Abstract
Stem/progenitor cells are involved in the regeneration of the renal tubules after damage due to a toxic insult. However, the mechanism involved in the regeneration of the tubules by the stem cells is not well understood due to the lack of immortal cell lines that represent the stem/progenitor cells of the kidney. A previous study from our laboratory has shown that the immortalized cell line RPTEC/TERT1 contains two populations of cells, one co-expressing CD24 and CD133, the other expressing CD24 only. The goal of the present study was to determine if both these populations could be sorted into separate independent cultures and if so, determine their characteristic features and response to the nephrotoxicant cadmium. The results of our study show that both the populations of cells could grow as independent cultures and maintain their phenotype after extended sub-culture. The CD133+/CD24+ co-expressing cells formed multicellular spheroids (nephrospheres), a characteristic feature of stem/progenitor cells, and formed branched tubule-like structures when grown on the surface of matrigel, whereas the CD133−/CD24+ cells were unable to form these structures. The CD133+/CD24+ cells were able to grow and undergo neurogenic, adipogenic, osteogenic, and tubulogenic differentiation, whereas the CD133−/CD24+ cells expressed some of the differentiation markers but were unable to grow in some of the specialized growth media. The CD133+/ CD24+ co-expressing cells had a shorter doubling time compared to the cells that expressed only CD24, and were more resistant to the toxic effects of the heavy metal, cadmium. In conclusion, the isolation and characterization of these two cell populations form the RPTEC/TERT1 cell line will facilitate the development of studies that determine the mechanisms involved in tubular damage and regeneration particularly after a toxic insult.
Keywords: Kidney, stem cell, cadmium, CD133, CD24, regeneration
1. Introduction
The proximal tubules of the kidney are a major site of toxic insult that is associated with hypoxia, aminoglycoside antibiotics, or exposure to environmental toxicants such as the heavy metal cadmium (Cd2+) which accumulates in the proximal tubule cells leading to overt renal damage. Cell culture is a frequently used tool to study the basic mechanisms underlying normal and disease processes that involve the tubular elements of the human kidney. The human kidney derived cell culture models, such as the HK-2 cell line and primary cultures of cortical renal epithelial cells, possess many, but not all, of the differentiation features associated with the human proximal tubule (Detrisac et al., 1984; Ryan et al., 1994). Recently, a human renal epithelial cell line was isolated from renal cortical tissue that was subsequently immortalized by transduction with human telomerase reverse transcriptase (hTERT) (Wieser et al. 2008). This immortalized cell line, referred to as RPTEC/TERT1, retains many of the differentiated features of human proximal tubule (HPT) cells, including the ability to form domes, a feature of vectorial active transport. A recent study by this laboratory using differential gene expression analysis demonstrated a strong identity between the RPTEC/TERT1 cells and primary mortal HPT cells isolated from renal cortical tissue (Shrestha et al. 2017). In contrast, the gene expression profile of HK-2 cells was markedly different from the RPTEC/TERT1 and HPT cells. This finding suggested that the RPTEC/TERT1 cells could be a convenient, easy to use, model to study the mechanisms of agents known to damage the tubular system of the human kidney.
One area of interest is the mechanism/s underlying the known ability of the kidney to regenerate renal tubules after insults. There is strong evidence which suggests the cells which participate in the renewal of the tubules are generated from within the kidney itself and are not recruited from non-renal sources (Lindgren et al. 2011; Smeets et al. 2011). It is not known if these cells are present within the kidney or result from dedifferentiation of existing cells. A number of studies have shown that the progenitor cells capable of regenerating renal tubules are characterized by the co-expression of cell surface markers CD133 and CD24 (Romagnani and Remuzzi 2014; Smeets et al. 2013; Romagnani et al. 2013; Angelotti et al. 2012; Lindgren et al. 2011; Sallustio et al. 2013; Ronconi et al. 2009). These cells proliferate in cell culture, maintain their phenotype and have the capacity to differentiate both in vivo and in vitro. In the developing human kidney, the CD133+ cells are a subset of CD24+ cells which constitute the metanephric mesenchyme-derived primorial nephron (Lazzeri et al. 2007). The fact that these studies define CD133+/CD24+ cells as a renal progenitor/stem cell population led this laboratory to determine if the RPTEC/TERT1 and the primary HPT cells also co-express CD133+/CD24+.
An analysis of the RPTEC/TERT1 and HPT cells demonstrated that both the cell populations contained a subset of cells co-expressing CD133 and CD24 as well as another population that expressed only CD24 (Shrestha et al. 2017). It was shown that RPTEC/TERT1 cells in culture were composed of 78.7% of cells co-expressing CD133 and CD24 (CD133+/CD24+) with the remaining 21.3% expressing only CD24 (CD133−/CD24+). The results were similar for the HPT cells with 61.8% CD133+/CD24+ cells and 38.1% CD133−/CD24+ cells. These findings raised a number of questions for pursuit in the present study. Thus, the current study was undertaken to determine if both the CD133+/CD24+ and CD133−/CD24+ cells could be sorted from the RPTEC/TERT1 cell line into separate cultures that could grow independently and maintain their expression of CD markers during extended subcultures. Furthermore, it was also determined if the three culture systems (RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+) could undergo tubulogenic, osteogenic, adipogenic, or neurogenic differentiation as shown previously for embryonic CD133+/CD24+ renal progenitor cells (Lazzeri et al. 2007) when grown in different cell culture media. The final goal of the study was to determine if the three cell cultures displayed differential toxicities when exposed to the known nephrotoxicant, Cd2+. This goal was undertaken since the proximal tubule cells of the kidney are considered a major target site of chronic Cd2+ exposure as they readily accumulate the metal (Navas-Acien et al., 2009, Ferraro et al., 2010, Sommar et al., 2013). In addition, studies have shown that these progenitor cells participate in the regeneration of the proximal tubules of the kidney during acute kidney injury (Lazzeri et al., 2007, Hansson et al. 2014).
2. Materials and Methods
2.1. Animal Studies
Athymic Nude-Foxn1nu mice from ENVIGO were used in these studies. The mice were housed four to a cage at 22°C under a 12-hour light/dark cycle. Food and water was available ad libitum. Confluent cultures of RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ cells were trypsinized and cell pellets were re-suspended in ice-cold phosphate buffered saline (PBS) and mixed with an equal volume of ice-cold Corning matrigel (Corning). The cell suspension (0.2 ml) was injected subcutaneously in the dorsal thoracic midline of mice using a 0.2 cc syringe. The matrigel nodules were harvested seven days post injection. The study adhered to all recommendations dictated in the Guide for the Care and Use of Laboratory Animals of the NIH. The specific protocol was approved by the University of North Dakota Animal Care Committee (IACUC#1612–2C).
2.2. Cell culture
Stock cultures of RPTEC/TERT1 cells were obtained from American Type Culture Collection and were grown using serum-free conditions as previously described by this laboratory (Detrisac et al., 1984; Kim et al., 2002). The growth formulation consisted of a 1:1 mixture of Dulbecco’s modified Eagles’ medium and Ham’s F-12 growth medium supplemented with selenium (5 ng/ml), insulin (5 µg/ml), transferrin (5 µg/ml), hydrocortisone (36 ng/ml), triiodothyronine (4 pg/ml), and epidermal growth factor (10 ng/ml).
For spheroid culture, the cells were detached using trypsin-EDTA (ethylenediaminetetraacetic acid) (0.05–0.02 %) or Accutase (BD Biosciences). The pelleted cells were suspended and 103 cells were transferred to a T-75 ultralow-attachment flask (Corning) and incubated at 37 °C with 5 % CO2. Spheroid formation was observed for 8–10 days and pictures were taken using the Zeiss light microscope.
2.3. RNA Isolation and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH) as described previously (Garrett et al., 1998). The level of expression of CD133, CD24, Aquaporin-1 (AQP-1), Calbindin (CAL), Enolase-2 (ENO-2), Nestin (NES), tau-protein, Peroxisome proliferator-activated receptor gamma (PPARG), Runt-related transcription factor-2 (RUNX2) was assessed using RT-qPCR and commercially available primers. The source of the primers along with their catalogue numbers is listed in Supplemental Materials, Table 1 (S1 Table). For analysis, 100 ng of total RNA was used to prepare complimentary DNA (cDNA) using the Bio-Rad iScript cDNA synthesis kit in a total volume of 20 µl. RT q-PCR was performed utilizing the SYBR Green kit (Bio-Rad Laboratories) with 2 µl of cDNA, 0.2 µM primers in a total volume of 20 µl in a CFX96 real-time detection system (Bio-Rad Laboratories). The relative levels of mRNA for each of the genes was assessed and normalized to the change in β-actin expression.
2.4. Fluorescence Activated Cell Sorting
Confluent cultures of the immortalized RPTEC/TERT1 cell line were washed two times with PBS and the cells were detached using Accutase and centrifuged at 2000 rpm at 4 °C for 5 min. The cell pellet was washed once and re-suspended in BD Pharmingen™ stain buffer (BSA). Cells were diluted to 1 × 106 cells/mL with the stain buffer. For every 100 µl of cell suspension, 10 µl of fluorescein isothiocyanate (FITC) conjugated CD133 and/or phycoerythrin (PE) conjugated CD24 antibody (Miltenyi Biotec Inc.) was added to the tubes. The cells were incubated for 30 min on ice in the dark and then washed two times with stain buffer and re-suspended in 1mL of stain buffer. Cells were sorted using BD FACS Aria (BD Biosciences). The singlet population was gated as FSC vs SSC following which the selected population was graphed as CD133 vs CD24 plot. Four quadrants were drawn to separate double positive vs single positive cell populations. Based on the compensation measurements, the threshold for positive signal was set to ≤ 1 × 103 for both markers at the x and y-axis. For each population, 105 cells were sorted directly into a 24-well plate containing warm culture media. The cells were allowed to grow to confluency and expanded for further experiments. After confirming purity and stability of sorted cells for 12 passages, the cells were cryopreserved in DMEM/F12 media containing 10% dimethyl sulfoxide (DMSO) for future use.
2.5. Transepithelial Resistance Measurement
RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ cells were seeded at a 2:1 split ratio in triplicate onto 0.4µm pore size, 1.1 cm2 polyethylene terephthalate inserts in 12-well plates (EMD Millipore). The transepithelial resistance (TER) was measured starting on the second day of seeding until day 10 using the EVOM Epithelial Voltohmmeter (World Precision Instruments) with a STX2 electrode set according to the manufacturer’s instructions. The resistance of the blank filter containing medium was subtracted from that obtained from filters with cell monolayers. The experiment was repeated three times in triplicates and the final result reported as the mean ± S.D.
2.6. Determination of cell growth and viability
Cell growth was determined by measuring the capacity of the cells to reduce MTT [3-(4,5-dimethylthiozol-2yl)-2,5-diphenyl tetrazolium bromide] to formazan. In brief, 40 µl of the MTT reagent (Sigma-Aldrich) was added each well of a 6-well plate containing cultured cells and incubated for 3.5 h at 37°C. The wells were washed 2 times with PBS and 1ml of acidic propanol was added to each well. Two hundred microliters from the well was transferred to a 96-well plate and the absorbance was measured at 570 nm using the Biotek spectrophometer. Cell viability was determined by visualization and counting of 4’,6-diamidino-2-phenylindole (DAPI)- stained nuclei as described previously by this laboratory (Garrett et al., 1998; Somji et al., 2004).
2.7. Effect of Cd+2 on cell growth and viability
Confluent cultures of RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ were exposed to 4.5 and 9µM Cd2+ for 34 days. Every 2 days, the cells were treated with fresh media containing 4.5 or 9µM Cd2+. The viable number of cells was determined by flow cytometry using BD FACS Aria (BD Biosciences). The data was analyzed using the FlowJo software. For each sample, 10,000 events were acquired. The results were gated to exclude doublets and identify the singlet population.
2.8. Immunohistochemistry
The matrigel nodules harvested from mice were fixed in 10% neutral-buffered formalin for 16–18 h. The fixed tissue samples were transferred to 70% ethanol and dehydrated in 100% ethanol. The dehydrated tissue samples were cleared in xylene, infiltrated, and embedded in paraffin. Serial sections were cut at 3–5 µm for use in immunohistochemical protocols. Prior to immunostaining, sections were immersed in preheated Target Retrieval Solution (Dako, Carpinteria, CA) and heated in a steamer for 20 min. The sections were allowed to cool to room temperature and immersed into Tris buffered saline (TBS) plus 5% Tween 20 (TBS-T) for 5 min. Blocking was performed by incubating the sections in Dako Peroxidase block solution. The slides were washed and incubated with the primary antibody overnight at 4 °C. The list of the primary antibodies used, along with their catalogue numbers and dilution is listed in Supplemental Materials Table 2 (S2 Table). After washing, the slides were incubated with DakoCytomation EnVision+ dual Link Antibody or Dako HRP-labelled antibody at room temperature for 30 min. Liquid diaminobenzidine (Dako) was used for visualization. Counter staining was performed for 8 min at room temperature using Ready-to-use Hematoxylin (Dako). Slides were rinsed with distilled water, dehydrated in graded ethanol, cleared in xylene, and cover-slipped.
2.9. Immunofluorescence staining
Cells were grown on coverslips and fixed with 3.7% formaldehyde for 15 min, followed by permeabilization using 0.1% Triton-X 100 for 10 min or were permeabilized and fixed with ice cold absolute methanol for 10 min. The coverslips were washed three times using PBS for 5 min and incubated with primary antibody for 40 min at 37 °C. The list of the primary antibodies used, along with their source, catalogue numbers and dilutions is provided in Supplemental Materials, Table 3 (S3 Table). The coverslips were washed three times with PBS for 3 min each. The primary antibody was detected by incubating cells with Alexa-Fluor 488 or Alexa-Fluor 568 secondary antibody for 30 min at 37 °C. The coverslips were washed three times with PBS and mounted with Prolong Diamond Antifade Mountant with DAPI (Life Technologies). The stained cells were observed and imaged using a Leica TCS SPE, DM5500 laser or Olympus FV3000 scanning confocal microscope. Two coverslips per sample were set up and a minimum of 5 fields per coverslip was examined.
2.10. In-vitro differentiation
All cells were cultured in serum-free DMEM/F12 media to confluency and then cultured under conditions that were favorable for tubulogenic, osteogenic, adipogenic, or neurogenic differentiation as described previously (Lazzeri et al, 2007, Sagrinati et al, 2006; Romagnani et al, 2005, Lazzeri et al, 2007). In brief, for osteogenic differentiation, RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ cells were cultured in α-MEM and 10% horse serum that contained 100 nM dexamethasone, 50 µM ascorbic acid (Sigma-Aldrich), and 2 mM β-glycero-phosphate (Santa Cruz Biotechnology, Santa Cruz, CA). The medium was changed twice a week for 2–3 weeks. For adipogenic differentiation, RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ cells were grown in DMEM high glucose (Gibco, Ireland) that contained 10% FBS, 1 µM dexamethasone, 0.5 µM 1-methyl-3-isobutylxanthine, 10 µg/ml insulin, and 100 µM indomethacin (Sigma-Aldrich). After 72 h, the medium was changed and the cells were maintained in DMEM hg, 10% FBS, and 10 µg/ml insulin until confluency. For neurogenic differentiation, RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ cells were grown in DMEM hg and 10% FBS for 24 h, following which the culture medium was replaced with DMEM hg, 10% FBS containing 20% B27 (Gibco), 10 ng/ml EGF (Peprotech, Rocky Hill, NJ), and 20 ng/ml basic fibroblast growth factor (Peprotech). Five days later, the medium was replaced with serum-free DMEM containing 5 µg/ml insulin, 200 µM indomethacin, and 0.5 mM 1-methyl-3-isobutylxanthine for 4–5 hr. For tubulogenic differentiation, RPTEC/TERT1, CD133+/CD24+, and CD133−/CD24+ cells were grown in REBM medium (Lonza, Basel, Switzerland) supplemented with 50 ng/ml hepatocyte growth factor (HGF) (Peprotech) for 2–3 weeks.
2.11. Statistical analysis
Statistical analysis consisted of one-way ANOVA with Tukey’s or Sidak’s multiple comparisons testing performed by GraphPad PRISM 7. All experiments were done in triplicates and unless otherwise stated, the data is plotted as the mean ±SD of triplicate determinations.
3. Results
3.1. Isolation of CD133+/CD24+ and CD133−/CD24+ renal epithelial cells
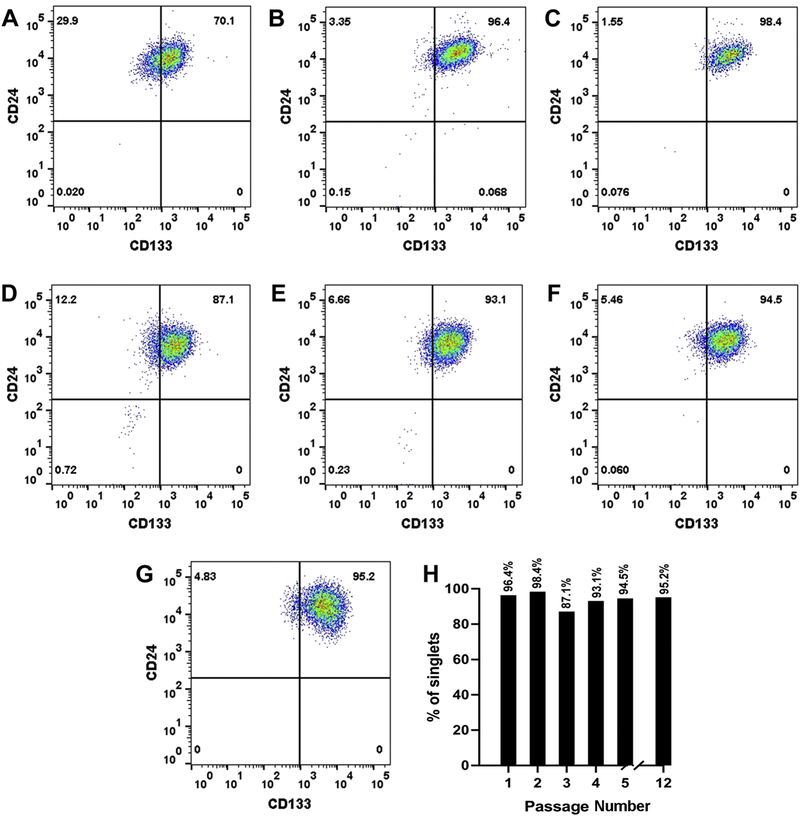
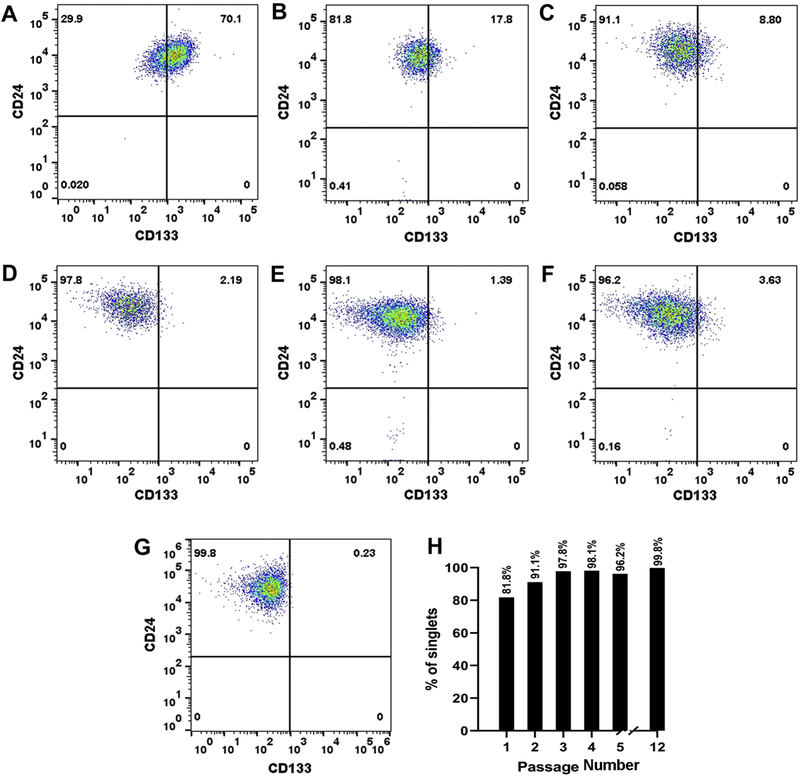
The RPTEC/TERT1 cell line was sorted to obtain populations of CD133+/CD24+ and CD133−/CD24+ cells using flow cytometry. The sorted populations were placed into a single well of a 24 well plate (1.9 cm2, passage 1, P1) and allowed to reach confluency. The cells were serially transferred from the 24 well plate, to single well of a 12 well plate (3.8 cm2, P2), followed by transfer to a single well of a 6 well plate (9.5 cm2, P3), a 25 cm2 tissue culture flask (P4), and thereafter to 75 cm2 tissue culture flasks (P5-P12). Flow cytometry was used to determine the purity of the cell population at each step of the isolation process. The results of this analysis showed that both the CD133+/CD24+ cells (Fig 1A–G) and the CD133−/CD24+ cells (Fig 2A–G) could be placed into culture with a purity consistently above 95% at later passages while retaining their morphology for over 12 serial passages (P1-P12) in culture. The CD133+/CD24+ cells and the CD133−/CD24+ cells are cultured in the same growth media as the RPTEC/TERT1 parent and routinely sub-cultured at a 1:3 ratio. Both the sorted populations can be preserved in liquid nitrogen and placed back into cell culture retaining their purity. The newly isolated CD133+/CD24+ cell line will now be referred to as Human Renal Tubular Precursor TERT (HRTPT), whereas the CD133−/CD24+ cell line will be referred to as the Human Renal Epithelial Cell 24TERT (HREC24T).
Fig. 1.
Flow cytometry analysis of CD133+ and CD24+ expressing cells. RPTEC/TERT1 cells were sorted based on the expression of CD133 and CD24 markers and the purity of the cells was determined at various passages of cell growth. (A). Analysis of the starting population of CD133+/CD24+ expressing RPTEC/TERT1 cells. (B-G). Analysis of CD133+/CD24+ co-expressing HRTPT cells at passage 1, 2, 3, 4, 5 and 6 respectively. (H). Bar diagram representing the percentage of CD133+/CD24+ expressing cells within the population of HRTPT cells at various passages of culture.
Fig. 2.
Flow cytometry analysis of HREC24T cells expressing CD24. The HREC24T cells were sorted based on the expression of CD133 and CD24 and the purity of the cells was determined at various passages of cell growth. (A). Analysis of the starting population of CD133−/CD24+ cells in the RPTEC/TERT1 cell line. (B-G). Analysis of CD133−/CD24+ expressing HREC24T cells at passage 1, 2, 3, 4, 5 and 6 respectively. (H). Bar diagram representing the percentage of CD133−/CD24+ expressing cells at various passages of culture.
The HRTPT, HREC24T, and parental RPTEC/TERT1cell lines were compared for their expression of CD24 and CD133 markers. The RPTEC/TERT1 cells showed a level of CD133 and CD24 mRNA expression expected from the flow cytometry analysis of their cell surface staining patterns (Fig. 3A and B, Fig. 1A). The expression of CD133 mRNA in the HRTPT cells was similar to the parent cell line (Fig 3A), whereas the expression of CD24 mRNA was significantly higher than the parent cells (Fig. 3B). The HREC24T showed a large increase in CD24 mRNA expression (Fig. 3B), and a marked decrease in CD133 mRNA expression compared to the parental cells (Fig. 3A). Immunofluorescence staining for CD133 and CD24 in the three cell lines showed that the RPTEC/TERT1 and the HRTPT cells had strong cell surface staining of both CD133 and CD24 (Fig. 3C and D). In contrast, the HREC24T cells demonstrated cell surface staining only for CD24 and there was no staining for CD133 (Fig. 3E). Thus, these results show that the HRTPT cell line is largely composed of cells expressing both CD133 and CD24 and the HREC24T cell line is largely composed of cells that express CD24 and do not express CD133.
Fig. 3.
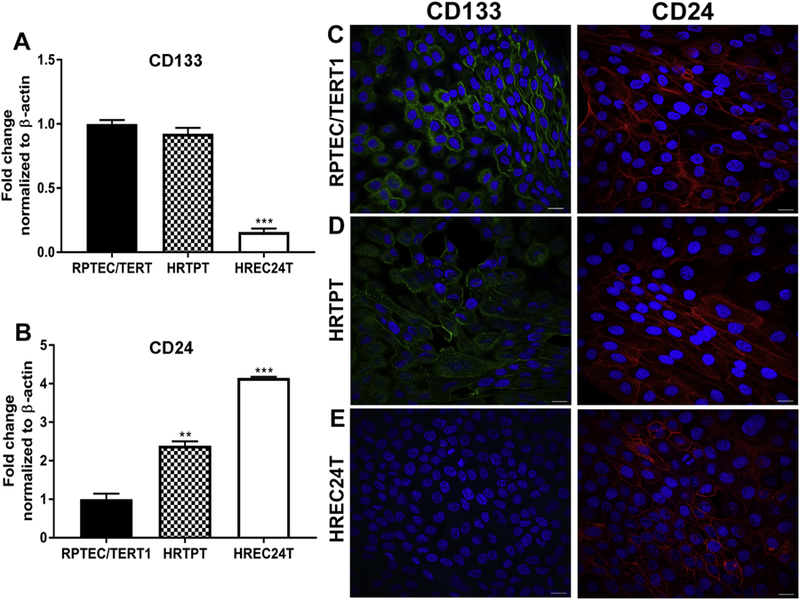
Expression of CD133 and CD24 in RPTEC/TERT1, HRTPT and HREC24T cell lines. RT-q PCR analysis of the expression level of (A) CD133 and (B) CD24 in the RPTEC/TERT1, HRTPT and HREC24T cell lines. Analysis was done in triplicates and plotted as the mean ±SD. ** indicates significantly different at a p-value of ≤0.01; *** indicates significantly different at a p-value of ≤ 0.05. (C-E). Immunofluorescent staining showing the localization of CD133 and CD24 in the (C) RPTEC/TERT1, (D) HRTPT and (E) HREC24T cells. Nuclei are stained with DAPI. Scale bar = 25 µm. Magnification x600.
All three cell lines displayed a very similar epithelial morphology (Fig. 4A–C) and formed “domes” which are noted as raised, out-of-focus areas of the cell monolayer when viewed by light microscopy. The formation of “domes” by epithelial cells is accepted as the cell monolayer’s ability to support vectorial active transport. A requirement for dome formation is the presence of tight junctions between cells comprising the monolayer. The “tightness” of these junctions can be determined by measuring the transepithelial resistance of the monolayer. The three cell lines displayed a transepithelial resistance of between 300 to 400 Ωcm2, consistent with the definition of a “leaky” epithelium (Fig. 4D). The HRTPT cells displayed a significantly higher transepithelial resistance than the other two cell lines, but clearly remained in the range defined as a “leaky” epithelium. The epithelial characteristic feature of these cell lines were further confirmed by determining the expression of adherens and tight junctional molecules. The data showed that there was an increased expression of E cadherin in all the cell lines whereas the expression of N cadherin was very low. This is shown in Supplemental Fig.1. In addition, the expression levels of claudins (CLDN1 and CLDN4) and occludins (OCLD) was also determined and it was found that all three cell lines expressed significant levels of these junctional molecules (Supplemental Fig.2).
Fig. 4.
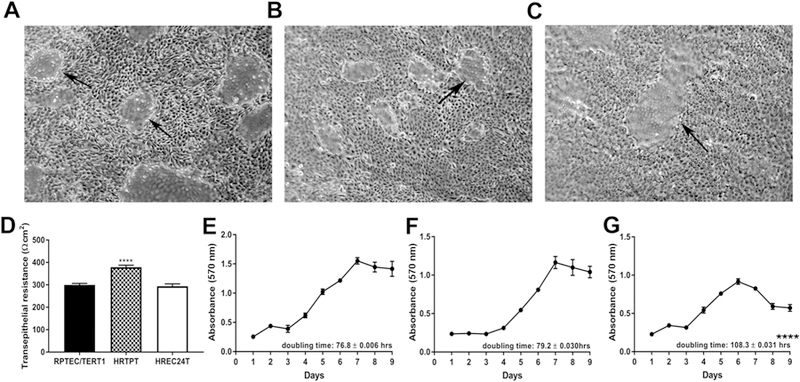
Characterization of the RPTEC/TERT1, HRTPT and HREC24T cell lines. (A-C). Light level microscopy showing the morphology of the (A) RPTEC/TERT1, (B) HRTPT and (C) HREC24T cells at confluency. Arrows indicate the presence of domes, which are indicative of vectorial active transport. (D) Transepithelial electrical resistance (TER) measurements of the RPTEC/TERT1, HRTPT and HREC24T cells. Resistance is expressed as Ohms-cm2. (E-G) Growth rates of the (E) RPTEC/TERT1, (F) HRTPT and (G) HREC24T cells. Doubling times are indicated at the lower right corner of the graph. **** indicates significantly different at a p-value of ≤ 0.0001. Analysis was done in triplicates and plotted as the mean ±SD.
Growth rates were also determined for each of the cell line with parental cells and HRTPT cells displaying similar growth rates with doubling times of 76.8 and 79.2 h respectively (Fig. 4E–F). In comparison, the HREC24T cells had a much longer doubling time of 108.3 h (Fig. 4G).
3.2. Multipotent Progenitor Properties of HRTPT and HREC24T Cells
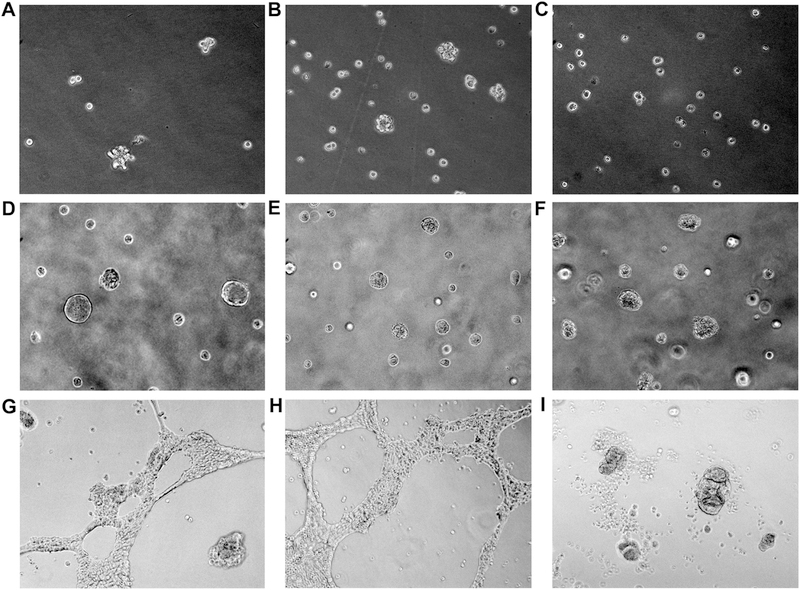
The progenitor and multipotent properties of the RPTEC/TERT1, HRTPT and HREC24T cells were determined using several different assays. The ability of the cells to form nephrospheres (spheroids) was determined by culturing the cells in low attachment tissue culture flasks. The ability of a cell line to generate these multicellular spheroids is an indication that the cells possess the property of self-renewal and differentiation. This concept is based on numerous studies of the self-renewal properties and differentiation potential of tumors (Garg, 2015; Ishiguro et al. 2017). The results showed that the RPTEC/TERT1 and HRTPT cells produced nephrospheres when sub-cultured into flasks that do not promote cell attachment (Fig. 5A and B). In contrast, the HREC24T cells did not form multi-cellular nephrospheres under identical conditions (Fig. 5C). The ability of the cells to form colonies when mixed with matrigel and plated into standard tissue culture wells was also determined and the results demonstrated that all the three cell lines formed colonies following 14 days of incubation (Fig. 5D–F). In addition, the ability of the cells to grow on plates coated with a thin layer of matrigel was also determined and the results showed that both the RPTEC/TERT1 and HRTPT cells formed branched “tubule-like” structures on the surface of the matrigel (Fig. 5G and H). In contrast, the HREC24T cells were unable to form branched “tubule-like” structures on the surface of the matrigel, but formed multicellular colonies of cells (Fig. 5I). The results provide evidence that cells co-expressing CD133 and CD24 possess the property of self-renewal and the ability to differentiate into “tubule-like” structures. The cells expressing CD24, but not 133, show no evidence of differentiation on matrigel and are unable to form nephrospheres, a characteristic feature of progenitor/stem type cells.
Fig. 5.
Spheroid formation and growth of RPTEC/TERT1, HRTPT and HREC24T cells in matrigel. (A-C). Phase contrast light level microscopy of spheroids generated from (A) RPTEC/TERT1, (B) HRTPT and (C) HREC24T cells when cultured in low attachment flasks. (D-F). Light level phase contrast microscopy of (D) RPTEC/TERT1, (E) HRTPT and (F) HREC24T cells when grown in matrigel. (G-I). Phase contrast light level microscopy of (G) RPTEC/TERT1, (H) HRTPT and (I) HREC24T cells plated on the surface of matrigel coated plates. A-C, magnification x200. D-I, magnification x100.
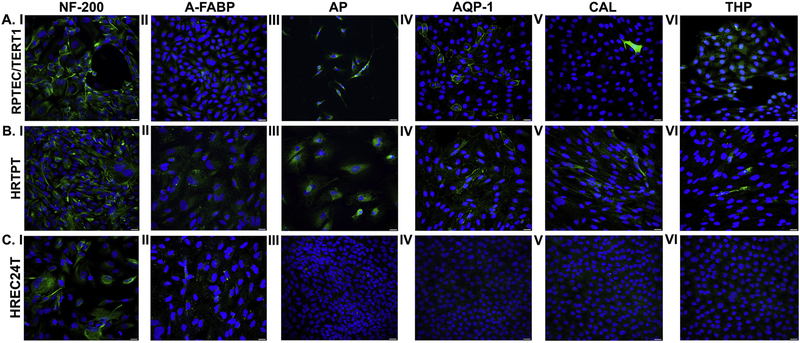
The ability of the three cell lines to undergo neurogenic, adipogenic, osteogenic, and tubulogenic differentiation was also determined using protocols published by Lazzeri and coworkers (2007) for embryonic renal cells. Both the cell lines (HRTPT, RPTEC/TERT1) that co-expressed CD133 and CD24 showed evidence of neurogenic, adipogenic, osteogenic and tubulogenic differentiation (Fig. 6A and B, I–VI). Immunofluorescence staining for neurofilament-200 (NF200), an intermediate filament present in neurons was seen in the cells co-expressing CD133 and CD24 when they were grown in growth medium that promotes neurogenic differentiation (Fig. 6A and B, I). Similarly, the co-expressing cell lines, when grown in medium promoting adipogenic differentiation, showed staining for fatty acid binding protein (FABP) (Fig. 6A and B, II). When these cell lines were grown in medium promoting osteogenic differentiation they showed staining for intestinal alkaline phosphatase (AP) (Fig. 6A and B, III) and when grown in cell culture medium that promotes tubulogenic differentiation, they stained positive for AQP-1, CAL, and Tamm-Horsfall glycoprotein (THP) (Fig. 6A and B, IV–VI). In addition to immunofluorescence staining, the expression of genes characteristic of the differentiated states (Lazzeri et al., 20007) was determined, and the results showed that there is an increase in expression of some of the genes that support the four differentiated states of the cell lines (Table 1). The graphical representation of the expression levels is shown in Supplemental Fig. 3. The two co-expressing cell lines were also able to grow and reach confluency in all four of the selective growth media. Overall, the results provide strong evidence that the two co-expressing cell lines possess the properties of self-renewal and multi-differentiation.
Fig. 6.
Immunofluorescent staining of neurogenic, adipogenic, osteogenic and tubulogenic markers in RPTEC/TERT1, HRTPT and HREC24T cells cultured in vitro. (A.1-C.1). Staining for neurogenic marker NF-200 in RPTEC/TERT1 (A.I), HRTPT (B.1) and HREC24T (C.I) cells grown in neurogenic differentiation media. (A.II-C.II). Staining for adipogenic marker A-FABP in RPTEC/TERT1 (A.II), HRTPT (B.II) and HREC24T (C.II) cells grown in adipogenic differentiation media. (A.III-C.III). Staining for osteogenic marker AP in RPTEC/TERT1 (A.III), HRTPT (B.III) and HREC24T (C.III) cells grown in oesteogenic differentiation media. Staining for tubulogenic markers AQP-1 (A.IV-C.IV), CAL (A.V-C.V) and THP (A.VI-C.IV) in RPTEC/TERT1, HRTPT and HREC24T cells grown in tubulogenic media. Scale bar = 25 µm. Magnification x400.
Table 1.
RT-qPCR analysis of mRNA expression of various differentiation markers in RPTEC/TERT1, HRTPT and HREC24T cells cultured in neurogenic, adipogenic, osteogenic and tubulogenic media.
| Genes | mRNA fold change compared to RPTEC/TERT1 cells |
|
|---|---|---|
| HRTPT | HREC24T | |
| Neurogenic ENO-2 NES MAPT |
2.779 ± 0.021 **** 14.13 ± 0.440 **** 1.951 ± 0.002 ** |
2.269 ± 0.022 **** 5.867 ± 0.251** 1.22 ± 0.138 |
| Adipogenic PPARG ADIPOQ |
0.418 ± 0.011 **** 2.238 ± 0.396 * |
0.316 ± 0.015 **** 0.002 |
| Osteogenic RUNX2 |
1.035 ± 0.014 |
0.794 ± 0.006 * |
| Tubulogenic AQP-1 CAL |
2.865 ± 0.076 *** 10.18 ± 0.779 *** |
1.514 ± 0.153 * 2.18 ± 0.053 |
indicates significantly different at p-value of ≤0.05.
indicates significantly different at p-value of ≤0.01.
indicates significantly different at p-value of ≤ 0.001.
indicates significantly different at p-value of ≤ 0.0001.
An identical analysis was performed on the HREC24T cells that only express CD24, and the morphology of the cells after six days of sub-culture is shown in Supplemental Fig. 4. In order to determine the expression of differentiation markers, the HREC24T cells were cultured in specific differentiation media, either at a 1:3 split ratio for the neurogenic and adipogenic differentiation or a 4:1 ratio for tubulogenic and osteogenic differentiation. This allowed a near confluent culture of cells to be utilized for immunofluorescence staining and gene expression studies. When these cells were assessed for the ability to differentiate using immunofluorescence staining, there was staining for NF-200 and FABP (Fig. 6C, I and II), however there was no staining for AP (Fig. 6C, III). Immunofluorescence staining for tubulogenic markers showed that the HREC24T cells did not express AQP-1, CAL or THP (Fig. 6C, IV–VI). The expression of mRNA for markers for each of the differentiated state was also determined for the HREC24T cells and the results are listed in Table1. These results suggest that the HREC24T cells possess some ability to differentiate, but they do not grow or grow very slowly in the differentiation-specific growth media.
The finding that the HREC24T cells would not proliferate on the tubulogenic growth medium was unexpected since the HREC24T cells proliferate on a serum-free growth medium. The serum-free growth media that supports the growth of all three cell lines is a 1:1 mixture of DMEM and F-12 supplemented with selenium, insulin, transferrin, hydrocortisone, triiodothyronine, and epidermal growth factor. The tubulogenic formulation is a commercial blend of basal medium, REMB, supplemented with hydrocortisone, human epidermal growth factor, hepatocyte growth factor, fetal bovine serum, epinephrine, insulin, triiodothyronine, transferrin and gentamicin/amphotericin-B. When the three cell lines grown in the non-tubulogenic serum-free growth medium were assessed for their expression of AQP-1, CAL and THP, it was shown that all the three cell lines stained for AQP-1, whereas only the HRTPT cells stained for CAL. There was no staining for THP in any of the cell lines (Supplemental Fig.5). It is presently unknown which factor/s affect the proliferative potential of the HREC24T cells, but the results do show that under certain conditions of growth the HREC24T cells are capable of proliferation.
3.3. Transplantation of RPTEC/TERT1, HRTPT and HREC24T Cells into immune-compromised mice
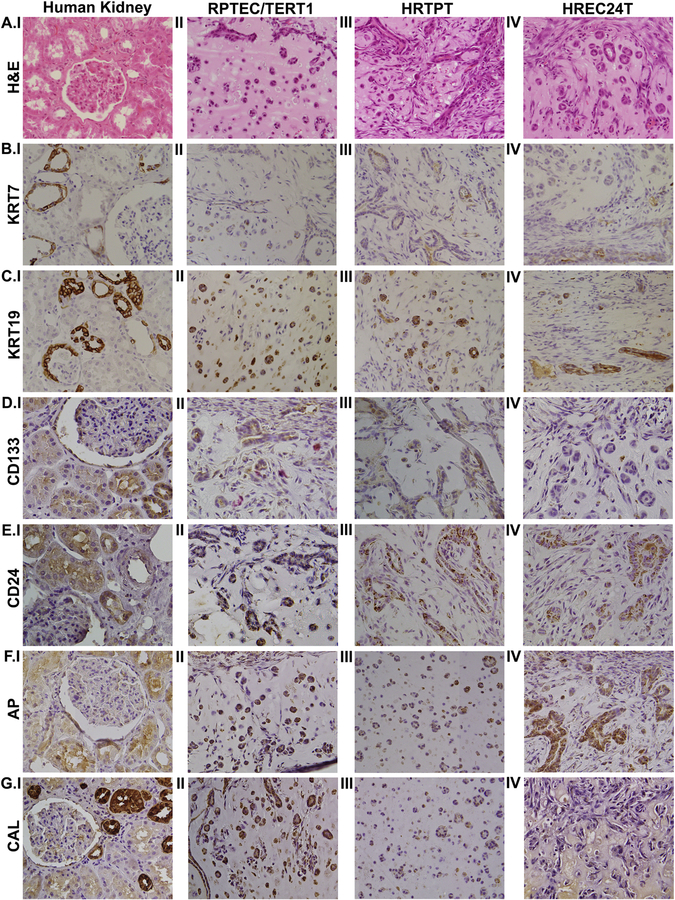
The three cell lines were mixed in matrigel and injected subcutaneously into immune compromised mice. The nodules produced following injection were harvested after 7 days, fixed in formalin, embedded in paraffin, and sectioned for examination. The hematoxylin and eosin (H&E) staining of the sections showed that all three cell lines formed tubular structures within the matrix provided by the matrigel (Fig. 7A, II–IV). No evidence of glomerular structures were noted on the H&E examination of sections for all 3 cell lines. Immunostaining for CK7 and CK19 was used to show that the tubular structures noted on H&E examination were composed of epithelial cells (Fig. 7B, II–IV and C, II–IV respectively). The immunostaining results for CD133 and CD24 showed that the cell lines that co-expressed CD133 and CD24 stained for both the proteins (Fig. 7D, II–III and E, II–III), whereas the CD133−/CD24+ cell line stained only for CD24 (Fig. 7E, IV) and did not stain for CD133 (Fig. 7D, IV). There was staining for AP in the nodules formed by all the three cell lines (Fig. 7F, II–IV). Staining for CAL was seen in the cell lines co-expressing CD133 and CD24 (Fig. 7G, II and III), whereas there was no staining for CAL in the cell line that only expressed CD24 (Fig. 7G, IV). Human kidney sections were used as a positive control (Fig. 7A–G, I)
Fig. 7.
Immunohistochemical analysis of subcutaneous matrigel nodules formed by the injection of RPTEC/TERT1, HRTPT and HREC24T cells in immune-compromised mice. (A.I - A.IV) H&E staining of human kidney (A.I), RPTEC/TERT1 nodules (A.II) and HRTPT nodules (A.III). (B.I-B.IV). Staining for KRT7 in human kidney (B.I), RPTEC/TERT1 nodules (B.II), HRTPT nodules (B.III) and HREC24T nodules (B.IV). Staining for KRT19 in human kidney (C.I), RPTEC/TERT1 nodules (C.II), HRTPT nodules (C.III) and HREC24T nodules (C.IV). Staining for CD133 in human kidney (D.I), RPTEC/TERT1 nodules (D.II), HRTPT nodules (D.III) and HREC24T nodules (D.IV). Staining for CD24 in human kidney (E.I), RPTEC/TERT1 nodules (E.II), HRTPT nodules (E.III) and HREC24T nodules (E.IV). Staining for AP in human kidney (F.I), RPTEC/TERT1 nodules (F.II), HRTPT nodules (F.III) and HREC24T nodules (F.IV). Staining for CAL in human kidney (G.I), RPTEC/TERT1 nodules (G.II), HRTPT nodules (G.III) and HREC24T nodules (G.IV). Magnification x200.
3.4. Effect of Cd2+ exposure on the viability of RPTEC/TERT1, HRTPT and HREC24T Cells
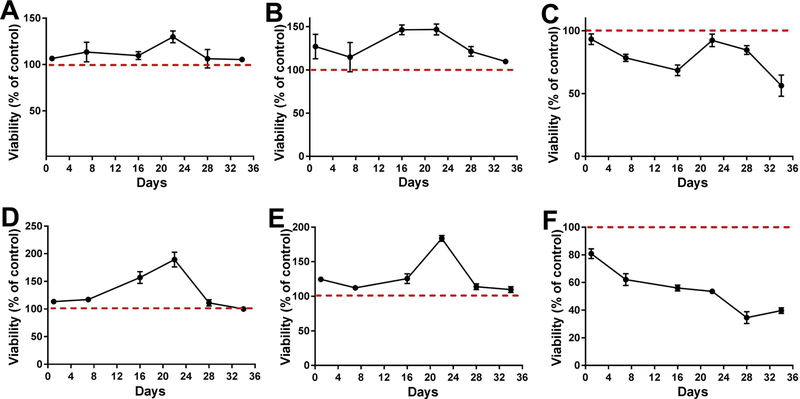
Preliminary experiments were performed on RPTEC/TERT1 cells to determine the optimal concentration of Cd2+ that would be required to determine if differences in toxicity existed between the cells that co-express CD133 and CD24 and those that express CD24, but not CD133. The RPTEC/TERT1 cells were exposed to various concentrations of Cd2+ (from 4.5µM to 45µM) for 16 days. The results of this analysis showed that 4.5µM and 9.0µM Cd2+ were optimal for this determination, as both these Cd2+ concentrations showed some toxicity and an effect on cell viability (Supplemental Figs. 6 and 7). The three cell lines were subsequently exposed to 4.5 and 9.0µM Cd2+ for 36 days and cell viability was determined by flow cytometry. The results of this analysis showed that the HREC24T cells were more sensitive to Cd2+ (Fig. 8C and F) than the RPTEC/TERT1 (Fig. 8A and D) and the HRTPT (Fig. 8B and E) cells that co-expressed CD133 and CD24.
Fig. 8.
Effect of cadmium on viability of RPTEC/TERT1, HRTPT and HREC24T cell lines. The RPTEC/TERT1, HRTPT and HREC24T cell lines were treated with 4.5 µM Cd2+ or 9µM Cd2+ for 34 days and the viable number of cells were determined by flow cytometric analysis. (A and D). Viability of RPTEC/TER1 cells after exposure to 4.5 (A) and 9 (D) µM Cd2+. (B and E). Viability of HRTPT cells after exposure to 4.5 (B) and 9 (E) µM Cd2+. (C and F). Viability of HREC24T cells after exposure to 4.5 (C) and 9 (F) µM Cd2+. The experiments were done in triplicates and the values are plotted as % of control (red dotted line). Analysis was done in triplicates and plotted as the mean ±SD.
4. Discussion
The immortalized culture of renal proximal tubule epithelial cells (RPTEC/TERT1) developed by Wieser and co-workers (2008) contain two distinct population of cells based on cell surface staining of CD133 and CD24 (Shrestha et al., 2017). The major population of cells co-express CD133 and CD24 (~79%), while a minor population express CD24 only (~21%). The initial goal of the present study was to determine if separate cultures of cells could be isolated where one co-expressed CD133 and CD24, while the other only expressed CD24. The results demonstrate that the two cell types can be separated into distinct cell cultures, one that expresses both CD133 and CD24 and the other that expresses only CD24. Flow cytometric analysis of the two cultures for the cell surface markers CD133 and CD24 at various passages show the two cultures to maintain their purity. While the analysis cannot prove an absolute purity of the co-expressing cells (CD133 and CD24) from the cells that express CD24 only, the results do support the isolation of highly pure cell cultures that co-express CD133 and CD24, and only CD24, respectively.
The newly isolated cell line HRTPT, that co-expresses both CD133 and CD24 should have significant utility in studies investigating the mechanisms underlying tubule cell regeneration and differentiation following renal injury. Renal epithelial cells co-expressing CD133 and CD24 have been identified as tubule progenitor/stem cells within the human kidney that are expressed following renal injury (Smeets et al., 2013; Romagnani and Remuzzi, 2014; Kusaba and Humphreys, 2014). The CD133+/CD24+ progenitor cells have also been identified and monitored during development of the human fetal kidney (Metsuyanim et al., 2009). An important study showed that CD133+/CD24+ progenitor cells isolated from the human fetal kidney could assist in renal recovery following acute renal failure (Lazzeri et al., 2007). In this study, the CD133+/CD24+ cells isolated from the fetal kidney were injected into immune compromised mice with acute renal failure from glycerol-induced rhabdomyolysis. The CD133+/CD24+ cells were shown to regenerate different portions of the nephron, to reduce tissue necrosis and fibrosis, and to significantly improve renal function. The majority of studies to date have involved in vivo analysis of human or animal renal tissue as noted above or the isolation and short-term culture of mortal renal epithelial cell cultures from human fetal kidney or the cortex from adult human kidneys (Lazzeri et al., 2007; Brossa et al., 2018). To the author’s knowledge, the HRTPT cells are the first to be isolated that express a highly pure population of CD133+/CD24+ cells that are immortal and capable of extended cell culture studies.
The HRTPT cells also possess the property of self-renewal and multi-differentiation potential expected of renal tubular progenitor cells. Our studies show the cells to undergo adipogenic, neurogenic, osteogenic, and tubulogenic differentiation when placed into growth media that promote their differentiation, and form colonies when mixed with matrigel and form “tubule-like” structures when placed onto a thin layer of matrigel. In addition, the HRTPT cells can also form multicellular structures referred to as “nephrospheres”, when cultured in low attachment flasks. In studies on human tumors and cancer cell lines, the “spheres” generated using this methodology are also referred to as cancer-initiating cells or cancer stem cells. They are accepted as a small subpopulation of cells within the tumor that drive cell proliferation and differentiation to produce and drive tumor heterogeneity (Ishiguro et al., 2017). It is presently unknown if the spheres generated from renal epithelial cells displaying the CD133+/CD24+ genotype represent a unique subpopulation of cells or if all cells in the culture have the ability to generate nephrospheres. In a study by Bombelli and co-workers (2018), spheres were directly isolated from the human kidney cortex and from primary cultures isolated from kidney cortex and it was shown that these cells were capable of multi lineage differentiation when cultured on decellularized human kidney scaffolds. Overall, the HRTPT cells should provide a new research avenue to explore the mechanisms underlying tubular regeneration following renal injury.
The HREC24T cell should also provide a new resource for research into the mechanism underlying renal tubule cell differentiation. The expression of CD24 in the absence of CD133 has been shown to occur in both the adult and fetal kidney (Lazzeri et al., 2007; Metsuyanim et al., 2009; Sagrinati et al., 2006; Smeets et al., 2013). In specimens of fetal human kidney (12, 13, and 18 weeks gestation), the expression of CD24 occurs in mature tubules and approximately 20% of the human fetal kidney is composed of CD24 positive cells (Metsuyanim et al., 2009). The authors predict that sorting cells from the human fetal kidney using CD24 would result in a heterogeneous population comprised predominantly of differentiated cells and to a much lesser extent of stem/progenitor cells. In human fetal kidney epithelial cell cultures isolated from the identical samples used for the in vivo analysis of the fetal kidney, the expression of CD24 and CD133 was different with CD133+/CD24+ cells accounting for 66.4% of the cells with the remainder being CD133−/CD24+. It is interesting that the distribution is highly similar to the distribution that was found previously by this laboratory for both the immortal RPTEC/TERT1 cells and mortal human proximal tubule (HPT) cell cultures (Shrestha et al., 2017). Although the two populations of cells (CD133+/CD24+ and CD133−/CD24+) could be separated from the RPTEC/TERT1 cell line into stable cell culture, there still remains a possibility that the CD133−/CD24+ cells have a role in supporting the CD133+/CD24+ cells or vice versa. In the adult kidney, cells expressing CD24 without CD133 have been localized to the distal tubules and collecting ducts (Sagrinati et al., 2006; Smeets et al., 2013).
The characterization of the HREC24T cells revealed several interesting features. The first was that the cells were unable to form nephrospheres when grown in low attachment flasks. As noted above, nephrospheres have been isolated from the human kidney and kidney-derived cell cultures and shown to possess the ability to differentiate (Bombelli et al., 2018). Similar to the present finding on nephrospheres isolated from the RPTEC/TERT1 and the HRTPT cell lines, the nephrospheres would be expected to harbor a population of CD133+/CD24+ cells. Even though the HREC24T cells do not produce nephrospheres, they are able to proliferate under standard culture conditions. The inability of the HREC24T cells to form nephrospheres may be related to the finding that the cells did not proliferate when sub-cultured into growth formulations favoring osteogenic and tubulogenic differentiation and proliferated very slowly in neurogenic and adipogenic differentiation media. The findings suggest that the cells are able to differentiate on selective growth media, but do not have the property of self-renewal. The inability to form nephrospheres is unlikely to be the only factor explaining the lack of cell growth on the selective media, since the HREC24T cells proliferate and show tubular differentiation on serum-free growth medium used for their routine growth and serial subculture. These cells can form colonies when mixed with soft agar, but are not able to form “tubule-like” structures when placed onto a thin coat of matrigel. The inability to form “tubule-like” structures is in contrast to cells expressing both CD133 and CD24. In addition, both the HRTPT and HREC24T cells can form tubules when mixed with matrigel and injected subcutaneously into immune compromised mice. Although there are some differences in the expression of markers associated with different nephron segments, both cell lines are able to differentiate and form tubular structures. Thus the HREC24T cells display both an ability to proliferate and differentiate under certain conditions. Some of the properties noted for the HREC24T cells suggests that the culture remains heterogeneous, being composed of tubule cells displaying different stages of differentiation. Metsuyanim and coworkers (2009) using fetal kidney cells predicted that sorting only based on CD24 would result in a heterogeneous population of cells comprised predominantly of differentiated tubule cells. Another possibility is the effect due to the cell line being immortalized using the human telomerase reverse transcriptase (hTERT) catalytic subunit. This may complicate the analysis of the self-renewal properties of the cells. Romagnani and Remuzzi (2014) present the argument that renal stem cells always co-express CD133 and CD24. The HRE24T cell line should provide a new avenue to study renal growth and differentiation in an environment free of cells co-expressing CD133 and CD24.
A preliminary study was also performed to determine if the cell lines co-expressing CD133 and CD24 were more resistant to the cytotoxic effects of the nephrotoxicant Cd2+, compared to the cells expressing only CD24. The rational to expect the CD133+/CD24+ to be more resistant is that they can regenerate tubules damaged by different disease states. For repair to occur, the CD133+/CD24+ cells would have to have increased resistance to the toxicant than the cells they are destined to replace in the tissue. In addition, it is widely accepted that cancer stem cells are more basal in character and more resistant to chemotherapeutic drugs that other cells within the tumor (Ishiguro et al., 2017). In line with this expectation, the HREC24T cells show significantly more toxicity when exposed to Cd2+ than either the RPTEC/TERT1 or the HRTPT cells, both of which co-express CD133 and CD24. Thus, the combination of the two new cell lines will facilitate studies to determine the mechanism involved in the resistance of the cells that co-express CD133 and CD24 to nephrotoxins that elicit tubular damage.
Supplementary Material
Highlights.
CD24 and CD133 proximal tubule progenitors repopulate the nephron after cell death.
The RPTEC/TERT1 cell line was cell sorted for these two markers
CD133+/CD24+ cells showed tubulogenic, adipogenic and osteogenic differentiation
CD133−/Cd24+ where not capable of differentiation
CD133+/CD24+ cells were more resistant to cadmium compared to CD133−/Cd24+
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Dr. Jane Dunlevy for training undergraduate and graduate students in confocal microscopy.
The research described was supported by funds provided by the Department of Pathology and the School of Medicine and Health Sciences, University of North Dakota. Undergraduate research, graduate student mentoring, core facilities for bioinformatics, microscopy, statistics, and gene expression were supported by the ND INBRE IDeA program P20 GM103442 from the National Institute of General Medical Sciences, NIH. The FACS analysis core is a joint venture supported by the ND INBRE and the NIH COBRE IDeA grant, 5P20GM113123 from the National Institute of General Medical Sciences, NIH.
Abbreviations:
- A-FABP
Adipocyte-Fatty acid binding protein
- AP
Alkaline phosphatase
- AQP-1
Aquaporin-1
- Cd2+
Cadmium
- CAL
Calbindin
- cDNA
complimentary DNA
- DAPI
4’,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- ENO-2
Enolase-2
- FSC
Forward Scatter
- H&E
Hematoxylin and eosin
- HPT
human proximal tubule
- HRTPT
Human Renal Tubular Precursor TERT
- HREC24T
Human Renal Epithelial Cell 24TERT
- hTERT
human telomerase reverse transcriptase
- KRT
keratin
- MTT
[3-(4,5-dimethylthiozol-2yl)-2,5-diphenyl tetrazolium bromide]
- NES
Nestin
- NF-200
Neurofilament-200
- PPARG
Peroxisome proliferator-activated receptor gamma
- PBS
phosphate buffered saline
- REBM
Renal Epithelial Cell Growth Basal Medium
- RPTEC/TERT1
renal proximal tubule epithelial cells
- RUNX2
Runt-related transcription factor-2
- SSC
Side Scatter
- THP
Tamm-Horsfall glycoprotein
- MAPT
tau-protein
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors declare that they have no competing interests.
References
- Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P, 2012. Stem. Cells 30, 1714–1725. [DOI] [PubMed] [Google Scholar]
- Bombelli S, Meregalli C, Scalia C, Bovo G, Torsello B, De Marco S, Cadamuro M, Viganò P, Strada G, Cattoretti G, Bianchi C, Perego RA, 2018. Nephrosphere-Derived cells are induced to multilineage differentiation when cultured on human decellularized kidney scaffolds. Am. J. Pathol 188, 184–195. [DOI] [PubMed] [Google Scholar]
- Brossa A, Papadimitriou E, Collino F, Incarnato D, Oliviero S, Camussi G, Bussolati B, 2017. Role of CD133 molecule in Wnt response and renal repair. Stem. Cells. Transl. Med 7, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA, 1984. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney. Int 25, 383–390. [DOI] [PubMed] [Google Scholar]
- Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, Gambaro G, 2010. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999–2006. BMC Public Health 10, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett SH, Somji S, Todd JH, Sens DA, 1998. Exposure of human proximal tubule cells to cd2+, zn2+, and Cu2+ induces metallothionein protein accumulation but not metallothionein isoform 2 mRNA. Environ. Health. Perspect 106(9), 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, 2015. Urothelial cancer stem cells and epithelial plasticity: current concepts and therapeutic implications in bladder cancer. Cancer. Metastasis. Rev 34, 691–701. [DOI] [PubMed] [Google Scholar]
- Hansson J, Hultenby K, Cramnert C, Pontén F, Jansson H, Lindgren D, Axelson H, Johansson ME, 2014. Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Human. Pathology 45, 382–393. [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K, 2017. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer. Sci 108, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba T, Humphreys BD, 2014. Controversies on the origin of proliferating epithelial cells after kidney injury. Pediatr. Nephrol 29, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P, 2007. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J. Am. Soc. Nephrol 18(12), 3128–3138. [DOI] [PubMed] [Google Scholar]
- Lindgren D, Bostrom AK, Nilsson K, Hansson J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson H, Johansson ME 2011. Isolation and characterization of progenitor-like cells from human kidney proximal tubules. Am. J. Pathol 178, 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsuyanim S, Harari-Steinberg O, Buzhor E, Omer D, Pode-Shakked N, Ben-Hur H, Halperin R, Schneider D, Dekel B 2009. Expression of stem cell markers in the human fetal kidney. PLoS ONE 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V, 2009. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am. J. Epidemiol 170, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S, 2005. CD14+CD34 low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ. Res 97(4):314–22. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Remuzzzi G 2013. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol 9, 137–146. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Remuzzi G 2014. CD133+ renal stem cells always co-express CD24 in adult human kidney tissue. Stem. Cell. Res 12, 828–829. [DOI] [PubMed] [Google Scholar]
- Ronconi E, Sagrinati C, Angelotti ML, Lazzen E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P 2009. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol 20, 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B 1994. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney. Int 45, 48–57. [DOI] [PubMed] [Google Scholar]
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P 2006. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidney. J. Am. Soc. Nephrol 17, 2443–2456. [DOI] [PubMed] [Google Scholar]
- Sallustio F, Costantino V, Cox SN, Loverre A, Divella C, Rizzi M, Schena FP 2013. Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR2-driven inhibin-A and microvesicle-shuttled decorin. Kidney. Int 83, 392–403. [DOI] [PubMed] [Google Scholar]
- Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JFM, Moeller MJ 2013. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J. Pathol 229, 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somji S, Garrett SH, Sens MA, Gurel V, Sens DA 2004. Expression of metallothionein isoform 3 (MT-3) determines the choice between apoptotic or necrotic cell death in Cd+2-exposed human proximal tubule cells. Toxicol Sci 80(2), 358–66. [DOI] [PubMed] [Google Scholar]
- Sommar JN, Svensson MK, Bjor BM, Elmstahl SI, Hallmans G, Lundh T, Schon SM, Skerfving S, Bergdahl IA, 2013. End-stage renal disease and low level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in Sweden. Environ. Health 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grallari-Voglauer R 2008. hTert alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am. J. Physiol. Renal. Physiol 295, F1365–1375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.