].
Table 3.
Antiplasmodial/antimalarial activities of investigated plants used for malaria treatment in Uganda and their active chemical constituents.
| Plant family | Scientific name | Part used | Extracting solvent | Means of traditional extraction | Report on antiplasmodial, IC50 (μg/ml)/antimalarial activity (Plasmodium strain) | Active chemical constituents | Reference(s) |
|---|---|---|---|---|---|---|---|
| Acanthaceae | Justicia betonica L. | Shoot | Methanol | Hot water | 69.6 (chloroquine sensitive, K39) | Justetonin (indole(3,2-b) quinoline alkaloid glycoside) | [20] |
| Water | >100 (chloroquine sensitive, K39) | ||||||
| Aloeaceae | Aloe dawei A. Berger (wild/cultivated) | Leaves | Ether | Cold water; mashing; hot water | Extract had anti-P. falciparum activity value of 7.97 (95% CI: 3.56 to 17.85) μg/ml with 50% schizonts suppression per 200 WBC (EC50) | Anthraquinones, aloin, lectins, | [19, 45] |
| Aloe kedongensis (wild) | Leaves | Methanol | Hot water | 87.7 (chloroquine sensitive, D6); 67.8 (chloroquine resistant, W2) | Anthrone, C-glucoside homonataloin, anthraquinones, aloin, lectins | [19, 46] | |
| Aloe ferox Mill | Leaves | Dichloromethane | Water | 21 (chloroquine sensitive, D10) | Mannans, polymannans, anthraquinones, aloin, lectins, anthrones | [19, 31, 47] | |
| Water | >100 (chloroquine sensitive, D10) | ||||||
| Anacardiaceae | Mangifera indica L. | Leaves | Chloroform:Methanol (1 : 1) | Hot water | Inhibited growth of P. falciparum by 50.4% at 20 μg/ml | Phenolics | [48, 49] |
| Stem bark | Ethanol | >50 (chloroquine resistant, FcB1) | |||||
| Rhus natalensis Bernh. Ex Krauss | Leaves | Ethanol | Hot water | 6.6 (P. falciparum) | Triterpenoids | [24] | |
| Apiaceae | Centella asiatica (L.) Urb. | Whole plant | Water | Water | 58.6 (chloroquine sensitive, D6); not detected (chloroquine resistant, W2) | Phenolics and flavonoids | [50] |
| Apocynaceae | Alstonia boonei De Wild. | Stem bark | Water | Hot water | 80.97% suppressive activity at 200 mg/kg (P. berghei) in combination with other two local herbs. | Alkaloids, triterpenoids | [51] |
| Carissa edulis (Forssk.) Vahl | Stem bark | Dichloromethane | Mashing; hot water | 33 (chloroquine sensitive, D10) | Lignan, nortrachelogenin | [52] | |
| Carissa spinarum Lodd. ex A. DC. | Root bark | Methanol | Hot water | 14.5 (chloroquine sensitive, D6) | Saponins, sesquiterpenes | [53] | |
| Catharanthus roseus G. Don | Leaves | Methanol | Hot water | 4.6 (chloroquine sensitive, D6); 5.3 (chloroquine resistant, W2) | Alkaloids, terpenoids, flavonoids, esquiterpenes | [54] | |
| Aristolochiaceae | Aristolochia elegans Mast. | Seeds | Methanol | Water | >50 (chloroquine sensitive, 3D7); undetectable (chloroquine resistant, W2) | Sesquiterpenoids, diterpenoids, monoterpenoids, alkaloids | [19, 55] |
| Asphodelaceae | Aloe vera (L.) Burm. f. | Leaves | Water | Cold water; mashing; hot water | Antiplasmodial activity in terms of EC50 values 0.289 to 1.056 μg/ml (chloroquine sensitive) | Aloin, anthraquinones, aloe-emodin | [56] |
| Asteraceae | Ageratum conyzoides L. | Whole plant | Methanol | Hot water | 11.5 (chloroquine sensitive, D6); 12.1 (chloroquine resistant, W2) | Flavonoids | [54] |
| Artemisia annua L. | Leaves | Water | Hot water | 1.1 (chloroquine sensitive, D10); 0.9 (chloroquine resistant, W2) | Sesquiterpenes and sesquiterpene lactones including artemisinin, flavonoids such as chrysoplenol-D, eupatorin, chyrsoplenetin | [19, 57] | |
| Artemisia afra Jacq. Ex Willd | Leaves | Methanol | Hot water | 9.1 (chloroquine sensitive, D6); 3.9 (chloroquine resistant, W2) | Acacetin, genkwanin, 7-methoxyacacetin | [54] | |
| Aspilia africana (Pers.) C. D. Adams | Leaves | Ethanol | Hot water | Significant chemo suppressive effect of 92.23% (400 mg/kg) on P. berghei | Saponins, terpenoids, alkaloids, resins, tannins, flavonoids, sterols | [19, 58] | |
| Baccharoides adoensis (Sch. Bip. ex Walp.) H. Rob. | Leaves | Petroleum ether | Hot water | 4.6 (chloroquine resistant, K1) | Flavonoids | [26] | |
| Aspilia africana L. | Leaves | Dichloromethane | Hot water; mashing | 8.5 (chloroquine sensitive, D10) | Flavonoids including quercetin 3,3′-dimethyl ether 7-0-α-L-rhamnopyranosyl-(1 ⟶ 6)-β-D-glucopyranose and quercetin 3,3′-dimethyl ether 7-0-β-D-glucopyranose | [52] | |
| Bothriocline longipes N. E. Br. | Leaves | Chloroform | Hot water | 3.7 (P. falciparum) | 5-alkylcoumarins, | [19, 24] | |
| Ethanol | 50 (P. falciparum) | ||||||
| Crassocephalum vitellinum | Leaves | Ethyl acetate | Hot water | 40.6% inhibition of P. falciparum at 10 μg/ml | Flavonoids | [32] | |
| Guizotia scabra Chiov. | Whole plant | Crude ethanol | Hot water | 49.09% growth inhibition at 100 μg/ml (chloroquine resistant, Dd2) | Lactones, eudesmanoline | [59] | |
| Melanthera scandens (Schumach. & Thonn.) Roberty | Leaves | Chloroform | Hot water | 68.83% chemo suppression activity (P. berghei) | Triterpenoid saponins | [60] | |
| Microglossa pyrifolia (Lam.)O. Ktze | Leaves | Hot water | <5 (both chloroquine sensitive, NF54 and resistant, FCR3) | E-phytol; 6e-geranylgeraniol-19-oic acid | [2, 28] | ||
| Schkuhria pinnata (lam.) | Whole plant | Water | Hot water | 22.5 (chloroquine sensitive, D6); 51.8 (chloroquine resistant, W2) | Schkuhrin I and schkuhrin II | [54] | |
| Methanol | 1.3 (chloroquine sensitive, D6); 6.8 (chloroquine resistant, W2) | ||||||
| Solanecio mannii (Hook. f.) C. Jeffrey | Leaves | Methanol | Water | 21.6 (chloroquine sensitive, 3D7); 26.2 (chloroquine resistant, W2) | Phytosterols, n-alkanes and N-hexacosanol, | [19, 55] | |
| Tagetes minuta L. | Leaves | Ethyl acetate | Water | 61.0% inhibition of P. falciparum at 10 μg/ml | [32] | ||
| Tithonia diversifolia A. Gray | Leaves | Methanol | Water | 1.2 (chloroquine sensitive, 3D7); 1.5 (chloroquine resistant, W2) | Tagitinin C, sesquiterpene lactones | [55] | |
| Vernonia adoensis Sch. Bip. ex Walp. | Leaves | Methanol | Hot water | 83.4% inhibition of parasitaemia, at 600 mg/kg (P. berghei) | Glycocides, glaucolides | [19, 61] | |
| Vernonia amygdalina Delile | Leaves | Methanol/dichloromethane | Hot water; cold water | 2.7 (chloroquine resistant, K1) | Coumarin, sesquiterpene lactones including vernolepin, vernolin, vernolide, vernodalin and hydroxyvernodalin, steroid glucosides | [19, 26] | |
| Vernonia cinerea (L.) Less. | Whole plant | Water | Hot water | >50 (chloroquine sensitive, 3D7); 37.2 (chloroquine resistant, K1) | Sesquiterpene lactone | [62] | |
| Vernonia lasiopus O. Hoffm. | Leaves | Methanol | Mashing; hot water | 44.3 (chloroquine sensitive, D6); 52.4 (chloroquine resistant, W2) | Sesquiterpene lactones, polysaccarides | [19, 54] | |
| Bignoniaceae | Markhamia lutea (Benth.) K. Schum. | Leaves | Ethyl acetate | Hot water | 71% inhibition of P. falciparum at 10 μg/ml | Phenylpropanoid glycosides, cycloartane triterpenoids | [32] |
| Spathodea campanulata Buch.-Harm. ex DC. | Stem bark | Ethyl acetate | Water | 28.9% inhibition of P. falciparum at 10 μg/ml | Quinone (lapachol) | [32] | |
| Caesalpiniaceae | Cassia didymobotrya Fres. | Leaves | Methanol | Hot water | 23.4 (chloroquine sensitive, D6); undetectable (chloroquine resistant, W2) | Alkaloids | [54] |
| Erythrophleum pyrifolia | Leaves | Ethanol | Hot water | >50 (P. falciparum) | [24] | ||
| Senna spectabilis (DC.) H. S. Irwin & Barneby | Leaves | Ethanol | Water | 59.29% growth inhibition at 100 mg/kg body weight dose (P. berghei) | Piperidine alkaloids | [63] | |
| Caesalpinioideae | Cassia hirsuta | Root back | Methanol | Water | 32.0 (chloroquine sensitive 3D7) | [64] | |
| Canelliaceae | Warbugia ugandensis Sprague | Stem back | Methanol | Hot water | 6.4 (chloroquine sensitive, D6); 6.9 (chloroquine resistant, W2) | Sesquiterpenes e.g. muzigadiolide | [27, 54] |
| Water | 12.9 (chloroquine sensitive, D6); 15.6 (chloroquine resistant, W2) | ||||||
| Caricaceae | Carica papaya L. | Leaves | Ethyl acetate | Hot water | 2.96 (chloroquine sensitive, D10); 3.98 (chloroquine resistant, DD2) | Alkaloids, saponins, tannins, glycosides | [65] |
| Methanol | 10.8 (chloroquine sensitive, D10) | ||||||
| Celastraceae | Maytenus senegalensis | Roots | Hot water | 1.9 (chloroquine sensitive, D6); 2.4 (chloroquine resistant, W2) | Terpenoids, pentacyclic triterpenes e.g. pristimerin | [66] | |
| Chenopodiaceae | Chenopodium ambrosioides L. | Leaves | Crude hydroalcoholic extract | Hot water | Inhibited the P. falciparum growth, exhibiting an IC50 of 25.4 μg/ml | Sesquiterpenes, monoterpenes | [67] |
| Combretaceae | Combretum molle G. Don | Stem back | Acetone | Water | 8.2 (chloroquine sensitive 3D7) | Phenolics, punicalagin | [68] |
| Cucurbitaceae | Cucurbita maxima Lam. | Seeds | Crude ethanol | Hot water | 50% reduction of parasitaemia levels in P. berghei infected mice at 500 mg/kg. | Phenols, terpenoids, alkaloids, tannins | [69] |
| Momordica foetida Schumach. | Shoot | Water | Hot water | 6.16 (chloroquine sensitive, NF54); 0.35 (chloroquine resistant, FCR3) | Saponins, alkaloid, cardiac glycosides | [28] | |
| Ebenaceae | Euclea latideus Staff | Root back | Hexane | Water | 38.2 (chloroquine sensitive, 3D7); 38.9 (chloroquine resistant, Dd2) | Triterpenoids lupeol, betulin, 3β-(5-hydroxyferuloyl)lup-20(30)-ene | [23] |
| Euphorbiaceae | Alchornea cordifolia (Schumach.) Mull. Arg. | Leaves | Water | Hot water | 4.8 (chloroquine resistant, K1) | Phenolics including ellagic acid | [70] |
| Bridelia micrantha Baill. | Stem bark | Methanol | Hot water | 19.4 (chloroquine sensitive, D6); 14.2 (chloroquine resistant, W2) | [50] | ||
| Clutia abyssinica Jaub. & Spach | Leaves | Methanol | Water | 7.8 (chloroquine sensitive, D6); 11.3 (chloroquine resistant, W2) | Diterpenes | [54] | |
| Croton macrostachyus Olive. | Leaves | Chloroform | Hot water | Chemotherapeutic effect of 66–82% in malaria mouse model | Triterpenoids including lupeol | [71] | |
| Fluegea virosa (Roxb. ExWillb.)Voigt | Leaves | Water/methanol | Hot water | 2 (chloroquine resistant, W2) | Bergenin | [72] | |
| Jatropha curcas L. | Leaves | Ethyl acetate | Hot water | 5.1 (chloroquine sensitive, NF54); 2.4 (chloroquine resistant, K1) | Alkaloids, saponnins, glycosides, tannins | [73] | |
| Phyllanthus (pseudo) niruri Mull. Arg. | Water | Hot water | Ranged from 2.9 to 4.1 (both chloroquine sensitive, 3D7 and resistant, Dd2) | Coumarins including 1-O-galloyl-6-O-luteoyl-a-D-glucose | [74] | ||
| Fabaceae | Cajanus cajan (L.) Druse | Leaves | Crude ethanol | Mashing | 29.0 (P. falciparum) | Cajachalcone; | [75] |
| Entada abyssinica Steud. ex A. Rich. | Seeds | Methanol | Hot water | >5 (chloroquine resistant, K1) | Flavonoids, terpenoids | [26, 32] | |
| Entada africana Guill. & Perr. | Leaves | Ethanol | Hot water | 26.4 (chloroquine sensitive, HB3); 28.9 (chloroquine resistant, FcM29) | Phenolics | [76] | |
| Erythrina abyssinica Lam. | Stem bark | Ethyl acetate | Hot water | 83.6% inhibition of P. falciparum at 10 μg/ml | Chalcones (5-prenylbutein, homobutein), flavanones including 5-deoxyabyssinin II, abyssinin III and abyssinone IV | [32] | |
| Indigofera emerginella Steud. ex A. Rich | Leaves | Ethanol | Hot water | 5.8 (P. falciparum) | [24] | ||
| Senna didymobotrya (Fresen.) H. S. Irwin & Barneby | Leaves | Methanol | Hot water | >100 (chloroquine sensitive, K39) | Quinones | [20, 29] | |
| Senna siamea (Lam.) H. S. Irwin & Barneby | Leaves | Ethanol | Mashing; hot water | 28.8 (chloroquine sensitive, 3D7); 48.3 (chloroquine resistant, W2) | Phenolic derivative, chrobisiamone a, anhydrobarakol | [77] | |
| Tamarindus indica L. | Stem bark | Water | Hot water | 25.1% chemo suppressive activity at 10 mg/kg (P. berghei) | Saponins (leaves), tannins (fruits) | [78] | |
| Flacourtiaceae | Trimeria bakeri Gilg. | Leaves | Petroleum ether | Hot water | 3.9 (P. falciparum) | Triterpenoids | [24] |
| Hypericaceae | Harungana madagascariensis Lam. | Stem bark | Water | Hot water | 9.64 (chloroquine resistant, K1) | Quinones including bazouanthrone, feruginin a, harunganin, harunganol a | [70] |
| Lamiaceae | Ajuga remota Benth. | Whole plant | Ethanol | Hot water | 55 (chloroquine sensitive, FCA/GHA); 57 (chloroquine resistant, W2) | Ajugarin-1, ergosterol-5,8-endoperoxide, 8-O-acetylharpagide, steroids | [79] |
| Clerodendrum myricoides R. Br. | Root bark | Methanol | Hot water | 4.7 (chloroquine sensitive, D6); 8.3 (chloroquine resistant, W2) | [50, 80] | ||
| Clerodendrum rotundifolium Oliv. | Leaves | Methanol | Mashing; hot water | <5 (both chloroquine sensitive, NF54 and resistant, FCR3) | Saponins, tannins | [28] | |
| Hoslundia opposita Vahl. | Leaves | Ethyl acetate | Hot water | 66.2% inhibition of P. falciparum at 10 μg/ml | Quinones, saponins, abietane diterpenes (3-O-benzoylhosloppone) | [32] | |
| Leonotis nepetifolia Schimp. exBenth | Leaves | Ethyl acetate | Water | 27.0% inhibition of P. falciparum at 10 μg/ml | [32] | ||
| Ocimum basilicum | Leaves | Ethanol | Hot water | 68.14 (chloroquine sensitive, CQ-s); 67.27 (chloroquine resistant, CQ-r) | [50, 80] | ||
| Ocimum gratissimum Willd. | Leaves/twigs | Dichloromethane | Hot water | 8.6 (chloroquine resistant, W2) | Flavonoids | [47, 49] | |
| Ocimum lamiifolium Hochst. | Leaves | Water | Water | Significantly suppressed parasitaemia, 22.2%, 26.8% and 35.5% at dose of 200, 400 and 600 mg·kg, respectively (P. berghei) | [81] | ||
| Plectranthus barbatus | Leaves/stem | Dichloromethane | Hot water | No activity | [23, 47] | ||
| Rosmarinus officinalis L. | Hot water | Essential oil at a concentration 15867 ng/ml had no antimalarial activity | [82] | ||||
| Tetradenia riparia (Hochst.) Codd | Root | Hot water | 13.2 (chloroquine-sensitive, NF54) | [83] | |||
| Lauranceae | Persea americana Mill. | Leaves | Ethanol | Hot water | 10.15 (chloroquine sensitive, 3D7); 44.94 (chloroquine resistant, W2) | Phenolics | [84] |
| Meliaceae | Azadirachta indica A. Juss. | Leaves | Hot water | 17.9 (chloroquine sensitive, D6); 43.7 (chloroquine resistant, W2) | Terpenoids, isoprenoids, gedunin | [49, 66] | |
| Melia azedarach | Leaves | Methanol | Hot water | 55.1 (chloroquine sensitive, 3D7); 19.1 (chloroquine resistant, W2) | [85] | ||
| Menispermaceae | Cissampelos mucronata A. Rich. | Root bark | Methanol | Hot water | 8.8 (chloroquine sensitive, D6); 9.2 (chloroquine resistant, W2) | Benzylisoquinoline alkaloids | [80] |
| Mimosaceae | Acacia nilotica | Stem bark | Methanol | Hot water | Dose of 100 mg/kg b/w produced parasitic (P. berghei) inhibition 77.7% | Tannins, flavonoids, terpenes | [86] |
| Albizia coriaria Welw. | Stem bark | Methanol | Hot water | 15.2 (chloroquine sensitive, D6); 16.8 (chloroquine resistant, W2) | Triterpenoids, lupeol, lupenone | [54] | |
| Albizia grandibracteata Taube | Leaves | Ethyl acetate | Hot water | 22.0% inhibition of P. falciparum at 10 μg/ml | [32] | ||
| Albizia zygia (DC.) Macbr. | Stem bark | Methanol | Water | 1.0 (chloroquine resistant, K1) | Flavonoids mainly 3′,4′,7-trihydroxyflavone | [87] | |
| Moraceae | Antiaris toxicaria Lesch. | Stem bark | Ethyl acetate | Hot water | 36.4% inhibition of P. falciparum at 10 μg/ml | [32] | |
| Ficus natalensis Hochst | Leaves | Hexane | Hot water | 6.7 (P. falciparum) | [88] | ||
| Milicia excels (Welw.) C. C. Berg. | Leaves | Ethanol | Hot water | 76.7% chemo suppressive activity at 250 mg/kg/day (P. berghei) | [89] | ||
| Moringaceae | Moringa oleifera Lam | Leaves | Methanol | Mashing; hot water | 9.8 (chloroquine sensitive, D6); not detected (chloroquine resistant, W2) | Flavonols | [49, 80] |
| Musaceae | Musa paradisiaca (NC) | Leaves | Ethyl acetate | Hot water | 75 (chloroquine sensitive, 3D7); 100 (chloroquine resistant, Dd2) | Flavonoids | [49, 90] |
| Myristicaceae | Pycnanthus angolensis (Welw.)Warb. | Leaves | 50% ethanol | Hot water | >1000 (chloroquine sensitive, 3D7) | Talaumidin | [91] |
| Myrsinaceae | Maesa lanceolata Forssk. | Twig | Dichloromethane:Methanol (1 : 1) | Hot water | 5.9 (chloroquine sensitive, D10) | Lanciaquinones, 2,5, dihydroxy-3-(nonadec-14-enyl)-1,4-benzoquinone | [24, 52, 55] |
| Myrtaceae | Psidium guajava L. | Stem back | Water | Hot water | 10–20 (chloroquine sensitive, D10) | Phenols, flavonoids, carotenoids, terpenoids | [49, 92] |
| Syzygium cordatum Hochst. | Twig | Dichloromethane:Methanol (1 : 1) | Hot water | 14.7 (chloroquine sensitive, D10) | [55] | ||
| Syzygium cumini (L.) Skeels | Stem back | Hot water | 0.25 to 27.1 (chloroquine-resistant strains) | [93] | |||
| Syzygium guineense (Willd.) DC. | Leaves | Crude ethanol | Hot water | 49.09% chemo suppression at 400 mg/kg (P. berghei) | [94] | ||
| Poaceae | Cymbopogon citratus Stapf. | Whole plant | Hot water | 99.89% suppression of parasitaemia at 1600 mg/kg | Flavonoids | [20, 49, 95] | |
| Zea mays L. | Husks | Ethyl acetate | Hot water | 9.3 (chloroquine sensitive, 3D7); 3.7 (chloroquine resistant, INDO) | Alkaloids, flavonoids and triterpenoids | [96] | |
| Polygalaceae | Securidaca longipedunculata Fresen. | Leaves | Dichloromethane | Hot water | 6.9 (chloroquine sensitive, D10) | Saponins, flavonoids, alkaloids, steroids | [92] |
| Rosaceae | Prunus africana (Hook. f.) Kalkman | Stem bark | Methanol | Hot water | 17.3 (chloroquine sensitive, D6); not detected (chloroquine resistant, W2) | Terpenoids | [54] |
| Rubiaceae | Hallea rubrostipulata (K. Schum.) J.-F. Leroy | Root | Ethanol | Water | 100 μg/ml extract had 65.54% growth inhibition (chloroquine resistant, Dd2) | Alkaloids | [59] |
| Pentas longiflora Oliv. | Root | Methanol | Hot water | 0.99 (chloroquine sensitive, D6); 0.93 (chloroquine resistant, W2) | Pyranonaphthoquinones, pentalongin (1) and psychorubrin (2), naphthalene derivative mollugin (3) | [97] | |
| Rutaceae | Citrus reticulata | Seeds (isolimonexic acid methyl ether) | Hot water | <4.76 (both chloroquine sensitive, D6 and resistant, W2) | Limonin, isolimonexic acid methyl ether, ichangin, deacetylnomilin, obacunone | [98] | |
| Citrus sinensis | 70% ethanol | Hot water | 53.27% suppression of parasitaemia at 700 mg/kg | Tannins, alkaloids, saponins, flavonoids | [20, 24, 99] | ||
| Teclea nobilis Delile | Bark | Ethyl acetate | Water | 54.7% inhibition of P. falciparum at 10 μg/ml | Quinonline alkaloids | [32] | |
| Toddalia asiatica Baill. | Root bark | Methanol | Water | 6.8 (chloroquine sensitive, D6); 13.9 (chloroquine resistant, W2) | Furoquinolines (nitidine, 5,6-dihydronitidine), coumarins | [80] | |
| Zanthoxylum chalybeum Engl. | Stem bark | Water | Hot water | 4.3 (chloroquine sensitive, NF54); 25.1 (chloroquine resistant, FCR3) | Chelerythine, nitidine, methyl canadine | [28] | |
| Salicaceae | Trimeria grandifolia ssp. tropica (Hochst.) Warb. | Leaves | Methanol | Hot water | >50 (chloroquine sensitive, 3D7) | [55] | |
| Sapindaceae | Blighia unijugata Baker | Leaves | Ethyl acetate | Hot water | 2.3% inhibition of P. falciparum at 10 μg/ml | [32] | |
| Simaroubaceae | Harrisonia abyssinica Olive. | Roots | Hot water | 4.4 (chloroquine sensitive, D6); 10.25 (chloroquine resistant, W2) | Limonoids, steroids | [66] | |
| Solanaceae | Solanum nigrum L. | Fruit | Methanol | Hot water | 10.3 (chloroquine sensitive, 3D7); 18.7 (chloroquine resistant, K1) | Steroidal alkaloids, flavonoids | [100] |
| Ulmaceae | Celtis africana L. | Stem bark | Ethyl acetate | Hot water | 37.5% inhibition of P. falciparum at 10 μg/ml | [32] | |
| Verbenaceae | Lantana camara | Leaves | Dichloromethane | Hot water | 8.7 (chloroquine sensitive, 3D7); 5.7 (chloroquine resistant, W2) | Sesquiterpenes, triterpenes, flavonoids | [30] |
| Lantana trifolia L. | Arial parts | Petroleum ether | Hot water | 13.2 (P. falciparum) | Steroids, terpenoids, alkaloids, saponins | [24] | |
| Ethanol | >50 (P. falciparum) | ||||||
| Zingiberaceae | Curcuma longa L. | Hot water; mashing | 5 mg/kg had a significantly high chemo suppressive activity of 56.8% (P. berghei) | Polyphenolic curcumin | [101] |
Flavonoids in a vast number of plants used for malaria treatment in Uganda are common to plants in the family Asteraceae such as B. longipes, A. conyzoides, and A. africana although other herbal plants from different families including C. roseus in Apocynaceae and A. zygia and A. nilotica in Mimosaceae also have them as active antiplasmodial constituents (Table 3). Flavonoids exhibit great antiplasmodial activity against different strains of the malaria parasite although the mechanism of antimalarial action is not clear [99]. Some studies suggest that flavonoids impede the influx of myoinositol and L-glutamine in erythrocytes that are infected [99]. Some flavonoids increase the level of oxidation of erythrocytes and inhibit protein synthesis in malaria parasites [99]. Furthermore, flavonoids are believed to inhibit fatty acid biosynthesis (FAS II) in Plasmodium [102].
Artemisinin resistance in P. falciparum has been reported in Vietnam, Cambodia, Muang Lao, and Thailand. A report published in 2018 showed over 30 separate cases in Southeast Asia of artemisinin resistance [36]. In case of resistance, parasitic clearance is slowed down and gametocytemia increases, resulting in greater selective pressure on other partner drugs to which resistance increases, thereby posing a great health threat. Thus, it is very important that the discovery of other drugs with novel mechanisms of action be prioritized by extensive exploration of the huge medicinal plant resources in Africa, which have been used by locals for effective malaria treatment yet have never been scientifically investigated for their antimalarial potential. Amoa Onguéné et al. [35] emphasized that it was indeed Africa's turn to offer a new antimalarial drug to humanity since artemisinin was discovered in Asia and quinine in Latin America.
5. Herbs and Plant Parts Used to Manage and Treat Malaria across Communities in Uganda
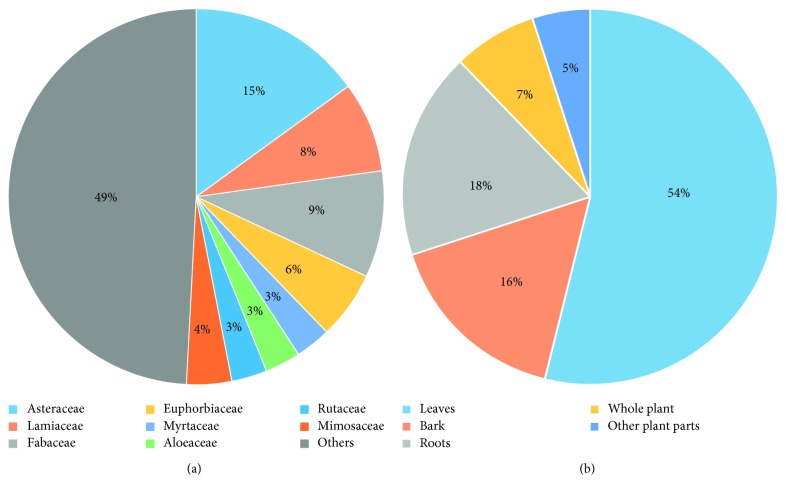
About 182 plant species from about 63 different plant families are used to treat malaria across several communities in Uganda (Table 1). Of the 63 plant families, species within the family Asteraceae are most widely used in the country to treat malaria, constituting up to 15% of all plant species used (Figure 3(a)). This is followed by species from Fabaceae (9%), Lamiaceae (8%), Euphorbiaceae (6%), and Mimosaceae (4%) families, with Myrtaceae, Aloeaceae, and Rutaceae families each contributing approximately 3% to the total number of species used for malaria treatment in Uganda (Figure 3(a)). The remaining families contribute only 49% of the total plant species used for malaria treatment (Figure 3(a)).
Figure 3.
(a) Composition of plant species in each family used to treat malaria. (b) Percentage use of plant parts for treatment of malaria.
The plant parts greatly used to treat malaria are leaves (54.4%) followed by roots (17.4%) and bark (16%); whole plants and other plant parts are used less commonly (Figure 3(b)). A particular herbal plant is commonly used singly though some times in combination with other herbs. The most common way of use is by boiling the medicinal plant part in water and then drinking the decoction; ingestion of fresh extracts and powdered forms of the herbs is also practiced (Table 1).
Different herbal remedies are used in different communities in different parts of the country depending on the geographical distribution of the medicinal plant species, for example, Warburgia ugandensis is particularly used in the eastern part of Uganda. However, herbal plant species such as Bidens pilosa L. are spread throughout the country and thus well known for malaria treatment across the country. In a study conducted by Ssegawa and Kasenene [20], no tree species in the forests of southern Uganda were more useful than Hallea rubrostipulata and Warburgia ugandensis in the treatment of malaria. These medicinal plants are known by different local names in different parts of the country as Uganda has diverse ethnic groups, including the Luo, Baganda, Itesots, and Banyankole/Bakiga.
Among all communities in Uganda, some measures are taken to control malaria, including draining of stagnant water, clearing and burning bushes, sleeping under insecticide-treated mosquito nets, and house spraying with insecticides.
6. Mode of Preparation and Use of Herbs in Treatment of Malaria in Uganda
The mode of preparation and use of herbs among different communities vary depending on the nature of the herb and plant parts used for malaria treatment [10]. Most commonly, the herbal medicines are prepared as water extracts in the form of decoction and infusion or as steam baths (Table 1) [19, 23]. The herbal plant water extract is made mostly by boiling a handful of the medicinal plant parts such as leaves in a litre of water and then given to the patient to take orally (Table 1) [23]. The dose of the extract given is dependent on the age of the patient and the “strength” of the herbal medicine although occasionally the weight of the patient [19, 23]. The quantity of extract given ranges from 100 to 500 ml, 100 to 250 ml, and 1 to 3 tea or tablespoons for adults, older children, and young children below 5 years of age, respectively, between 1 and 3 times a day for about a week or until when patient has recovered [19, 25]. The extracts are mostly prepared from single herbal plants or from combination of two herbal plants, for example, a decoction of Tamarindus indica and Mangifera indica is common [25].
In some cases, the medicinal plant parts are dried then pulverized to powder and 2–5 tablespoons of the power added to water and boiled to make a decoction. Some medicinal plant parts such as bark of M. indica stem and roots of V. lasiopus and their powders are boiled for long until the water is half the initial amount [25]. The herbal plant powder can also be added to cold or hot water and stirred and then drunk as recommended [10].
Medicine for malaria treatment from a herb such as B. pilosa can be made by squeezing a handful of its freshly picked leaves and drinking 1–3 teaspoons of the extract a day (Table 1) [23]. Occasionally, malaria herbal medicines can be obtained by preparing different plant parts in combination, for example, an infusion can be made from fresh leaves and pounded fresh roots of V. amygdalina [25]. This is then taken orally in a recommended dose. A handful of medicinal plant parts such as leaves can be squeezed and mixed with cold or warm water for bath, for example, leaves of B. adoensis [25]. Some common herbs are also eaten as vegetables as a prophylactic measure against malaria while others are planted in pots around houses or burnt to drive away mosquitoes (Table 2).
7. Antimalarial Activities and Toxicity of Herbs Used in Uganda for Malaria Treatment
Some studies have been performed on antiplasmodial/antimalarial activities of some of the herbal plants used in Uganda to treat malaria by using various strains of malarial parasites to confirm effectiveness as malaria treatment [26, 28]. Furthermore, a broad range of phytochemicals responsible for biological activities in some of the antimalarial herbs have been isolated and identified [23]. Of the 182 plant species used in Uganda for the treatment of malaria, 112 plant species (64%) have been investigated for antimalarial activities, of which 108 plants showed positive results and only four plant species did not give positive results when tested for antimalarial activities (Table 1). For about 70 plant species (39%) that are used among different communities in Uganda for the treatment of malaria, there was no record of investigation for antimalarial activities (Table 1).
The antimalarial activity of herbal plants is due to the presence of a number of metabolically active compounds [23]. These compounds may occur in the form of alkaloids, sesquiterpenes, quinones, triterpenoids, flavonoids, quassinoids, limonoids, terpenes, chalcones, coumarins, or other miscellaneous forms [85]. The solvent of extraction largely determines the concentrations of the active metabolites in the extract. For example, methanolic extracts of the herbal plants are in general more active in vitro than water extracts probably due to the presence of higher amounts of more active lipophilic compounds (Table 3) [54].
The levels of activity of the antimalarial plant extracts depend on the concentration of the active antimalarial secondary metabolites [54]. For example, gedunin, a very active compound against Plasmodium present in leaves of A. indica had an IC50 of 0.02 μg/ml against P. falciparum, but its concentration in the plant is in very low and thus moderate activity of its extract (Table 3) [23, 54].
The synergistic effect of the interaction of the different active secondary metabolites is a main contributing factor to the high levels of antiplasmodial activity of some of the herbal plant extracts, for example, in A. afra, none of the isolated flavonoids and sesquiterpenes had a high activity, yet the plant extract had an IC50 of 3.9 μg/ml against P. falciparum suggesting a synergistic effect of the compounds in the extract [54]. The presence of particular active compounds in the herbal plant extracts is key in enhancing its antimalarial property. The compound 6E-geranylgeraniol-19-oic-acid a diterpene isolated from M. pyrifolia aqueous extract was considered responsible for its antiplasmodial activity; nitidine isolated from Z. chalybeum had an IC50 as low as 0.17 μg/ml against P. falciparum 3D7 [10]; and pristimerin with an IC50 0.5 mg/ml against P. falciparum was the main active ingredient in M. senegalensis extract, making it have a very high antiplasmodial activity [54]. The presence of a moderate amount of a minimum of two secondary metabolites in the extract could explain the efficacy of the herbal extracts for malaria treatment [10]. The pathogenic strains used may be different for different in vitro studies; thus, resistance of the parasite to the active metabolites could cause a variation in the level of antimalarial activity of the extracts [10]. Herbal plants with no antiplasmodial activity suggest the absence of the metabolically active compounds against the Plasmodium parasites in their extracts [23]. Table 4 indicates a list of herbal plants used for malaria treatment in Uganda with high antiplasmodial activities (IC50<5 μg/ml in one of its solvent extracts or high percentage inhibition of plasmodia) that could be potentially investigated further.
Table 4.
Top 17 herbal plants used locally in Uganda for malaria treatment with highest antimalarial/antiplasmodial activities (arranged alphabetically).
| Plant family | Plant species | Plant part | Extracting solvent | Report on antiplasmodial, IC50 (μg/ml)/antimalarial activity (Plasmodium strain) | Active chemical constituents | Toxicity/safety information | Reference(s) |
|---|---|---|---|---|---|---|---|
| Asteraceae | Artemisia afra Jacq. Ex Willd | Leaves | Methanol | 3.9 (chloroquine resistant, W2) | Acacetin, genkwanin, 7-methoxyacacetin | Cytotoxicity was observed in Vero cells | [54, 103] |
| Artemisia annua L. | Leaves | Water | 0.9 (chloroquine resistant, W2); 1.1 (chloroquine sensitive, D10) | Sesquiterpenes and sesquiterpene lactones including artemisinin | Generally safe and effective; nausea may occur on drinking herbal extract; artemisinin, an active compound in the extract is safe for pregnant women at least during second and third trimesters | [19, 57, 104] | |
| Aspilia africana (Pers.) C. D. Adams | Leaves | Ethanol | Significant chemo suppressive effect of 92.23% (400 mg/kg) on P. berghei | Saponins, terpenoids, alkaloids, resins, tannins, flavonoids, sterols | No signs of toxicity in mice even at a dose as high as 5000 mg/kg | [19, 58] | |
| Jatropha curcas L. | Leaves | Ethyl acetate | 2.4 (chloroquine resistant, K1) | Alkaloids, saponnins, glycosides, tannins | Moderate toxicity on thrombocyte line and a protective effect on cardiovascular system; no signs of toxicity in mice following oral administration of 5000 mg/kg body weight (bw) dose | [73, 105] | |
| Microglossa pyrifolia (Lam.)O. Ktze | Leaves | Dichloromethane | 1.5 (chloroquine sensitive, 3D7; 2.4 chloroquin resistant, W2) | E-phytol; 6e-geranylgeraniol-19-oic acid | Relatively high cytotoxicity against cells from the human foetal lung fibroblast cell line | [2, 28, 55] | |
| Schkuhria pinnata (lam.) | Whole plant | Methanol | 1.3 (chloroquine sensitive, D6) | Schkuhrin I and schkuhrin II | Methanol extract: low cytotoxicity against human cells; aqueous extracts: no observed toxicity observed in mice | [32, 54] | |
| Tithonia diversifolia A. Gray | Leaves | Methanol | 1.2 (chloroquine sensitive, 3D7); 1.5 (chloroquine resistant, W2) | Tagitinin C, sesquiterpene lactones | Aerial parts are cytotoxic against cells from the human foetal lung fibroblast cell line | [55] | |
| Vernonia amygdalina delile | Leaves | Methanol/dichloromethane | 2.7 (chloroquine resistant, K1) | Coumarin, sesquiterpene lactones including vernolepin, vernolin, vernolide, vernodalin and hydroxyvernodalin, steroid glucosides | Petroleum ether extract shows strong cytotoxicity | [19, 26, 32] | |
| Caricaceae | Carica papaya L. | Leaves | Ethyl acetate | 2.96 (chloroquine sensitive, D10); 3.98 (chloroquine resistant, DD2) | Alkaloids, saponins, tannins, glycosides | No serious toxicity reported, carpaine, an active compound against P. falciparum had high selectivity and was nontoxic to normal RBCs | [65, 106] |
| Celastraceae | Maytenus senegalensis | Roots | 1.9 (chloroquine sensitive, D6); 2.4 (chloroquine resistant, W2) | Terpenoids, pentacyclic triterpenes, e.g., pristimerin | No toxicity observed in ethanol extract | [66, 107] | |
| Cucurbitaceae | Momordica foetida Schumach. | Shoot | Water | 0.35 (chloroquine resistant, FCR3); 6.16 (chloroquine sensitive, NF54) | Saponins, alkaloid, phenolic glycosides including 5,7,4′-Trihydroxyflavanone and kaempferol | No pronounced toxicity against human hepatocellular (HepG2) and human urinary bladder carcinoma (ECV-304, derivative of T-24) cells | [26, 28, 108] |
| Euphorbiaceae | Alchornea cordifolia (Schumach.) Mull. Arg. | Leaves | Water | 4.8 (chloroquine resistant, K1) | Phenolics including ellagic acid | No mortality in mice in acute toxicity test | [70, 109] |
| Fluegea virosa (Roxb. ExWillb.)Voigt | Leaves | Water/methanol | 2 (chloroquine resistant, W2) | Bergenin | Nontoxic, extracts exposed to murine macrophages did not slow or inhibit growth of cells | [72, 110] | |
| Phyllanthus (pseudo) niruri Mull. Arg. | Water | Ranged from 2.9 to 4.1 (both chloroquine sensitive, 3D7 and resistant, Dd2) | Coumarins including 1-O-galloyl-6-O-luteoyl-a-D-glucose | No toxicity was observed; thus, LD50 of the aqueous extract is >5000 mg/kg. b.w. | [74, 111] | ||
| Lamiaceae | Clerodendrum rotundifolium Oliv. | Leaves | Methanol | 0.02 (chloroquine sensitive, CQS); 1.56 (chloroquine resistant, CQR) | Iridoid glycosides such as serratoside A, serratoside B and monomelittoside, diterpenoids including uncinatone, clerodin, and sugiol | Not explored | [28, 33] |
| Mimosaceae | Albizia zygia (DC.) Macbr. | Stem bark | Methanol | 1.0 (chloroquine resistant, K1) | Flavonoids, mainly 3′,4′,7-trihydroxyflavone | The aqueous extract is relatively safe on subacute exposure | [87, 112] |
| Rubiaceae | Pentas longiflora Oliv. | Root | Methanol | 0.99 (chloroquine sensitive, D6); 0.93 (chloroquine resistant, W2) | Pyranonaphthoquinones, pentalongin (1) and psychorubrin (2), naphthalene derivative mollugin (3) | Low cytotoxicity | [97] |
| Rutaceae | Citrus reticulata | Seeds (isolimonexic acid methyl ether) | <4.76 (both chloroquine sensitive, D6 and resistant, W2) | Limonin, isolimonexic acid methyl ether, ichangin, deacetylnomilin, obacunone | Dermal 50% lethal dose (LD50) of undiluted leaf oil is >2 g/kg in rabbits; seed extract causes respiratory distress and strong spleen contraction | [34, 113] |
Although herbs are generally considered safer when used for treatment compared to conventional drugs, some of the herbs used traditionally to treat malaria in Uganda may be efficacious, but there is a need to have them used with caution as some may be toxic (Table 4). There is a variation in degree of toxicity depending on the sensitivity of animals, tissue or cells used, type of extract, nature of the test substance, dose, and mode of administration [114]. According to Lacroix et al. [32] one third of the herbs for malaria treatment in Uganda they investigated had significant antiplasmodial activity with low toxicity. Some of the plant parts with good antiplasmodial/antimalarial activities with no or low toxicity include leaves of A. annua, leaves of A. africana, S. pinnata whole plant, leaves of C. papaya, and leaves of F. virosa amongst others (Table 4). There are however extracts of some plants used for malaria treatment with very good activity against Plasmodium but with high toxicity; such plant extracts include petroleum ether leaf extract of V. amygdalina and dichloromethane leaf extract of M. pyrifolia (Table 4) [32, 55]. Clerodendrum rotundifolium is on those plants that have very good antimalarial/antiplasmodial activities but have not been investigated for their toxicity (Table 4) [33].
8. Traditional Health Care Practice and Policy Framework in Uganda
The health care system of Uganda consists of the public, private-profit oriented, and private-nonprofit oriented sectors. There is quite a large sector of informal health care including traditional medicine practitioners, drug shops, medicine vendors, and complementary and alternative practitioners. The contribution of traditional health practitioners to Uganda's health care system was not valued until lately [115]. The negative perspective could be traced back to the colonial times when culture including use of traditional medicine such as herbs for treatment was considered primitive and so discouraged [115]. Efforts are now being made to promote the use of traditional medicine since the government has realized that traditional health practitioners are key contributors to its primary health care system [115]. The Ministry of Health created a public-private partnership with the traditional health practitioners following a recommendation that they be brought into the mainstream health system [115, 116].
A policy on Traditional and Complementary Medicine was created to regulate traditional medicine practice focusing on research and development while emphasizing the propagation, protection, and sustainable use of medicinal plant resources [115, 116]. For collaboration between the mainstream health care sector and traditional health practitioners, the Ministry of Health submitted a bill for the creation of the National Council of Indigenous and Complementary Medicine Practitioners, a semiautonomous body that shall as well protect their intellectual property rights [115, 116].
The National Drug Authority (NDA) is a body that ensures quality control of all medical products including herbal medicines in Uganda under the government statute and policy of 1993 [117]. In Uganda, there is no special regulatory measure for herbal medicines in that the same laws and policies for conventional pharmaceuticals also apply to the herbal medicinal products. A policy was introduced in 2002 to have herbal medicines registered, but so far, no registration of any herbal medicine has been made [117].
Herbal medicines though vastly used in Uganda are not sufficiently regulated. A system to license and track traditional health practitioners or their products is still lacking in the country, and the efforts to have the TCM integrated in the mainstream health care system is still a long way from being realized.
9. Conclusion
Uganda is rich in indigenous plant resources that are used by its people to treat malaria. Communities in different regions of the country use different herbs within their geographical range, though a few common herbs are used by different communities across the country. Many herbs used for malaria treatment among several communities have not been investigated for their efficacy, and yet they could be potential sources for antimalarial remedies including drugs. Few studies have been conducted to document herbs for malaria treatment in the country, especially in the northern region. Some of the plants investigated for antimalarial/antiplasmodial activities have been found to lack efficacy, toxicity, and safety study aspects. Some plants used in the local communities had very strong antimalarial activities and could be investigated further for the identification and validation of the potential therapeutic antimalarial compounds. This review is critical in that it clearly highlights herbal plants documented in Uganda for malaria treatment but have never been investigated for their antimalarial potential, thus providing guidance for further research on potential natural plant resources that could be sources of novel compounds with therapeutic properties for the treatment of malaria.
Acknowledgments
This study was supported under the framework of International Cooperation Program (Korea-South Africa Cooperative Research Project for Excavation of Candidate Resources of Complementary and Alternative Medicine) managed by National Research Foundation of Korea (grant nos. 2017093655 and KIOM:D17470). Additionally, this study was equally supported by grants from Development of Foundational Techniques for the Domestic Production of Herbal Medicines (K18405) and Applicational Development of Standardized Herbal Resources (KSN1911420), from the Korea Institute of Oriental Medicine (KIOM), through the Ministry of Science and ICT, Republic of Korea.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Denis Okello carried out the data search and was the main contributor in writing the manuscript. Youngmin Kang technically designed and helped in writing the manuscript. Both authors read and approved the final manuscript.
References
- 1.World Health Organisation. World Malaria Report 2017. Geneva, Switzerland: World Health Organisation; 2017. [Google Scholar]
- 2.Pan W. H., Xu X. Y., Shi N., Tsang S. W., Zhang H. J. Antimalarial activity of plant metabolites. International Journal of Molecular Sciences. 2018;19(5):p. 1382. doi: 10.3390/ijms19051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asua V., Mugenyi L., Rosenthal P. J., et al. Plasmodium species infecting children presenting with malaria in Uganda. The American Journal of Tropical Medicine and Hygiene. 2017;97(3):753–757. doi: 10.4269/ajtmh.17-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation. World Malaria Report 2018. Geneva, Switzerland: World Health Organisation; 2018. [Google Scholar]
- 5.Barber B. E., Grigg M. J., William T., Yeo T. W., Anstey N. M. The treatment of plasmodium knowlesi malaria. Trends in Parasitology. 2017;33(3):242–253. doi: 10.1016/j.pt.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organisation. World Malaria Report 2005. Geneva, Switzerland: World Health Organisation; 2005. [Google Scholar]
- 7.Njoroge G., Bussmann R. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among Kikuyus (Central Kenya) Journal of Ethnobiology and Ethnomedicine. 2006;2(1):p. 8. doi: 10.1186/1746-4269-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tugume P., Kakudidi E. K., Buyinza M., et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central forest reserve, Uganda. Journal of Ethnobiology and Ethnomedicine. 2016;12(1):p. 5. doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharafzadeth S., Alizadeti O. Some medicinal plants cultivated in Iran. Journal of Applied Pharmaceutical Science. 2012;2(1):134–137. [Google Scholar]
- 10.Adia M. M., Anywar G., Byamukama R., et al. Medicinal plants used in malaria treatment by prometra herbalists in Uganda. Journal of Ethnopharmacology. 2014;155(1):580–588. doi: 10.1016/j.jep.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 11.Komakech R., Kang Y., Lee J.-H., Omujal F. A review of the potential of phytochemicals from prunus africana (hook f.) kalkman stem bark for chemoprevention and chemotherapy of prostate cancer. Evidence-Based Complementary and Alternative Medicine. 2017;2017:10. doi: 10.1155/2017/3014019.3014019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uganda Ministry of Health. National Malaria Annual Report 2017-2018. Kampala, Uganda: National Malaria Control Dividion, Surveillance Monitoring and Evaluation Unit, Uganda Ministry of Health; 2019. [Google Scholar]
- 13.Betson M., Clifford S., Stanton M., Kabatereine N. B., Stothard J. R. Emergence of nonfalciparum plasmodium infection despite regular artemisinin combination therapy in an 18-month longitudinal study of Ugandan children and their mothers. The Journal of Infectious Diseases. 2018;217(7):1099–1109. doi: 10.1093/infdis/jix686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larocca A., Moro Visconti R., Marconi M. Malaria diagnosis and mapping with M-health and geographic information systems (GIS): evidence from Uganda. Malaria Journal. 2016;15(1):p. 520. doi: 10.1186/s12936-016-1546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly B., Berrang-Ford L., Labbe J., et al. Plasmodium falciparum malaria parasitaemia among indigenous batwa and non-indigenous communities of Kanungu district, Uganda. Malaria Journal. 2016;15(1):p. 254. doi: 10.1186/s12936-016-1299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betson M., Sousa-Figueiredo J. C., Atuhaire A., et al. Detection of persistent plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology. 2014;141(14):1880–1890. doi: 10.1017/s003118201400033x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oguttu D. W., Matovu J. K. B., Okumu D. C., et al. Rapid reduction of malaria following introduction of vector control interventions in Tororo district, Uganda: a descriptive study. Malaria Journal. 2017;16(1):p. 227. doi: 10.1186/s12936-017-1871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raouf S., Mpimbaza A., Kigozi R., et al. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clinical Infectious Diseases. 2017;65(3):453–460. doi: 10.1093/cid/cix251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strangeland T., Alele P. E., Katuura E., Lye K. A. Plants used to treat malaria in Nyakayojo sub-county, Western Uganda. Journal of Ethnopharmacology. 2011;137(1):154–166. doi: 10.1016/j.jep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Ssegawa P., Kasenene J. M. Plants for malaria treatment in Southern Uganda: traditional use, preference and ecological viability. Journal of Ethnobiology. 2007;27(1):110–131. doi: 10.2993/0278-0771(2007)27[110:pfmtis]2.0.co;2. [DOI] [Google Scholar]
- 21.Tabuti J. R. Herbal medicines used in the treatment of malaria in budiope county, Uganda. Journal of Ethnopharmacology. 2008;116(1):33–42. doi: 10.1016/j.jep.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Cock I. E. The genus aloe: phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. Progress in Drug Research, Arthritis and Gastro-Intestinal Diseases. 2015;70:179–235. doi: 10.1007/978-3-0348-0927-6_6. [DOI] [PubMed] [Google Scholar]
- 23.Philip K., Elizabeth M., Cheplogoi P., Samuel K. Ethnobotanical survey of antimalarial medicinal plants used in Butebo county, Eastern Uganda. European Journal of Medicinal Plants. 2017;21(4):1–22. doi: 10.9734/ejmp/2017/35368. [DOI] [Google Scholar]
- 24.Katuura E., Waako P., Ogwal-Okeng J., Bukenya-Ziraba R. Traditional treatment of malaria in Mbarara district, Western Uganda. African Journal of Ecology. 2007;45(1):48–51. doi: 10.1111/j.1365-2028.2007.00737.x. [DOI] [Google Scholar]
- 25.Anywar G., van’t Klooster C. I. E. A., Byamukama R., et al. Medicinal plants used in the treatment and prevention of malaria in Cegere sub-county, Northern Uganda. Ethnobotany Research and Applications. 2016;14:505–516. doi: 10.17348/era.14.0.505-516. [DOI] [Google Scholar]
- 26.Obbo C. J. D., Kariuki S. T., Gathirwa J. W., Olaho-Mukani W., Cheplogoi P. K., Mwangi E. M. In vitro antiplasmodial, antitrypanosomal and antileishmanial activities of selected medicinal plants from Ugandan flora: refocusing into multi-component potentials. Journal of Ethnopharmacology. 2019;229:127–136. doi: 10.1016/j.jep.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Okello D., Komakech R., Matsabisa M. G., Kang Y.-M. A review on the botanical aspects, phytochemical contents and pharmacological activities of Warburgia ugandensis. Journal of Medicinal Plants Research. 2018;12(27):448–455. doi: 10.5897/jmpr2018.6626. [DOI] [Google Scholar]
- 28.Adia M. M., Emami S. N., Byamukama R., Faye I., Borg-Karlson A.-K. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. Journal of Ethnopharmacology. 2016;186:14–19. doi: 10.1016/j.jep.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Muregi F. W., Chhabra S. C., Njagi E. N., et al. In vitro antiplasmodial activity of some plants used in Kisii, Kenya against malaria and their chloroquine potentiation effects. Journal of Ethnopharmacology. 2003;84(2-3):235–239. doi: 10.1016/s0378-8741(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 30.Kamatenesi M. M., Acipa A., Oryem-Origa H. Medicinal plants of Otwal and Ngai sub counties in Oyam district, Northern Uganda. Journal of Ethnobiology and Ethnomedicine. 2011;7(1):p. 7. doi: 10.1186/1746-4269-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabuti J. R., Kukunda C. B., Kaweesi D., Kasilo O. M. Herbal medicine use in the districts of Nakapiripirit, Pallisa, Kanungu, and Mukono in Uganda. Journal of Ethnobiology and Ethnomedicine. 2012;8(1):p. 35. doi: 10.1186/1746-4269-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacroix D., Prado S., Kamoga D., et al. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. Journal of Ethnopharmacology. 2011;133(2):850–855. doi: 10.1016/j.jep.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Kaur H., Mukhtar H. M., Singh A., Mahajan A. Antiplasmodial medicinal plants: a literature review on efficacy, selectivity and phytochemistry of crude plant extracts. Journal of Biologically Active Products from Nature. 2018;8(5):272–294. doi: 10.1080/22311866.2018.1526651. [DOI] [Google Scholar]
- 34.Nanyunja R. K. Indigenous Knowledge of the Abundance of Medicinal and Food Plants in Mount Moroto Forest Reserve. Kampala, Uganda: Institute of Environmental & Natural Resources, Makerere University; 2003. [Google Scholar]
- 35.Amoa Onguéné P., Ntie-Kang F., Lifongo L., Ndom J., Sippl W., Mbaze L. The potential of anti-malarial compounds derived from african medicinal plants, part I: a pharmacological evaluation of alkaloids and terpenoids. Malaria Journal. 2013;12(1):p. 449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tse E. G., Korsik M., Todd M. H. The past, present and future of anti-malarial medicines. Malaria Journal. 2019;18(1):p. 93. doi: 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishna S., Uhlemann A. C., Haynes R. K. Artemisinins: mechanisms of action and potential for resistance. Drug Resistance Updates. 2004;7(4-5):233–244. doi: 10.1016/j.drup.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Mok S., Liong K.-Y., Lim E.-H., et al. Structural polymorphism in the promoter of pfmrp2 confers Plasmodium falciparum tolerance to quinoline drugs. Molecular Microbiology. 2014;91(5):918–934. doi: 10.1111/mmi.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shandilya A., Chacko S., Jayaram B., Ghosh I. A plausible mechanism for the antimalarial activity of artemisinin: a computational approach. Scientific Reports. 2013;3(1):p. 2513. doi: 10.1038/srep02513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbengue A., Bhattacharjee S., Pandharkar T., et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520(7549):683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staines H. M., Krishna S. Treatment and Prevention of Malaria. Basel, Switzerland: Springer; 2012. [Google Scholar]
- 42.World Health Organization. Model List of Essential Medicines, 19th List. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 43.Esu E., Effa E. E., Opie O. N., Uwaoma A., Meremikwu M. M. Artemether for severe malaria (Review) The Cochrane Database of Systematic Reviews. 2014;9(9) doi: 10.1002/14651858.CD010678.pub2.CD010678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foley M., Tilley L. Quinoline antimalarials: mechanisms of action and resistance. International Journal for Parasitology. 1997;27(2):231–240. doi: 10.1016/s0020-7519(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 45.Bbosa S., Kyegombe D. B., Lubega A., Musisi N., Ogwal-Okeng J., Odyek O. Anti-Plasmodium falciparum activity of Aloe dawei and Justicia betonica. African Journal of Pharmacy and Pharmacology. 2013;7(31):2258–2263. doi: 10.5897/ajpp12.479. [DOI] [Google Scholar]
- 46.Kigondu E. V. M., Rukunga G. M., Keriko J. M., et al. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. Journal of Ethnopharmacology. 2009;123(3):504–509. doi: 10.1016/j.jep.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Owuor B. O., Ochanda J. O., Kokwaro J. O., et al. In vitro antiplasmodial activity of selected luo and kuria medicinal plants. Journal of Ethnopharmacology. 2012;144(3):779–781. doi: 10.1016/j.jep.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 48.Shah K. A., Patel M. B., Patel R. J., Parmar P. K. Mangifera indica (mango) Pharmacognosy Reviews. 2010;4(7):42–48. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asase A., Akwetey G. A., Achel D. G. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West district of Ghana. Journal of Ethnopharmacology. 2010;129(3):367–376. doi: 10.1016/j.jep.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Murugan K., Aarthi N., Kovendan K., et al. Mosquitocidal and antiplasmodial activity of Senna occidentalis (cassiae) and ocimum basilicum (lamiaceae) from Maruthamalai hills against Anopheles stephensi and Plasmodium falciparum. Parasitology Research. 2015;114(10):3657–3664. doi: 10.1007/s00436-015-4593-x. [DOI] [PubMed] [Google Scholar]
- 51.Idowu E. T., Ajaegbu H. C., Omotayo A. I., Aina O. O., Otubanjo O. A. In vivo anti-plasmodial activities and toxic impacts of lime extract of a combination of Picralima nitida, Alstonia boonei and Gongronema latifolium in mice infected with chloroquine-sensitive Plasmodium berghei. African Health Sciences. 2015;15(4):1262–1270. doi: 10.4314/ahs.v15i4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarkson C., Maharaj V. J., Crouch N. R., et al. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. Journal of Ethnopharmacology. 2004;92(2-3):177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Fatima A., Singh P. P., Agarwal P., Irchhaiya R., Alok S., Verma A. Treatment of various diseases by Carissa spinarum L.–a promising shrub. IJPSR. 2013;4(7):2489–2495. [Google Scholar]
- 54.Muthaura C. N., Keriko J. M., Mutai C., et al. Antiplasmodial potential of traditional phytotherapy of some remedies used in treatment of malaria in Meru-Tharaka Nithi County of Kenya. Journal of Ethnopharmacology. 2015;175:315–323. doi: 10.1016/j.jep.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Muganga R., Angenot L., Tits M., Frédérich M. Antiplasmodial and cytotoxic activities of rwandan medicinal plants used in the treatment of malaria. Journal of Ethnopharmacology. 2010;128(1):52–57. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 56.Kumar R., Duffy S., Avery V. M., Davis R. A. Synthesis of antimalarial amide analogues based on the plant serrulatane diterpenoid 3,7,8-trihydroxyserrulat-14-en-19-oic acid. Bioorganic & Medicinal Chemistry Letters. 2017;27(17):4091–4095. doi: 10.1016/j.bmcl.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 57.Lemma M. T., Ahmed A. M., Elhady M. T., et al. Medicinal plants for in vitro antiplasmodial activities: a systematic review of literature. Parasitology International. 2017;66(6):713–720. doi: 10.1016/j.parint.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Christian A. G., Mfon A. G., Dick E. A., David-Oku E., Linus A. J., Chukwuma E. B. Antimalarial potency of the leaf extract of Aspilia africana (Pers.) C.D. Adams. Asian Pacific Journal of Tropical Medicine. 2012;5(2):126–129. doi: 10.1016/s1995-7645(12)60010-8. [DOI] [PubMed] [Google Scholar]
- 59.Nondo R., Moshi M., Erasto P., et al. Evaluation of the cytotoxic activity of extracts from medicinal plants used for the treatment of malaria in Kagera and Lindi regions, Tanzania. Journal of Applied Pharmaceutical Science. 2015;5(4):007–012. doi: 10.7324/japs.2015.50402. [DOI] [Google Scholar]
- 60.Okokon J. E., Antia B. S., Mohanakrishnan D., Sahal D. Antimalarial and antiplasmodial activity of husk extract and fractions of zea mays. Pharmaceutical Biology. 2017;55(1):1394–1400. doi: 10.1080/13880209.2017.1302966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zemicheal G., Mekonnen Y. Antiplasmodial activity of Vernonia adoensis aqueous, methanol and chloroform leaf extracts against chloroquine sensitive strain of Plasmodium berghei in vivo in mice. BMC Research Notes. 2018;11(1):p. 736. doi: 10.1186/s13104-018-3835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soma A., Sanon S., Gansane A., et al. Antiplasmodial activity of Vernonia cinerea Less (Asteraceae), a plant used in traditional medicine in burkina faso to treat malaria. African Journal of Pharmacy and Pharmacology. 2017;11(5):87–93. doi: 10.5897/ajpp2016.4703. [DOI] [Google Scholar]
- 63.Ekasari W., Wahyuni T. S., Arwaty H., Putri N. T. Determination of effective dose of antimalarial from cassia spectabilis leaf ethanol extract in plasmodium berghei-infected mice. African Journal of Infectious Diseases. 2018;12(1S):111–115. doi: 10.21010/ajid.v12i1s.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mesia G. K., Tona G. L., Nanga T. H., et al. Antiprotozoal and cytotoxic screening of 45 plant extracts from Democratic Republic of Congo. Journal of Ethnopharmacology. 2008;115(3):409–415. doi: 10.1016/j.jep.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 65.Melariri P., Campbell W., Etusim P., Smith P. Antiplasmodial properties and bioassay-guided fractionation of ethyl acetate extracts from carica papaya leaves. Journal of Parasitology Research. 2011;2011:7. doi: 10.1155/2011/104954.104954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nanyingi M. O., Kipsengeret K. B., Wagate C. G., Langat B. K., Asaava L. L., Midiwo J. O. In vitro and in vivo antiplasmodial activity of Kenyan medicinal plants. In: Midiwo J. O., Clough J., editors. Aspects of African Biodiversity: Proceedings of the Pan-Africa Chemistry Network. Cambridge, UK: RCS Publishing; 2010. pp. 20–28. [Google Scholar]
- 67.Cysne D. N., Fortes T. S., Reis A. S., et al. Antimalarial potential of leaves of Chenopodium ambrosioides L. Parasitology Research. 2016;115(11):4327–4334. doi: 10.1007/s00436-016-5216-x. [DOI] [PubMed] [Google Scholar]
- 68.Asres K., Bucar F., Knauder E., Yardley V., Kendrick H., Croft S. L. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytotherapy Research. 2001;15(7):613–617. doi: 10.1002/ptr.897. [DOI] [PubMed] [Google Scholar]
- 69.Amorim C. Z., Marques A. D., Cordeiro R. S. B. Screening of the antimalarial activity of plants of the cucurbitaceae family. Memórias Do Instituto Oswaldo Cruz. 1991;86(suppl 2):177–180. doi: 10.1590/s0074-02761991000600040. [DOI] [PubMed] [Google Scholar]
- 70.Memvanga P. B., Tona G. L., Mesia G. K., Lusakibanza M. M., Cimanga R. K. Antimalarial activity of medicinal plants from the Democratic Republic of Congo: a review. Journal of Ethnopharmacology. 2015;169:76–98. doi: 10.1016/j.jep.2015.03.075. [DOI] [PubMed] [Google Scholar]
- 71.Bantie L., Assefa S., Teklehaimanot T., Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of croton macrostachyus hocsht. (euphorbiaceae) against plasmodium berghei in mice. BMC Complementary and Alternative Medicine. 2014;14(1):p. 79. doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaou A. M., Mahiou-Leddet V., Hutter S., et al. Antimalarial activity of crude extracts from nine african medicinal plants. Journal of Ethnopharmacology. 2008;116(1):74–83. doi: 10.1016/j.jep.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Abiodun O., Gbotosho G., Ajaiyeoba E., et al. In vitro antiplasmodial activity and toxicity assessment of some plants from Nigerian ethnomedicine. Pharmaceutical Biology. 2011;49(1):9–14. doi: 10.3109/13880209.2010.490224. [DOI] [PubMed] [Google Scholar]
- 74.Mustofa M., Sholikhah E. N., Wahyuono S. In vitro and in vivo antiplasmodial activity and cytotoxicity of extracts of Phyllanthus niruri L. herbs traditionally used to treat malaria in Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health. 2007;38(4):609–615. [PubMed] [Google Scholar]
- 75.Duker-Eshun G., Jaroszewski J. W., Asomaning W. A., Oppong-Boachie F., Brogger Christensen S. Antiplasmodial constituents of Cajanus cajan. Phytotherapy Research. 2004;18(2):128–130. doi: 10.1002/ptr.1375. [DOI] [PubMed] [Google Scholar]
- 76.Ezenyi I. C., Ranarivelo L., Oluwakanyinsola S. A., Emeje M. Analgesic, anti-inflammatory, and heme biomineralization inhibitory properties of entada africana ethanol leaf extract with antiplasmodial activity against Plasmodium falciparum. Journal of Basic and Clinical Physiology and Pharmacology. 2014;25(2):217–223. doi: 10.1515/jbcpp-2013-0066. [DOI] [PubMed] [Google Scholar]
- 77.Komlaga G., Agyare C., Dickson R. A., et al. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. Journal of Ethnopharmacology. 2015;172:333–346. doi: 10.1016/j.jep.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 78.Nguta J. M., Mbaria J. M. Brine shrimp toxicity and antimalarial activity of some plants traditionally used in treatment of malaria in Msambweni district of Kenya. Journal of Ethnopharmacology. 2013;148(3):988–992. doi: 10.1016/j.jep.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 79.Kuria K. A. M., De Coster S., Muriuki G., et al. Antimalarial activity of Ajuga remota benth (labiatae) and Caesalpinia volkensii harms (Caesalpiniaceae): in vitro confirmation of ethnopharmacological use. Journal of Ethnopharmacology. 2001;74(2):141–148. doi: 10.1016/s0378-8741(00)00367-6. [DOI] [PubMed] [Google Scholar]
- 80.Muthaura C. N., Keriko J. M., Mutai C., et al. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the kwale community of the Kenyan Coast. Journal of Ethnopharmacology. 2015;170:148–157. doi: 10.1016/j.jep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 81.Kefe A., Giday M., Mamo H., Erko B. Antimalarial properties of crude extracts of seeds of brucea antidysenterica and leaves of Ocimum lamiifolium. BMC Complementary and Alternative Medicine. 2016;16:p. 118. doi: 10.1186/s12906-016-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheljazkov V. D., Astatkie T., Zhalnov I., Georgieva T. D. Method for attaining rosemary essential oil with differential composition from dried or fresh material. Journal of Oleo Science. 2015;64(5):485–496. doi: 10.5650/jos.ess14258. [DOI] [PubMed] [Google Scholar]
- 83.Kumari A., Baskaran P., Chukwujekwu J. C., de Kock C. A., Smith P. J., Van Staden J. The changes in morphogenesis and bioactivity of Tetradenia riparia, Mondia whitei and Cyanoptis speciosa by an aeroponic system. Industrial Crops and Products. 2016;84:199–204. doi: 10.1016/j.indcrop.2016.01.046. [DOI] [Google Scholar]
- 84.Komlaga G., Cojean S., Dickson R. A., et al. Antiplasmodial activity of selected medicinal plants used to treat malaria in Ghana. Parasitology Research. 2016;115(8):3185–3195. doi: 10.1007/s00436-016-5080-8. [DOI] [PubMed] [Google Scholar]
- 85.Batista R., De Jesus Silva A., Jr., de Oliveira A. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules. 2009;14(8):3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alli L., Adesokan A., Salawu A. Antimalarial activity of fractions of aqueous extract of Acacia nilotica root. Journal of Intercultural Ethnopharmacology. 2016;5(2):180–185. doi: 10.5455/jice.20160331064817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ndjakou Lenta B., Vonthron-Senecheau C., Fongang Soh R., et al. In vitro antiprotozoal activities and cytotoxicity of some selected cameroonian medicinal plants. Journal of Ethnopharmacology. 2007;111(1):8–12. doi: 10.1016/j.jep.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 88.Bwalya A. G. London, UK: University College London (UCL), School of Pharmacy; 2014. Evaluation of the in vitro biological activities and phytochemical profiling of eight ficus species collected in Zambia. Doctoral Thesis. [Google Scholar]
- 89.Areola J. O., Omisore N. O., Babalola O. O. Antiplasmodial activity of stem-bark extract of Milicia excelsa (welw.) c.c.berg against rodent malaria parasites (Plasmodium berghei) in mice. Ife Journal of Science. 2016;18(4):905–911. [Google Scholar]
- 90.Bagavan A., Rahuman A. A., Kaushik N. K., Sahal D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitology Research. 2011;108(1):15–22. doi: 10.1007/s00436-010-2034-4. [DOI] [PubMed] [Google Scholar]
- 91.Ohashi M., Amoa-Bosompem M., Kwofie K. D., et al. In vitro antiprotozoan activity and mechanisms of action of selected G hanaian medicinal plants against trypanosoma, leishmania, and plasmodium parasites. Phytotherapy Research. 2018;32(8):1617–1630. doi: 10.1002/ptr.6093. [DOI] [PubMed] [Google Scholar]
- 92.Pillay P., Maharaj V. J., Smith P. J. Investigating South African plants as a source of new antimalarial drugs. Journal of Ethnopharmacology. 2008;119(3):438–454. doi: 10.1016/j.jep.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Simões-Pires C. A., Vargas S., Marston A., et al. Ellagic acid derivatives from Syzygium cumini stem bark: investigation of their antiplasmodial activity. Natural Product Communications. 2009;4(10):1371–1376. doi: 10.1177/1934578x0900401012. [DOI] [PubMed] [Google Scholar]
- 94.Tadesse S. A., Wubneh Z. B. Antimalarial activity of Syzygium guineense during early and established plasmodium infection in rodent models. BMC Complementary and Alternative Medicine. 2017;17(1):p. 21. doi: 10.1186/s12906-016-1538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chukwuocha U. M., Fernández-Rivera O., Legorreta-Herrera M. Exploring the antimalarial potential of whole Cymbopogon citratus plant therapy. Journal of Ethnopharmacology. 2016;193:517–523. doi: 10.1016/j.jep.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 96.Okokon J. E., Etebong E. O., Udobang J. A., Obot J. Antiplasmodial and antiulcer activities of Melanthera scadens. Asian Pacific Journal of Tropical Biomedicine. 2012;2(1):16–20. doi: 10.1016/s2221-1691(11)60182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Endale M., Alao J. P., Akala H. M., et al. Antiplasmodial quinones from Pentas longiflora and Pentas lanceolata. Planta Medica. 2012;78(1):31–35. doi: 10.1055/s-0031-1280179. [DOI] [PubMed] [Google Scholar]
- 98.Khalil A. T., Maatooq G. T., El Sayed K. A. Limonoids from Citrus reticulata. Zeitschrift Für Naturforschung C. 2003;58(3-4):165–170. doi: 10.1515/znc-2003-3-403. [DOI] [PubMed] [Google Scholar]
- 99.Chinwuba P., Akah P. A., Iiodigwe E. E. In vivo antiplasmodial activity of the ethanol stem extract and fractions of Citrus sinensis in mice. Merit Research Journal of Medicine and Medical Sciences. 2015;3(4):140–146. [Google Scholar]
- 100.Haddad M. H. F., Mahbodfar H., Zamani Z., Ramazani A. Antimalarial evaluation of selected medicinal plant extracts used in Iranian traditional medicine. Iranian Journal of Basic Medical Sciences. 2017;20(4):415–422. doi: 10.22038/IJBMS.2017.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busari Z. A., Dauda K. A., Morenikeji O. A., et al. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Frontiers in Pharmacology. 2017;8:p. 622. doi: 10.3389/fphar.2017.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freundlich J. S., Anderson J. W., Sarantakis D., et al. Synthesis, biological activity, and X-ray crystal structural analysis of diaryl ether inhibitors of malarial enoyl acyl carrier protein reductase. Part 1: 4′-substituted triclosan derivatives. Bioorganic & Medicinal Chemistry Letters. 2005;15(23):5247–5252. doi: 10.1016/j.bmcl.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 103.Liu N. Q., Van Der Kooy F., Verpoorte R. Artemisia afra: a potential flagship for African medicinal plants? South African Journal of Botany. 2009;75(2):185–195. doi: 10.1016/j.sajb.2008.11.001. [DOI] [Google Scholar]
- 104.Yarnell E. Artemisia annua (sweet annie), other artemisia species, artemisinin, artemisinin derivatives, and malaria. Journal of Restorative Medicine. 2014;3(1):69–84. doi: 10.14200/jrm.2014.3.0105. [DOI] [Google Scholar]
- 105.Sawadogo S., Sanou S. D., Dabiré A. P., et al. In vivo evaluation of Jatropha curcas L (euphorbiaceae) leaves acute and subacute toxicity in mice. Journal of Scientific Research. 2018;10(2):187–193. doi: 10.3329/jsr.v10i2.35267. [DOI] [Google Scholar]
- 106.Teng W.-C., Chan W., Suwanarusk R., et al. In vitro antimalarial evaluations and cytotoxicity investigations of carica papaya leaves and carpaine. Natural Product Communications. 2019;14(1):33–36. doi: 10.1177/1934578x1901400110. [DOI] [Google Scholar]
- 107.Malebo H. M., Wiketye V., Katani S. J., et al. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malaria Journal. 2015;14(1):p. 79. doi: 10.1186/s12936-014-0525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Froelich S., Onegi B., Kakooko A., Siems K., Schubert C., Jenett-Siems K. Plants traditionally used against malaria: phytochemical and pharmacological investigation of Momordica foetida. Revista Brasileira de Farmacognosia. 2007;17(1):1–17. doi: 10.1590/s0102-695x2007000100002. [DOI] [Google Scholar]
- 109.Banzouzi J.-T., Prado R., Menan H., et al. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. Journal of Ethnopharmacology. 2002;81(3):399–401. doi: 10.1016/s0378-8741(02)00121-6. [DOI] [PubMed] [Google Scholar]
- 110.Singh S. V., Manhas A., Kumar Y., et al. Antimalarial activity and safety assessment of Flueggea virosa leaves and its major constituent with special emphasis on their mode of action. Biomedicine & Pharmacotherapy. 2017;89:761–771. doi: 10.1016/j.biopha.2017.02.056. [DOI] [PubMed] [Google Scholar]
- 111.Asare G., Addo P., Bugyei K., et al. Acute toxicity studies of aqueous leaf extract of Phyllanthus niruri. Interdisciplinary Toxicology. 2011;4(4):206–210. doi: 10.2478/v10102-011-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okpo S. O., Igwealor C. O., Eze G. I. Sub-acute toxicity study on the aqueous extract of Albizia zygia stem bark. Journal of Pharmacy & Bioresources. 2016;13(1):32–41. doi: 10.4314/jpb.v13i1.5. [DOI] [Google Scholar]
- 113.Mandal S., Mandal M. Essential Oils in Food Preservation, Flavor and Safety. Amsterdam, Netherlands: Elsevier; 2016. Tangerine (Citrus reticulata L. var.) oils; pp. 803–811. [Google Scholar]
- 114.Abdelgadir H. A., Van Staden J. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): a review. South African Journal of Botany. 2013;88:204–218. doi: 10.1016/j.sajb.2013.07.021. [DOI] [Google Scholar]
- 115.Falk E. Traditional Medicine; Sharing Experiences from the Field. Jeonju, Republic of Korea: ICHCAP; 2017. [Google Scholar]
- 116.The republic of Uganda. National Medicines Policy. Kampala, Uganda: Ministry of Health; 2015. [Google Scholar]
- 117.Giri R. P., Gangawane A. K., Giri S. G. Regulation on herbal product used as medicine around the world: a review. IRJET. 2018;5(10) [Google Scholar]