Abstract
Mitochondrial toxicity has been proposed as a potential cause of developmental defects in humans. We evaluated 51 organophosphate and carbamate pesticides using the U.S. EPA ToxCast and Tox21 databases. Only a small number of them bind directly to cholinesterases in the parent form. The hydrophobicity of organophosphate pesticides is correlated significantly to TSPO binding affinity, mitochondrial membrane potential reduction in HepG2 cells, and developmental toxicity in Caenorhabditis elegans and Danio rerio (p < 0.05). Structural analysis suggests that in some cases the Krebs cycle is a potential target of organophosphate and carbamate exposure at early life stages. The results support the hypothesis that mitochondrial effects of some organophosphate pesticides—particularly those that require enzymatic activation to the oxon form—may augment the documented effects of disruption of acetylcholine signaling. This study provides a proof of concept for applying new approach methodologies to interrogate mechanisms of action for cumulative risk assessment.
1. Introduction
Organophosphates (OPs) and carbamates are two important classes of chemical agents that are used in pest management. A major concern for the use of these pesticides is potential human health hazards resulting from cumulative exposure during development [1-3]. Adverse effects of OP and carbamate exposure have been reported at concentrations below acetylcholinesterase (AChE) inhibition [4, 5]. This has been linked to the trophic role of AChE in brain development [6]. Another potential target for these developmental effects is mitochondria. Low doses of chlorpyrifos can result in oxidative stress in rodent models [7]. Exposure to the active metabolite chlorpyrifos-oxon leads to over-expression of gene sets involved in mitochondrial dysfunction and oxidative stress in the rat cerebellum [8]. In addition, the antioxidant vitamin E ameliorates the anti-proliferative effect of chlorpyrifos in PC12 cells [9].
Mitochondria play an important role in early stages of animal development via generation of low levels of reactive oxygen species that can act as signaling molecules. One example is axis specification through H2O2 signaling [10]. At later developmental stages, redox signaling regulates neuroprogenitor cell proliferation, differentiation, and function in the hippocampus [11, 12]. Prenatal exposure to mitochondrial toxicants results in microvascular impairments in adult rats [13]. A number of in vitro and animal studies have shown that exposure to OPs and carbamates can result in oxidative stress [9, 14-18], which, together with altered macronutrient metabolism, has been linked to structural birth defects in mice [8, 9]. Yet, it is unclear (1) if there is a bona fide, specific mitochondrial effect of OPs and carbamates; 2) if so, what the molecular target(s) of mitochondrial toxicity is for OPs and carbamates; and (3) whether mitochondrial toxicity contributes to their noncholinergic disruption of development.
New approach methodologies (NAMs) refer to any in silico or in vitro method that is alternative to mammalian testing in chemical hazard assessment [19]. For example, the U.S. EPA ToxCast program is building high-throughput screening (HTS) datasets to profile in vitro and in vivo bioactivity of chemical libraries [20]. Large collections of toxicity data are now available for different classes of pesticides, enabling a novel approach to the study of mitochondrial toxicity of OPs and carbamates. An earlier study revealed that 73% of the ToxCast Phase II library of 676 chemicals could disrupt mitochondrial respiration in primary cultures of renal proximal tubule cells [21]. A quantitative structure-activity relationship (QSAR) study of the Tox21 library of 8,300 chemicals identified the molecular features associated with changes in mitochondrial membrane potential in HepG2 cells [22]. A follow-up mechanistic analysis identified four lesser-known mitochondrial toxicants to be further characterized in vivo [23]. Thus, the use of NAMs enables an analysis of the importance of AChE inhibition in comparison with mitochondrial and other mechanisms of action.
The goal of this study was to examine the concordance of in vitro mitochondrial and cholinesterase activities with in vivo developmental toxicity, among OPs and carbamates. We evaluated the 32 OPs and 19 carbamates in the ToxCast Phase I and II libraries for in vitro binding affinities with several targets, changes in mitochondrial membrane potential in HepG2 cells, and developmental toxicity in Caenorhabditis elegans and Danlo rerlo. We also characterized the physicochemical and structural features that contribute to biological differences in vitro and in vivo. In previous single-chemical and cumulative risk assessments, mitochondrial dysfunction and oxidative stress have not been considered a starting point for extrapolation to determine the risk of environmentally relevant levels of human exposure [24-26]. Yet, the present study suggests that in some cases, the mitochondrial effects of OP pesticides may augment the OP-mediated disruption of acetylcholine signaling in animal developmental toxicity. As a proof of concept, this study illustrates the usefulness of QSAR-, cell-, and small organism-based NAMs in interrogating mechanisms of action for cumulative risk assessment.
2. Materials and Methods
2.1. Examination set of organophosphates and carbamates
We examined 32 OPs and 19 carbamates in Phase I and II of the U.S. EPA ToxCast project. According to the Compendium of Pesticide Common Names (http://www.alanwood.net/pesticides/index.html), 38 of these chemicals are used as insecticides, 25 as acaricides, and 10 as nematicides. Of note, while these active ingredients are mostly used in formulations with other chemicals, such as solvents, surfactants, and synergists, in this study we examined the active ingredients only. Thirty eight were evaluated by U.S. EPA in 2006 and 2007 for their commonality of toxic effects and mechanisms [24, 26]. Structural information was obtained from the supplemental materials of Richard et al (2016; [27]). The logarithm of Poctanol-water (log Pow) values were predicted with OPEn structure—activity/property Relationship App (OPERA) models [28]. Molecular weight and predicted log Pow values were acquired at the U.S. EPA Chemistry Dashboard V3.0 website (https://comptox.epa.gov/dashboard/) [29]. A full list of these 51 pesticides and properties is provided in Supplemental Material S1.
2.2. Analytical visualization of physical chemistry and Tanimoto similarity
The molecular weight and log Pow of acetylcholine, cholesterol, 32 OPs, and 19 carbamates were visualized in a scatter plot using GraphPad Prism version 7.0. The two-dimensional structures of the pesticides were compared to acetylcholine, sarin, cholesterol, PK-11195, and substrates of the Krebs cycle (i.e. succinyl CoA, fumarate, malate, oxaloacetate, citrate, isocitrate, and alpha-ketoglutaric acid) using substructure keys [30]. Tanimoto similarity was calculated in a score of 1 - 100 using the PubChem score matrix (https://pubchem.ncbi.nlm.nih.gov/score_matrix) [31]. The similarity scores were subjected to two-directional hierarchical clustering in R version 3.5.1 using Euclidean distance for measure and Ward’s method for cluster analysis [32, 33].
2.3. In vitro binding affinity assay, hierarchical clustering, and network visualization
We evaluated the 51 pesticides at a concentration range of 0.023 - 50 μM (or 0.009 - 20 μM for cytochrome P450 enzyme (CYP) assays) across 330 cell-free enzymatic and ligand-binding high-throughput assays [34, 35]. We excluded 90 assays in the same dataset with missing data. The technical and biological details of each assay are described at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). Briefly, the assays captured the binding activity of a pesticide with an enzyme reporter specific to each target, which was measured as absorbance signals using colorimetric technology. The molecular targets of these assays included 76 G-protein coupled receptors (GPCRs), 18 nuclear receptors, 10 CYPs, and 3 ChEs. Calculation of the half-maximal activity concentrations (AC50s) was described in details by Judson et al [36]. The raw AC50 data were obtained at the ToxCast website and the AC50 value (in μM) of each pesticide was transformed to an affinity score (A), where
The affinity score for an AC50 value of 1,000 μM was defined as zero, since any higher concentration in vitro would no longer be environmentally or pharmacologically relevant. The affinity scores were subjected to two-directional hierarchical clustering in R version 3.5.1 using Euclidean distance for measure and Ward’s method for cluster analysis [32, 33]. The binding pairs and affinity scores were visualized in a quantitative bipartite network as previously described [37, 38].
2.4. Mitochondrial membrane potential assay in HepG2 cells and developmental toxicity assays in Caenorhabditis elegans and Danio rerio
We analyzed 32 OPs and 18 carbamates in the U.S. EPA’s ToxCast Phase I and II library that were assayed for (1) changes in mitochondrial membrane potential in HepG2 cells and (2) developmental toxicity in C. elegans and D. rerio [39, 40]. In the HepG2 cell assay, the pesticides were tested at a concentration range 1.18 nM - 92.2 μM [22]. Changes in mitochondrial membrane potential were captured with a derivative of the JC-1 dye [22, 41]. There are important caveats associated with JC-1 and related dyes [42, 43]. However, in this case, we think that the approach generated reasonable data for a high-throughput analysis, because the authors (1) used a short exposure time and standardized conditions for cross-chemical comparisons, (2) used hepatic rather than neuronal cells to test for cytotoxicity, and (3) included carbonyl cyanide-p-trifluoromethoxyphenylhydrazone as a positive control. In the C. elegans assay, age-synchronized populations of 50 LI larvae were exposed at 20°C to each pesticide at a concentration range of 0.5 - 200 μM [39]. After a 48-hr exposure, the populations were assessed for mean length. In the D. rerio assay, the embryos were exposed individually at 26°C to each pesticide at a concentration range of 1 nM - 80 μM [40]. After a 5-day exposure, the embryos were evaluated for death and overt structural defects.
2.5. Concordance analysis of chemical and biological properties across multiple experimental platforms
Calculation of AC05S in the HepG2 cell and D. rerio assays was described by Judson et al [40]. The raw data were obtained at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). Calculation of AC50S in the C. elegans assay was described by Boyd et al [39]; the raw data were obtained from the supplemental materials of the same publication. The AC50 values of each pesticide in the HepG2 cell, C. elegans, and D. rerio assays were transformed to toxicity scores (T) as described in Section 2.3. The affinity scores, toxicity scores, molecular weight, and log Pow of OPs and carbamates were statistically tested as two separate experiments with Spearman’s rank correlation [44] followed by multiple testing correction with Holm’s method in R version 3.5.1 [33, 45]. The AC50 values for HepG2 cell viability were acquired at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). The cytotoxicity limit of each pesticide (i.e. the concentration three median absolute deviations below the median of cytotoxicity AC50S [36]) was acquired at the U.S. EPA Chemistry Dashboard V3.0.8 website (https://comptox.epa.gov/dashboard/) [29]. The raw data, calculated scores, and cytotoxicity limits are presented in Supplemental Material S2 and S3.
2.6. Chemical exposures in C. elegans and mitochondrial DNA damage assay
Chlorpyrifos (ethyl; CAS Registry Number: 2921-88-2) was purchased from Sigma (St Louis, MO). Aflatoxin B1 was used as a positive control in the assay for mitochondrial DNA (mtDNA) damage [46]. The chemicals were dissolved in dimethyl sulfoxide (DMSO) and added to treatment wells at a maximum amount of 1 % (v/v) in K medium (51 mM sodium chloride, 32 mM potassium chloride, 3 mM calcium chloride, 3 mM magnesium sulfate, 13 μM cholesterol) [47, 48]. The germline-deficient JK1107 strain (glp-1) of C. elegans was used to evaluate the DNA damage in somatic cells. It was obtained from the Caenorhabditis Genetics Center (University of Minnesota) and treated with the solutions in 12-well plates. Each well contained 1 ml of the treatment solution, 300 adults, and the OP50 strain of Escherichia coll as a food source. Synchronized glp-1 worms, grown at 15°C for 40 h and sterilized for 18 h at 25°C, were exposed to 3, 30, and 100 μM chlorpyrifos and aflatoxin B1 for 24 hr as previously described [46]. The highest exposure level chosen for each chemical was the highest tested that resulted in no lethality [49]. Six worms were picked and pooled in a single tube per biological replicate. Four biological replicates were taken per treatment (n = 4). Mitochondrial DNA damage was evaluated using a QPCR-based assay as previously described [49-52]. The measurement of this assay was relative to the DMSO control sample, which was defined as having 0 lesion/10 kb by convention, with a detection limit of 0.2 lesion/10 kb.
3. Results
3.1. Organophosphates and carbamates in ToxCast Phase I and II libraries are intermediate between acetylcholine and cholesterol in size and hydrophobicity
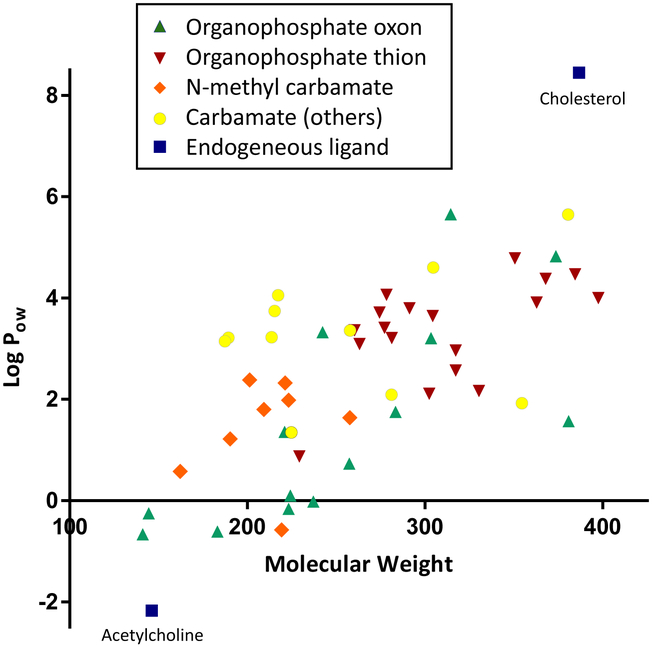
We began by analyzing the physicochemical properties of the compounds, since they are strong predictors of a compound’s biological activities [53, 54]. A previous investigation found that log Pow was correlated to developmental toxicity in D. rerio in the ToxCast Phase I library, which included many pesticides [40]. Figure 1 is a scatterplot of molecular weight and Log Pow of acetylcholine, cholesterol, 32 OPs, and 19 carbamates. Acetylcholine is the third smallest and most hydrophilic compound in this analysis (MW = 146.209; Log Pow = − 2.17) and cholesterol is the second largest and most hydrophobic (MW = 386.664; Log Pow = 8.45). On average, OP thions are larger and more hydrophobic than OP oxons, and N-methyl carbamates are more hydrophilic than other carbamates. Acetylcholine and cholesterol share limited chemoinformatic/structural similarity with any OP and carbamate (S≤ 56). An undirected quantitative network of Tanimoto similarity revealed no distinct partition of OP thions, OP oxons, or N- methyl carbamates (data not shown), suggesting that these structural features had limited contributions to the molecular similarity in this chemical set.
Figure 1. Molecular weight and hydrophobicity of organophosphates and carbamates in ToxCast Phase I and II libraries in comparison with endogenous ligands.
The molecular weight and logarithm of Poctanol-water (log Pow) of acetylcholine, cholesterol, 14 organophosphate oxons, 18 organophosphate thions, 8 N-methyl carbamates, and 11 other carbamates were visualized in a scatter plot. Acetylcholine is the third smallest and most hydrophilic compound and cholesterol is the second largest and most hydrophobic.
3.2. Cholinesterases only account for a small fraction of the molecular targets of parent organophosphates and carbamates
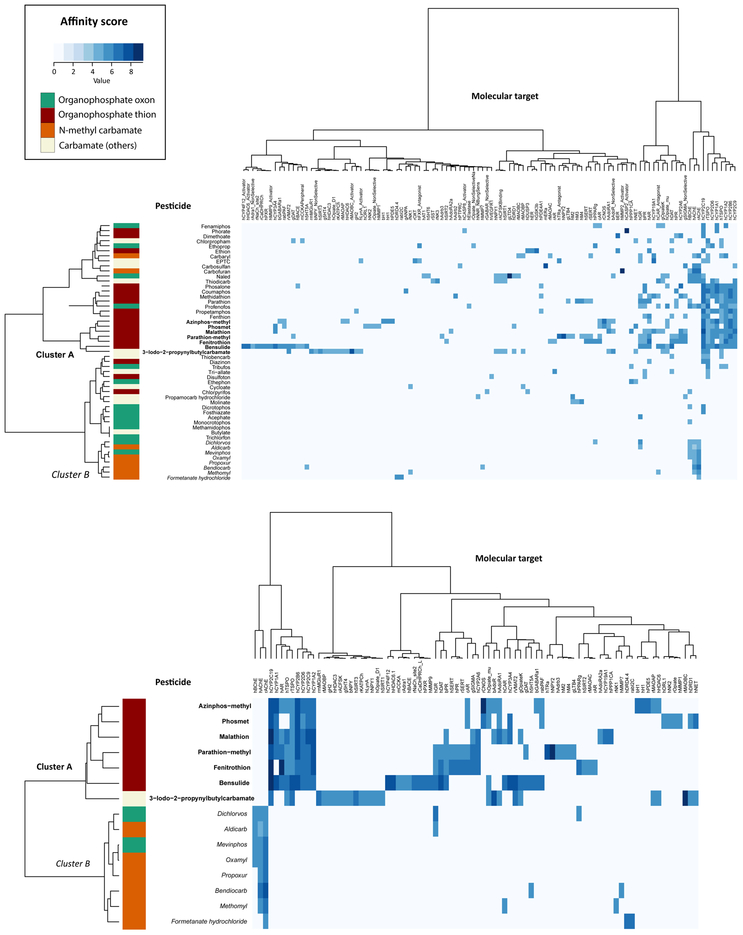
Figure 2A shows the two-way hierarchical clustering of all compounds by affinity scores (see Section 2.3). Many OP and carbamate pesticides are AChE inhibitors. However, in these experiments, less than half of them bound to ChEs, and many interacted with other targets. Forty eight of the 51 pesticides bound to 110 of the 330 molecular targets. Human CYP2C19 was the most promiscuous target, binding to 25 of the 51 pesticides. It was followed by rat mitochondrial translocator protein (23 pesticides), human mitochondrial translocator protein (18), human CYP1A2 (16), and rat AChE (rAChE; 14). Seventeen pesticides bound to cholinesterases and mitochondrial translocator proteins at concentrations below cytotoxicity limits (Supplemental Material S3), although it should be noted that the binding assays are cell-free, limiting the interpretability of the concentration comparisons. The most promiscuous pesticide was bensulide, binding to 30 molecular targets. It was followed by 3-iodo-2-propynyl butylcarbamate (26 targets), parathion-methyl (25), azinphos-methyl (21), and fenitrothion (21). The lack of AChE binding by many of these OPs and carbamates is likely due to the fact that cholinergic pesticides are engineered to (1) bind to insect AChE better than mammalian AChE [55], and (2) inhibit AChE either directly as parent compounds or indirectly as metabolites [56, 57]. This is supported by the enrichment of OP thions in what we term “Cluster A” (Figure 2), since the replacement of sulphur by oxygen (i.e. P=S → P=0) increases the specificity of OP pesticides for AChE. Cluster A binds to human and rat mitochondrial translocator proteins and human CYP1A1, CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP2D6; but not human AChE (hAChE), rAChE, and human butyrylcholinesterase (hBChE). We also identified another group of 2 OP oxons and 6 N-methyl carbamates that bind to the ChEs, but not mitochondrial translocator proteins and CYPs (i.e. “Cluster B”). This is consistent with the enrichment of the carbamate core in a previous univariate analysis of the same chemical-assay dataset [35]. For improved visualization of Clusters A and B, they were re-clustered in a separate analysis with their 84 molecular targets in Figure 2B.
Figure 2A and 2B. Unsupervised two-directional heat map from hierarchical clustering of 32 organophosphates and 19 carbamates across 110 molecular targets.
The heat map was generated with the “gplot” package in R version 3.5.1, using Euclidean distance for measure and Ward’s method for linkage analysis. The intensity of blue color indicates the value of affinity score. Organophosphate (OP) thions are enriched in Cluster A (in bold), which bind to mitochondrial translocator proteins (TSPOs) and cytochrome P450 (CYP) 1A1, 1A2, 2B6, 2C9, 2C19, and 2D6; but not acetylcholinesterases (AChEs) and butyrylcholinesterase (BChE). Another group of 2 OP oxons and 6 N-methyl carbamates bind to the ChEs, but not TSPOs and CYPs (i.e. Cluster B; in italic). For improved visualization of these two groups, Cluster A and B were re-clustered with their 84 molecular targets in Figure 2B. Animal species of these targets are specified by a first-letter initial (b: bovines; g: guinea pigs; h: humans; m: mouse; r: rats; and rab: rabbits) preceding the gene symbol.
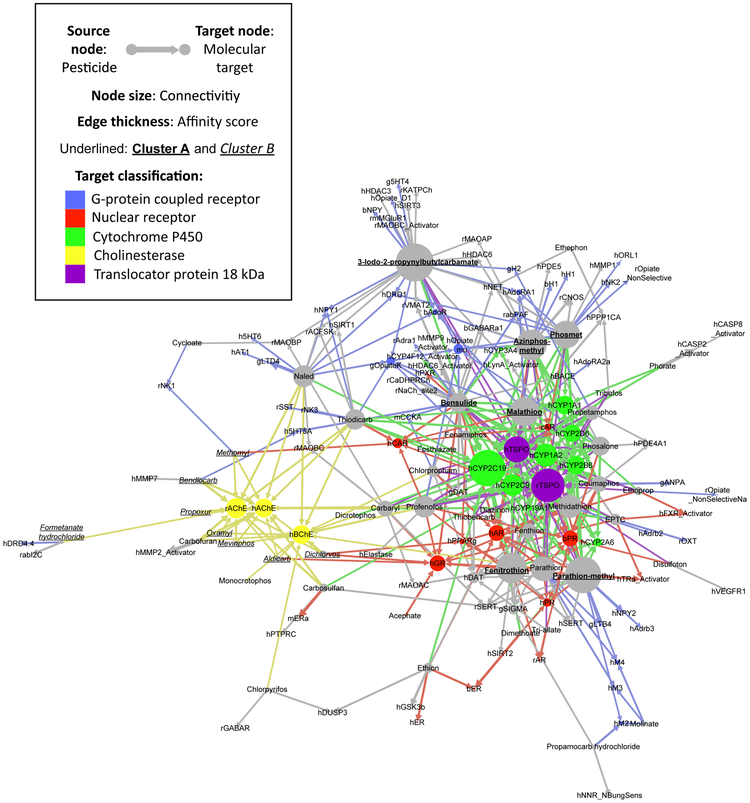
Network visualization is a powerful tool to complement biological intuition and reduce bias, by making visually explicit mathematical relationships present in raw data. This approach has been used to discover structural similarity of skin sensitizing chemicals and novel mechanisms of action of male reproductive toxicants [38, 58]. Figure 3 is a quantitative bipartite network that shows the 412 binding pairs of 48 pesticides and 110 molecular targets. Cluster A was connected to 10 CYPs, including human CYP2B6 and CYP3A4. Several nuclear receptors were also located in close proximity with this cluster, including human and chimpanzee androgen receptors (hAR and cAR) and bovine progesterone receptor (bPR). Cluster B was connected to hAChE, rAChE, and hBChE on the opposite side. The molecular weight and and log Pow of Cluster A (MW = 263.20 - 397.50; log Pow = 2.1 - 4.0) was larger than B (MW = 162.21 - 257.72; log Pow = −0.58 - 2.0). Fourteen OPs and 10 carbamates bound to 38 GPCRs, which can be seen at the periphery of Figure 3. Nine OPs and 2 carbamates bound to 5 opioid receptors from rats, humans, and guinea pig; and 4 OPs and a carbamate bound to 3 human adenosine receptors. Although we were able to generate novel, data-driven, and testable hypotheses of mechanisms of action based on these specific receptors, determining the toxicological significance of these in vitro interactions will require additional experimentation.
Figure 3. Quantitative bipartite network that shows in vitro binding affinity of 30 organophosphates and 18 carbamates to 110 molecular targets.
The network was constructed with ForceAtlas layout using Gephi version 0.8.2 beta, with 5 target classifications in different colors. The locality of the pesticides and their targets was determined by affinity score and connectivity, as visualized in edge thickness and node size. Cluster A (see Figure 2B) was connected to mitochondrial translocator proteins (TSPOs), nuclear receptors, and cytochrome P450 (CYP) enzymes. Cluster B was connected to acetylcholinesterases (AChEs) and butyrylcholinesterase (BChE) on the opposite side. Animal species of these targets were specified by a first-letter initial (b: bovines; g: guinea pigs; h: humans; m: mouse; r: rats; and rab: rabbits) preceding the gene symbol.
3.3. The hydrophobicity of organophosphate pesticides is correlated significantly to mitochondrial translocator protein binding affinity, mitochondrial membrane potential reduction, and developmental toxicity in C. elegans and D. rerio
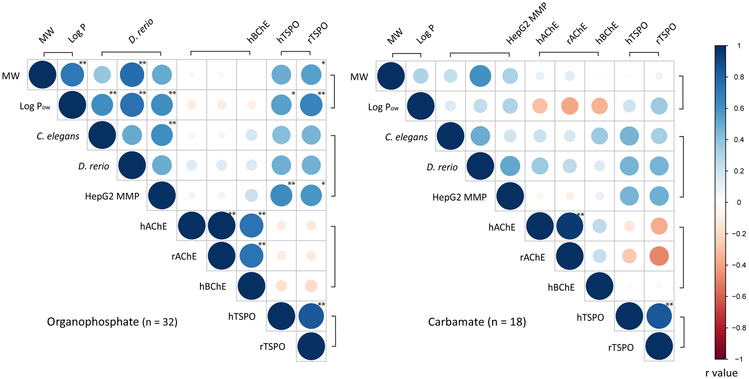
We next used the ToxCast and Tox21 datasets to test for concordance between mitochondrial, cholinergic, and developmental effects of OPs and carbamates across multiple experimental platforms. Figure 4A and 4B show the correlation matrices for (1) developmental toxicity in C. elegans and D. rerio, (2) reduction in mitochondrial membrane potential in HepG2 cells, (3) in vitro binding affinity to ChEs and mitochondrial translocator proteins, and (4) molecular weight and log Pow. Exposure to all but one of the 51 pesticides resulted in various degrees of developmental toxicity in either C. elegans or D. rerio (Supplemental Material S2). Exposure to 7 pesticides resulted in mitochondrial membrane potential reduction (AC50 value) at concentrations below the AC50 values for viability of HepG2 cells at the same time point (24 h of exposure; Supplemental Material S3). None resulted in mitochondrial membrane potential increase, although it should be noted that this assay was optimized for detection of mitochondrial membrane potential reduction, rather than increase, limiting the interpretability of the lack of detection of increased mitochondrial membrane potential. No significant correlation was found between the physicochemical properties and biological activities of carbamate pesticides (Figure 4B; p > 0.05). Yet, the hydrophobicity of OP pesticides was correlated significantly to mitochondrial translocator protein binding affinity, mitochondrial membrane potential reduction, and developmental toxicity (Figure 4A; p < 0.05). Specifically, mitochondrial membrane potential reduction was correlated significantly to developmental toxicity in C. elegans (p < 0.01), but not D. rerio (p > 0.05). This correlation is consistent with an earlier report of mitochondrial membrane potential reduction and C. elegans developmental toxicity with OP flame retardants [59]. The binding affinity of OP pesticides to rat and human mitochondrial translocator proteins was correlated significantly to mitochondrial membrane potential reduction in HepG2 cells (p > 0.05), but not to developmental toxicity in C. elgeans and D. rerio (p > 0.05). This is potentially due to the functional divergence of mitochondrial translocator proteins in nematode, fish, and mammalian species [60]. Taken together, the results suggest that mitochondria are involved in the mechanism of action for some (but not all) of the OP pesticides.
Figure 4A and 4B. Correlation matrices of molecular weight and octanol-water partition coefficients (MW and Log Pow); toxicity scores of developmental effects in Caenorhabditis elegans and Danio rerio and mitochondrial membrane potential (MMP) reduction in HepG2 cells; and affinity scores of acetylcholinesterase in humans and rats (hAChE and rAChE), butyrylcholinesterase in humans (hBChE), and mitochondrial translocator proteins in humans and rats (hTSPO and rTSPO) of 32 organophosphates and 18 carbamates.
The affinity scores, toxicity scores, molecular weight, and log Pow of OPs, carbamates were examined with Spearman's rank correlation [44], followed by multiple testing correction with Holm’s method in R version 3.5.1 [33, 45]. The hydrophobicity of organophosphate pesticides was correlated significantly to TSPO binding affinity, MMP reduction, and developmental toxicity in C. elegans and D. rerio. * p < 0.05; ** p < 0.01
3.4. Energy metabolism as a potential site of toxic action for some organophosphates and carbamates
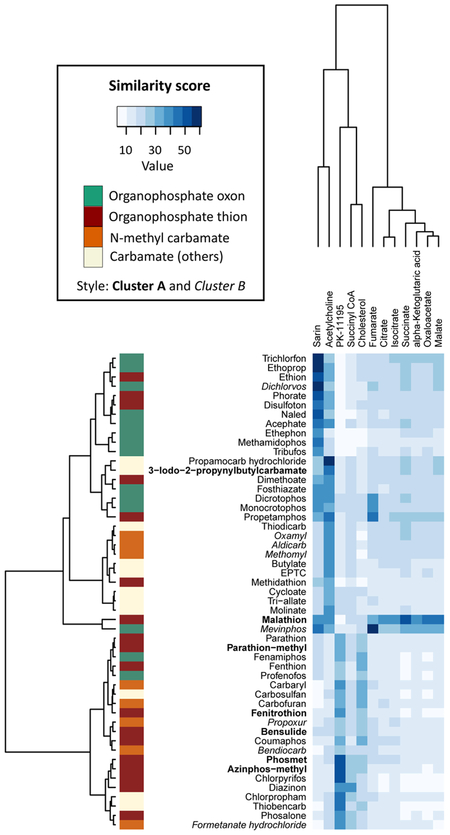
Loss of mitochondrial membrane potential is expected to result in alterations in energy metabolism or from alterations in upstream energetic biochemistry. For example, decreased provision of reducing equivalents from the Krebs cycle to the electron transport chain could reduce mitochondrial membrane potential, assuming constant use of the proton gradient. There is evidence for altered energy metabolism following co-exposure to dichlorvos, malathion, and pirimicarb in mice [61]. As a preliminary NAM-based test of this hypothesis, we compared the molecular structures of acetylcholine, cholesterol, sarin (a chemical warfare agent that targets AChE), PK-11195 (a pharmaceutical ligand of mitochondrial translocator proteins), and substrates of the Krebs cycle to the 51 OP and carbamate pesticides (Figure 5). Overall, the pesticides showed limited Tanimoto similarity to these chemicals with a mean score of 19.9 (out of 100; standard deviation = 9.6). Yet, the structural clustering sorted the pesticides into three groups: (1) 28 pesticides with acetylcholine and sarin; (2) 21 pesticides with cholesterol, PK-11195, and succinyl coenzyme A; and (3) malathion and mevinphos with fumarate and other Krebs cycle intermediates. None of these groups was enriched in Cluster A and B pesticides, suggesting that a molecular initiating event is not essentially triggered by structurally similar chemicals on an exposome scale, at least at the structural resolution scale permitted by our analysis. Nonetheless, the results support the notion of considering energy metabolism as a site of toxic action for some OP and carbamate pesticides at early life stages, in particular for those chemicals showing similarity with Krebs cycle intermediates (Figure 5). Previous studies have shown that exposure to chlorpyrifos, triphenyl phosphate, and tributyl phosphate results in metabolic disruption of the Krebs cycle in D. rerio and rats [62-64]. Further metabolomic studies should examine the tissue-specific effects of OP and carbamate metabolites on a developmental scale.
Figure 5. Unsupervised two-directional heat map from hierarchical clustering of 32 organophosphates and 19 carbamates with acetylcholine, cholesterol, sarin, PK-11195, and Krebs cycle intermediates by structural similarity.
The heat map was generated with the “gplot” package in R version 3.5.1, using Euclidean distance for measure and Ward’s method for linkage analysis. The chemicals were clustered into three groups based on two-dimensional structural similarity: (1) 28 pesticides with acetylcholine and sarin; (2) 21 pesticides with cholesterol, PK-11195, and succinyl coenzyme A; and (3) malathion and mevinphos with fumarate and other Krebs cycle intermediates. Sarin is a chemical warfare agent that targets acetylcholinesterase. PK-11195 is a pharmaceutical ligand of mitochondrial translocator proteins The intensity of blue color indicates the value of similarity score, as computed with the PubChem score matrix (https://pubchem.ncbi.nlm.nih.gov/score_matrix).
3.5. Exposure to chlorpyrifos produces mitochondrial DNA damage at concentrations that result in acetylcholine inhibition in C. elegans
Early-life exposure to mitochondrial genotoxicants has been associated to adverse health outcomes in a later stage of life [65]. We conducted a pilot study to characterize mtDNA damage after exposure to chlorpyrifos in C. elegans (Supplemental Material S4). Exposure to 100 μM chlorpyrifos resulted in a mtDNA lesion level of 0.5 / 10 kb. The same concentration also produced paralysis in glp-1 adults (data not shown) and was 10-fold higher than the concentrations inhibiting L1 larval growth (5 μM for both chlorpyrifos and chlorpyrifos-oxon; [39]). In addition, the mtDNA lesion levels were close to the detection limit (0.1 / 10 kb) for both aflatoxin B1 and chlorpyrifos at 3 and 30 μM. These results demonstrate that exposure to chlorpyrifos can result in mtDNA damage, but it is unclear whether this takes place at concentrations below AChE inhibition. Noticeably, exposures to chlorpyrifos and diazinon have been shown to inhibit mitochondrial bioenergetics in D. rerio embryos only at high levels of exposure (i.e. > 25 μM) [66].
4. Discussion
Mitochondrial toxicants are prevalent across the chemical landscape of environmental exposure [21]. Mitochondrial dysfunctions and oxidative stress are disruptive to cell differentiation at the early stages of development [67]. Here, we take advantage of the diverse chemical structures captured in the U.S. EPA and Tox21 HTS datasets to evaluate the potential for mitochondrial toxicity in the entire class of cholinergic pesticides. We show that (1) the binding of AChE and BChE only accounts for a fraction of the in vitro binding portfolio of the 32 OPs and 19 carbamates (parent compounds); (2) the mitochondrial effects of OPs are in concordance with developmental toxicity in C. elegans; and (3) exposure to chlorpyrifos produces mtDNA damage at concentrations that result in AChE inhibition. Taken together, this study results in the generation of three hypotheses, that: (1) mitochondria are a common biological target of developmental exposure to some OP pesticides; (2) the developmental effects of OP pesticide exposure are caused by multi-target interactions with mitochondria; and (3) energy metabolism is a potential site of toxic action for OP and carbamate exposure at early life stages.
The non-cholinergic toxicity of OPs and carbamates has attracted significant research interest in recent years, both in developmental, neurotoxicological, and other contexts [68, 69]. This is motivated by the observations that AChE inhibition alone cannot account for the wide variety of adverse outcomes of repeated exposures, many of which occur without acute signs of poisoning (i.e. salivation, lacrimation, urination, and defecation; or SLUD), even at levels below those that detectably inhibit AChE [69]. Chemoproteomic studies have shown that exposure to OPs and carbamates can result in off-target inhibition of serine hydrolases, which are pivotal to lipid metabolism [70, 71]. Mitochondrial lipid metabolism is regulated by membrane transporters, and mitochondrial translocator proteins have been hypothesized to play a role in lipid homeostasis and mitochondrial function [72, 73]. In this study, OPs and carbamates were found to bind not only to mitochondrial translocator proteins but also to androgen, estrogen, and glucocorticoid receptors and cholesterol-metabolizing CYP2s, many of which co-localize in mitochondria [74, 75]. In addition, some of the pesticides (and, potentially, their metabolites) are structurally similar to Krebs cycle intermediates (including succinyl coenzyme A, a key substrate in beta-oxidation of fatty acids in mitochondria [76]). Taken together, these findings support the hypothesis that developmental OP and carbamate exposure can disrupt mitochondrial metabolism through targeting multiple receptors and transporters at low specificity (i.e. promiscuously), thereby resulting in disruption of energetics and redox signaling in developing cells.
Exposure to OP and N-methyl carbamate insecticides disrupts acetylcholine signaling in both insects and humans. Since these pesticides also share the same toxic effects in human infants and children, the cumulative exposure of these pesticides is often evaluated using a dose-additive approach based on a common mechanism [77]. In this study, we identify mitochondria effects with more than half of the OP pesticides tested in vitro. In a single-chemical risk assessment, mitochondrial toxicity may be not a valid point of departure since each OP pesticide typically only exists at a low concentration in the environment. However, it remains unclear whether the cumulative effects of these pesticides in mitochondria can augment the disruption of acetylcholine signaling in brain development (as suggested by other studies of non-cholinergic OP toxicity [9, 66, 78]). This warrants further mechanistic examination to refine the current common mechanism groups of OP pesticides [24]. An important caveat is that since many of these in vitro effects were mediated by OP thions, it is unclear whether the thion would persist long enough in vivo (i.e., avoid conversion to the oxon) to cause these effects. Mechanistic follow-up of the predictions made in this study will be important, with particular attention to situations (e.g., developmental stages) in which parent compound effects may be more important due, for example, to reduced roles for cholinesterases, or reduced enzymatic conversion either to active or inactive metabolites. Further studies should examine energetic and redox effects as well as broader mitochondrial phenotypes such as biogenesis and morphology.
Previous QSAR studies have investigated how structural features are associated with AChE inhibition [79, 80], developmental toxicity [81], and mitochondrial toxicity [22]. Common mechanisms of action of mitochondrial toxicants include oxidative stress and mtDNA damage, interaction with mitochondrial proteins, alteration in mitochondrial lipid composition, inhibition of the electron transport chain, and induction of apoptosis [22, 65]. In this study, the phosphorothioate group was identified as a novel toxicophore in mitochondria (i.e. a structural feature associated with mitochondrial toxicity; [82]). As the hydrophobicity of an OP compound increases with the size of the rest of its structural moieties, the phosphorothioate group becomes more bioavailable and toxic to the double-membraned mitochondria [65], cuticle-covered C. elegans [83, 84], and chorion-protected D. rerio [85]. It is possible that the phosphorothioate group acts similarly to malonic acid, which reduces mitochondrial membrane potential through inhibiting succinate dehydrogenase [86, 87]; however, this hypothesis has yet to be proven experimentally. Given the structural similarity of benzophenone and mitochondrial translocator protein ligands [88], the concordance of TPSO binding and mitochondrial membrane potential reduction with OPs is consistent with the earlier finding of the benzophenone core as a mitochondrial toxicophore [22].
Conclusion
The present study is the first to apply NAMs to evaluate two common mechanism groups of OP and carbamate pesticides. While previous assessments have evaluated these pesticides based on commonality of toxic effects and mechanisms (i.e. cholinergic toxicity and AChE inhibition), this study provides a proof of concept for applying QSAR-, cell-, and small organism-based NAMs to (1) refine the existing groups based on dissimilar mechanisms of action and (2) define new common mechanism groups based on structural features. Future mechanistic studies should examine the tissue-specific effects of OP and carbamate metabolites in mitochondria at different developmental stages and identify the molecular initiating events and AOPs of developmental toxicity caused by metabolic disruption in mitochondria.
Supplementary Material
Supplemental Material S1. Thirty-two organophosphates and nineteen carbamates in the U.S. EPA ToxCast Phase I and II libraries. Thirty eight of these 51 pesticides were evaluated by U.S. EPA in the OP and carbamate cumulative risk assessments as common mechanism groups (CMG) in 2006—2007 [24, 26]. Chemical identifiers and structural information were obtained in the supplemental materials of Richard et al (2016; [27]). The logarithm of Poctanol-water (log Pow) values were predicted with OPEn structure—activity/property Relationship App (OPERA) models. Molecular weight and predicted log Pow values were acquired at the U.S. EPA Chemistry Dashboard V3.0 website (https://comptox.epa.gov/dashboard/; accessed on October 17, 2018).
Supplemental Material S2. Half-maximal activity concentrations (AC50) and calculated scores for the in vitro binding affinity assays of cholinesterases and mitochondrial translocator proteins, mitochondrial membrane potential assay in HepG2 cells, and developmental toxicity assays in Caenorhabditis elegans and Danio rerio. The raw AC50 data of the in vitro binding affinity, HepG2 cell, and D. rerio assays were obtained at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). The raw data of the C. elegans assays were obtained in the supplemental materials of Boyd et al (2016) [39], Calculation of AC50S was described in details by Judson et al and Boyd et al [36, 39].The AC50 values were transformed to an affinity (A) or toxicity score (T), where A (or T)= − log10 (AC50 (in μM) ÷1,000,000). The score for an AC50 value of 1,000 μM was defined as zero. The technical and biological details of each assay are described at the U.S. EPA ToxCast Data Release website and Boyd et al (2016) [39]. Carbofuran was not available (NA) in the D. rerio data set and therefore excluded in the correlation analysis for carbamates (Figure 4B). hAChE and rAChE, human and rat acetylcholinesterase; hBChE, human butyrylcholinesterase; hTSPO and rTSPO, human and rat mitochondrial translocator proteins; MMP, mitochondrial membrane potential.
Supplemental Material S3. Half-maximal activity concentrations (AC50) for the in vitro binding affinity assays of cholinesterases and mitochondrial translocator proteins, mitochondrial membrane potential and cell viability assays in HepG2 cells, and cytotoxicity limits for thirty-two organophosphates and nineteen carbamates. The raw AC50 data of the in vitro binding affinity, HepG2 cell, and D. rerio assays were obtained at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). The cytotoxicity limit of each pesticide was acquired at the U.S. EPA Chemistry Dashboard V3.0.8 website (https://comptox.epa.gov/dashboard/; accessed on May 29, 2019) [29]. The AC50 values below viability AC50s (for HepG2 cells) or cytotoxicity limits (for cell-free binding assays) were highlighted in colors. CMG, common mechanism group; hAChE and rAChE, human and rat acetylcholinesterase; hBChE, human butyrylcholinesterase; hTSPO and rTSPO, human and rat mitochondrial translocator proteins; MMP, mitochondrial membrane potential.
Supplemental Material S4. Mitochondrial DNA damage produced by exposures to aflatoxin B1 and chlorpyrifos in C. elegans. Young adult glp-1 nematodes were exposed in liquid medium for 24 h (n = 4). Aflatoxin B1 was tested as a positive control. Two-way analysis of variance (ANOVA) was used to assess the dose and chemical effects. The standard error of the mean (SEM) was shown in the error bars.
Highlights.
We evaluated two common mechanism groups of pesticides for in vitro binding affinity to multiple receptors and proteins, mitochondrial membrane potential changes in HepG2 cells, and developmental toxicity in Caenorhabditis elegans and Danio rerio.
Our results support the hypothesis that in some cases mitochondrial toxicity of organophosphate pesticides may augment the disruption of acetylcholine signaling in animal developmental toxicity.
This study provides a proof of concept for applying new approach methodologies to refine the existing groups based on dissimilar mechanisms of action and define new common mechanism groups based on structural features.
Acknowledgements
We would like to thank Windy A. Boyd and Nisha S. Sipes for technical consultation and P42ES010356
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe C, et al. , Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ Res, 2016. 150: p. 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shelton JF, et al. , Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect, 2014. 122(10): p. 1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertz-Picciotto I, et al. , Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms. PLoS medicine, 2018. 15(10): p. e1002671–e1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr RL, et al. , Decreased anxiety in juvenile rats following exposure to low levels of chlorpyrifos during development. Neurotoxicology, 2017. 59: p. 183–190. (JNM) for funding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Gimenez B, et al. , Developmental Exposure to Pesticides Alters Motor Activity and Coordination in Rats: Sex Differences and Underlying Mechanisms. Neurotox Res, 2018. 33(2): p. 247–258. [DOI] [PubMed] [Google Scholar]
- 6.Slotkin TA, et al. , Nonenzymatic role of acetylcholinesterase in neuritic sprouting: regional changes in acetylcholinesterase and choline acetyltransferase after neonatal 6-hydroxydopamine lesions. Neurotoxicol Teratol, 2009. 31(3): p. 183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopjar N, et al. , Evaluation of chlorpyrifos toxicity through a 28-day study: Cholinesterase activity, oxidative stress responses, parent compound/metabolite levels, and primary DNA damage in blood and brain tissue of adult male Wistar rats. Chem Biol Interact, 2018. 279: p. 51–63. [DOI] [PubMed] [Google Scholar]
- 8.Cole TB, et al. , Repeated developmental exposure of mice to chlorpyrifos oxon is associated with paraoxonase 1 (PON1)-modulated effects on cerebellar gene expression. Toxicol Sci, 2011. 123(1): p. 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slotkin TA, et al. , Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect, 2007. 115(9): p. 1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffman JA, et al. , Oral-aboral axis specification in the sea urchin embryo III. Role of mitochondrial redox signaling via H(2)O(2). Developmental biology, 2009. 330(1): p. 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert-Chatelain E, et al. , A cannabinoid link between mitochondria and memory. Nature, 2016. 539(7630): p. 555–559. [DOI] [PubMed] [Google Scholar]
- 12.Le Belle JE, et al. , Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell, 2011. 8(1): p. 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton PA, et al. , Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology, 2015. 9(8): p. 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma P, et al. , Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem Toxicol, 2013. 58: p. 177–83. [DOI] [PubMed] [Google Scholar]
- 15.Hassani S, et al. , Protective effects of curcumin and vitamin E against chlorpyrifos-inducedlung oxidative damage. Hum Exp Toxicol, 2015. 34(6): p. 668–76. [DOI] [PubMed] [Google Scholar]
- 16.Ambali SF, et al. , Vitamin E protects Wistar rats from chlorpyrifos-induced increase in erythrocyte osmotic fragility. Food Chem Toxicol, 2010. 48(12): p. 3477–80. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Fuentes G, et al. , Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp Biochem Physiol C Toxicol Pharmacol, 2015. 172–173: p. 19-25. [DOI] [PubMed] [Google Scholar]
- 18.Samir AMZ, et al. , Carbamate Toxicity and Protective effect of vit. A and vit. E on some biochemical aspects of male albino rats. Egyptian J Hosp Med, 2000. 1: p. 60–77. [Google Scholar]
- 19.European Chemicals Agency (ECHA), New Approach Methodologies in Regulatory Science. 2016, ECHA: Helsinki, Finland: p. 1–65. [Google Scholar]
- 20.Dix DJ, et al. , The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci, 2007. 95(1): p. 5–12. [DOI] [PubMed] [Google Scholar]
- 21.Wills LP, et al. , Assessment of ToxCast Phase II for Mitochondrial Liabilities Using a High-Throughput Respirometric Assay. Toxicological Sciences, 2015. 146(2): p. 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attene-Ramos MS, et al. , Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ Health Perspect, 2015. 123(1): p. 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia M, et al. , Comprehensive Analyses and Prioritization of Tox21 10K Chemicals Affecting Mitochondrial Function by in-Depth Mechanistic Studies. Environ Health Perspect, 2018. 126(7): p. 077010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Environmental Protection Agency (US EPA), Organophosphosphorus Cumulative Risk Assessment - Update. 2006, U.S. EPA Office of Pesticide Programs: Washington, DC: p. 1–522. [Google Scholar]
- 25.U.S. Environmental Protection Agency (US EPA), Chlorpyrifos: Revised Human Health Risk Assessment for Registration Review. 2014, U.S. EPA Office of Chemical Safety and Pollution Prevention: Washington, DC: p. 1–531. [Google Scholar]
- 26.U.S. Environmental Protection Agency (US EPA), Revised N-Methyl Carbamate Cumulative Risk Asssessment. 2007, U.S. EPA Office of Pesticide Programs: Washington, DC: p. 1–277. [Google Scholar]
- 27.Richard AM, et al. , ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem Res Toxicol, 2016. 29(8): p. 1225–51. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri K, et al. , OPERA models for predicting physicochemical properties and environmental fate endpoints. Journal of Cheminformatics, 2018. 10: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams AJ, et al. , The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminform, 2017. 9(1): p. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X and Reynolds CH, Performance of similarity measures in 2D fragment-based similarity searching: comparison of structural descriptors and similarity coefficients. J Chem Inf Comput Sci, 2002. 42(6): p. 1407–14. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, et al. , PubChem Substance and Compound databases. Nucleic Acids Res, 2016. 44(D1): p. D1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward JH, Hierarchical Grouping to Optimize an Objective Function. Journal of the American Statistical Association, 1963. 58(301): p. 236–244. [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing. 2018. [cited 2018 July 9]; Available from: http://www.R-project.org/.
- 34.Knudsen TB, et al. , Activity profiles of 309 ToxCast chemicals evaluated across 292 biochemical targets. Toxicology, 2011. 282(1-2): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 35.Sipes NS, et al. , Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem Res Toxicol, 2013. 26(6): p. 878–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Judson R, et al. , Editor’s Highlight: Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space. Toxicol Sci, 2016. 152(2): p. 323–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastian M, Heymann S, and Jacomy M, Gephi: an open source software for exploring and manipulating networks, in Proceedings of the Third International Conference on Weblogs and Social Media, ICWSM 2009: San Jose, California. [Google Scholar]
- 38.Leung MC, et al. , Systems Toxicology of Male Reproductive Development: Profiling 774 Chemicals for Molecular Targets and Adverse Outcomes. Environ Health Perspect, 2016. 124(7): p. 1050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd WA, et al. , Developmental Effects of the ToxCast Phase I and Phase II Chemicals in Caenorhabditis elegans and Corresponding Responses in Zebrafish, Rats, and Rabbits. Environ Health Perspect, 2016. 124(5): p. 586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padilla S, et al. , Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod Toxicol, 2012. 33(2): p. 174–87. [DOI] [PubMed] [Google Scholar]
- 41.Sakamuru S, et al. , Application of a homogenous membrane potential assay to assess mitochondrial function. Physiological genomics, 2012. 44(9): p. 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connolly NMC, et al. , Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ, 2018. 25(3): p. 542–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholls DG, Fluorescence Measurement of Mitochondrial Membrane Potential Changes in Cultured Cells. Methods Mol Biol, 2018. 1782: p. 121–135. [DOI] [PubMed] [Google Scholar]
- 44.Spearman C, The Proof and Measurement of Association between Two Things. The American Journal of Psychology, 1904. 15(1): p. 72–101. [PubMed] [Google Scholar]
- 45.Holm S, A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics, 1979. 6(2): p. 65–70. [Google Scholar]
- 46.Leung MC, et al. , Caenorhabditis elegans generates biologically relevant levels of genotoxic metabolites from aflatoxin B1 but not benzo[a]pyrene in vivo. Toxicol Sci, 2010. 118(2): p. 444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams PL and Dusenbery DB, Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health, 1988. 4(4): p. 469–78. [DOI] [PubMed] [Google Scholar]
- 48.Boyd WA, et al. , Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol Teratol, 2010. 32(1): p. 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Hunt CP, et al. , Exposure to mitochondrial genotoxins and dopaminergic neurodegeneration in Caenorhabditis elegans. PLoS One, 2014. 9(12): p. e114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunter SE, et al. , The QPCR assay for analysis of mitochondrial DNA damage, repair, and relative copy number. Methods, 2010. 51(4): p. 444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furda AM, et al. , Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. Methods Mol Biol, 2012. 920: p. 111–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer JN, QPCR: a tool for analysis of mitochondrial and nuclear DNA damage in ecotoxicology. Ecotoxicology, 2010. 19(4): p. 804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson MA and Maggiora GM, Concepts and Applications of Molecular Similarity. 1990, New York: John Wiley & Sons; pp. 1–393. [Google Scholar]
- 54.McKinney JD, et al. , The practice of structure activity relationships (SAR) in toxicology. Toxicol Sci, 2000. 56(1): p. 8–17. [DOI] [PubMed] [Google Scholar]
- 55.Casida JE and Durkin KA, ANTICHOLINESTERASE INSECTICIDE RETROSPECTIVE. Chemico-biological interactions, 2013. 203(1): p. 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chambers JE, Meek EC, and Chambers HW, Chapter 65 - The Metabolism of Organophosphorus Insecticides, in Hayes’ Handbook of Pesticide Toxicology (Third Edition), Krieger R, Editor. 2010, Academic Press: New York: p. 1399–1407. [Google Scholar]
- 57.Colovic MB, et al. , Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol, 2013. 11(3): p. 315–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luechtefeld T, et al. , Analysis of publically available skin sensitization data from REACH registrations 2008-2014. Altex, 2016. 33(2): p. 135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behl M, et al. , Editor’s Highlight: Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphenyl Ethers to Caenorhabditis elegans. Toxicol Sci, 2016. 154(2): p. 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan J, et al. , Structural and functional evolution of the translocator protein (18 kDa). Curr Mol Med, 2012. 12(4): p. 369–86. [DOI] [PubMed] [Google Scholar]
- 61.Wang P, et al. , Combined subchronic toxicity of dichlorvos with malathion or pirimicarb in mice liver and serum: a metabonomic study. Food Chem Toxicol, 2014. 70: p. 222–30. [DOI] [PubMed] [Google Scholar]
- 62.Wang D, et al. , Neonatal triphenyl phosphate and its metabolite diphenyl phosphate exposure induce sex- and dose-dependent metabolic disruptions in adult mice. Environ Pollut, 2018. 237: p. 10–17. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, et al. , Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol, 2019. 216: p. 19–28. [DOI] [PubMed] [Google Scholar]
- 64.Neerathilingam M, et al. , 1H NMR-based metabonomic investigation of tributyl phosphate exposure in rats. Toxicol Lett, 2010. 199(1): p. 10–6. [DOI] [PubMed] [Google Scholar]
- 65.Meyer JN, et al. , Mitochondria as a target of environmental toxicants. Toxicol Sci, 2013. 134(1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao F, et al. , Biological impacts of organophosphates chlorpyrifos and diazinon on development, mitochondrial bioenergetics, and locomotor activity in zebrafish (Danio rerio). Neurotoxicol Teratol, 2018. 70: p. 18–27. [DOI] [PubMed] [Google Scholar]
- 67.Chandler KJ, et al. , Evaluation of 309 environmental chemicals using a mouse embryonic stem cell adherent cell differentiation and cytotoxicity assay. PLoS One, 2011. 6(6): p. e18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terry AV, Functional Consequences of Repeated Organophosphate Exposure: Potential Non-Cholinergic Mechanisms. Pharmacology & therapeutics, 2012. 134(3): p. 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slotkin TA, Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol, 2004. 198(2): p. 132–51. [DOI] [PubMed] [Google Scholar]
- 70.Nomura DK and Casida JE, Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. J Agric Food Chem, 2011. 59(7): p. 2808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long JZ and Cravatt BF, The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease. Chemical reviews, 2011. 111(10): p. 6022–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li F, et al. , Translocator Protein 18 kDa (TSPO): An Old Protein with New Functions? Biochemistry, 2016. 55(20): p. 2821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glatz JF, Luiken JJ, and Bonen A, Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev, 2010. 90(1): p. 367–417. [DOI] [PubMed] [Google Scholar]
- 74.Levin ER and Hammes SR, Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol, 2016. 17(12): p. 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Avadhani NG, et al. , Bimodal Targeting of Cytochrome P450s to Endoplasmic Reticulum and Mitochondria: The Concept of Chimeric Signals. The FEBS journal, 2011. 278(22): p. 4218–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansford RG and Johnson RN, The steady state concentrations of coenzyme A-SH and coenzyme A thioester, citrate, and isocitrate during tricarboxylate cycle oxidations in rabbit heart mitochondria. J Biol Chem, 1975. 250(21): p. 8361–75. [PubMed] [Google Scholar]
- 77.U.S. Environmental Protection Agency (US EPA), Guidance on Cumulative Risk Assessment of Pesticide Chemicals That Have a Common Mechanism of Toxicity 2002, U.S. EPA Office of Pesticide Programs: Washington, DC: p. 1–81. [Google Scholar]
- 78.Colovic MB, et al. , In vitro evaluation of neurotoxicity potential and oxidative stress responses of diazinon and its degradation products in rat brain synaptosomes. Toxicol Lett, 2015. 233(1): p. 29–37. [DOI] [PubMed] [Google Scholar]
- 79.Lee S and Barron MG, A mechanism-based 3D-QSAR approach for classification and prediction of acetylcholinesterase inhibitory potency of organophosphate and carbamate analogs. J Comput Aided Mol Des, 2016. 30(4): p. 347–63. [DOI] [PubMed] [Google Scholar]
- 80.Mladenovic M, et al. , The Targeted Pesticides as Acetylcholinesterase Inhibitors: Comprehensive Cross-Organism Molecular Modelling Studies Performed to Anticipate the Pharmacology of Harmfulness to Humans In Vitro. Molecules, 2018. 23(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu S, et al. , Framework for identifying chemicals with structural features associated with the potential to act as developmental or reproductive toxicants. Chem Res Toxicol, 2013. 26(12): p. 1840–61. [DOI] [PubMed] [Google Scholar]
- 82.U.S. Environmental Protection Agency (US EPA), Guidance for Identifying Pesticide Chemicals and Other Substances That Have a Common Mechanism of Toxicity 1999, U.S. EPA Office of Pesticide Programs: Washington, DC: p. 1–14. [Google Scholar]
- 83.Watanabe M, et al. , A mutation in a cuticle collagen causes hypersensitivity to the endocrine disrupting chemical, bisphenol A, in Caenorhabditis elegans. Mutat Res, 2005. 570(1): p. 71–80. [DOI] [PubMed] [Google Scholar]
- 84.Partridge FA, et al. , The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev Biol, 2008. 317(2): p. 549–59. [DOI] [PubMed] [Google Scholar]
- 85.Henn K and Braunbeck T, Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol, 2011. 153(1): p. 91–8. [DOI] [PubMed] [Google Scholar]
- 86.Thorn MB, Proceedings of the Biochemical Society. Biochemical Journal, 1953. 53(1): p. i.2–ix.l. [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez-Gomez FJ, et al. , Malonate induces cell death via mitochondrial potential collapse and delayed swelling through an ROS-dependent pathway. Br J Pharmacol, 2005. 144(4): p. 528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tuccinardi T, et al. , A virtual screening study of the 18 kDa translocator protein using pharmacophore models combined with 3D-QSAR studies. ChemMedChem, 2009. 4(10): p. 1686–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material S1. Thirty-two organophosphates and nineteen carbamates in the U.S. EPA ToxCast Phase I and II libraries. Thirty eight of these 51 pesticides were evaluated by U.S. EPA in the OP and carbamate cumulative risk assessments as common mechanism groups (CMG) in 2006—2007 [24, 26]. Chemical identifiers and structural information were obtained in the supplemental materials of Richard et al (2016; [27]). The logarithm of Poctanol-water (log Pow) values were predicted with OPEn structure—activity/property Relationship App (OPERA) models. Molecular weight and predicted log Pow values were acquired at the U.S. EPA Chemistry Dashboard V3.0 website (https://comptox.epa.gov/dashboard/; accessed on October 17, 2018).
Supplemental Material S2. Half-maximal activity concentrations (AC50) and calculated scores for the in vitro binding affinity assays of cholinesterases and mitochondrial translocator proteins, mitochondrial membrane potential assay in HepG2 cells, and developmental toxicity assays in Caenorhabditis elegans and Danio rerio. The raw AC50 data of the in vitro binding affinity, HepG2 cell, and D. rerio assays were obtained at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). The raw data of the C. elegans assays were obtained in the supplemental materials of Boyd et al (2016) [39], Calculation of AC50S was described in details by Judson et al and Boyd et al [36, 39].The AC50 values were transformed to an affinity (A) or toxicity score (T), where A (or T)= − log10 (AC50 (in μM) ÷1,000,000). The score for an AC50 value of 1,000 μM was defined as zero. The technical and biological details of each assay are described at the U.S. EPA ToxCast Data Release website and Boyd et al (2016) [39]. Carbofuran was not available (NA) in the D. rerio data set and therefore excluded in the correlation analysis for carbamates (Figure 4B). hAChE and rAChE, human and rat acetylcholinesterase; hBChE, human butyrylcholinesterase; hTSPO and rTSPO, human and rat mitochondrial translocator proteins; MMP, mitochondrial membrane potential.
Supplemental Material S3. Half-maximal activity concentrations (AC50) for the in vitro binding affinity assays of cholinesterases and mitochondrial translocator proteins, mitochondrial membrane potential and cell viability assays in HepG2 cells, and cytotoxicity limits for thirty-two organophosphates and nineteen carbamates. The raw AC50 data of the in vitro binding affinity, HepG2 cell, and D. rerio assays were obtained at the U.S. EPA ToxCast Data Release website (https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data; invitrodb_v2). The cytotoxicity limit of each pesticide was acquired at the U.S. EPA Chemistry Dashboard V3.0.8 website (https://comptox.epa.gov/dashboard/; accessed on May 29, 2019) [29]. The AC50 values below viability AC50s (for HepG2 cells) or cytotoxicity limits (for cell-free binding assays) were highlighted in colors. CMG, common mechanism group; hAChE and rAChE, human and rat acetylcholinesterase; hBChE, human butyrylcholinesterase; hTSPO and rTSPO, human and rat mitochondrial translocator proteins; MMP, mitochondrial membrane potential.
Supplemental Material S4. Mitochondrial DNA damage produced by exposures to aflatoxin B1 and chlorpyrifos in C. elegans. Young adult glp-1 nematodes were exposed in liquid medium for 24 h (n = 4). Aflatoxin B1 was tested as a positive control. Two-way analysis of variance (ANOVA) was used to assess the dose and chemical effects. The standard error of the mean (SEM) was shown in the error bars.