Abstract
Activation of the renin-angiotensin system (RAS) contributes to the pathogenesis of cardiovascular diseases. Sodium potassium ATPase (NKA) expression and activity are often regulated by angiotensin II (Ang II). This study is aimed at investigating whether DR-Ab, an antibody against 4th extracellular region of NKA, can protect Ang II-induced cardiomyocyte hypertrophy. Our results showed that Ang II treatment significantly reduced NKA activity and membrane expression. Pretreatment with DR-Ab preserved cell size in Ang II-induced cardiomyopathy by stabilizing the plasma membrane expression of NKA and restoring its activity. DR-Ab reduced intracellular ROS generation through inhibition of NADPH oxidase activity and protection of mitochondrial functions in Ang II-treated H9c2 cardiomyocytes. Pharmacological manipulation and Western blotting analysis demonstrated the cardioprotective effects were mediated by the activation of the AMPK/Sirt-3/PPARγ signaling pathway. Taken together, our results suggest that dysfunction of NKA is an important mechanism for Ang II-induced cardiomyopathy and DR-Ab may be a novel and promising therapeutic approach to treat cardiomyocyte hypertrophy.
1. Introduction
Cardiovascular disorders are one of the most common diseases in adults and the leading cause of death worldwide [1]. Pathological activation of renin-angiotensin system (RAS) is a keyfactor in several cardiovascular diseases [2]. Angiotensin (Ang) II, a critical component of RAS, presents in both systemic circulation and local organs such as the brain, blood vessel, kidney, and heart [2, 3]. Multiple studies reported that increased Ang II leads to hypertension and also directly promotes cardiomyocyte death, hypertrophy, and remodeling [2]. They have proved that Ang II is involved in cardiomyocyte damage [4–6]. Unscrambling the underline mechanisms of Ang II may supply a new therapeutic target for the prevention and treatment of these diseases.
In most mammalian cells, sodium potassium ATPase (NKA) is an energy-transducing ion pump across the plasma membrane [7]. In the past decade, NKA has also been proved to be an ion-pumping-independent receptor function that confers a ligand-like effect of cardiotonic steroids (CTS) on protein/lipid kinases, intracellular Ca2+ oscillation, and ROS production [8, 9]. However, drugs targeted at NKA are mainly CTS which was used to treat chronic heart failure, a kind of cardiovascular diseases. These chemical drugs also often cause severe toxic effects, such as cardiac arrhythmias and atrioventricular block, gastrointestinal disorders, nervous system disorders, anorexia, blurred vision, nausea, and vomiting [10]. In recent years, we and other groups have demonstrated that antibody targeted at DR region (897DVEDSYGQQWTYEQR911, amino acid sequence number showed as in rat), the 4th extracellular domain of α-subunit of NKA, can activate NKA's function [10, 11]. Our previous studies have already proved that DR-Ab produces cardioprotection and protects isoproterenol-induced mouse cardiac injury [10, 12]. Therefore, this antibody was a kind of ideal tool to study the NKA function in relative studies.
Recently, extensive studies have demonstrated that Ang II has a close relationship with NKA. Rasmussen's group reported that Ang II induced NKA inhibition in cardiac myocytes via PKC-dependent activation of NADPH oxidase [13]. Massey et al. also reported that Ang II-dependent phosphorylation of the rat kidney NKA at specific sites can regulate how the NKA releases bound cardiac glycoside [14]. Moreover, Ang II inhibits the NKA activity accompanied with the involvement of an increase in NADPH oxidase-derived O2∗- [15]. Thus, the present study was designed to study the effects of DR-Ab in Ang II-induced cardiac myocyte damage and its underlying mechanism.
2. Material and Methods
2.1. Chemicals and Reagents
Antibodies against p22phox, p47phox, Na+/K+-ATPase alpha 1 (NKA α1), PPAR-γ, Sirt-3, β-actin, GAPDH, β-tubulin, and the horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Antibodies against phosphorylated and total AMPK were purchased from Cell Signaling Technology (Beverly, MA, USA). The specific primers were synthesized by Integrated DNA Technologies Pte. Ltd. (Singapore). Antibody against α-actinin was obtained from Abcam (Cambridge, MA, USA). Mitochondrial membrane potential assay kit with JC-1 and the kits for measurement of ATP were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Dihydroethidium (DHE) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). MitoSOX™ was purchased from Invitrogen (Carlsbad, CA, USA). AMPK inhibitor compound C, a selective Sirt3 inhibitor 3-TYP, and PPARγ antagonists GW9662 were obtained from Cayman Chemical Company (Ann Arbor, MI, USA). DR-Ab was generated and identified in our lab as previously described [12, 16].
2.2. Cell Culture
Embryonic rat heart-derived cells (H9c2, passage 15) preserved by our lab were cultured in high-glucose Dulbecco's modified Eagle's medium (4.5 g/l glucose) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin-streptomycin, Gibco) in humidified air containing 5% CO2 at 37°C.
Primary neonatal mouse cardiomyocytes: the cardiomyocytes were isolated from 1- to 3-day-old C57BL/6 neonatal mice as described previously [17–19]. In short, the hearts were placed into ice-cold Hanks' balanced saline solution (HBSS; Life Technologies). After removal of atrial and aortic appendages, the cardiomyocytes were collected by using 0.2 mg/ml collagenase type II (Worthington Biochemical, Lakewood, NJ) and 0.6 mg/ml pancreatin (Sigma, MAK030, St. Louis, MO) at common cellular incubator. The supernatant-containing suspended cells were cultured in minimum essential medium with 10% fetal bovine serum for 2 h to remove nonmyocytes. Then, the culture medium was changed to minimum essential medium containing 10% FBS with 1% antibiotics after seeding for 48h. Cardiomyocytes were seeded 3 days prior to use.
All primary cell culture protocols were performed strictly according to the principles and guidance of Institutional Animals Care and Use Committee at the National University of Singapore.
2.3. Intracellular and Mitochondrial ROS Measurement
After fixing collected H9c2 cells, they were incubated with DHE (10 μM) and DCFH-DA (10 μM) in a dark and humidified incubator at 37°C for 30min as previously described [20] and changed the solution to phosphate-buffered saline (PBS) and observed on microscope immediately.
Mitochondrial ROS production was measured with a fluorogenic dye named MitoSOX Red (Invitrogen, Darmstadt, Germany). Cells were loaded with 1 μM MitoSOX Red for 30 min at 37°C protecting from light and washed cells with PBS and then observed on microscope (DMi 8; Leica, Microsystems, Germany).
The fluorescence signals were captured and analyzed with the Image-Pro Plus 6.0 (Version 6.0, Media Cybernetics, Bethesda, MD, USA) in same parameters.
2.4. Western Blotting Analysis
After washing twice with PBS, the cells were lysed with ice-cold lysis buffer. The cell lysate was centrifuged at 10,000 g for 10 min at 4°C. Equal amount of proteins was electrophoresed, transferred, blotted, and then incubated with required primary antibodies at 4°C overnight. After washing with TBST buffer three times, the membranes were incubated with appropriate secondary horseradish peroxidase- (HRP-) conjugated antibodies. Then, membranes were detected using an ECL Advanced Western Blot Detection Kit (Millipore Darmstadt, Germany). The integrated optical density was quantified with the Image-Pro Plus 6.0 software.
2.5. Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was dectected with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) (Beyotime Institute of Biotechnology, Shanghai, China). The H9c2 cells were stained with JC-1 and observed with a fluorescence microscope (DMi 8; Leica, Microsystems, Germany).
2.6. Real-Time PCR
Total RNA extraction was performed with TRIzol (Life Technologies, USA) according to the manufacturer's instructions, and then RevertAid First-Strand cDNA Synthesis Kit (Thermo Scientific, USA) was used for reverse transcription. Following, GoTaq® quantitative PCR (qPCR) Master Mix (Promega, USA) was used for quantitative PCR with indicated primers on a VIIA(TM) 7 System (Applied Biosystems). Data were analyzed by normalization against GAPDH. The primers used are indicated as in Table 1.
Table 1.
| Gene (rat) | Primer sequences (5′-3′) |
| GAPDH | Forward: AGGAGTAAGAAACCCTGGAC |
| Reverse: CTGGGATGGAATTGTGAG | |
| ANF | Forward: CCGTATACAGTGCGGTGTCC |
| Reverse: CAGAGAGGGAGCTAAGTGCC | |
| BNP | Forward: AGCTGCTTTGGGCAGAAGAT |
| Reverse: AAAACAACCTCAGCCCGTCA | |
| β-MHC | Forward: GACAACGCCTATCAGTACATG |
| Reverse: TGGCAGCAATAACAGCAAAA | |
| ND1 | Forward: AAGCGGCTCCTTCTCCCTACAAAT |
| Reverse: GAAGGGAGCTCGATTTGTTTCTGC | |
| Cytb | Forward: GCAGCTTAACATTCCGCCCAATCA |
| Reverse: TGTTCTACTGGTTGGCCTCCGATT | |
| mt-co1 | Forward: AAGGTTTGGTCCTGGCCTTA |
| Reverse: GGCAAGGCGTCTTGAGCTAT | |
| CPT-1β | Forward: TCAAGGTTTGGCTCTATGAGGGCT |
| Reverse: TCCAGGGACATCTTGTTCTTGCCA | |
| CPT-2 | Forward: TCCTGCATACCAGCAGATGAACCA |
| Reverse: TATGCAATGCCAAAGCCATCAGGG | |
| LCAD | Forward: AATGGGAGAAAGCCGGAGAAGTGA |
| Reverse: GATGCCGCCATGTTTCTCTGCAAT | |
| MCAD | Forward: CTGCTCGCAGAAATGGCGATGAAA |
| Reverse: CAAAGGCCTTCGCAATAGAGGCAA | |
|
| |
| Gene (mouse) | Primer sequences (5′-3′) |
| β-Actin | Forward: CCGTGAAAAGATGACCCAGA |
| Reverse: CTGGGATGGAATTGTGAG | |
| ANP | Forward: ACCTGCTAGACCACCTGGAG |
| Reverse: CCTTGGCTGTTATCTTCGGTACCGG | |
| BNP | Forward: GAGGTCACTCCTATCCTCTGG |
| Reverse: GCCATTTCCTCCGACTTTTCTC | |
| β-MHC | Forward: CCGAGTCCCAGGTCAACAA |
| Reverse: CTTCACGGGCACCCTTGGA | |
2.7. Plasma Membrane Extraction
EZ-Link NHS-SS-biotin (Pierce Chemical Co., USA) was used to label surface protein for 1 h. Cells were washed with PBS containing 100 mM glycine and then lysed in lysis buffer. After protein quantitative, equal proteins (150–300 μg) were added to Streptavidin (Pierce Chemical Co.) beads at 4°C overnight. Next day, beads were washed thoroughly, resuspended in 30 μl loading buffer, and analyzed using Western blots.
2.8. Isolation of Endosomes
The preparation of endosomes was fractioned on a floatation gradient. In brief, the treated cells were washed by cold PBS and homogenization buffer (250 mM sucrose and 3 mM imidazole, pH 7.4). After centrifuging for 10 min at 2000×g in 4 °C, the supernatant was adjusted to 40.6% sucrose, followed by incubation of 35% sucrose supplemented with 3 mM imidazole and 0.5 mM EDTA and homogenization buffer. The samples were centrifuged at 210,000×g for 1.5 h; the endosomes were then obtained at the homogenization buffer—35% sucrose interface. The endosome fraction was identified by immunoblots for Rab 7 as previously described [12, 21].
2.9. Measurement of NKA Activity
NKA activity was determined according to previous study [22, 23]. H9c2 cells were homogenized in buffer A containing 20 mM HEPES, 250 mM sucrose, 2 mM EDTA, 1 mM MgCl2, pH 7.4, and then centrifuged at 20,000 g for 30 min. Consequently, resuspended the pellet in buffer A again and quantified the protein. One 50 μl aliquot of homogenate was mixed with 50 μl of reaction buffer 1 (200 mM Tris-HCl, 30 mM MgCl2, 200 mM NaCl, 60 mM KCl, 10 mM EGTA, pH 7.5). Another 50 μl aliquot was mixed with reaction buffer 2 (buffer 1+1 mM ouabain). To prevent protein degradation, 100 μg/ml PMSF, 2 μg/ml apronitin, and 2 μg/ml pepstatin A were added in. After 1 mM of ATP was added, the mixtures were incubated for 10 min at 37°C and then stopped by adding 10 μl of 100% (w/v) trichloroacetic acid. After incubating them on ice for 1 h, they were centrifuged at 20,000 g for 30 min. The supernatant without phosphate was assayed with the Phosphate Colorimetric Kit (Sigma, MAK030, St. Louis, MO) at 650 nm.
2.10. Immunofluorescence Staining
Immunofluorescent staining was performed as described previously [24]. The collected H9c2 cardiomyocytes or primary neonatal mouse cardiomyocytes were fixed in freshly made -20°C ethanol at room temperature for 10 min and then permeabilized with 0.1% Triton X-100. After blocking with 5% BSA at room temperature for 1 h, the cells were incubated with the mouse anti-NKA antibody or mouse anti-α-actinin overnight at 4°C. Next, the cells were washed with PBS three times, and then incubated with goat anti-mouse cross-adsorbed secondary antibody, Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature, and the nucleus was stained with DAPI. Goat anti-mouse IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA). The images were captured with a fluorescence microscope (Leica DMi8, Leica, Wetzlar, Germany).
2.11. Statistical Analysis
Data were expressed as mean ± SD. One-way or two-way ANOVA followed by the post hoc Bonferroni test was used for multiple comparisons. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. DR-Ab Improves Ang II-Induced Cardiomyocyte Hypertrophy through Preservation of NKA Activity
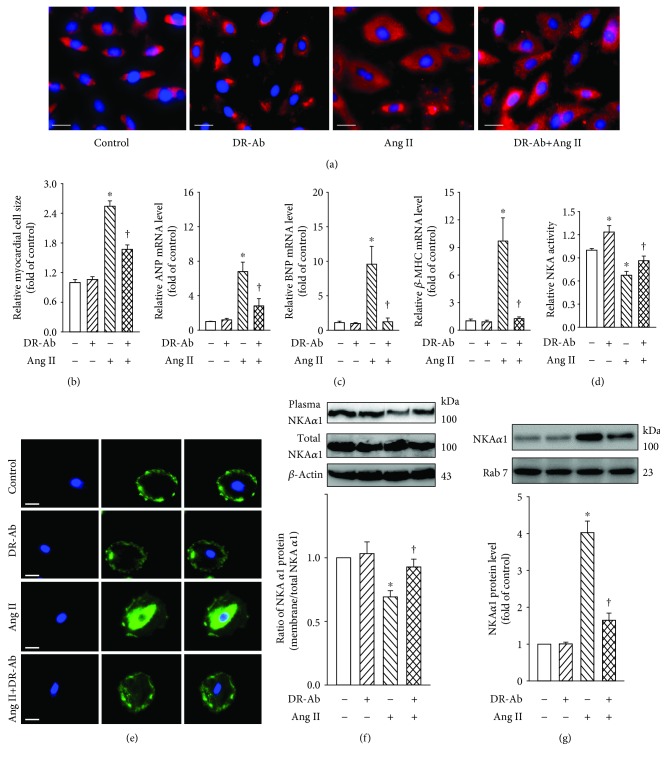
The immunofluorescence staining of α-actin was performed to reveal the H9c2 cardiomyocyte morphology (Figure 1(a)) and primary cultured neonatal mouse cardiomyocytes (). It was found that Ang II (100 nM, 48 h) treatment significantly increased the cell size of cardiomyocytes, and this effect was attenuated by pretreatment with DR-Ab (2 μM, 30 min) (Figure 1(b) and ). We also examined the mRNA expression of various hypertrophic biomarkers like atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and beta-myosin heavy chain (β-MHC). Similar to what we observed in myocyte morphology, pretreatment with DR-Ab significantly attenuated Ang II-stimulated the above three hypertrophic biomarkers (Figure 1(c) and Figures ).
Figure 1.
Effects of DR-Ab on Ang II-induced H9c2 cardiomyocyte hypertrophy. DR-Ab (2 μM) was given 30 min before treatment with Ang II (100 nM) for 48 h. (a, b) Representative immunofluorescence staining (a) and group data (b) showing that DR-Ab reversed enlarged cell size caused by Ang II. Red: α-actinin. Blue: DAPI. Scale bar, 25 μm. n = 6. (c) qRT-PCR analysis showing the mRNA levels of ANP, BNP, and β-MHC. n = 4. (d–g) DR-Ab reversed Ang II-induced loss of plasma membrane NKA α1 (e, f), increase of endosome NKA α1 (g), and downregulation of NKA activity (d). n = 4~6. Scale bar, 30 μm. Blue: DAPI. Green: NKA α1 staining. ∗p < 0.05 versus control group, †p < 0.05 versus Ang II alone group.
To study the underlying mechanisms, we first determined the effect of DR-Ab on NKA activity. As shown in Figure 1(d), DR-Ab attenuated Ang II-impaired NKA activity in the H9c2 cardiomyocytes (Figure 1(d)). We further examined the plasma membrane and total expression of NKA with Western blots and immunostaining. As shown in Figures 1(e)–1(g), treatment with Ang II reduced plasma membrane NKA expression (Figures 1(e) and 1(f) and ) and increased endosome NKA expression (Figures 1(g) and ), but had minor effect on its total protein expression. Pretreatment with DR-Ab reversed the effect of Ang II on plasma and endosome NKA expression. Taken together, our experiments indicated that DR-Ab inhibits plasma membrane NKA endocytosis. Our data imply that membrane NKA expression and activity are important in regulation of cell size when RAS is upregulated.
3.2. DR-Ab Alleviates Ang II-Induced Intracellular ROS Generation in H9c2 Cells
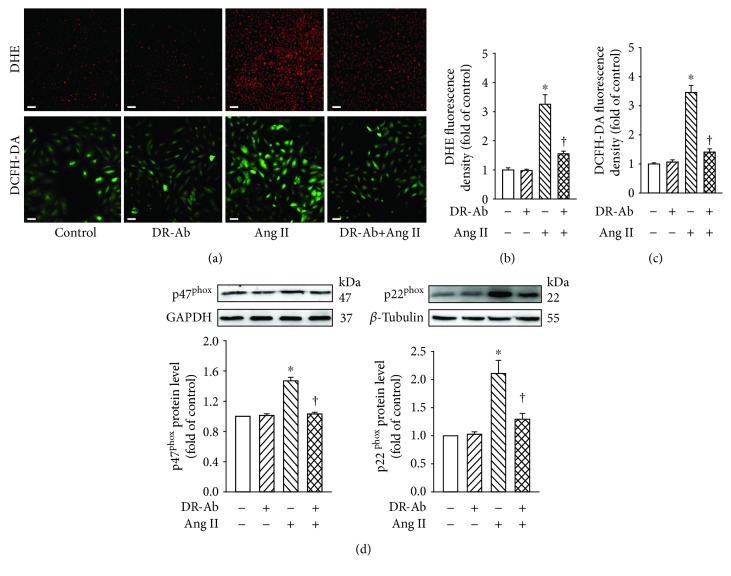
Oxidative stress plays an important role in Ang II-induced cardiomyopathy [2]. We first detected whether DR-Ab can affect Ang II-induced intracellular ROS production by using both DHE and DCFH-DA staining kits. As shown in Figures 2(a)–2(c), Ang II (100 nM, 48 h) significantly increased the generation of superoxide, hydroxyl, peroxyl, and other ROS. Pretreatment with DR-Ab (2 μM, 30 min), which itself had no obvious effect, significantly reduced Ang II-induced intracellular ROS generation (Figures 2(a)–2(c)).
Figure 2.
Effects of DR-Ab on Ang II-induced intracellular ROS generation in H9c2 cells. DR-Ab (2 μM) was given 30 min before treatment with Ang II (100 nM) for 48 h. (a–c) Representative immunofluorescence image (a) and group data (b, c) showing that DR-Ab decreased ROS generation caused by Ang II. Red: DHE staining (a, upper). Scale bar, 100 μm. Green: DCFH-DA staining (a, lower). Scale bar, 50 μm. n = 6. (d) Effect of DR-Ab on the protein level of two subunits of NADPH oxidase: p22phox and p47phox. n = 4‐6. ∗p < 0.05 versus control group, †p < 0.05 versus Ang II alone group.
To examine the involvement of NADPH oxidase, we detected the protein expression of two subunits of NADPH oxidase (NOX2): p22phox and p47phox. Western blotting analysis showed that treatment with Ang II upregulated the protein expression of these two proteins and this effect was attenuated by pretreatment with DR-Ab in both H9c2 and neonatal mouse cardiomyocytes (Figures 2(d) and ). Our data suggest that DR-Ab may inhibit NADPH oxidase activity in pathological situations.
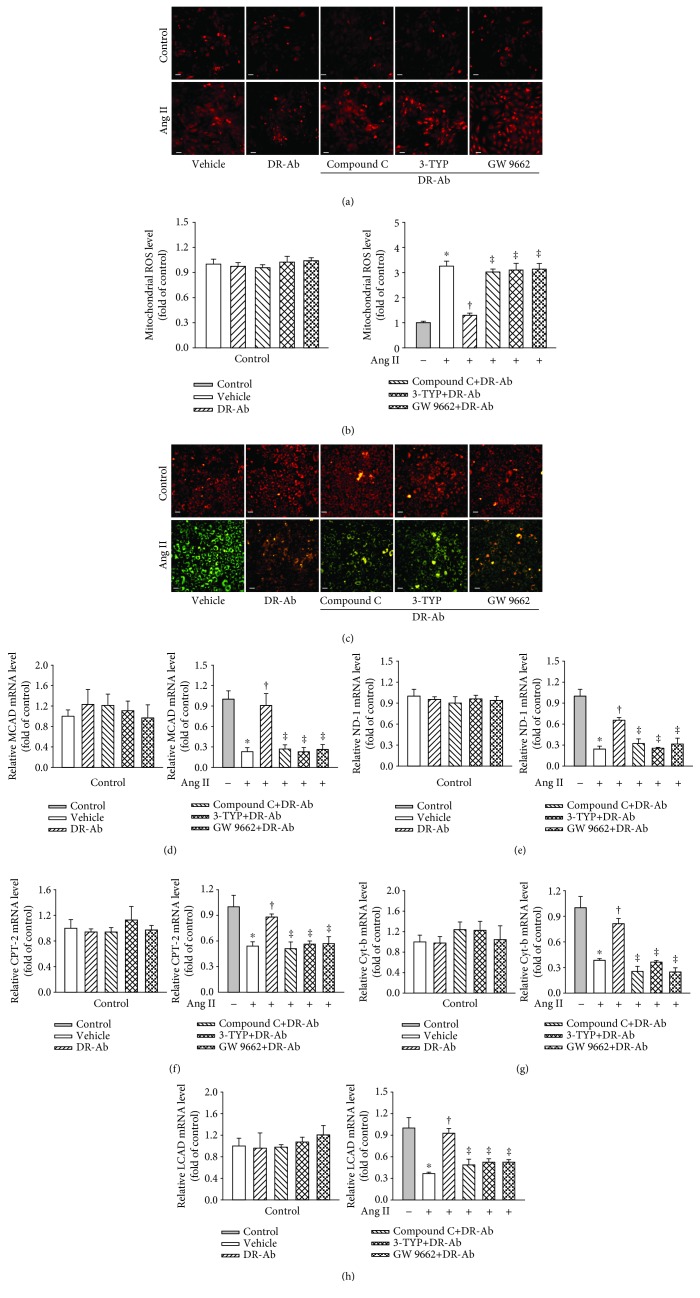
3.3. DR-Ab Prevents Ang II-Induced Mitochondrial ROS and Energy Metabolic Dysfunction
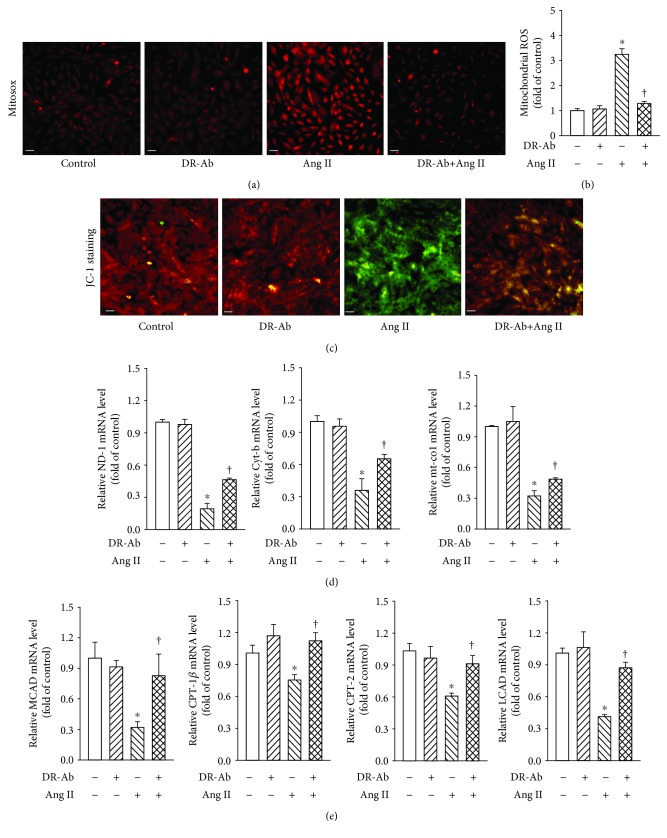
We further studied mitochondrial ROS generation with MitoSOX™ Red staining. As shown in Figures 3(a) and 3(b), Ang II significantly increased mitochondrial ROS generation in the mitochondria, and this effect was reversed by pretreatment with DR-Ab. The mitochondrial permeability transition, an important step in the induction of cellular apoptosis, was also determined using the unique fluorescent cationic dye, JC-1. It was found that Ang II induced loss of red JC-1aggregate fluorescence, and only green monomer fluorescence was detected in the cytoplasm of these cells (Figure 3(c)). This effect was also reversed by DR-Ab treatment.
Figure 3.
Effect of DR-Ab on Ang II-induced mitochondrial ROS (mit-ROS) generation and energy metabolic dysfunction. (a, b) Representative image (a) and group data (b) showing that DR-Ab decreased Ang II-induced mit-ROS generation. Red: Mit-ROS. Scale bar, 50 μm. n = 4‐6. (c) Representative JC-1 staining showing that DR-Ab reversed mitochondrial membrane potential loss caused by Ang II. Red: aggregate. Green: monomer. Scale bar, 50 μm. (d) qRT-PCR analysis showing that DR-Ab increased the mRNA expression of mitochondrial encoded genes (ND-1, Cyt-b, and mt-co1) in Ang II-treated cells. n = 4. (e) qRT-PCR analysis showing the effect of DR-Ab on the mRNA expression of fatty acid oxidation related genes (CPT-1β, CPT-2, LCAD, and MCAD). n = 4. ∗p < 0.05 versus control group, †p < 0.05 versus Ang II alone group.
We continued to study the mRNA levels of mitochondrial DNA-encoded genes including ND-1, cyt-b, and mt-co1. Real-time PCR analysis showed that pretreatment with DR-Ab significantly attenuated Ang II-suppressed expression of these genes (Figure 3(d)). Our data imply that DR-Ab recovered impaired mitochondrial function induced by Ang-II.
Fatty acid oxidation (FAO) is one of the pivotal mechanisms involved in the development of cardiomyopathy [25]. We also studied whether DR-Ab can affect fatty acid metabolism in Ang-II-induced H9C2 cardiomyocyte damage. As shown in Figure 3(e), Ang II-significantly reduced the mRNA expression of FAO-related genes including CPT-1β, CPT-2, long-chain acyl-CoA dehydrogenase (LCAD), and medium-chain acyl-CoA dehydrogenase (MCAD), and these effects were reversed by pretreatment with DR-Ab. These data suggest that DR-Ab may improve Ang II-induced impaired fatty acid oxidation.
3.4. DR-Ab Protects H9c2 Cardiomyocytes against Ang II-Induced Hypertrophy via Activation of AMPK/Sirt-3/PPARγ Signaling Pathway
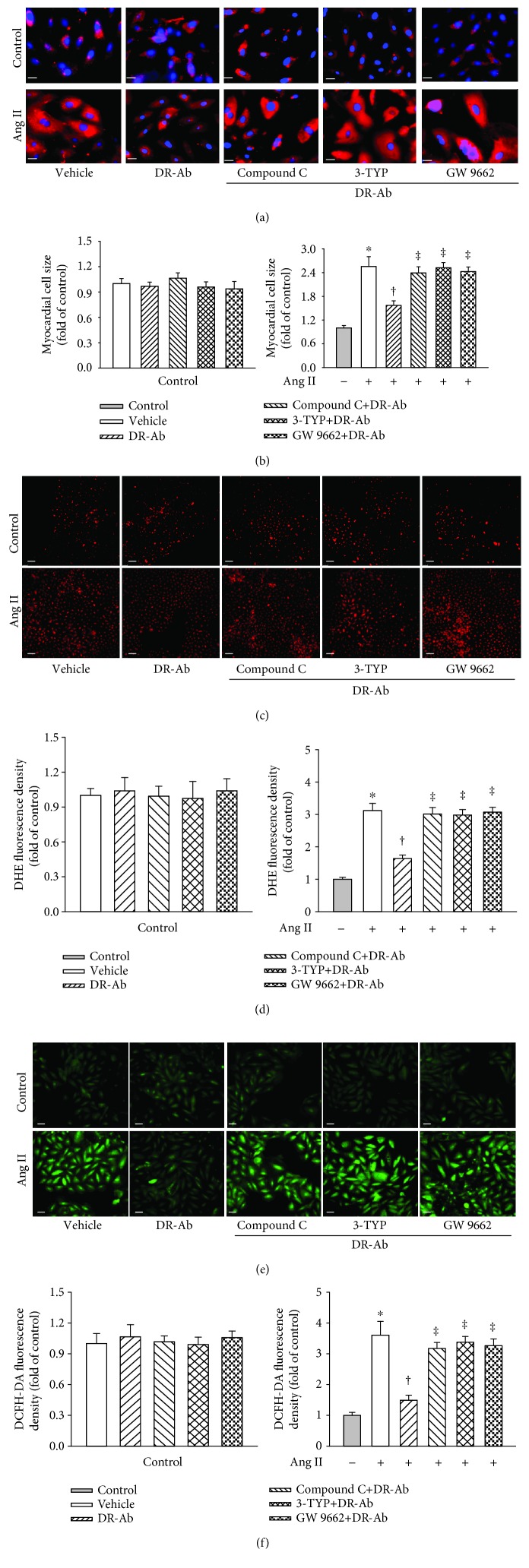
It is well known that the AMPK/Sirt-3/PPARγ signaling pathway participates in Ang II-induced cardiomyocyte hypertrophy [26–34]. In this study, we also tested the involvement of this pathway in the effect of DR-Ab. We first repeated the effects of DR-Ab on cell morphology (Figures 4(a) and 4(b)), intracellular (Figures 4(c)–4(f)) and mitochondrial ROS (Figures 5(a) and 5(b)) generation, mitochondrial membrane potential loss (Figure 5(c)), and mitochondrial function-related gene level (Figures 5(d)–5(h)) in the presence and absence of compound C, an AMPK inhibitor, 3-TYP, a selective Sirt3 inhibitor, and GW9662, a PPARγ antagonist. As shown in Figures 4 and 5, all these inhibitors abolished the protective effects of DR-Ab. Our data suggest that the AMPK/Sirt-3/PPARγ signaling pathway mediates the cardioprotective effects of DR-Ab.
Figure 4.
Effect of DR-Ab on myocyte hypertrophy and intracellular ROS generation in Ang II-treated H9c2 in the presence and absence of compound C (40 μM, a selective AMPK inhibitor), 3-TYP (50 μM, a selective Sirt3 inhibitor), or GW9662 (10 μM, a PPARγ antagonist). Cells were treated with these inhibitors for 30 min before DR-Ab (2 μM, 30 min) and Ang II (100 nM, 48 h). (a, b) Representative immunofluorescence staining (a) and group data (b) showing that blockade of AMPK, Sirt3, or PPARγ abolished the effect of DR-Ab on cell size. Red: α-actinin. Blue: DAPI. Scale bar, 25 μm. n = 4‐6. (c–f) Representative image (c, e) and group data (d, f) showing that blockade of AMPK, Sirt3, or PPARγ promoted the intracellular ROS which were decreased by DR-Ab in Ang II-induced cells. (c) Red: DHE relative fluorescence density. Scale bar, 100 μm. (e) Green: DCFH-DA staining. Scale bar, 50 μm. n = 4‐6. ∗p < 0.05 versus control, †p < 0.05 versus Ang II alone group, ‡p < 0.05 versus Ang II+DR-Ab group.
Figure 5.
Effects of DR-Ab on mit-ROS production and energy metabolic dysfunction in Ang II-treated cells in the presence and absence of inhibitors of AMPK, Sirt3, or PPARγ. (a, b) Representative image (a) and statistic data (b) showing that blockade of AMPK, Sirt3, or PPARγ with their inhibitors abolished the protective effect of DR-Ab on mit-ROS production. Red: mit-ROS. Scale bar, 50 μm. n = 4‐6. (c) JC-1 staining showing that blockade of AMPK, Sirt3, or PPARγ reversed the effect of DR-Ab on mitochondrial membrane potential. Red: aggregate. Green: monomer. Scale bar, 50 μm. (d–h) qRT-PCR analysis showing that blockade of AMPK, Sirt3, or PPARγ abolished the effects of DR-Ab on the mRNA expression of ND-1, Cyt-b, CPT-2, LCAD, and MCAD. n = 4‐6. ∗p < 0.05 versus control, †p < 0.05 versus Ang II alone group, ‡p < 0.05 versus Ang II+DR-Ab group.
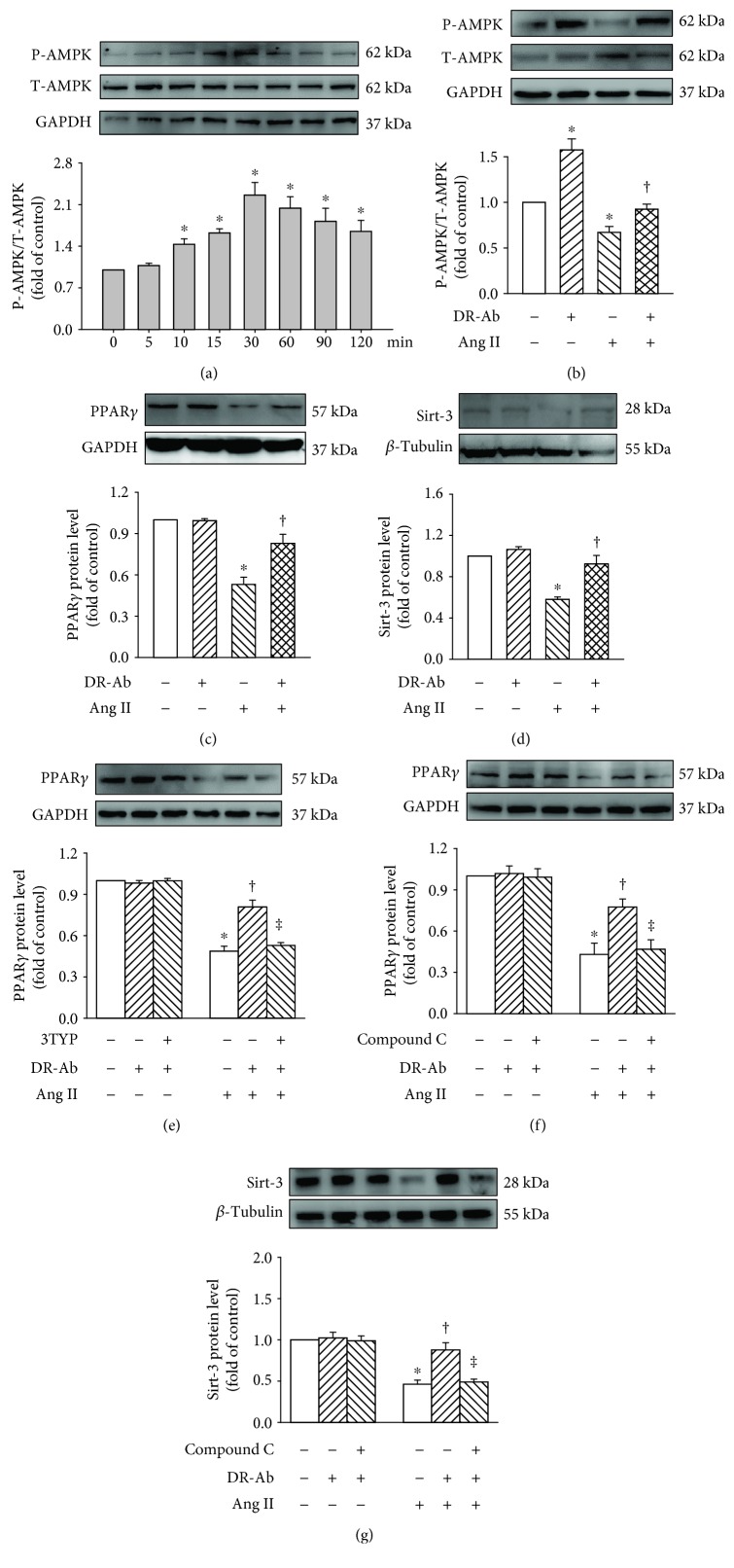
To further confirm the involvement of this signaling pathway, we observed the effect of DR-Ab on AMPK phosphorylation (P-AMPK). A time-course study showed that DR-Ab obviously increased P-AMPK level and the strongest effect was observed when cells were treated with DR-Ab for 30 min (Figure 6(a)). For this reason, DR-Ab reversed Ang II-suppressed P-AMPK (Figure 6(b)). To study the signaling cascade, compound C, an AMPK inhibitor, was used. As shown in Figures 6(f) and 6(g), compound C abolished the effect of DR-Ab on both Sirt-3 and PPARγ. Moreover, Ang II treatment significantly reduced the protein levels of PPARγ and Sirt-3 (Figures 6(c) and 6(d)). These effects were significantly attenuated by incubation with DR-Ab. Interestingly, treatment with 3-TYP, a selective Sirt3 inhibitor, reversed the effect of DR-Ab on PPARγ protein expression (Figure 6(e)). Taken together, DR-Ab protects H9c2 cardiomyocytes against Ang II-induced hypertrophy may via activate the AMPK/Sirt-3/PPARγ signaling pathway.
Figure 6.
Western blotting analysis showing that DR-Ab stimulated AMPK/Sirt-3/PPARγ signaling pathway. (a) Time-course study showing the effect of DR-Ab on AMPK phosphorylation. (b–d) DR-Ab reversed the effect of Ang II on p-AMPK (b), PPARγ (c), & Sirt-3 (d). n = 4‐6. (e) 3-TYP eliminated DR-Ab effect on the PPARγ level in Ang II-treated cells. n = 4. (f, g) Compound C abolished the effect of DR-Ab on the protein expression of PPARγ (f) and Sirt-3 (g) in Ang II-treated cells. n = 4‐6. ∗p < 0.05 versus control group, †p < 0.05 versus Ang II alone group, ‡p < 0.05 versus Ang II+ DR-Ab.
4. Discussion
Ang II, a key component of renin-angiotensin system (RAS), is crucial in cardiovascular physiology and pathology [35]. The increased circulating Ang II level and activated RAS are closely associated with cardiovascular diseases such as cardiac hypertrophy [36] and heart failure [37]. Therefore, Ang II is widely used to mimic the pathology of clinical cardiac hypertrophy. An important function of NKA is to regulate cell volume [38, 39]. Recently, NKA expression and activity were also found to be closely regulated by Ang II [14, 40–42]. For instance, Ang II can inhibit NKA activity via induction of NADPH oxidase-derived O2∗−15. Molkentin's group also reported that overexpression of NKA successfully protects the heart against pathological cardiac hypertrophy and remodeling [43]. We previously reported that DR-Ab protects the heart against oxidative and ischemic injury [10, 12]. In this study, we demonstrated for the first time that DR-Ab prevented Ang II-induced myocyte hypertrophy through observing myocyte size, cell morphology, ROS generation, and mitochondrial functions.
We first investigated whether Ang II can regulate NKA expression and function in this study. It was found that Ang II treatment significantly reduced both plasma expression and activity of NKA. To study whether Ang II-induced pathology is caused by impairment of NKA, we pretreated the cells with DR-Ab which stimulates NKA activity. As expected, DR-Ab improved cardiomyocytes hypertrophy induced by Ang II. The enlarged cell size induced by Ang II was recovered to nearly normal cell after treatment with DR-Ab. Our results suggest that DR-Ab protect cells against Ang II-induced cells injury through preservation of membrane NKA activity, which helps to maintain resting potential, ion transport, and regulates cellular volume.
Oxidative stress plays an important role in Ang II-induced cardiomyopathy [44]. Recent studies have revealed that NKA is one of the target proteins of ROS [45, 46]. Moreover, it was found that NADPH oxidase is crucial for the inhibited NKA activity in cardiac myocytes treated with Ang II [13]. For the above reasons, we first examined whether DR-Ab can protect cardiomyocytes through inhibition of NADPH oxidase. We found that DR-Ab markedly attenuated Ang II-induced intracellular ROS generation through inhibition of NADPH oxidase. This effect was achieved by inhibition of the upregulated protein expression of p22phox and p47phox caused by Ang II.
Mitochondrial dysfunction also produces high levels of ROS. Multiple experiments were therefore performed to test the mitochondrial functions. We found that Ang II treatment largely increased mitochondrial ROS production and decreased mitochondrial membrane potential. This is consistent with previous studies [47]. We further studied its effects on mitochondrial DNAs, which encode proteins for the electron transport chain and then produce the majority of cellular energy [48, 49]. Our results showed that Ang II notably decreased the mRNA levels of ND-1, cyt-b, and mt-co1. Metabolic derangement is a signature in pathological cardiac hypertrophy [50]. Ang II also significantly inhibited the mRNA levels of CPT-1β, CPT-2, LCAD, and MCAD, all of which are important in mitochondrial oxidative phosphorylation and fatty acid metabolism. Interestingly, all the above effects caused by Ang II were significantly reversed by the pretreatment with DR-Ab.
DR-Ab is an antibody targeted at the 4th extracellular domain of α-subunit of NKA. It remains unclear why and how DR-Ab protects mitochondrial functions by binding to NKA. As NKA also serve as a signaling protein [28], we studied the signaling mechanisms underlying the protective effect of DR-Ab. Previous studies revealed that Ang II-induced cardiac hypertrophy is mediated by AMPK- [29–31, 51, 52], Sirt3- [32–34], and PPARγ- [26–28] dependent mechanisms. Therefore, there is a close relationship between NKA and AMPK [53–56]. On the one hand, activation of AMPK has been reported to regulate the activity and cell surface abundance of NKA [57]. On the other hand, ouabain blocks the carbachol-induced phosphorylation and activation of AMPK [58]; thus, activation of NKA also stimulates AMPK activity [59]. For this reason, we studied the effect of DR-Ab on AMPK activity and found that DR-Ab promoted AMPK phosphorylation. Activation of AMPK has been shown to stimulate Sirt-3 [60–62], and then activated Sirt-3 affects PPARγ [32] which has been proved in participating in Ang II-induced myocyte hypertrophy [26–28]. In our study, we confirmed the involvement of the Sirt-3/PPARγ pathway with pharmacological manipulation. Western botting analysis also confirmed that activation of Sirt-3/PPARγ is secondary to that of AMPK. Our data suggest that the AMPK/Sirt-3/PPARγ signaling pathway mediates the protective effects of DR-Ab against Ang II-induced H9c2 cardiomyocyte damage.
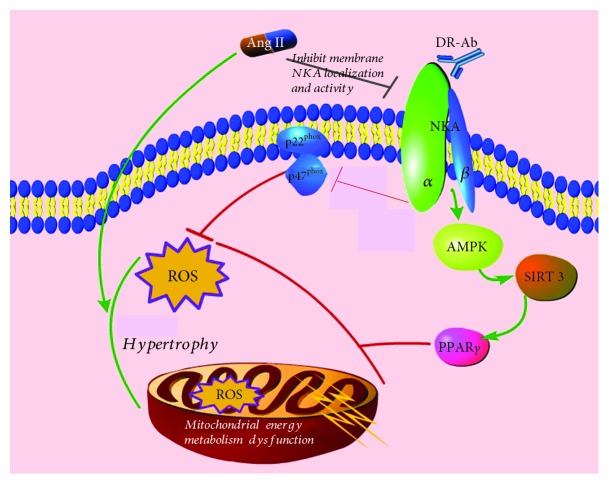
In summary, as shown in Figure 7, we found for the first time that DR-Ab prevents Ang II-induced H9c2 cardiomyocyte hypertrophy. This protective effect is mediated by activation of the AMPK/Sirt-3/PPARγ signaling pathway and stabilization of membrane NKA expression. Our results suggest a novel mechanism and therapeutic strategy in the treatment of cardiac hypertrophy and associated oxidative injury.
Figure 7.
Schematic illustration showing the mechanisms for the protective effects of DR-Ab. DR-Ab protects against Ang II-induced cell injury by stabilization of membrane NKA and stimulation of its activity. This helps to maintain the normal intracellular ion homeostasis, thus reserves the cell size. DR-Ab inhibits NADPH oxidase activity by downregulation of p22phox and p47phox expression. Meanwhile, DR-Ab inhibits mitochondrial ROS generation and preserves mitochondrial function through stimulation of the AMPK/Sirt-3/PPARγ signaling pathway.
Acknowledgments
This work was supported by research grants from the Singapore National Medical Research Council (NMRC/CIRG/1432/2015 and NMRC/1274/2010) and the National Nature Science Foundation of China (NSFC 81872865).
Data Availability
The derived data used to support the findings of this study are included within the article. The raw data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
This work was designed by Jin-Song Bian, Hai-Jian Sun, and Siping Xiong. The cells were cultured by Mengyuan Zhu and Zhiyuan Wu. Most experiments were performed by Hai-Jian Sun, Siping Xiong, Lei Cao, and Tengteng Liu. Zhiyuan Wu and Mengyuan Zhu performed the statistical analysis. Siping Xiong and Hai-Jian Sun wrote the manuscript. Jin-Song Bian were consultants for the study and helped with manuscript editing. Siping Xiong and Hai-Jian Sun contributed equally to this work.
Supplementary Materials
Figure S1: Effects of DR-Ab on Ang II-induced hypertrophy in the neonatal mouse cardiomyocytes. DR-Ab (2 μM) was given 30 min before treatment with Ang II (100 nM) for 48 h. (A-B) Representative immunofluorescence staining (A) and group data (B) showing that DR-Ab reversed enlarged cell size caused by Ang II. Green: NKA α1. Blue: DAPI. Scale bar, 30 μm. n = 6. (C-E) qRT-PCR analysis showing the mRNA levels of ANP, BNP, and β-MHC. n = 4. (F-G) DR-Ab reversed Ang II-induced loss of plasma membrane NKA α1 (A&F) and increase of endosome NKA α1 (G). n = 4‐6. (H-I) Effect of DR-Ab on the protein level of two subunits of NADPH oxidase: p22phox and p47phox. n = 4‐6. ∗p < 0.05 versus control group, †p < 0.05 versus Ang II alone group.
References
- 1.Naghavi M., Abajobir A. A., Abbafati C., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xin Y., Bai Y., Jiang X., et al. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ss/Fyn pathway. Redox Biology. 2018;15:405–417. doi: 10.1016/j.redox.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer M., McMurray J. J. V. Importance of endogenous compensatory vasoactive peptides in broadening the effects of inhibitors of the renin-angiotensin system for the treatment of heart failure. Lancet. 2017;389(10081):1831–1840. doi: 10.1016/S0140-6736(16)30969-2. [DOI] [PubMed] [Google Scholar]
- 4.Herichova I., Szantoova K. Renin-angiotensin system: upgrade of recent knowledge and perspectives. Endocrine Regulations. 2013;47(1):39–52. doi: 10.4149/endo_2013_01_39. [DOI] [PubMed] [Google Scholar]
- 5.Ren X. S., Tong Y., Ling L., et al. NLRP3 gene deletion attenuates angiotensin II-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cellular Physiology and Biochemistry. 2018;44(6):2269–2280. doi: 10.1159/000486061. [DOI] [PubMed] [Google Scholar]
- 6.Sun H. J., Li P., Chen W. W., Xiong X. Q., Han Y. Angiotensin II and angiotensin-(1-7) in paraventricular nucleus modulate cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS One. 2012;7(12, article e52557) doi: 10.1371/journal.pone.0052557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingrel J. B., Kuntzweiler T. Na+,K(+)-ATPase. The Journal of Biological Chemistry. 1994;269(31):19659–19662. [PubMed] [Google Scholar]
- 8.Liu J., Tian J., Haas M., Shapiro J. I., Askari A., Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. The Journal of Biological Chemistry. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 9.Cui X., Xie Z. Protein interaction and Na/K-ATPase-mediated signal transduction. Molecules. 2017;22(6):p. 990. doi: 10.3390/molecules22060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J., Koh X., Hua F., Li G., Larrick J. W., Bian J. S. Cardioprotection induced by Na+/K+-ATPase activation involves extracellular signal-regulated kinase 1/2 and phosphoinositide 3-kinase/Akt pathway. Cardiovascular Research. 2011;89(1):51–59. doi: 10.1093/cvr/cvq263. [DOI] [PubMed] [Google Scholar]
- 11.Xu K. Y., Takimoto E., Fedarko N. S. Activation of (Na+ + K+)-ATPase induces positive inotropy in intact mouse heart in vivo. Biochemical and Biophysical Research Communications. 2006;349(2):582–587. doi: 10.1016/j.bbrc.2006.08.070. [DOI] [PubMed] [Google Scholar]
- 12.Hua F., Wu Z., Yan X., et al. DR region of Na+-K+-ATPase is a new target to protect heart against oxidative injury. Scientific Reports. 2018;8(1, article 13100) doi: 10.1038/s41598-018-31460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White C. N., Figtree G. A., Liu C. C., et al. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. American Journal of Physiology-Cell Physiology. 2009;296(4):C693–C700. doi: 10.1152/ajpcell.00648.2008. [DOI] [PubMed] [Google Scholar]
- 14.Massey K. J., Li Q., Rossi N. F., Mattingly R. R., Yingst D. R. Angiotensin II-dependent phosphorylation at Ser11/Ser18 and Ser938 shifts the E2 conformations of rat kidney Na+/K+-ATPase. The Biochemical Journal. 2012;443(1):249–258. doi: 10.1042/BJ20111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahaman S. M., Dey K., Chakraborti T., Chakraborti S. Angiotensin II inhibits Na+/K+ATPase activity in pulmonary artery smooth muscle cells via glutathionylation and with the involvement of a 15.6 kDa inhibitor protein. Indian Journal of Biochemistry & Biophysics. 2015;52:119–124. [PubMed] [Google Scholar]
- 16.Xiong S., Yang X., Yan X., et al. Immunization with Na+/K+ ATPase DR peptide prevents bone loss in an ovariectomized rat osteoporosis model. Biochemical Pharmacology. 2018;156:281–290. doi: 10.1016/j.bcp.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Iwatsubo K., Minamisawa S., Tsunematsu T., et al. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. The Journal of Biological Chemistry. 2004;279(39):40938–40945. doi: 10.1074/jbc.M314238200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J., Xiao H., Shen J., Wang N., Zhang Y. Different roles of β-arrestin and the PKA pathway in mitochondrial ROS production induced by acute β-adrenergic receptor stimulation in neonatal mouse cardiomyocytes. Biochemical and Biophysical Research Communications. 2017;489(4):393–398. doi: 10.1016/j.bbrc.2017.05.140. [DOI] [PubMed] [Google Scholar]
- 19.Durham K. K., Chathely K. M., Mak K. C., et al. HDL protects against doxorubicin-induced cardiotoxicity in a scavenger receptor class B type 1-, PI3K-, and Akt-dependent manner. American Journal of Physiology-Heart and Circulatory Physiology. 2018;314(1):H31–H44. doi: 10.1152/ajpheart.00521.2016. [DOI] [PubMed] [Google Scholar]
- 20.Lu Q. B., Wan M. Y., Wang P. Y., et al. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biology. 2018;14:656–668. doi: 10.1016/j.redox.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X., Xun M., Dou X., Wu L., Han Y., Zheng J. Regulation of Na+-K+-ATPase effected high glucose-induced myocardial cell injury through c-Src dependent NADPH oxidase/ROS pathway. Experimental Cell Research. 2017;357(2):243–251. doi: 10.1016/j.yexcr.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Ritter L., Kleemann D., Hickmann F. H., et al. Disturbance of energy and redox homeostasis and reduction of Na+, K+-ATPase activity provoked by in vivo intracerebral administration of ethylmalonic acid to young rats. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2015;1852(5):759–767. doi: 10.1016/j.bbadis.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Li S., Hu L., et al. Mechanisms underlying action of Xinmailong injection, a traditional Chinese medicine in cardiac function improvement. African Journal of Traditional, Complementary, and Alternative Medicines. 2017;14(2):241–252. doi: 10.21010/ajtcam.v14i2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H. J., Chen D., Wang P. Y., et al. Salusin-βis involved in diabetes mellitus-induced endothelial dysfunction via degradation of peroxisome proliferator-activated receptor gamma. Oxidative Medicine and Cellular Longevity. 2017;2017:14. doi: 10.1155/2017/6905217.6905217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y., Li Y., Liu C., et al. The role of angiopoietin-like protein 4 in phenylephrine-induced cardiomyocyte hypertrophy. Bioscience Reports. 2019;39(7) doi: 10.1042/BSR20171358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan L., Zhang J. D., Wang B., et al. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity. PLoS One. 2013;8(9, article e72548) doi: 10.1371/journal.pone.0072548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang W., Li N., Ai D., Niu X. L., Guan Y. F., Zhu Y. Activation of peroxisome proliferator-activated receptor-γ downregulates soluble epoxide hydrolase in cardiomyocytes. Clinical and Experimental Pharmacology and Physiology. 2011;38(6):358–364. doi: 10.1111/j.1440-1681.2011.05492.x. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y., Xue B. J., Wei S. G., et al. Activation of central PPAR-γ attenuates angiotensin II-induced hypertension. Hypertension. 2015;66(2):403–411. doi: 10.1161/HYPERTENSIONAHA.115.05726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen T., Kröller-Schön S., Schönfelder T., et al. α1AMPK deletion in myelomonocytic cells induces a pro-inflammatory phenotype and enhances angiotensin II-induced vascular dysfunction. Cardiovascular Research. 2018;114(14):1883–1893. doi: 10.1093/cvr/cvy172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan Q., Song P., Ding Y., Zou M. H. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. British Journal of Pharmacology. 2017;174(13):2140–2151. doi: 10.1111/bph.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mia S., Castor T., Musculus K., Voelkl J., Alesutan I., Lang F. Role of AMP-activated protein kinase α1 in angiotensin-II-induced renal Tgfß-activated kinase 1 activation. Biochemical and Biophysical Research Communications. 2016;476(4):267–272. doi: 10.1016/j.bbrc.2016.05.111. [DOI] [PubMed] [Google Scholar]
- 32.Guo X., Yan F., Shan X., et al. SIRT3 inhibits Ang II-induced transdifferentiation of cardiac fibroblasts through β-catenin/PPAR-γ signaling. Life Sciences. 2017;186:111–117. doi: 10.1016/j.lfs.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Meng G., Liu J., Liu S., et al. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. British Journal of Pharmacology. 2018;175(8):1126–1145. doi: 10.1111/bph.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L., Yin A., Zhang Q., Zhong T., O’Rourke S. T., Sun C. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac hypertrophy via a Sirt3-dependent mechanism. American Journal of Physiology-Heart and Circulatory Physiology. 2017;312(5):H980–H991. doi: 10.1152/ajpheart.00768.2016. [DOI] [PubMed] [Google Scholar]
- 35.van Kats J. P., Methot D., Paradis P., Silversides D. W., Reudelhuber T. L. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice: Direct and indirect effects of angiotensin II on the heart. The Journal of Biological Chemistry. 2001;276(47):44012–44017. doi: 10.1074/jbc.M106132200. [DOI] [PubMed] [Google Scholar]
- 36.Sadoshima J., Xu Y., Slayter H. S., Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75(5):977–984. doi: 10.1016/0092-8674(93)90541-W. [DOI] [PubMed] [Google Scholar]
- 37.Serneri G. G. N., Boddi M., Cecioni I., et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circulation Research. 2001;88(9):961–968. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 38.Russo M. A., Morgante E., Russo A., Rossum G. D. ., Tafani M. Ouabain-induced cytoplasmic vesicles and their role in cell volume maintenance. BioMed Research International. 2015;2015:13. doi: 10.1155/2015/487256.487256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dijkstra K., Hofmeijer J., van Gils S. A., van Putten M. J. A. M. A biophysical model for cytotoxic cell swelling. The Journal of Neuroscience. 2016;36(47):11881–11890. doi: 10.1523/JNEUROSCI.1934-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massey K. J., Li Q., Rossi N. F., Keezer S. M., Mattingly R. R., Yingst D. R. Phosphorylation of rat kidney Na-K pump at Ser938 is required for rapid angiotensin II-dependent stimulation of activity and trafficking in proximal tubule cells. American Journal of Physiology-Cell Physiology. 2016;310(3):C227–C232. doi: 10.1152/ajpcell.00113.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Vicente A., Garvin J. L. Angiotensin II-induced hypertension increases plasma membrane Na pump activity by enhancing Na entry in rat thick ascending limbs. American Journal of Physiology-Renal Physiology. 2013;305(9):F1306–F1314. doi: 10.1152/ajprenal.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naderi M. M., Boroujeni S. B., Sarvari A., et al. The effect of angiotensin on the quality of in vitro produced (IVP) sheep embryos and expression of Na+/K+/ATPase. Avicenna Journal of Medical Biotechnology. 2016;8:9–15. [PMC free article] [PubMed] [Google Scholar]
- 43.Correll R. N., Eder P., Burr A. R., et al. Overexpression of the Na+/K+ ATPase α2 but not α1 isoform attenuates pathological cardiac hypertrophy and remodeling. Circulation Research. 2014;114(2):249–256. doi: 10.1161/CIRCRESAHA.114.302293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou G., Li X., Hein D. W., et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. Journal of the American College of Cardiology. 2008;52(8):655–666. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Comellas A. P., Dada L. A., Lecuona E., et al. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circulation Research. 2006;98(10):1314–1322. doi: 10.1161/01.RES.0000222418.99976.1d. [DOI] [PubMed] [Google Scholar]
- 46.Petrushanko I. Y., Yakushev S., Mitkevich V. A., et al. S-Glutathionylation of the Na,K-ATPase catalytic α subunit is a determinant of the enzyme redox sensitivity. The Journal of Biological Chemistry. 2012;287(38):32195–32205. doi: 10.1074/jbc.M112.391094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong W., Hua J., Liu Z., et al. PTEN induced putative kinase 1 (PINK1) alleviates angiotensin II-induced cardiac injury by ameliorating mitochondrial dysfunction. International Journal of Cardiology. 2018;266:198–205. doi: 10.1016/j.ijcard.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 48.Srirattana K., St John J. C. Transmission of dysfunctional mitochondrial DNA and its implications for mammalian reproduction. In: Sutovsky P., editor. Cellular and Molecular Basis of Mitochondrial Inheritance, vol 231. Springer; 2019. pp. 75–103. (Advances in Anatomy, Embryology, and Cell Biology). [DOI] [PubMed] [Google Scholar]
- 49.Gao D., Zhu B., Sun H., Wang X. Mitochondrial DNA methylation and related disease. Advances in Experimental Medicine and Biology. 2017;1038:117–132. doi: 10.1007/978-981-10-6674-0_9. [DOI] [PubMed] [Google Scholar]
- 50.Choi Y. S., de Mattos A. B. M., Shao D., et al. Preservation of myocardial fatty acid oxidation prevents diastolic dysfunction in mice subjected to angiotensin II infusion. Journal of Molecular and Cellular Cardiology. 2016;100:64–71. doi: 10.1016/j.yjmcc.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beauloye C., Bertrand L., Horman S., Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovascular Research. 2011;90(2):224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 52.Stuck B. J., Lenski M., Bohm M., Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. The Journal of Biological Chemistry. 2008;283(47):32562–32569. doi: 10.1074/jbc.M801904200. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Q., Zhou Q. Y., Liu D., et al. Advanced glycation end-products impair Na+/K+-ATPase activity in diabetic cardiomyopathy: role of the adenosine monophosphate-activated protein kinase/sirtuin 1 pathway. Clinical and Experimental Pharmacology and Physiology. 2014;41(2):127–133. doi: 10.1111/1440-1681.12194. [DOI] [PubMed] [Google Scholar]
- 54.Ingwersen M. S., Kristensen M., Pilegaard H., Wojtaszewski J. F. P., Richter E. A., Juel C. Na,K-ATPase activity in mouse muscle is regulated by AMPK and PGC-1α. The Journal of Membrane Biology. 2011;242(1):1–10. doi: 10.1007/s00232-011-9365-7. [DOI] [PubMed] [Google Scholar]
- 55.Benziane B., Björnholm M., Lantier L., Viollet B., Zierath J. R., Chibalin A. V. AMP-activated protein kinase activator A-769662 is an inhibitor of the Na+-K+-ATPase. American Journal of Physiology-Cell Physiology. 2009;297(6):C1554–C1566. doi: 10.1152/ajpcell.00010.2009. [DOI] [PubMed] [Google Scholar]
- 56.Vadász I., Dada L. A., Briva A., et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. The Journal of Clinical Investigation. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benziane B., Björnholm M., Pirkmajer S., et al. Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells. The Journal of Biological Chemistry. 2012;287(28):23451–23463. doi: 10.1074/jbc.M111.331926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soltoff S. P., Hedden L. Regulation of ERK1/2 by ouabain and Na-K-ATPase-dependent energy utilization and AMPK activation in parotid acinar cells. American Journal of Physiology-Cell Physiology. 2008;295(3):C590–C599. doi: 10.1152/ajpcell.00140.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukhopadhyay R., Venkatadri R., Katsnelson J., Arav-Boger R. Digitoxin suppresses human cytomegalovirus replication via Na+, K+/ATPase α1 subunit-dependent AMP-activated protein kinase and autophagy activation. Journal of Virology. 2018;92(6) doi: 10.1128/JVI.01861-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J., Liu S., Xu F., et al. Trilobatin protects against oxidative injury in neuronal PC12 cells through regulating mitochondrial ROS homeostasis mediated by AMPK/Nrf2/Sirt3 signaling pathway. Frontiers in Molecular Neuroscience. 2018;11:p. 267. doi: 10.3389/fnmol.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L. Y., Wang Y., Terkeltaub R., Liu-Bryan R. Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthritis and Cartilage. 2018;26(11):1539–1550. doi: 10.1016/j.joca.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao X. L., Yu C. Z. Vosaroxin induces mitochondrial dysfunction and apoptosis in cervical cancer HeLa cells: involvement of AMPK/Sirt3/HIF-1 pathway. Chemico-Biological Interactions. 2018;290:57–63. doi: 10.1016/j.cbi.2018.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Effects of DR-Ab on Ang II-induced hypertrophy in the neonatal mouse cardiomyocytes. DR-Ab (2 μM) was given 30 min before treatment with Ang II (100 nM) for 48 h. (A-B) Representative immunofluorescence staining (A) and group data (B) showing that DR-Ab reversed enlarged cell size caused by Ang II. Green: NKA α1. Blue: DAPI. Scale bar, 30 μm. n = 6. (C-E) qRT-PCR analysis showing the mRNA levels of ANP, BNP, and β-MHC. n = 4. (F-G) DR-Ab reversed Ang II-induced loss of plasma membrane NKA α1 (A&F) and increase of endosome NKA α1 (G). n = 4‐6. (H-I) Effect of DR-Ab on the protein level of two subunits of NADPH oxidase: p22phox and p47phox. n = 4‐6. ∗p < 0.05 versus control group, †p < 0.05 versus Ang II alone group.
Data Availability Statement
The derived data used to support the findings of this study are included within the article. The raw data used to support the findings of this study are available from the corresponding author upon request.