Abstract
Colorectal cancer (CRC) influences individual health worldwide with high morbidity and mortality. Melatonin, which shows multiple physiological functions (e.g., circadian rhythm, immune modulation, and antioncogenic action), can be present in almost all organisms and found in various tissues including gastrointestinal tract. Notably, melatonin disruption is closely associated with the elevation of CRC incidence, indicating that melatonin is effective in suppressing CRC development and progression. Mechanistically, melatonin favors in activating apoptosis and colon cancer immunity, while reducing proliferation, autophagy, metastasis, and angiogenesis, thereby exerting its anticarcinogenic effects. This review highlights that melatonin can be an adjuvant therapy and be beneficial in treating patients suffering from CRC.
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and a major cause of cancer-related mortality around the world [1–3]. Multiple factors are associated with the occurrence and the development of CRC, including genetic makeup, population aging/gender, dietary behaviors, poor physical activity, and smoking [4–6]. According to the clinical situations of the patients with CRC, the status of CRC treatments (e.g., surgical therapy, radiotherapy, chemotherapy, targeted therapy, and immunotherapy) develops rapidly [7]. Even though different and novel therapies are available, in almost >25% of patients with metastatic cancer systemic therapy remains the treatment option [8]. For example, treating CRC by conducting chemotherapy causes cytotoxicity and agents resistance (e.g., 5-FU, capecitabine, cetuximab, and panitumumab) which calls for the development of more effective and novel alternative agents and/or adjuvants [9, 10]. Fortunately, melatonin is under consideration for its low toxicity and high efficacy.
Melatonin (a natural substance derived from tryptophan, and for its synthesis, refer to [11, 12]), which was initially isolated from the bovine pineal gland, shows a wide distribution from bacteria to humans [13–15]. Interestingly, melatonin also has turned out to be found in other tissues, such as lymphocytes, Harderian gland, liver, and gastrointestinal tract [16–19]. Melatonin is highly pleiotropic and regulates numerous physiological functions including circadian rhythms [20], antioxidative protection [21, 22], immune modulation [12, 23], and, with particular relevance to this article, antioncogenic and oncostatic actions [24, 25]. Given melatonin could be produced in the gastrointestinal tract, in which the total level of melatonin is ∼400 times than those in the pineal gland [26], and the protective effects of melatonin in the gastrointestinal tract (e.g., enhancing immune functions of the gut, reducing peristalsis [17], and altering intestinal microbiota community [27, 28]), and the antitumor function of melatonin, it is not surprising that melatonin could inhibit the gastrointestinal cancers including colon [29, 30]. Actually, the circadian rhythm change of blood melatonin is disordered in patients with CRC and melatonin disruption elevates the CRC incidence in humans [31, 32]. Previous studies confirmed that melatonin blocks colon carcinogenesis [33, 34]. Moreover, CGP 52608 (functions as a ligand for melatonin nuclear RZR/ROR receptor) could promote colon cancer cell apoptosis [35], and CGP 55644 (a RZR/ROR receptor antagonist) lowers the efficacy of melatonin in blocking colon tumor proliferation [36]. Altogether, these aforementioned results suggest that melatonin may inhibit CRC development and progression in humans.
Here, firstly, we summarize the cross-link between melatonin disorder and CRC occurrence; thereafter, we discuss several potential mechanisms (e.g., suppression of cancer cell proliferation, autophagy, metastasis and angiogenesis, and activation of apoptosis and cancer immunity) by which melatonin limits CRC development and progression.
2. Melatonin Disruption and CRC Incidence
The fluctuation of melatonin level in day and night is associated with the circadian rhythms and highly affects individual development and health [37]. Indeed, melatonin disruption is closely correlated with CRC. Epidemiologic surveys showed that the CRC incidence increased significantly in humans who have ever performed rotating shift work and/or worked at night [38–40]. Besides, Kvetnaia [41] found that the level of melatonin was increased in male patients with CRC; however, the amplitude of rhythm and secretion of melatonin in patients with CRC was significantly lowered [42, 43]. Likewise, constant illumination could cause crypt foci aberrance and promote the rodent colon cell proliferation [29]. Experimental study also reported that the melatonin concentration of serum in female rats with colon cancer was elevated compared with controls [44].
Collectively, these findings indicate that melatonin disruption is related to the elevation of CRC incidence and melatonin could be of high potential to modulate CRC development and progression.
3. Melatonin in CRC Cell Proliferation, Apoptosis, and Autophagy
Excessive proliferation of malignant tumors always favors in tumor progression; thus, it is meaningful to develop agents with high efficacy to inhibit CRC cell proliferation to limit CRC development and progression. The colon 38 is a transplantable adenocarcinoma originally induced in the colon of C57BL/6 mice by 1,2-dimethylhydrazine. Indeed, melatonin can inhibit murine colon 38 cancer cell proliferation [36] and reduce the multiplicity of colon tumors induced by 1,2-dimethylhydrazine (DMH) in rats [45]. Mechanistically, melatonin mainly inhibits cancer cell proliferation via (1) decreasing DNA synthesis and (2) promoting cell differentiation. It has been shown that the utilization of melatonin was significantly correlated with reduced DNA synthesis in colonic cancer cells [46, 47]. Moreover, melatonin could increase the number of highly differentiated cells to inhibit DMH-induced colon carcinoma cell proliferation [48].
The imbalance between the apoptosis and proliferation leads to malignancy development; therefore, it is another strategy to inhibit CRC development and progression by promoting cancer cell apoptosis. Actually, melatonin could induce Caco-2 cells [49] and human CRC cell apoptosis [34, 50]. 2-Hydroxymelatonin (a main melatonin metabolite in plants) could also increase CRC cell apoptosis [51]. Mechanistically, melatonin activates apoptosis through altering cell cycle program by increasing G1-phase arrest [34]. Intriguingly, it was shown that melatonin significantly contributed to 5-FU (a chemotherapeutic agent) inhibition of cell proliferation by activating apoptosis and cell cycle arrest [52]. Besides, endothelin-1 (ET-1), a peptide that serves as a survival factor in colon cancer, can promote proliferation while inhibiting apoptosis in carcinoma cells; melatonin was found to induce apoptosis by reducing ET-1 expression, thereby limiting the development and progression of colon cancer [53].
The overproliferating cancer cells compete for nutrients during the process of carcinogenesis, indicating that cancer cells may alter their metabolic states to survive. Indeed, autophagy could allow cancer cells to survive under stress (e.g., nutrients deprivation) [54]. Interestingly, melatonin can promote or inhibit autophagy (probably due to the antioxidant activity of melatonin) under specific conditions [55–58]. A series of autophagy-related proteins, such as microtubule-associated protein 1 light chain 3B (LC3B), p62, and Beclin-1, have been employed as markers of autophagy. [59] Previous study showed that melatonin treatment decreased the progression of colitis-associated colon carcinogenesis (CACC) by downregulating the process of autophagy as revealed by the expression pattern of various autophagy markers such as Beclin-1, LC3B-II/LC3B-I ratio, and p62. Melatonin intervention ameliorated inflammation and oxidative stress to inhibit autophagy, thereby blocking the progression of colitis-associated colon carcinogenesis [60].
Summarily, the inhibition of proliferation/autophagy and the activation of apoptosis could contribute to the antioncogenic effects of melatonin in inhibition of CRC.
4. Melatonin in CRC Metastasis, Angiogenesis, and Immunity
The cancer metastasis leads to the majority of cancer deaths because the advanced tumors are prone to invasion, migration, and metastasis, complicating the surgery and reducing its effectiveness [61, 62]. Melatonin's efficacy on migration in colonic cells has been well established. Accumulating evidence suggests that melatonin can inhibit cancer metastasis [63, 64]. It was shown that melatonin also significantly contributed to 5-FU inhibition of colon cancer cell migration [52]. Liu et al. [65] reported that melatonin decreased RKO colon cancer cell migration involving the p38/MAPK (mitogen-activated protein kinase) signaling pathway. Likewise, Zou et al. [66] also found that melatonin reduced human CRC cell proliferation and migration via the inactivation of p38 MAPK signaling. Moreover, melatonin has been suggested to decrease the depth of colon cancer invasion in vivo [48].
Angiogenesis serves an important role not only in physiological processes, but also in pathological conditions, including cancer [67, 68], and it favors in promoting aggressive tumor activity (e.g., tumor growth, metastasis, and invasion) [69]. Actually, the antioncogenic effects of melatonin in the suppression of CRC angiogenesis have also been investigated. Melatonin could destabilize hypoxia-inducible factor (HIF)-1α and/or suppress HIF-1α transcriptional activity in colon cancer cell [70], resulting in a reduction in the expression of vascular endothelial growth factor (VEGF), which functions as the most important angiogenesis growth factor that promotes cancer progression [71, 72]. Additionally, ET-1, a survival factor in colon cancer, is associated with the activation of angiogenesis [73]. Melatonin could also block the release of ET-1 from CRC cells, leading to inhibit angiogenesis, thereby limiting the CRC development and progression [53].
The cross-link between cancer and immune system plays a crucial role in the modulation of cancer development and progression [74, 75]. Melatonin has immune system activation property (e.g., altering macrophage and/or T-cell polarization and function) [12, 23]. Notably, circadian disturbances induce selective proinflammatory responses in the rat colonic mucosa, suggesting that melatonin may modulate cancer immunity to inhibit CRC development [76]. Indeed, melatonin is effective in restraining neoplastic growth in various tumors and cancers, including CRC, by enhancing TH cell immune response by producing interleukin (IL)-2, IL-10, and interferon-gamma (IFN-γ) [77]. Previous study demonstrated that melatonin exposure could decrease mitotic and apoptotic indices in the colonic adenocarcinomas and lower the expression of inflammatory mediators like nuclear factor-κB (NF-κB), tumor necrosis factor (TNF)-α, IL-1β, and STAT3 in the epithelial malignancies [33]. Besides, melatonin was confirmed to enhance splenic zone expansion and augment CD8+ lymphocytes and Fas-positive cell proliferation in DMH-induced colon carcinogenesis of rats [78].
Collectively, the published results document that melatonin blocks metastasis and angiogenesis and augments cancer immunity, thereby inhibiting CRC development and progression.
5. Concluding Remarks
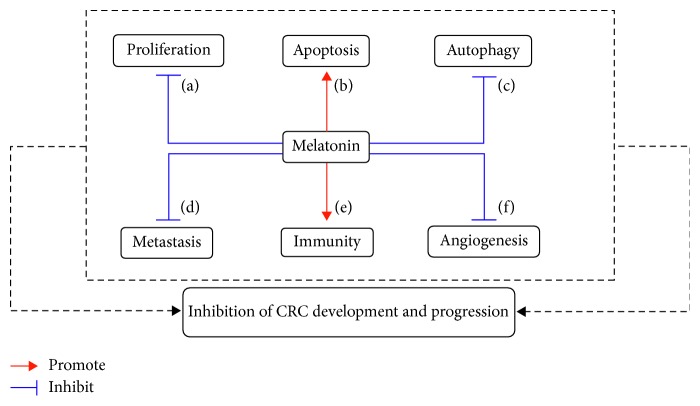
CRC is a prevalent cancer all over the world. Melatonin disruption has been reported in patients suffering from CRC, which heralds that melatonin could be a promising agent to block CRC development and progression. Mechanistically, melatonin mainly inhibits CRC cell proliferation and autophagy, metastasis, and angiogenesis, while promoting apoptosis and enhancing cancer immunity (Figure 1). Given the mechanisms of melatonin are carried out by various other means (e.g., epigenetic modulation), and cancer development always accompany with epigenetic alteration, it is of great interest to investigate whether melatonin could inhibit CRC progression through epigenetic modification. Additionally, intestinal microbiota are closely associated with the CRC onset [79–81]; it is also interesting to study that melatonin affects CRC development that involves in shifting intestinal microbiota structure in the future.
Figure 1.
Potential mechanisms connecting to melatonin limit the development and progression of colorectal cancer (CRC). (a) Inhibiting CRC cell proliferation; (b) promoting CRC cell apoptosis; (c) reducing CRC cell autophagy; (d) blocking CRC metastasis; (e) activating CRC immunity; (f) suppressing angiogenesis.
Acknowledgments
This work was partially supported by Grant nos. 2017YFD0500203, 2017YFD 0500105, and 2016YDF0500905 from the National Key Research and Development Program of China, grants from the Chinese National Science Foundation Grant (Nos. 31672579, 30571374, 30771603, 31092136, 31270171), and a project funded by the Priority Academic Program of Development Jiangsu High Education Institution and it grants from the Yangzhou Science and Technology Bureau International Cooperation Project (YZ2018154).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Hucong Wu, Jiaqi Liu, and Yi Yin contributed equally to this work.
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Roncucci L., Mariani F. Prevention of colorectal cancer: how many tools do we have in our basket? European Journal of Internal Medicine. 2015;26(10):752–756. doi: 10.1016/j.ejim.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Stigliano V., Sanchez-Mete L., Martayan A., Anti M. Early-onset colorectal cancer: a sporadic or inherited disease? World Journal of Gastroenterology. 2014;20(35):12420–12430. doi: 10.3748/wjg.v20.i35.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J., Tan Z., Hollis-Hansen K., Zhang Y., Yu C., Li Y. Epidemiological trends in colorectal cancer in China: an ecological study. Digestive Diseases and Sciences. 2017;62(1):235–243. doi: 10.1007/s10620-016-4362-4. [DOI] [PubMed] [Google Scholar]
- 5.Johnson C. M., Wei C., Ensor J. E., et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes & Control. 2013;24(6):1207–1222. doi: 10.1007/s10552-013-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuipers E. J., Grady W. M., Lieberman D., et al. Colorectal cancer. Nature Reviews Disease Primers. 2015;1(1):p. 25. doi: 10.1038/nrdp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Chen Z., Li J. The current status of treatment for colorectal cancer in China. Medicine. 2017;96(40) doi: 10.1097/md.0000000000008242.e8242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta A., Patel B. M. Therapeutic opportunities in colon cancer: focus on phosphodiesterase inhibitors. Life Sciences. 2019;230:150–161. doi: 10.1016/j.lfs.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D., Humblet Y., Siena S., et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. New England Journal of Medicine. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E., Peeters M., Siena S., et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of Clinical Oncology. 2007;25(13):1658–1664. doi: 10.1200/jco.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 11.Tan D.-X., Hardeland R., Back K., Manchester L. C., Alatorre-Jimenez M. A., Reiter R. J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. Journal of Pineal Research. 2016;61(1):27–40. doi: 10.1111/jpi.12336. [DOI] [PubMed] [Google Scholar]
- 12.Ren W., Liu G., Chen S., et al. Melatonin signaling in T cells: functions and applications. Journal of Pineal Research. 2017;62(3) doi: 10.1111/jpi.12394.e12394 [DOI] [PubMed] [Google Scholar]
- 13.Dubbels R., Reiter R. J., Klenke E., et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. Journal of Pineal Research. 1995;18(1):28–31. doi: 10.1111/j.1600-079x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 14.Tosches M. A., Bucher D., Vopalensky P., Arendt D. Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell. 2014;159(1):46–57. doi: 10.1016/j.cell.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan D.-X., Zheng X., Kong J., et al. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. International Journal of Molecular Sciences. 2014;15(9):15858–15890. doi: 10.3390/ijms150915858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acuña-Castroviejo D., Escames G., Venegas C., et al. Extrapineal melatonin: sources, regulation, and potential functions. Cellular and Molecular Life Sciences. 2014;71(16):2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubenik G. A. Gastrointestinal melatonin: localization, function, and clinical relevance. Digestive Diseases and Sciences. 2002;47(10):2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 18.Conti A., Conconi S., Hertens E., Skwarlo-Sonta K., Markowska M., Maestroni G. J. M. Evidence for melatonin synthesis in mouse and human bone marrow cells. Journal of Pineal Research. 2000;28(4):193–202. doi: 10.1034/j.1600-079x.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- 19.Raikhlin N. T., Kvetnoy I. M., Tolkachev V. N. Melatonin may be synthesised in enterochromaffin cells. Nature. 1975;255(5506):344–345. doi: 10.1038/255344a0. [DOI] [PubMed] [Google Scholar]
- 20.Reiter R. J. Melatonin: the chemical expression of darkness. Molecular and Cellular Endocrinology. 1991;79(1–3):C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 21.Poeggeler B., Reiter R. J., Tan D.-X., Chen L.-D., Manchester L. C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. Journal of Pineal Research. 1993;14(4):151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 22.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27(2):119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y., Chen S., Zeng S., et al. Melatonin in macrophage biology: current understanding and future perspectives. Journal of Pineal Research. 2019;66(2) doi: 10.1111/jpi.12547.e12547 [DOI] [PubMed] [Google Scholar]
- 24.Hill S. M., Frasch T., Xiang S., Yuan L., Duplessis T., Mao L. Molecular mechanisms of melatonin anticancer effects. Integrative Cancer Therapies. 2009;8(4):337–346. doi: 10.1177/1534735409353332. [DOI] [PubMed] [Google Scholar]
- 25.Favero G., Moretti E., Bonomini F., Reiter R. J., Rodella L. F., Rezzani R. Promising antineoplastic actions of melatonin. Frontiers in Pharmacology. 2018;9:p. 1086. doi: 10.3389/fphar.2018.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bubenik G. A. Thirty four years since the discovery of gastrointestinal melatonin. Journal of Physiology and Pharmacology. 2008;59:33–51. [PubMed] [Google Scholar]
- 27.Ren W., Wang P., Yan J., et al. Melatonin alleviates weanling stress in mice: involvement of intestinal microbiota. Journal of Pineal Research. 2018;64(2) doi: 10.1111/jpi.12448.e12448 [DOI] [PubMed] [Google Scholar]
- 28.Yin J., Li Y., Han H., et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. Journal of Pineal Research. 2018;65(4) doi: 10.1111/jpi.12524.e12524 [DOI] [PubMed] [Google Scholar]
- 29.Kannen V., Marini T., Zanette D. L., et al. The melatonin action on stromal stem cells within pericryptal area in colon cancer model under constant light. Biochemical and Biophysical Research Communications. 2011;405(4):593–598. doi: 10.1016/j.bbrc.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Guo W., Chen W., et al. Melatonin potentiates the antiproliferative and pro-apoptotic effects of ursolic acid in colon cancer cells by modulating multiple signaling pathways. Journal of Pineal Research. 2013;54(4):406–416. doi: 10.1111/jpi.12035. [DOI] [PubMed] [Google Scholar]
- 31.Anisimov V. N., Vinogradova I. A., Panchenko A. V., Popovich I. G., Zabezhinski M. A. Light-at-night-induced circadian disruption, cancer and aging. Current Aging Science. 2012;5:170–177. doi: 10.2174/1874609811205030002. [DOI] [PubMed] [Google Scholar]
- 32.Anisimov V. N. Light pollution, reproductive function and cancer risk. Neuroendocrinology Letters. 2006;27:35–52. [PubMed] [Google Scholar]
- 33.Tanaka T., Yasui Y., Tanaka M., Tanaka T., Oyama T., Rahman K. W. Melatonin suppresses AOM/DSS-induced large bowel oncogenesis in rats. Chemico-Biological Interactions. 2009;177(2):128–136. doi: 10.1016/j.cbi.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y., Won J., Lee Y., et al. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. Journal of Pineal Research. 2014;56(3):264–274. doi: 10.1111/jpi.12119. [DOI] [PubMed] [Google Scholar]
- 35.Winczyk K., Pawlikowski M., Karasek M. Melatonin and RZR/ROR receptor ligand CGP 52608 induce apoptosis in the murine colonic cancer. Journal of Pineal Research. 2001;31(2):179–182. doi: 10.1034/j.1600-079x.2001.310213.x. [DOI] [PubMed] [Google Scholar]
- 36.Winczyk K., Pawlikowski M., Guerrero J. M., Karasek M. l. Possible involvement of the nuclear RZR/ROR-alpha receptor in the antitumor action of melatonin on murine colon 38 cancer. Tumor Biology. 2002;23(5):298–302. doi: 10.1159/000068569. [DOI] [PubMed] [Google Scholar]
- 37.Pechanova O., Paulis L., Simko F. Peripheral and central effects of melatonin on blood pressure regulation. International Journal of Molecular Sciences. 2014;15(10):17920–17937. doi: 10.3390/ijms151017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C.-L., Liu T.-C., Wang Y.-N., Chung C.-H., Chien W.-C. The association between sleep disorders and the risk of colorectal cancer in patients: a population-based nested case-control study. In Vivo. 2019;33(2):573–579. doi: 10.21873/invivo.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papantoniou K., Devore E. E., Massa J., et al. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. International Journal of Cancer. 2018;143(11):2709–2717. doi: 10.1002/ijc.31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parent M.-E., El-Zein M., Rousseau M.-C., Pintos J., Siemiatycki J. Night work and the risk of cancer among men. American Journal of Epidemiology. 2012;176(9):751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 41.Kvetnaia T. V. Melatonin for diagnosis of cancer and assessment of prognosis in elderly patients. Advances in Gerontology = Uspekhi Gerontologii. 2003;12:132–142. [PubMed] [Google Scholar]
- 42.Khoory R., Stemme D. Plasma melatonin levels in patients suffering from colorectal carcinoma. Journal of Pineal Research. 1988;5(3):251–258. doi: 10.1111/j.1600-079X.1988.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 43.Kos-Kudla B., Ostrowska Z., Kozlowski A., et al. Circadian rhythm of melatonin in patients with colorectal carcinoma. Neuroendocrinology Letters. 2002;23:239–242. [PubMed] [Google Scholar]
- 44.Anisimov V. N., Kvetnoy I. M., Chumakova N. K., et al. Melatonin and colon carcinogenesis. Experimental and Toxicologic Pathology. 1999;51(1):47–52. doi: 10.1016/s0940-2993(99)80062-1. [DOI] [PubMed] [Google Scholar]
- 45.Anisimov V. N., Popovich I. G., Shtylik A. V., et al. Melatonin and colon carcinogenesis. Experimental and Toxicologic Pathology. 2000;52(1):71–76. doi: 10.1016/s0940-2993(00)80022-6. [DOI] [PubMed] [Google Scholar]
- 46.Ryabykh T. P., Nikolayeva T. G., Bodrova N. B. Effects of the biorhythm regulator melatonin on DNA synthesis in short-term human malignant tumors. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk. 2000;(8):30–33. [PubMed] [Google Scholar]
- 47.Farriol M., Venereo Y., Orta X., Castellanos J. M., Segovia-Silvestre T. In vitro effects of melatonin on cell proliferation in a colon adenocarcinoma line. Journal of Applied Toxicology. 2000;20(1):21–24. doi: 10.1002/(sici)1099-1263(200001/02)20:1<21::aid-jat623>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 48.Anisimov V., Popovich I. G., Zabezhinski M. A. Melatonin and colon carcinogenesis: I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2-dimethylhydrazine in rats. Carcinogenesis. 1997;18(8):1549–1553. doi: 10.1093/carcin/18.8.1549. [DOI] [PubMed] [Google Scholar]
- 49.Batista A. P. C., da Silva T. G., Teixeira Á. A. C., et al. Ultrastructural aspects of melatonin cytotoxicity on Caco-2 cells in vitro. Micron. 2014;59:17–23. doi: 10.1016/j.micron.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Yun C. W., Kim S., Lee J. H., Lee S. H. Melatonin promotes apoptosis of colorectal cancer cells via superoxide-mediated ER stress by inhibiting cellular prion protein expression. Anticancer Research. 2018;38(7):3951–3960. doi: 10.21873/anticanres.12681. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y., Zhou R., Park S.-Y., et al. 2-Hydroxymelatonin, a predominant hydroxylated melatonin metabolite in plants, shows antitumor activity against human colorectal cancer cells. Molecules. 2017;22(3):p. 453. doi: 10.3390/molecules22030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Y., Xiao X., Zhang C., et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. Journal of Pineal Research. 2017;62(2) doi: 10.1111/jpi.12380.e12380 [DOI] [PubMed] [Google Scholar]
- 53.León J., Casado J., Jiménez Ruiz S. M., et al. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-κβ. Journal of Pineal Research. 2014;56(4):415–426. doi: 10.1111/jpi.12131. [DOI] [PubMed] [Google Scholar]
- 54.Sato K., Tsuchihara K., Fujii S., et al. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Research. 2007;67(20):9677–9684. doi: 10.1158/0008-5472.Can-07-1462. [DOI] [PubMed] [Google Scholar]
- 55.Guo Y., Wang J., Wang Z., Yang Y., Wang X., Duan Q. Melatonin protects N2a against ischemia/reperfusion injury through autophagy enhancement. Journal of Huazhong University of Science and Technology [Medical Sciences] 2010;30(1):1–7. doi: 10.1007/s11596-010-0101-9. [DOI] [PubMed] [Google Scholar]
- 56.Coto-Montes A., Tomas-Zapico C. Could melatonin unbalance the equilibrium between autophagy and invasive processes? Autophagy. 2006;2(2):126–128. doi: 10.4161/auto.2.2.2351. [DOI] [PubMed] [Google Scholar]
- 57.Kongsuphol P., Mukda S., Nopparat C., Villarroel A., Govitrapong P. Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. Journal of Pineal Research. 2009;46(2):199–206. doi: 10.1111/j.1600-079X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 58.Nopparat C., Porter J. E., Ebadi M., Govitrapong P. The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. Journal of Pineal Research. 2010;49(4):382–389. doi: 10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 59.Gao J., Wang W. Knockdown of galectin-1 facilitated cisplatin sensitivity by inhibiting autophagy in neuroblastoma cells. Chemico-Biological Interactions. 2019;297:50–56. doi: 10.1016/j.cbi.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 60.Trivedi P. P., Jena G. B., Tikoo K. B., Kumar V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Molecular Carcinogenesis. 2016;55(3):255–267. doi: 10.1002/mc.22274. [DOI] [PubMed] [Google Scholar]
- 61.Thakur R. K., Yadav V. K., Kumar A., et al. Non-metastatic 2 (NME2)-mediated suppression of lung cancer metastasis involves transcriptional regulation of key cell adhesion factor vinculin. Nucleic Acids Research. 2014;42(18):11589–11600. doi: 10.1093/nar/gku860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Q., Zhang Y., Feng L., Jiang Y. Upregulated long noncoding RNA LINC01296 indicates a dismal prognosis for pancreatic ductal adenocarcinoma and promotes cell metastatic properties by affecting EMT. Journal of Cellular Biochemistry. 2019;120(1):552–561. doi: 10.1002/jcb.27411. [DOI] [PubMed] [Google Scholar]
- 63.Gonçalves N. D. N., Colombo J., Lopes J. R., et al. Effect of melatonin in epithelial mesenchymal transition markers and invasive properties of breast cancer stem cells of canine and human cell lines. PloS one. 2016;11(3) doi: 10.1371/journal.pone.0150407.e0150407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang S., Qi Y., Zhang H., et al. Melatonin inhibits cell growth and migration, but promotes apoptosis in gastric cancer cell line, SGC7901. Biotechnic & Histochemistry. 2013;88(6):281–289. doi: 10.3109/10520295.2013.769633. [DOI] [PubMed] [Google Scholar]
- 65.Liu Z., Zou D., Yang X., et al. Melatonin inhibits colon cancer RKO cell migration by downregulating rho-associated protein kinase expression via the p38/MAPK signaling pathway. Molecular Medicine Reports. 2017;16(6):9383–9392. doi: 10.3892/mmr.2017.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou D.-B., Wei X., Hu R.-L., et al. Melatonin inhibits the migration of colon cancer RKO cells by down-regulating myosin light chain kinase expression through cross-talk with p38 MAPK. Asian Pacific Journal of Cancer Prevention. 2015;16(14):5835–5842. doi: 10.7314/apjcp.2015.16.14.5835. [DOI] [PubMed] [Google Scholar]
- 67.Ushio-Fukai M., Alexander R. W. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD(P)H oxidase. Molecular and Cellular Biochemistry. 2004;264(1/2):85–97. doi: 10.1023/B:MCBI.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 68.Gacche R. N., Meshram R. J. Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth. Progress in Biophysics and Molecular Biology. 2013;113(2):333–354. doi: 10.1016/j.pbiomolbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Park J.-H., Yoon J., Park B. Pomolic acid suppresses HIF1α/VEGF-mediated angiogenesis by targeting p38-MAPK and mTOR signaling cascades. Phytomedicine. 2016;23(14):1716–1726. doi: 10.1016/j.phymed.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Park S.-Y., Jang W.-J., Yi E.-Y., et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1α stabilization under hypoxia. Journal of Pineal Research. 2010;48(2):178–184. doi: 10.1111/j.1600-079X.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 71.Gelfand M. V., Hagan N., Tata A. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife. 2014;3:p. 40. doi: 10.7554/eLife.03720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abusnina A., Keravis T., Zhou Q., Justiniano H., Lobstein A., Lugnier C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thrombosis and Haemostasis. 2015;113(2):319–328. doi: 10.1160/th14-05-0454. [DOI] [PubMed] [Google Scholar]
- 73.Abdel-Gawad I. A., Hassanein H. M., Bahgat N. A., et al. Study of endothelin-1 and vascular endothelial growth factor in patients with cancer colon. Journal of the Egyptian National Cancer Institute. 2008;20:216–223. [PubMed] [Google Scholar]
- 74.Toso A., Revandkar A., Di Mitri D., et al. Enhancing chemotherapy efficacy in pten -deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Reports. 2014;9(1):75–89. doi: 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 75.Dong B., Minze L. J., Xue W., Chen W. Molecular insights into the development of T cell-based immunotherapy for prostate cancer. Expert Review of Clinical Immunology. 2014;10(11):1547–1557. doi: 10.1586/1744666x.2014.962515. [DOI] [PubMed] [Google Scholar]
- 76.Polidarová L., Houdek P., Sumová A. Chronic disruptions of circadian sleep regulation induce specific proinflammatory responses in the rat colon. Chronobiology International. 2017;34(9):1273–1287. doi: 10.1080/07420528.2017.1361436. [DOI] [PubMed] [Google Scholar]
- 77.Srinivasan V., Pandi-Perumal S. R., Brzezinski A., Bhatnagar K. P., Cardinali D. P. Melatonin, immune function and cancer. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery. 2011;5:109–123. doi: 10.2174/187221411799015408. [DOI] [PubMed] [Google Scholar]
- 78.Kossoy G., Ben-Hur H., Popovich I., Zabezhinski M., Anisimov V., Zusman I. Melatonin and colon carcinogenesis. IV. Effect of melatonin on proliferative activity and expression of apoptosis-related proteins in the spleen of rats exposed to 1,2-dimethylhydrazine. Oncology Reports. 2000;7:1401–1405. doi: 10.3892/or.7.6.1401. [DOI] [PubMed] [Google Scholar]
- 79.Tilg H., Adolph T. E., Gerner R. R., Moschen A. R. The intestinal microbiota in colorectal cancer. Cancer Cell. 2019;33(6):954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Huang P., Liu Y. A reasonable diet promotes balance of intestinal microbiota: prevention of precolorectal cancer. BioMed Research International. 2019;2019 doi: 10.1155/2019/3405278.3405278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong F., Cai Y. Study insights into gastrointestinal cancer through the gut microbiota. BioMed Research International. 2019;2019:p. 8. doi: 10.1155/2019/8721503.8721503 [DOI] [PMC free article] [PubMed] [Google Scholar]