Abstract
Objective:
To examine whether childhood body mass index (BMI) trajectories are prospectively associated with later eating disorder (ED) diagnoses.
Method:
Using a subsample from the Avon Longitudinal Study of Parents and Children (N = 1,502), random-coefficient growth models were used to compare premorbid BMI trajectories of individuals who later developed anorexia nervosa (n = 243), bulimia nervosa (n = 69), binge-eating disorder (n = 114), and purging disorder (n = 133) and a control group without EDs or ED symptoms (n = 966). BMI was tracked longitudinally from birth to 12.5 years of age and EDs were assessed at 14, 16, and 18 years of age.
Results:
Distinct developmental trajectories emerged for EDs at a young age. The average growth trajectory for individuals with later anorexia nervosa veered significantly below that of the control group before 4 years of age for girls and 2 years for boys. BMI trajectories were higher than the control trajectory for all other ED groups. Specifically, the mean bulimia nervosa trajectory veered significantly above that of controls at 2 years for girls, but boys with later bulimia nervosa did not exhibit higher BMIs. The mean binge-eating disorder and purging disorder trajectories significantly diverged from the control trajectory at no older than 6 years for girls and boys.
Conclusion:
Premorbid metabolic factors and weight could be relevant to the etiology of ED. In anorexia nervosa, premorbid low weight could represent a key biological risk factor or early manifestation of an emerging disease process. Observing children whose BMI trajectories persistently and significantly deviate from age norms for signs and symptoms of ED could assist the identification of high-risk individuals.
Keywords: Avon Longitudinal Study of Parents and Children (ALSPAC), eating disorders, premorbid body mass index, development, prospective
Appetite and weight dysregulation are hallmark trans-diagnostic features of eating disorders (EDs). Body mass index (BMI) plays a key role in the differential diagnosis and course of illness. Low BMI is a central diagnostic feature of anorexia nervosa (AN), a key marker of illness severity and prognosis, and a nosologic feature that distinguishes AN from other EDs.1,2 Individuals with AN who later develop bulimia nervosa (BN) have a higher illness-related BMI than individuals with AN restricting subtype.3 In contrast, patients with binge-eating disorder (BED) often present with BMIs in the overweight/obese range.4
Despite its definitional role in ED diagnoses, studies examining whether child and adolescent BMI predicts later ED have yielded conflicting results. Prospective longitudinal studies have suggested that high BMI in early adolescence increases later risk for dysfunctional eating attitudes.5-7 In some studies, premorbid childhood and adolescent overweight and obesity were associated with adult BN and BED8-10 and more common in individuals with BN than with AN.11,12 However, other studies have failed to demonstrate an association between premorbid BMI and later BN and BED.13-15 Premorbid BMI also can vary by diagnosis: in contrast to BN and BED, low BMI in adolescent girls and young adults can predict later AN in adulthood.13,14 However, it is unclear whether low BMI in these girls represents existing undetected subthreshold AN or whether low BMI is a risk factor that predates disorder onset.
Because of the higher female prevalence of EDs, most research studies on premorbid BMI were conducted using data from female participants. Although some evidence suggests higher premorbid and prepubertal BMIs for boys with EDs (especially for AN or atypical AN) compared with girls,16-18 whether there are notable sex differences in the premorbid BMIs of patients with an ED remains unclear.19
Our overarching goal was to examine whether child-hood BMI trajectories represent a prospective risk factor for the development of 4 EDs. We described premorbid BMI trajectories (from birth through 12.5 years of age) in individuals who later developed AN, BN, BED, and purging disorder (PD; presence of recurrent compensatory behaviors such as vomiting without binge-eating episodes). We compared these trajectories with the BMI trajectories of individuals with no ED or ED symptoms (ie, control group). Examining developmental trajectories of BMI has advantages over previous research measuring child or adolescent BMI at only 1 time point. First, doing so allows us to characterize the developmental progression of BMI by age relative to ED status. Second, the method allows us to identify whether and at which time point trajectories diverge across disorders.
HYPOTHESES
Using data from the Avon Longitudinal Study of Parents and Children (ALSPAC), a large prospective longitudinal cohort, we used random coefficient models to describe BMI trajectories for individuals with AN, BN, BED, PD, or no ED. We hypothesized that adolescents who developed AN would have a consistently lower BMI trajectory curve throughout child-hood compared with adolescents who developed BN, BED, or PD or unaffected adolescents, controlling for sociodemographic variables (sex and parental social class).
METHOD
Participants
The ALSPAC is a longitudinal, population-based, prospective study of women and their children. All pregnant women living in the geographic area of Avon, United Kingdom who expected to deliver from April 1, 1991 to December 31, 1992 were invited to participate in the study. All participating women gave informed and written consent. The ALSPAC is representative of the geographic area of Avon and is broadly nationally representative, although ethnic minorities are under-represented.20 The ALSPAC website contains details of all the data that are available through a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary). A total of 14,541 pregnancies were enrolled, resulting in 14,062 live births and 13,617 singleton children who were alive at 1 year of age.20 An additional 713 children enrolled in the cohort at 7 years are not included in these analyses because of missing data by design (ie, child BMI in early life).20 Our analytic sample included 1,502 individuals with at least 1 BMI measurement from birth to 12.5 years who could be confidently coded as having an ED diagnosis at 14, 16, or 18 years or as having no diagnosis or risk symptoms at any of these ages. Specifically, 4,037 participants with no diagnosed ED but who exhibited only ED behaviors or cognitions (such as binge eating, restrictive eating, or purging less than once a month, or high weight and shape concern in the absence of ED behaviors) or met criteria for another specified feeding and eating disorder (apart from PD) at any time point were excluded from the analyses.
Of the 1,502 individuals included in our study, 58% were girls and 97% were white. Average maternal age at birth was 29.73 years (standard deviation 4.51) and mean maternal BMI before pregnancy was 22.37 kg/m2 (standard deviation 3.32). The 1,502 children in the sample presented for an average of 5.98 waves (standard deviation 2.42, range 1–12) of BMI longitudinal follow-up before 12.5 years, resulting in 8,980 longitudinal BMI observations. We restricted BMI observations to 12.5 years of age to ensure that data on BMI preceded any ED assessment (Table 1).
TABLE 1.
Study Design by Sex and Eating Disorder (ED) Diagnostic Status
| BMI Measurementsa | |||||
|---|---|---|---|---|---|
| Subjects | nb | Mean | SD | Min | Max |
| AN | |||||
| Girls | 180 | 6.10 | 2.35 | 1 | 12 |
| Boys | 63 | 6.03 | 1.63 | 1 | 12 |
| BN | |||||
| Girls | 64 | 6.38 | 3.19 | 1 | 12 |
| Boys | 5 | 5.80 | 3.77 | 2 | 12 |
| BED | |||||
| Girls | 89 | 5.16 | 2.66 | 1 | 12 |
| Boys | 25 | 5.96 | 3.18 | 1 | 12 |
| PD | |||||
| Girls | 120 | 5.39 | 2.73 | 1 | 12 |
| Boys | 13 | 6.85 | 2.67 | 2 | 12 |
| No ED | |||||
| Girls | 438 | 5.94 | 2.28 | 1 | 12 |
| Boys | 528 | 6.18 | 2.34 | 1 | 12 |
| Total | |||||
| Girls | 868 | 5.84 | 2.47 | 1 | 12 |
| Boys | 634 | 6.16 | 2.33 | 1 | 12 |
Note: AN = anorexia nervosa; BED = binge-eating disorder; BMI = body mass index; BN = bulimia nervosa; PD = purging disorder; Max = maximum; Min = minimum.
BMI measurements per participant before 12.5 years of age.
Number of subjects in each row sums to more than the sample total because 23 girls met criteria for more than 1 ED diagnosis over the course of the 3 assessment points (4 AN and BN, 2 AN and BED, 3 AN and PD, 5 BN and BED, 6 BN and PD, and 3 BED and PD). Participants with more than 1 ED were classified according to which disorder came first.
Measures
Body Mass Index.
Child and adolescent BMI (weight in kilograms/[height in centimeters/100]2) was obtained from weight and height collected from questionnaires sent to mothers on average every year and face-to-face assessments (carried out on average every 2 years). The correlation between directly measured height and weight and maternal report was 0.91.21 We used measured BMI for trajectory analyses. Because all analyses were conducted by sex and comparisons across participants were made at discrete time points when they were all the same age, we did not further standardize BMI measures (ie, z-scores or BMI percentiles).
ED Status.
ED assessments were conducted at 14, 16, and 18 years. ED diagnoses were obtained using DSM-5 criteria as presented in Table 2.21 We used self-reported ED behaviors for the 12 months before each data collection point, collected from questions adapted from the Youth Risk Behavior Surveillance System questionnaire previously validated in a population-based sample of adolescents.22,23 Weight and shape concern were ascertained using 3 questions from the McKnight Risk Factor Survey to derive a dichotomous variable.24,25 Parental report of fear of fatness and restrictive eating obtained using the Development and Wellbeing Assessment26,27 was used to supplement self-reported fear of fatness and restrictive eating when diagnosing AN, given evidence that young people often deny AN symptoms and that the use of parental report can overcome this.28
TABLE 2.
Criteria Used to Diagnose Eating Disorders
| Wave 14 | Wave 16 | Wave 18 | |
|---|---|---|---|
| Anorexia Nervosa | |||
| Self-report |
|
|
|
| Parental report |
|
|
N/A |
| Bulimia Nervosa |
|
|
|
| Binge Eating Disorder |
|
|
|
| Purging Disorder |
|
|
|
Note: AND indicates algorithm. BMI = body mass index; N/A = not applicable.
Underweight was determined using age, sex, and BMI-specific cutoffs (based on UK reference data) corresponding to World Health Organization grade 1 thinness.
Participants were categorized as meeting criteria for an ED diagnosis if they met criteria for ED at 14, 16, or 18 years. Participants with no ED diagnoses and no ED behaviors at any assessment were categorized as having no ED. Table 1 presents ED frequencies by sex. Twenty-two participants met criteria for 2 and 1 met criteria for 3 different ED diagnoses over the 3 ED assessment time points. Participants with more than 1 ED were classified according to which disorder came first.
Confounders.
Maternal age at birth, maternal ED history, maternal history of other psychiatric disorders, gestational age, birthweight, race/ethnicity, and social class—as reported by the mother at enrollment—were included as confounders. Social class was obtained by questionnaire at enrollment and categorized as manual and non-manual (87%). Age at menarche was not included as a confounder because boys were included in the analyses; however, a sensitivity analysis and our previous work concluded that results are not sensitive to the inclusion of this covariate.29 Because maternal BMI can arguably be considered a confounder or a predictor in the causal pathway between BMI trajectory and ED status, we tested the sensitivity of model results with and without maternal prepregnancy BMI included as a covariate.
Ethical approval for the study was obtained from the ALSPAC ethics and law committee and the local research ethics committees, including the institutional review board of the University of North Carolina at Chapel Hill.
Data Analysis
Growth Models.
We conducted parametric and nonparametric analyses of growth trajectories as a function of child sex and eventual ED status. Nonparametric analyses were conducted in R software (R Foundation, Vienna, Austria) by plotting 95% confidence bands for BMI as a function of child age in months and ED status separately by sex. Significant differences in mean BMI across ED categories exist when confidence bands do not overlap.
Parametric growth models were conducted using linear mixed models (MIXED procedure) in SAS 9.4 (SAS Institute, Cary, NC). First, unconditional growth models were specified to determine the appropriate functional form of growth trajectories by sex and the appropriate covariance structure for random effects. Second, covariates were added, followed by ED status and ED-by-age interactions. The results indicate whether differences are present at birth (intercept) and whether groups differ with respect to rate of change over time. Third, we tested sex-by-ED status and interactions of sex, ED status, and age to evaluate whether the effect of ED status on BMI growth trajectories varied as a function of sex. These models were probed to identify the ages at which conditional mean BMI values for each ED group differ significantly from the no-ED group.
Missing Data.
Missing BMI measurements were handled with a full information maximum likelihood estimator under the assumption that missing observations were conditionally missing at random after accounting for observed BMI measures and controls. Missing covariates were handled using multiple imputation with 20 chained imputations under a fully conditional specification using SAS MI and MIANALYZE procedures.
RESULTS
Nonparametric Growth Charts
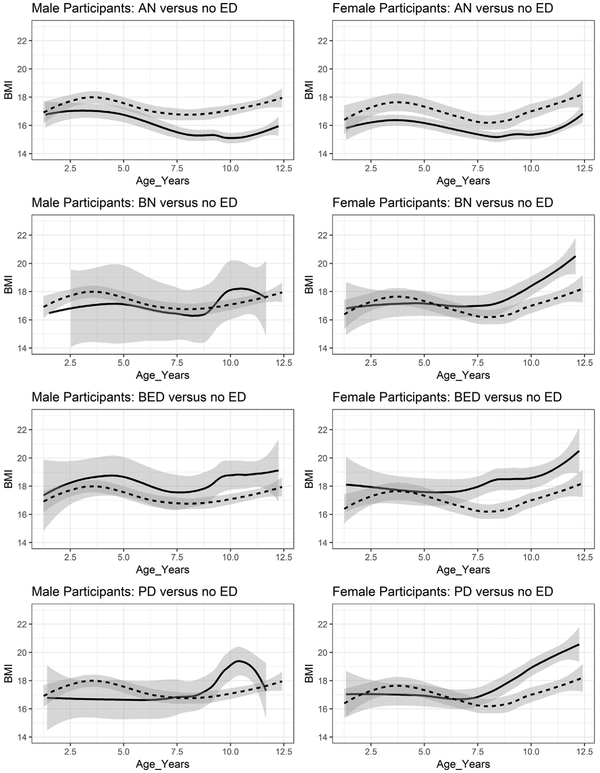
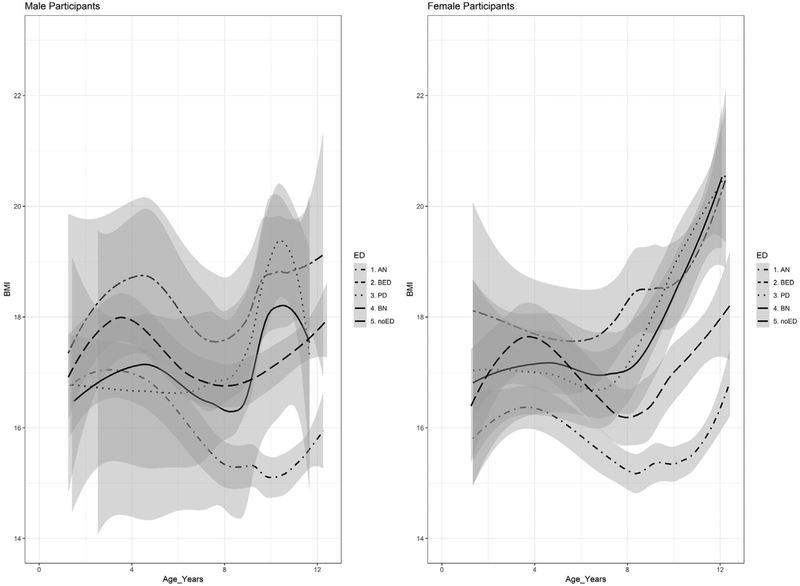
Figures 1 and 2 display BMI values as a function of age, sex, and ED diagnostic status and a nonparametric 95% confidence band for the ED group mean conditional on age. Each ED group is contrasted with the no-ED control in Figure 1, and all groups are overlaid in Figure 2.
FIGURE 1.
Average Male and Female Body Mass Index (BMI) Values by Age in Years and Eating Disorder (ED) Diagnostic Status With Nonparametric 95% Confidence Bands
Note: Each ED diagnostic status is shown separately and contrasted with the no-ED control group. The control group is depicted with dotted lines, and the ED groups are depicted with solid lines. AN = anorexia nervosa; BED = binge-eating disorder; BN = bulimia nervosa; PD = purging disorder.
FIGURE 2.
Average Male and Female Body Mass Index (BMI) Values by Age in Years and Eating Disorder (ED) Diagnostic Status (Overlaid) With Nonparametric 95% Confidence Bands
Note: The no-ED group is represented by the solid line. AN = anorexia nervosa; BED = binge-eating disorder; BN = bulimia nervosa; PD = purging disorder.
Boys.
On average, boys with AN had significantly lower BMIs than no-ED controls by 7 years. As a result of sparseness for the BN category in boys, confidence bands were too wide to detect meaningfully significant differences between boys with BN and the control group, although BMI means for the BED and PD groups were significantly higher than those for the no-ED group starting at 9.5 years.
Girls.
Confidence bands for mean BMI began to diverge significantly for the AN group and no-ED control group at 3 years, with lower BMIs observed in girls with AN. The confidence bands overlapped slightly at approximately 7 years but then diverged significantly again before 8 years. Although the BN, BED, and PD groups did not differ significantly from one another, the mean BMI of girls with BED began to be significantly higher than that of the no-ED controls at 7 years and the PD and BN trajectories began to diverge from the no-ED controls at 9 years.
Parametric Growth Models
Results from the final parametric growth models with and without controlling for maternal BMI are listed in Table 3. The significant interaction between age and ED status mirrors the results displayed in Figures 1 and 2. Because results are not sensitive to the inclusion of maternal prepregnancy BMI, we interpret the model with maternal BMI included as a covariate. A linear growth model fit better than models with quadratic or cubic time trends. The random effects followed a variance components structure, meaning that there was significant interindividual variation in BMI at birth and in rate of change over time, but that these 2 random effects did not correlate with each other.
TABLE 3.
Parameter Estimates for Final Growth Model
| Controlling for Maternal BMI |
Not Controlling for Maternal BMI |
|||||
|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | |
| Intercept | 14.178 | 0.401 | <.001 | 14.238 | 0.404 | <.0001 |
| Age (mo) | 0.025 | 0.001 | <.001 | 0.025 | 0.001 | <.0001 |
| Male | 0.398 | 0.199 | .046 | 0.392 | 0.200 | .050 |
| Months × male | −0.004 | 0.002 | .022 | −0.004 | 0.002 | .025 |
| Non-white | −0.136 | 0.357 | .703 | −0.215 | 0.360 | .550 |
| Birthweight | 0.001 | 0.000 | <.001 | 0.001 | 0.000 | <.0001 |
| Gestational age | −0.075 | 0.043 | .080 | −0.084 | 0.043 | .052 |
| Maternal ED | 0.689 | 0.302 | .022 | 0.635 | 0.304 | .037 |
| Maternal psychiatric history | 0.386 | 0.256 | .131 | 0.370 | 0.258 | .151 |
| Social class | 0.087 | 0.204 | .669 | 0.036 | 0.204 | .861 |
| Maternal BMI | 0.057 | 0.019 | .003 | — | — | — |
| Maternal age | −0.004 | 0.014 | .806 | −0.005 | 0.014 | .747 |
| AN | 0.243 | 0.271 | .371 | 0.213 | 0.272 | .434 |
| BN | 0.568 | 0.372 | .127 | 0.632 | 0.373 | .090 |
| BED | −1.054 | 0.388 | .007 | −1.001 | 0.389 | .010 |
| PD | −0.231 | 0.335 | .491 | −0.141 | 0.335 | .674 |
| Months × AN | −0.016 | 0.003 | <.001 | −0.016 | 0.003 | <.0001 |
| Months × BN | 0.007 | 0.004 | .052 | 0.007 | 0.004 | .056 |
| Months × BED | 0.027 | 0.004 | <.001 | 0.027 | 0.004 | <.0001 |
| Months × PD | 0.015 | 0.003 | <.001 | 0.015 | 0.003 | <.0001 |
| Male × AN | −0.894 | 0.518 | .084 | −0.920 | 0.519 | .076 |
| Male × BN | −3.784 | 1.424 | .008 | −3.869 | 1.427 | .007 |
| Male × BED | 1.692 | 0.723 | .019 | 1.759 | 0.725 | .015 |
| Male × PD | −1.102 | 0.818 | .178 | −1.126 | 0.821 | .171 |
| Months × male × AN | 0.007 | 0.005 | .173 | 0.006 | 0.005 | .181 |
| Months × male × BN | 0.035 | 0.014 | .011 | 0.034 | 0.014 | .013 |
| Months × male × BED | −0.019 | 0.007 | .007 | −0.019 | 0.007 | .006 |
| Months × male × PD | 0.024 | 0.008 | .003 | 0.024 | 0.008 | .003 |
Note: Results are aggregated from multiply imputed data. Covariates were mean centered. Sex and race/ethnicity are dummy coded (1 = male; 0 = female; 1 = white; 0 = non-white). Age is centered at birth. AN = anorexia nervosa; BED = binge-eating disorder; BMI = body mass index; BN = bulimia nervosa; PD = purging disorder; SE = standard error.
The addition of ED status contributed significantly to model fit compared with the covariates-only model. There were few significant group differences at birth: boys who went on to develop BN had significantly lower intercepts than boys without BN with no ED (B = −3.22, standard error [SE] 1.38), but this trend was not significant by 2 years and is suspect given the small number for this group. Similarly, girls who went on to develop BED has significantly lower intercepts than girls with no ED (B = −1.05, SE 0.39), but this trend became nonsignificant by 2 years and reversed itself by 6 years.
Anorexia Nervosa.
For boys and girls, the AN group diverged from the no-ED group at a young age. Even after controlling for a host of potential confounds, girls who went on to develop AN had significantly lower BMIs by 4 years (B = −0.505, SE 0.22), and boys who went on to develop AN had significantly lower BMIs by 2 years (B = −0.87, SE 0.38). These differences increased over time.
Bulimia Nervosa.
Although there were no significant differences in BMI for boys with BN after 2 years, BMIs diverged significantly for girls with BN compared with no-ED controls at 2 years (B = 0.74, SE 0.33), and this gap continued over time.
Binge-Eating Disorder.
Girls who went on to develop BED developed significantly higher mean BMIs than the no-ED controls at 6 years (B = 0.88, SE 0.29), and boys who went on to develop BED diverged from the no-ED control group at 4 years (B = 1.03, SE 0.51), and the gap between the BED and no-ED groups continued to expand with age for boys and girls.
Purging Disorder.
The girls who went on to develop PD had significantly higher mean BMIs than the no-ED controls by 5 years (B = 0.58, SE 0.26), and boys who went on to develop PD had significantly higher BMIs by 6 years (B = 1.48, SE 0.64). BMIs for the PD group continued to diverge from the no-ED group over time.
DISCUSSION
To our knowledge, this is the largest study examining premorbid BMI trajectories for EDs. Our analyses included ALSPAC data obtained from birth through 18 years, allowing for the assessment of premorbid BMI trajectories up to 12.5 years, before ED onset, while accounting for potential confounders.
Distinct developmental trajectories emerged for AN, BN, BED, and PD at a young age compared with the no-ED control group. The crude mean BMI growth trajectories for the AN group and all other groups began to diverge significantly starting at approximately 7 years of age for boys and much earlier for girls. After adjusting for potential confounders, the average growth trajectories for girls who developed AN departed significantly from those of girls who did not develop AN by approximately 4 years of age and at 2 years for boys. This distinction of AN premorbid BMI trajectory from others at such a young age provides evidence for the involvement of low premorbid BMI in the etiology of AN independent of disordered eating and subthreshold AN symptoms before AN diagnosis. Our findings are consistent with reports of significant negative genetic correlations between BMI and obesity and AN and support metabolic involvement in the etiology of AN.30,31
Although the mean trajectories for BN, BED, and PD were indistinguishable from one another, all were substantially higher than the mean trajectory for AN and the no-ED controls by middle childhood. Our results concur with previous research suggesting shared risk factors for BED, BN, and PD9,32 and evidence of a possible causal role played by obesity genes in relation to binge eating.19 Furthermore, it could be important to monitor youth whose BMI trajectories are notably and persistently above the age norms for psychological and behavioral symptoms, that might point to loss of control eating, binge eating, and purging behaviors.33
Our results came from a large longitudinal population cohort for which extensive demographic and anthropometric data were available. Use of prospective data is a key strength of our study and allowed for examination of premorbid BMI in individuals who go onto develop EDs. Although studies examining premorbid BMI in EDs in clinical settings often include only girls because of prevalence differences between sexes, our study included girls and boys. Availability of maternal information allowed for controlling for important confounders such as mothers’ psychiatric history. In addition, investigation of childhood and adolescent BMI trajectories provides key information about ED risk factors during crucial developmental years and could have important clinical implications for detection, diagnosis, and prevention.
Despite the strengths of our study, certain limitations should be considered. For instance, because our analyses required at least 1 BMI measurement from birth to 12.5 years and completed self-report ED questionnaires for all 3 time points (ie, 14, 16, and 18 years of age), our sample was restricted to a subset of ALSPAC children. Hence, after excluding youth with subthreshold EDs and ED behaviors/cognitions, 1,502 individuals were eligible for the present study, and we were unable to take advantage of the entire ALSPAC sample. Furthermore, ED symptoms and BMI at certain time points were obtained through self-report rather than clinical records, and no information was available for eating behaviors until 13 or 14 years. Thus, we could not assess ED status before 13 years or examine the symptoms of avoidant/restrictive food intake disorder at any time point. However, we would expect the prevalence of EDs before 12.5 years—when we censored BMI trajectories—to be relatively low. Of note, the mean BMI trajectories began to diverge very early for some EDs (ie, 3 years for girls who went onto develop AN). This observation—coupled with the typical ED age of onset often occurring at the time of pubertal onset34—suggests that differences are not likely to be an artifact of early ED onset, but there might be early metabolic or appetite dysregulation factors at play for ED susceptibility, particularly in the case of AN and BED. However, we cannot exclude the possibility that contextual factors or changes resulting from eating experiences early in childhood (eg, avoidant/restrictive food intake disorder) could have played a role in the findings reported in our study. Participants in the ALSPAC are predominantly white, and although we adjusted for ethnicity in our analyses, results might not be generalizable to multiethnic populations.
Although reliable differences emerged in BMI trajectory among groups of children who developed different ED presentations, our results relate to group means and not to the prediction of individual ED outcomes based on a single person’s growth curve data. That is, the 95% CI for the predicted BMI trajectory of a single child will be wider than the 95% confidence band for an entire group; therefore, caution is advised against particularization of our findings to an individual child with lower weight. Nevertheless, falling off the growth curve is a warning sign for a range of poor health outcomes.35 Observing children whose BMI trajectories persistently and significantly deviate from the age norms for signs of disordered eating behaviors could aid in identifying those who are at high risk when considered in combination with other indicators. We would recommend that, during routine recording of BMI on growth charts by pediatricians or general practitioners, persistent high or low BMI trajectories signal the need for sensitive screening for loss of control eating, binge eating, weight dissatisfaction, and dieting. This could facilitate early detection and appropriate intervention.
Our observations have clinical implications. Adequate weight restoration is a critical first step in recovery from AN. During treatment, providers typically rely on premorbid growth charts and BMI percentile ranges—particularly for adolescents and young adults—to determine a patient’s percentage of ideal body weight and weight goal for recovery.36 A key question raised by these findings is how much weight gain should be considered “enough.” Target weight is a key treatment component that can dictate level of care. In general, higher BMI and higher fat percentage at discharge from inpatient treatment have been associated with better outcomes and lower likelihood of relapse.37-39 Our results raise the question of whether setting a lower weight goal consistent with a patient’s premorbid BMI trajectory is adequate or whether weight goals should reflect a return to a BMI trajectory of individuals without EDs. Additional research is needed to incorporate these findings to clinical decision making.
Our results provide important clues about the etiology of EDs and their relation with premorbid BMI. Especially for AN, premorbid persistent low weight could be a key biological risk factor and a trait- rather than an ED-related state characteristic.
Acknowledgments
Dr. Yilmaz is supported by National Institutes of Health grant K01MH109782. Dr. Gottfredson is supported by National Institutes of Health grant DA035153A. Dr. Zerwas is supported by a National Institute of Mental Health (NIMH) career development grant (K01MH100435). Dr. Bulik acknowledges funding from the Swedish Research Council (VR Dnr: 538-2013-8864). Dr. Micali was funded by a Clinician Scientist Award by the National Institute for Health Research, UK (DHCS/08/08/012). The UK Medical Research Council and the Wellcome Trust (grant reference 102215/2/13/2) and the University of Bristol provide core support for ALSPAC.
Dr. Gottfredson served as the statistical expert for this research.
The authors are deeply grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the entire ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. Dr. Micali had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, thereby serving as the guarantor for the contents of this article.
Disclosure: Dr. Bulik received a grant from Shire. Drs. Yilmaz, Gottfredson, Zerwas, and Micali report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Zeynep Yilmaz, University of North Carolina at Chapel Hill.
Nisha C. Gottfredson, University of North Carolina at Chapel Hill.
Stephanie C. Zerwas, University of North Carolina at Chapel Hill.
Cynthia M. Bulik, University of North Carolina at Chapel Hill; Karolinska Institutet, Stockholm, Sweden.
Nadia Micali, Icahn School of Medicine at Mount Sinai, New York, NY; University of Geneva, Switzerland; Institute of Child Health, University College London, UK.
REFERENCES
- 1.American Psychiatric Association. Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am J Psychiatry. 2006;163(suppl):4–54. [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: Author; 2013. [Google Scholar]
- 3.Nishimura H, Komaki G, Ando T, et al. Psychological and weight-related characteristics of patients with anorexia nervosa-restricting type who later develop bulimia nervosa. Biopsychosoc Med. 2008;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Zwaan M Binge eating disorder and obesity. Int J Obes Relat Metab Disord. 2001;25(suppl 1):S51–S55. [DOI] [PubMed] [Google Scholar]
- 5.Westerberg-Jacobson J, Edlund B, Ghaderi A. Risk and protective factors for disturbed eating: a 7-year longitudinal study of eating attitudes and psychological factors in adolescent girls and their parents. Eat Weight Disord. 2010;15:e208–e218. [DOI] [PubMed] [Google Scholar]
- 6.Westerberg J, Edlund B, Ghaderi A. A 2-year longitudinal study of eating attitudes, BMI, perfectionism, asceticism and family climate in adolescent girls and their parents. Eat Weight Disord. 2008;13:64–72. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz SA, Witt AA, Gillberg C, Rastam M, Wentz E, Lowe MR. Childhood body mass index in adolescent-onset anorexia nervosa. Int J Eat Disord. 2016;49:1002–1009. [DOI] [PubMed] [Google Scholar]
- 8.Goncalves S, Machado BC, Martins C, Hoek HW, Machado PP. Retrospective correlates for bulimia nervosa: a matched case-control study. Eur Eat Disord Rev. 2016;24:197–205. [DOI] [PubMed] [Google Scholar]
- 9.Allen KL, Byrne SM, Crosby RD. Distinguishing between risk factors for bulimia nervosa, binge eating disorder, and purging disorder. J Youth Adolesc. 2015;44:1580–1591. [DOI] [PubMed] [Google Scholar]
- 10.Hilbert A, Pike KM, Goldschmidt AB, et al. Risk factors across the eating disorders. Psychiatry Res. 2014;220:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado BC, Goncalves SF, Martins C, et al. Anorexia nervosa versus bulimia nervosa: differences based on retrospective correlates in a case-control study. Eat Weight Disord. 2015;21:185–197. [DOI] [PubMed] [Google Scholar]
- 12.Villarejo C, Fernandez-Aranda F, Jimenez-Murcia S, et al. Lifetime obesity in patients with eating disorders: increasing prevalence, clinical and personality correlates. Eur Eat Disord Rev. 2012;20:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stice E. Interactive and mediational etiologic models of eating disorder onset: evidence from prospective studies. Annu Rev Clin Psychol. 2016;12:359–381. [DOI] [PubMed] [Google Scholar]
- 14.Tyrka AR, Waldron I, Graber JA, Brooks-Gunn J. Prospective predictors of the onset of anorexic and bulimic syndromes. Int J Eat Disord. 2002;32:282–290. [DOI] [PubMed] [Google Scholar]
- 15.Killen JD, Taylor CB, Hayward C, et al. Weight concerns influence the development of eating disorders: a 4-year prospective study. J Consult Clin Psychol. 1996;64:936–940. [DOI] [PubMed] [Google Scholar]
- 16.Welch E, Ghaderi A, Swenne I. A comparison of clinical characteristics between adolescent males and females with eating disorders. BMC Psychiatry. 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raevuori A, Keski-Rahkonen A, Hoek HW. A review of eating disorders in males. Curr Opin Psychiatry. 2014;27:426–430. [DOI] [PubMed] [Google Scholar]
- 18.Gueguen J, Godart N, Chambry J, et al. Severe anorexia nervosa in men: comparison with severe AN in women and analysis of mortality. Int J Eat Disord. 2012;45:537–545. [DOI] [PubMed] [Google Scholar]
- 19.Reed ZE, Micali N, Bulik CM, Davey Smith G, Wade KH. Assessing the causal role of adiposity on disordered eating in childhood, adolescence, and adulthood: a Mendelian randomization analysis. Am J Clin Nutr. 2017;106:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micali N, Solmi F, Horton NJ, et al. Adolescent eating disorders predict psychiatric, high-risk behaviors and weight outcomes in young adulthood. J Am Acad Child Adolesc Psychiatry. 2015;54:652–659.e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kann L, Warren CW, Harris WA, et al. Youth risk behavior surveillance—United States, 1995. J Sch Health. 1996;66:365–377. [DOI] [PubMed] [Google Scholar]
- 23.Field AE, Taylor CB, Celio A, Colditz GA. Comparison of self-report to interview assessment of bulimic behaviors among preadolescent and adolescent girls and boys. Int J Eat Disord. 2004;35:86–92. [DOI] [PubMed] [Google Scholar]
- 24.Shisslak CM, Renger R, Sharpe T, et al. Development and evaluation of the McKnight Risk Factor Survey for assessing potential risk and protective factors for disordered eating in preadolescent and adolescent girls. Int J Eat Disord. 1999;25:195–214. [DOI] [PubMed] [Google Scholar]
- 25.Field AE, Camargo CA Jr, Taylor CB, Berkey CS, Roberts SB, Colditz GA. Peer, parent, and media influences on the development of weight concerns and frequent dieting among preadolescent and adolescent girls and boys. Pediatrics. 2001;107:54–60. [DOI] [PubMed] [Google Scholar]
- 26.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- 27.House J, Eisler I, Simic M, Micali N. Diagnosing eating disorders in adolescents: a comparison of the eating disorder examination and the development and well-being assessment. Int J Eat Disord. 2008;41:535–541. [DOI] [PubMed] [Google Scholar]
- 28.Swanson SA, Aloisio KM, Horton NJ, et al. Assessing eating disorder symptoms in adolescence: is there a role for multiple informants? Int J Eat Disord. 2014;47:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micali N, Daniel RM, Ploubidis GB, De Stavola BL. Maternal prepregnancy weight status and adolescent eating disorder behaviors: a longitudinal study of risk pathways. Epidemiology. 2018;29:579–589. [DOI] [PubMed] [Google Scholar]
- 30.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan L, Yilmaz Z, Gaspar H, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry. 2017;174:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micali N, Martini MG, Thomas JJ, et al. Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: a population-based study of diagnoses and risk factors. BMC Med. 2017;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Micali N, De Stavola B, Ploubidis G, Simonoff E, Treasure J, Field AE. Adolescent eating disorder behaviours and cognitions: gender-specific effects of child, maternal and family risk factors. Br J Psychiatry. 2015;207:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yilmaz Z, Hardaway JA, Bulik CM. Genetics and epigenetics of eating disorders. Adv Genomics Genet. 2015;5:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parry-Jones WL. Target weight in children and adolescents with anorexia nervosa. Acta Paediatr Scand Suppl. 1991;373:82–90. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan AS, Walsh BT, Olmsted M, et al. The slippery slope: prediction of successful weight maintenance in anorexia nervosa. Psychol Med. 2009;39:1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer LE, Roberto CA, Glasofer DR, et al. Does percent body fat predict outcome in anorexia nervosa? Am J Psychiatry. 2007;164:970–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodell LP, Mayer LE. Percent body fat is a risk factor for relapse in anorexia nervosa: a replication study. Int J Eat Disord. 2011;44:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]