Abstract
Background:
Caffeine consumption by children and adolescents has risen dramatically in recent years, yet the lasting effects of caffeine consumption during adolescence remain poorly understood.
Aim:
These experiments explore the effects of adolescent caffeine consumption on cocaine self-administration and seeking using a rodent model.
Methods:
Sprague-Dawley rats consumed caffeine for 28 days during the adolescent period. Following the caffeine consumption period, the caffeine solution was replaced with water for the remainder of the experiment. Age-matched control rats received water for the duration of the study. Behavioral testing in a cocaine self-administration procedure occurred during adulthood (postnatal days 62–82) to evaluate how adolescent caffeine exposure influenced the reinforcing properties of cocaine. Cocaine seeking was also tested during extinction training and reinstatement tests following cocaine self-administration.
Results:
Adolescent caffeine consumption increased the acquisition of cocaine self-administration and increased performance on different schedules of reinforcement. Consumption of caffeine in adult rats did not produce similar enhancements in cocaine self-administration. Adolescent caffeine consumption also produced an upward shift in the U-shaped dose response curve on cocaine self-administration maintained on a within-session dose-response procedure. Adolescent caffeine consumption had no effect on cocaine seeking during extinction training or reinstatement of cocaine seeking by cues or cocaine.
Conclusions:
These findings suggest that caffeine consumption during adolescence may enhance the reinforcing properties of cocaine, leading to enhanced acquisition that may contribute to increased addiction vulnerability.
Keywords: Development, psychostimulant, reinforcement, adenosine, vulnerability, substance use
Introduction
Caffeine is found naturally in coffee, tea and chocolate and is increasingly added as a supplement to other products including soda and energy drinks (Heckman et al., 2010; Higgins et al., 2010). A majority of adults consume caffeinated products daily, making caffeine the most commonly used psychostimulant in the world (Frary et al., 2005; Rath, 2012). Consumption of caffeine among adolescents has risen in recent years and daily caffeine consumption in 9–17 year-olds has more than doubled since 1980 (Frary et al., 2005; Temple, 2009; Warzak et al., 2011). Recent estimates demonstrate that 75% of children aged five years or older in the USA consume caffeine on a daily basis (Ahluwalia and Herrick, 2015). While moderate caffeine consumption in adults is considered relatively safe, there has been little examination of the long-term consequences of adolescent caffeine consumption (Temple, 2009).
Caffeine is a psychoactive drug that increases alertness, arousal, and psychomotor activation. The effects of caffeine result from nonselective blockade of both adenosine A1 and A2A receptors throughout the brain (Fredholm et al., 1999). Although there is some discrepancy in the neuroanatomical specificity of dopamine release resulting from adenosine receptor antagonism, it appears that caffeine and selective adenosine A1 receptor antagonism leads to an increase in dopamine release in the dorsal portion of the nucleus accumbens shell and prefrontal cortex (Acquas et al., 2002; Borycz et al., 2007; De Luca et al., 2007; Solinas et al., 2002). These effects are generally milder than many other psychostimulant drugs such as amphetamines and cocaine, but these similarities raise questions about the development of cross-sensitization. This may be especially important for adolescent consumption of caffeine since adolescent exposure to drugs of abuse is associated with an increased risk for developing a substance-use disorder (Andersen, 2003; Carlezon and Konradi, 2004). In fact, caffeine intake is associated with illicit drug use and the development of substance dependence, especially in young adults (Arria et al., 2017; Kendler et al., 2006; Miller, 2008; Peacock et al., 2017). A primary goal of these studies is to evaluate the effects of adolescent caffeine consumption on subsequent cocaine self-administration and seeking during withdrawal.
There is some debate about whether children and adolescents are more sensitive to the effects of caffeine compared with adults, and a number of factors including body weight, amount consumed, and source of caffeine appear to be important (Leviton, 1992; Nehlig et al., 1992; Temple, 2009). The use of animal models allows for controlled experimental designs to test distinct aspects of the effects of caffeine. For example, compared with adult rats, adolescent rats are more sensitive to the acute psychomotor-activating effects of low doses of caffeine and less sensitive to caffeine’s motor-depressant effects that are observed following higher caffeine doses (Marin et al., 2011). Adolescent rats also display exaggerated tolerance and withdrawal following chronic caffeine consumption compared with adult rats (Rhoads et al., 2011). Together, these findings suggest that adolescents can consume larger amounts of caffeine that may support greater dependence on caffeine. More directly related to the current study, there is also evidence that chronic caffeine exposure during adolescence produces behavioral cross-sensitization with the psychostimulant drugs, methylphenidate and cocaine (Boeck et al., 2009; O’Neill et al., 2015). Interestingly, in both studies, testing for cross-sensitization occurred in adulthood and in the absence of caffeine, suggesting that adolescent caffeine exposure may produce lasting changes in the brain likely to increase vulnerability to substance use. Based on these findings, we hypothesize that caffeine consumption during adolescence will enhance cocaine self-administration as measured by acquisition and performance on various schedules of reinforcement. Likewise, we predict that prior adolescent caffeine consumption will enhance cocaine seeking during withdrawal from cocaine self-administration.
Materials and methods
Animals
Male Sprague-Dawley rats (Envigo, Indianapolis, Indiana, USA) were received on either postnatal day (P) 23 or P60 and double housed with food and water ad libitum. All experimental procedures were conducted during the light period of a 12-hour light/dark cycle and were completed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Colorado Boulder.
Drugs
The non-selective adenosine receptor antagonist, caffeine (1, 3, 7-trimethylxanthine) was purchased from Fisher Scientific (Waltham, Massachusetts, USA). Cocaine hydrochloride was obtained from Sigma-Aldrich (St Louis, Missouri, USA). Cocaine hydrochloride was dissolved in sterile-filtered bacteriostatic saline and caffeine was dissolved in tap water.
Caffeine consumption procedure
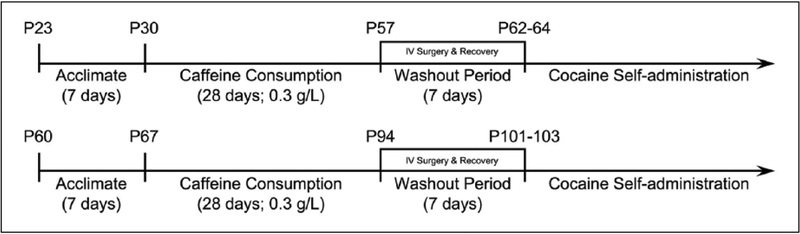
The caffeine consumption procedure began on P30 for adolescent studies and P67 for adult studies. Caffeine-consuming rats were given access to a single bottle containing caffeine in water (0.3 g/L) for 28 days (Figure 1). Age-matched control groups continued to receive water throughout the procedure. The 28-day consumption period was chosen since it broadly encompasses the rat developmental period just prior to puberty (approximately P40) through young adulthood (Spear, 2000). This consumption period was intended to model a human caffeine consumption period from late childhood, through the teenage years and into young adulthood (Andersen, 2003; Spear, 2000). Caffeine and water consumption were monitored throughout the procedure. Following the 28-day caffeine consumption period, the caffeine solution was replaced with water for the remainder of the experiment. Cocaine and sucrose pellet self-administration was initiated at least seven days after the last caffeine exposure. Thus, all behavioral testing was performed in the absence of caffeine during a period corresponding to adulthood (P62+) (Spear, 2000).
Figure 1.
Timeline of caffeine consumption model and behavioral testing. Caffeine (0.3 g/L) was introduced into the drinking water of adolescent rats on postnatal day (P) 30 and adult rats at P67. Caffeine consumption endured for 28 continuous days. Caffeine was removed from the drinking water at P57 (adolescent) or P94 (adult) and rats remained caffeine free for the remainder of the study. Rats were surgically implanted with an intravenous jugular catheter during a seven-day washout period and began cocaine self-administration after recovery from surgery.
Surgical and self-administration procedures
Surgical implantation of indwelling intravenous catheters occurred during the washout period (Figure 1). Catheters were implanted into the jugular vein under halothane anesthesia (1–2.5%) according to previously published procedures (O’Neill et al., 2012). Catheters were flushed daily with 0.1 mL heparinized saline and rats were allowed 4–7 days recovery in their home cage before self-administration procedures began.
Experiment 1: acquisition of cocaine self-administration and performance on fixed ratio (FR) and progressive ratio (PR) schedules
All self-administration procedures were conducted in operant conditioning chambers (Med-Associates, St Albans, Vermont, USA) equipped with a house light, two response levers, two stimulus cue lights located directly above each lever, a sucrose pellet magazine, and an infusion pump system for drug delivery. To test the effects of adolescent caffeine consumption on the acquisition of cocaine self-administration, sated rats were allowed to lever press for an intravenous cocaine infusion (0.5 mg/kg/100 μL injection) on a FR1 reinforcement schedule in two-hour sessions for five days/week for a total of 10 sessions. Self-delivered cocaine infusions were administered over five seconds concurrent with the illumination of a cue light above the cocaine-paired lever and were followed by a 15 s time-out period (TO 20 s) when the house light remained off and responding produced no consequence. Inactive lever responses produced no consequence throughout testing, and experimenter-delivered cocaine priming injections were never given.
Acquisition of cocaine self-administration was determined by a rat responding preferentially on the cocaine-paired lever (>75% responses on the cocaine-paired lever) and having greater than 10 self-delivered cocaine infusions over two consecutive sessions. After completing 10 sessions on the FR1:TO20 s schedule, rats that successfully acquired cocaine self-administration and demonstrated stability of intake (cocaine intake varied <20% over three consecutive days) were advanced to an FR5:TO20 s schedule where five cocaine-paired lever responses were required for a five-second cocaine/cue delivery. After three FR5:TO20 s sessions, rats were advanced to a PR schedule of reinforcement where the progression for response/injection ratios was determined according to (5e(injection number×0.2))–5 (e.g. 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, etc.).
Experiment 2: acquisition of sucrose pellet self-administration
The effect of adolescent caffeine consumption was tested on the acquisition of sucrose pellet self-administration in a separate groups of rats. Rats were trained to lever-press for 45 mg banana-flavored sucrose pellets (Bio-Serv, Flemington, New Jersey, USA) in standard operant test chambers on an FR1:TO20 s schedule of reinforcement under food-restricted conditions. Self-administration sessions were terminated after two hours or the acquisition of 50 sucrose pellets. Animals were tested for 10 consecutive sessions and acquisition was determined by a rat responding preferentially on the sucrose-paired lever (>75% responses on the sucrose-paired lever) and self-administering 50 sucrose pellets in one session.
Experiment 3: performance on a FR dose-response cocaine self-administration procedure
A separate group of rats were trained on a within-session dose-response procedure to evaluate the effects of adolescent caffeine consumption on stable maintenance responding for cocaine. Procedures for cocaine self-administration were similar to those stated above, however, in order to facilitate the acquisition of cocaine self-administration, rats were initially trained to lever press for sucrose pellets. During the caffeine washout period, rats were food-restricted for 18 h prior to a sucrose self-administration session during which rats could lever press for banana-flavored sucrose pellets on a FR1 reinforcement schedule. After successfully reaching a criterion (100 sucrose pellet deliveries in one session), rats were fed ad libitum for the remainder of the experiment. Rats failing to meet the criterion within three sessions are removed from the procedure. Rats were surgically implanted with an intravenous catheter at least 24 h after returning to ad libitum feeding conditions.
After recovery from surgery, rats self-administered intravenous cocaine on an FR1:TO20 s schedule in a minimum of five daily two-hour sessions. The within-session dose response procedure was implemented when animals had acquired cocaine self-administration (see above) and established stability in cocaine intake (cocaine intake varied <20% over three consecutive days). To evaluate cocaine intake across a range of doses, each session was divided into five sequential 30-minute periods where different cocaine infusion doses were delivered throughout the session on a FR1:TO20 s schedule. The ascending limb of the dose-response curve was evaluated in a one cohort of rats where lever responses were reinforced with a low unit cocaine (0.03125 mg/kg) dose that was increased at 30-minute intervals throughout the session (0.03125, 0.0625, 0.125, 0.25, and 0.50 mg/kg/infusion). The descending limb of the dose-response curve was evaluated in a separate cohort of rats where lever responses were reinforced with a high unit cocaine dose (0.75 mg/kg) that was decreased at 30-minute intervals throughout the session (0.75, 0.50, 0.25, 0.125, 0 mg/kg/infusion). Overall, seven doses were evaluated with three doses (0.125, 0.25, and 0.50 mg/kg/infusion) overlapping between the cohorts of rats run on the ascending and descending procedures.
Experiment 4: extinction and reinstatement of cocaine seeking
We next identified how adolescent caffeine consumption influenced the extinction and reinstatement of cocaine seeking following cocaine self-administration. Procedures for cocaine self-administration were similar to those stated in Experiment 3 above. After recovery from surgery, rats self-administered intravenous cocaine on an FR1:TO20 s schedule during 12 daily cocaine self-administration sessions. Rats then returned to the operant conditioning chambers for six daily two-hour extinction training sessions. During extinction training sessions, responses on the lever previously paired with cocaine infusions no longer resulted in cocaine or the cocaine-associated cue, and responses on the inactive lever continued to have no programmed consequence.
Reinstatement of cocaine seeking was conducted in two separate three-hour sessions. The first reinstatement test session evaluated cocaine seeking induced by the presentation of the cocaine-associated cue light. The test session was initiated with a two-hour period of extinction conditions, which was immediately followed by a one-hour reinstatement test period. During the test period, five non-contingent presentations of the cocaine-associated cue light (5 s) were delivered every two minutes over the first 10 min of the session. Responses at the previously cocaine-paired lever also resulted in a five-second illumination of the cocaine-associated cue light. The second reinstatement test session evaluated cocaine seeking induced by a cocaine priming injection. This test session was also initiated with a two-hour period of extinction conditions immediately followed by the administration of cocaine (15 mg/kg, intraperitoneal (i.p.)) and a one-hour reinstatement test period. During this test, responses at the previously cocaine-paired lever had no programmed consequences and did not result in either cocaine or cocaine-associated cue delivery. Reponses on the inactive lever never had programmed consequence during either extinction conditions or the reinstatement testing period.
Data analysis
Body weight and consumption data (average g/day and average ml/day, respectively) were analyzed using a two-way mixed-design analysis of variance (ANOVA) with consumption group (between) and weeks (within) as factors. Cocaine self-administration on the FR1, FR5, and PR schedules was evaluated using the number of infusions in a mixed-design ANOVA with consumption group (between) and sessions (within) as factors. On the PR schedule, final ratios, or breakpoints, were recorded, but were not analyzed statistically because final ratios are derived from an escalating (exponential) function and the data tend to violate assumptions of homogeneity of variance. Therefore, the number of infusions self-administered was used in all statistical analyses. Self-administration of sucrose pellets was analyzed similarly with a two-way ANOVA using latency to acquire 100 pellets and total pellets acquired as the dependent measures. To supplement the cocaine self-administration data, we also evaluated average cocaine intake using a between-subjects t-test for each schedule of reinforcement. Performance on the within-session dose-response procedure was evaluated by averaging the number of infusions, rate of lever responding and cocaine intake (mg/kg) for each dose over the final five sessions after an individual rat displayed stability in intake during the procedure (cocaine intake varied <20% over three consecutive days). A two-way ANOVA was used to evaluate the effects of consumption group and dose on each of these dependent measures. Lever responding during extinction training was analyzed by two-way mixed-design ANOVA with consumption group and session as the factors. Similarly, lever responding during reinstatement testing was analyzed by a two-way mixed-design ANOVA with consumption group and session (extinction vs. reinstatement) as the factors. Responding on the active and inactive levers were analyzed separately by a two-way ANOVA. In all cases, significant interactions and main effects (p<0.05) were followed by either post-hoc or planned comparisons using one-way ANOVAs and/or Tukey’s post-hoc test.
Results
Caffeine consumption
The volume of fluid consumed (mL) and body weights (g) were recorded throughout the caffeine consumption procedure in adolescent and adult rats and are reported as averages for each week of the caffeine consumption procedure (Figure 2). Total caffeine consumption (mg/kg) was calculated for the caffeine group based on the amount of fluid consumed, the concentration of the caffeine-containing solution and the body weight of each rat. Figure 2 displays data from the cohort of adolescent (n=24/group) and adult (n=10/group) rats used in the cocaine acquisition experiment. There was a significant increase in body weight across time in both adolescent and adult rats (adolescent: F3,138=3454, p<0.0001; adult: F3,54=659.8, p<0.0001) and no differences were observed between the water and caffeine consuming groups in either age cohort. Significant increases in body weight were observed in both adults and adolescent rats across each week (Tukey’s, p<0.05). There was a significant main effect of fluid intake across time for both adolescent and adult rats (adolescent: F3,66=458.90, p<0.0001; adult: F3,24=6.72, p<0.005). There was also a significant interaction in fluid consumption between the consumption group and time for both age cohorts (adolescent: F3,66=4.07, p<0.05; adult: F3,24=3.23, p<0.05), demonstrating that greater amounts of fluid were consumed by the caffeine group during some weeks (Figure 2). The average intake of caffeine (mg/kg) varied between adolescent and adult rats (age: F1,18=65.53, p<0.001) and across time (F3,54=131.70, p<0.0001). Analysis of the significant interaction of time and age (F3,54=11.12, p<0.0001) suggests that adolescent rats consumed higher amounts of caffeine throughout the procedure (Figure 2). Average caffeine consumption over the entire procedure averaged 28.03±2.50 mg/kg in adolescent rats, while caffeine consumption in adult rats averaged approximately 23.25±1.46 mg/kg. Analogous consumption data (data not shown) were collected for each cohort of rats that were used in each experimental procedure, and all data is comparable to previously published results using this procedure (O’Neill et al., 2015, 2016).
Figure 2.
Caffeine consumption during adolescence and adulthood over the 28-day procedure. Body weight was not significantly altered during the caffeine consumption procedure in either (a) adolescent or (c) adult rats. (b) Fluid consumption progressively increased during the procedure in adolescent rats. Tukey’s post-hoc test revealed that the adolescent caffeine group consumed significantly greater fluid (ml) during the third and fourth week of the procedure (*p<0.05). (d) Fluid consumption was relatively stable in adult rats, but a Tukey’s post-hoc test revealed that consumption was significantly greater in the caffeine group during the first week of the procedure(*p<0.05). (e) A comparison of adolescent and adult caffeine consumption reveals that adolescent rats consumed significantly more caffeine (mg/kg) than adult rats each week throughout the caffeine consumption procedure (Tukey’s post-hoc test: #p<0.05).
Adolescent caffeine consumption increases acquisition of cocaine self-administration
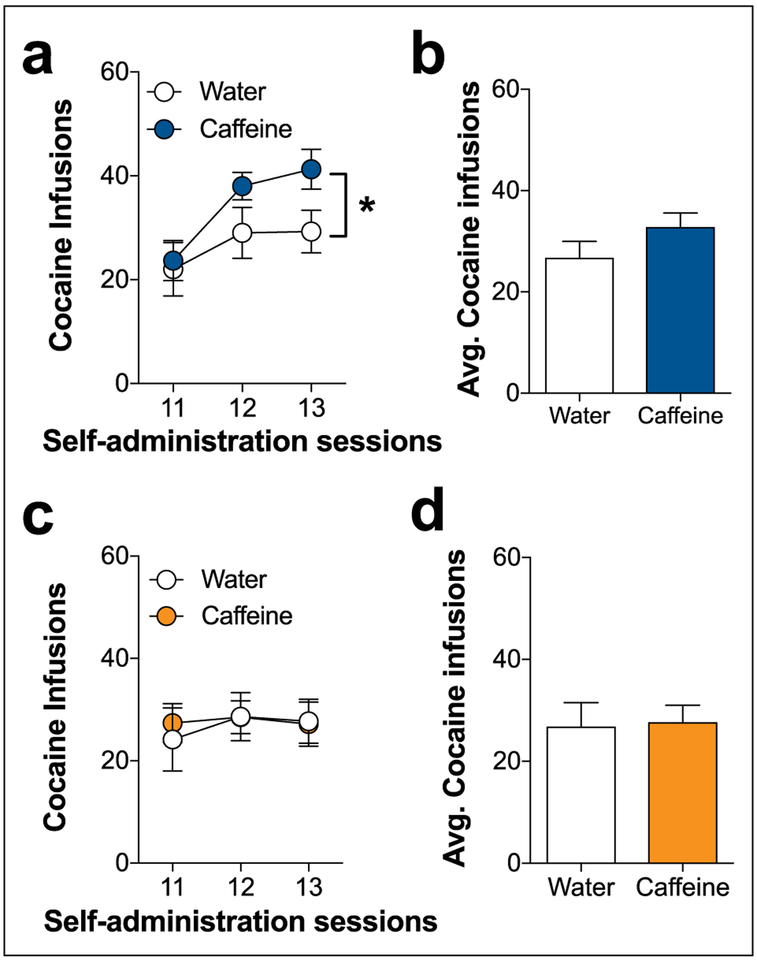
We first determined whether caffeine consumption during the adolescent period altered the acquisition of cocaine self-administration using an operant intravenous self-administration procedure. Figure 3 demonstrates the successful acquisition of FR1 responding, as indicated by cocaine infusions over the first 10 sessions in both consumption groups (sessions: F9,180=4.389, p<0.0001). In particular, lever responding during sessions 6, 7, 8, 9, and 10 were significantly greater than session 1 responding across both groups (Tukey’s, p<0.05). Rats that consumed caffeine during adolescence displayed a significant increase in cocaine infusions on the FR1 schedule compared to the water control group (consumption group: F1,180=26.75, p<0.0001). Overall, rats that consumed caffeine during adolescence reached the acquisition criterion (>75% cocaine-paired lever responses and >10 self-delivered cocaine infusions in two consecutive sessions) at an accelerated rate compared to the water-consuming rats (Figure 3(b)). Cocaine intake stabilized over the last five FR1 sessions. Average intake across these sessions suggests higher cocaine intake in rats that consumed caffeine during adolescence (t18=2.919, p<0.01).
Figure 3.
Effects of caffeine consumption on the acquisition of cocaine self-administration in sated rats on a fixed ratio (FR) 1 schedule of reinforcement. (a) Rats that consumed caffeine (n=11) during adolescence showed significantly greater self-administration of cocaine across the 10 sessions compared with the water control group (n=10; *p<0.05, main effect of consumption group). (b) Adolescent caffeine consumption was associated with a faster acquisition to reach criterion compared with the water control group. (c) Average cocaine intake during the last five FR1 sessions reveals that the adolescent caffeine-consuming group had higher levels of stabilized cocaine intake. (d) Rats that consumed caffeine (n=10) or water (n=11) during adulthood did not significantly differ in the self-administration of cocaine across the 10 FR1 sessions. (e). There were no differences in the percentages of rats that reached the acquisition criterion between the adult caffeine and water groups. (f) Average cocaine intake during the last five FR1 sessions was not significantly different between adult animals that consumed caffeine and the water control group.
Enhanced acquisition and cocaine intake on an FR1 schedule were not observed in an analogous study where caffeine was consumed during adulthood (Figure 3(a)). Thus, both groups acquired cocaine self-administration (sessions: F9,190=2.615, p<0.01) with greater responding observed during sessions 7, 8, 9, and 10 compared with session 1 across both groups (Tukey’s, p<0.05). Caffeine consumption in adult animals did not alter cocaine self-administration compared with the water control group. Likewise, the percentage of rats reaching the acquisition criterion across the FR1 sessions was similar between the consumption groups (Figure 3(b)). There was also no significant difference in the stabilized cocaine intake across the last five FR1 sessions (Figure 3(c)). These findings suggest that adolescent, but not adult, caffeine consumption enhances the acquisition of cocaine self-administration.
To further evaluate the effect of caffeine consumption on cocaine self-administration, we increased the reinforcement schedule to an FR5 and PR schedule. Figure 4 illustrates an overall increase in the number of cocaine self-infusions in the rats that consumed caffeine as adolescents (consumption group: F1,45=5.158, p<0.05), but not in rats that consumed caffeine as adults when analyzed across all three sessions. However, average cocaine intake on the FR5 schedule did not differ in either the caffeine consuming adolescent or adult animals (Figure 4). Adolescent caffeine consumption also increased performance on the PR schedule, both across the three test sessions (Figure 5, consumption group: F1,99=22.23, p<0.0001), and when intake was averaged (t32=2.697, p<0.02). Similar enhancements were not observed on FR or PR performance following adult caffeine consumption (Figures 4 and 5). Together, these findings suggest that adolescent, but not adult, caffeine consumption enhances the reinforcing nature of cocaine as indicated by enhanced acquisition and PR performance.
Figure 4.
Effects of caffeine consumption on cocaine self-administration on a fixed-ratio (FR) 5 schedule of reinforcement. (a) Rats that consumed caffeine (n=11) during adolescence showed significantly greater self-administration of cocaine across the three sessions compared with the water control group (n=7; *p<0.05, main effect of consumption group). (b) There were no differences between the consumption groups when the three FR5 sessions were averaged. (c) Rats that consumed caffeine (n=11) during adulthood did not significantly differ in cocaine self-administration maintained on a FR5 schedule compared with the water-consuming control group (n=10). (d) There were also no significant differences between the adult consumption groups when cocaine infusions were averaged across the three FR5 sessions.
Figure 5.
Effects of caffeine consumption on cocaine self-administration on a progressive ratio (PR) schedule of reinforcement. (a) Rats that consumed caffeine (n=19) during adolescence showed significantly greater number of cocaine infusions and higher breakpoints across the three PR sessions compared with the water control group (n=15; *p<0.05, main effect of consumption group). (b) When cocaine infusions were averaged across the three PR sessions there was also a significant increase in the rats that consumed caffeine during adolescence. (c) Rats that consumed caffeine (n=10) during adulthood did not significantly differ from rats in the water group (n=10) in the number of cocaine infusions or breakpoints when behavior was maintained on a PR schedule. (d) There were also no significant differences between the adult consumption groups when cocaine infusions were averaged across the three PR sessions.
Adolescent caffeine consumption does not affect acquisition of sucrose pellet self-administration
There was some concern that caffeine consumption during adolescence may enhance the ability to learn and perform a positively reinforced operant behavior. Therefore, we evaluated the effects of adolescent caffeine consumption on the acquisition of sucrose pellet self-administration. Figure 6 illustrates the latencies to acquire the maximum 50 pellets during 10 two-hour sessions that were run identically to the cocaine self-administration sessions. Both water- and caffeine-consuming groups successfully learned the operant response over sessions (Sessions: F9,126=12.72, p<0.0001) as evidenced by shorter latencies during sessions 9 and 10 compared with sessions 1, 2, 3, 4, 5, and 6 across both groups (Tukey’s, p<0.05). There were no group differences in the latencies to acquire 50 sucrose pellets in any of the sessions. Similarly, the percentage of rats reaching the acquisition criterion across the sessions was comparable between the consumption groups (Figure 6).
Figure 6.
Effects of caffeine consumption on sucrose pellet self-administration. (a) Rats that consumed caffeine (n=10) during adolescence showed similar performance on the sucrose self-administration procedure compared with rats that consumed water (n=10) as revealed by the latency to acquire 50 pellets, a measure that is inversely related to performance. (b) Similarly, there were no group differences in the percentage of animals meeting acquisition criteria.
Adolescent caffeine consumption enhances performance on a FR dose-response procedure
To further evaluate the effect of caffeine consumption on cocaine self-administration, we utilized a within-session dose-response procedure to assess stable maintenance responding across a range of doses. To facilitate acquisition of cocaine self-administration in both groups, rats were trained to lever press for banana-flavored sucrose pellets on an FR1 schedule. When the acquisition criteria (100 pellet deliveries in one session) was achieved, rats were implanted with jugular catheters and allowed to lever press for 0.5 mg/kg/infusion cocaine on an FR1 schedule in two-hour sessions. Once rats had reached the acquisition criterion and established stabilized intake, they were transitioned to either a within-session ascending or descending dose-response procedure. Consistent with the within-session procedure, Figure 7 illustrates the resulting inverted-U shaped dose-response on response rates (F1,117=10.29, p<0.001) and number of infusions (F1,117=9.97, p<0.001). In both measures for both groups, the peak dose of 0.125 mg/kg/infusion was significantly different from all other doses (Tukey’s, p<0.05). For both consumption groups, cocaine intake significantly increased as the infusion dose increased (F1,117=58.85, p<0.001). All infusion doses were significantly different from the others (Tukey’s, p<0.05) except 0.03125 mg/kg/infusion and 0.0625 mg/kg/infusion, which were not significantly different from one another. Rats that consumed caffeine during adolescence displayed significantly increased response rates (consumption group: F1,117=10.88, p<0.01), number of infusions (consumption group: F1,117=11.44, p<0.005), and cocaine intake (consumption group: F1,117=16.13, p<0.001). These findings further suggest that adolescent caffeine consumption enhances the reinforcing properties of cocaine resulting in upward shifts in FR1 responding reinforced by different cocaine doses.
Figure 7.
Effects of adolescent caffeine consumption on cocaine self-administration maintained on a within-session dose response procedure. The within-session dose response procedure produced the characteristic U-shaped curve using (a) lever presses and (b) infusions. There was a main effect of consumption group such that rats that consumed caffeine (n=9) during adolescence displayed a general increase in (a) lever pressing and (b) cocaine infusions compared with the water control group (n=7; *p<0.05, main effect of consumption group). (c) Adolescent consumption of caffeine also produced an upward shift in the total cocaine intake (mg/kg) across all the doses tested compared with the water control group (*p<0.05, main effect of consumption group).
Adolescent caffeine consumption does not affect extinction responding or reinstatement of cocaine seeking
The effects of adolescent caffeine consumption were also tested on non-reinforced cocaine seeking during extinction learning and subsequent reinstatement testing. Rats acquired cocaine self-administration on an FR1 schedule following sucrose-reinforced lever training as described above. Following 12 daily two-hour sessions, rats underwent extinction training in six sessions. Figure 8 illustrates robust initial drug seeking that subsides over the extinction sessions (sessions: F5,70=13.77, p<0.001). Extinction responding did not significantly differ between the water- and caffeine-consuming groups. Following extinction training, rats were tested for reinstatement of cocaine seeking using both a cocaine-associated cue or a non-contingent cocaine injection (Figure 8). Both stimuli induced robust cocaine seeking as evidenced by the significant increase in lever responding at the previously drug-paired lever (cue: F1,14=14.45, p<0.005 and cocaine: F1,14=38.97, p<0.001), but there were no differences between the consumption groups.
Figure 8.
Effects of adolescent caffeine consumption on cocaine seeking during extinction training and during cue- and cocaine-primed reinstatement tests. (a) Both water (n=8) and caffeine (n=9) consumption groups displayed equivalent decrease in responding on the previously drug-paired (top) and inactive (bottom) levers during extinction training (^p<0.05, Tukey’s post-hoc comparison, significant from all other sessions; #p<0.05, Tukey’s post-hoc comparison, significant from session 1). (b) Cocaine seeking resulting from contingent delivery of a previously cocaine-paired cue was robust compared with extinction responding in both the water and caffeine consumption groups (*p<0.05, main effect of cue-reinstatement). (c) Reinstatement of cocaine seeking induced by systemic cocaine administration (15 mg/kg, intraperitoneal (i.p.)) produced robust cocaine seeking in both the water and caffeine consumption groups (*p<0.05 main effect of cocaine-reinstatement).
Discussion
Caffeine intake is positively correlated with substance use disorders and has been shown to increase illicit drug use and other risky behaviors in young adults (Arria et al., 2017; Kendler et al., 2006; Miller, 2008; Peacock et al., 2017). We utilized a controlled experimental design to test effects of caffeine consumption during adolescence on cocaine self-administration and cocaine seeking. Our findings suggest that adolescent caffeine consumption enhances the acquisition of cocaine self-administration on an FR1 schedule of reinforcement in sated male rats. Tests of reinforcement efficacy using a PR schedule of reinforcement and a within-session dose-response procedure also reveal enhanced performance in rats that consumed caffeine during adolescence. Enhancements in the acquisition of cocaine self-administration and performance on a PR schedule were not observed when caffeine was consumed during adulthood. These findings support prior work demonstrating that adolescent, but not adult, caffeine consumption enhances both locomotor and reward sensitivity to psychostimulant drugs (Boeck et al., 2009; O’Neill et al., 2015). However, a recent study demonstrates that neither adult nor adolescent caffeine exposure cross-sensitize with methamphetamine-induced locomotor activity suggesting that caffeine may produce cross-sensitization in a psychostimulant-specific manner (Franklin et al., 2017). Adolescent caffeine consumption did not modify the acquisition or performance on a sucrose self-administration procedure indicating that these changes are specific to drug-related rewards. Lastly, adolescent caffeine consumption did not alter cocaine seeking during extinction training sessions or cocaine seeking induced by cocaine-associated cues or a cocaine prime. Together, our results indicate that adolescent caffeine use may increase vulnerability to initiate psychostimulant use.
Acquisition of drug self-administration is thought to reflect the initial reinforcing effects of a drug and may be predictive of transitions to higher levels of drug intake (Piazza et al., 2000). Enhanced acquisition of drug self-administration behavior may arise from a variety of factors including impulsivity, deprivation states, alternative reinforcers, and the dose of the drug during the self-administration procedure (Carroll and Meisch, 2011). Developmental exposure to drugs used to treat attention-deficit hyperactivity disorder, including psychostimulants and atomoxetine, have also been shown to enhance the acquisition of cocaine self-administration (Harvey et al., 2011; Jordan et al., 2016; Lacy et al., 2018; Somkuwar et al., 2013). Our findings demonstrate that developmental exposure to caffeine is also sufficient to enhance the acquisition of cocaine self-administration. It is plausible that caffeine-induced enhancements in the acquisition of cocaine self-administration arise from general augmentation of operant learning. This seems unlikely given that adolescent caffeine consumption does not enhance the acquisition of sucrose self-administration. Rather, it seems more likely that adolescent caffeine consumption increases the sensitivity to the reinforcing and/or stimulant properties of cocaine. Indeed, adolescent caffeine consumption produces behavioral cross-sensitization with the psychostimulant drugs, methylphenidate, and cocaine (Boeck et al., 2009; O’Neill et al., 2015), but not methamphetamine (Franklin et al., 2017). Adolescent caffeine consumption also produces a leftward shift in the dose-response curve for cocaine-induced place preferences (O’Neill et al., 2015). Our current observations using the PR and FR dose-response procedures substantiate that rats that consumed caffeine during adolescence self-administer cocaine at higher rates across a range of doses.
Caffeine is known to potentiate the effects of other psychostimulant drugs when they are administered concurrently. For example, both acute and chronic caffeine treatments augment psychostimulant-induced locomotion and reward-related behavior (Andén and Jackson, 1975; Gasior et al., 2000; Jaszyna et al., 1998; Justinova et al., 2009; Misra et al., 1986; Prieto et al., 2015, 2016; Schenk et al., 1990, 1994, 1996; White and Keller, 1984; Worley et al., 1994). Caffeine also partially generalizes and enhances the discriminative-stimulus effects of cocaine and other psychostimulants, suggesting that interoceptive cues of these drugs may overlap (Gauvin et al., 1989, 1990; Harland et al., 1989; Munzar et al., 2002; Young et al., 1998). These direct interactive effects between caffeine and other psychostimulant drugs are largely due to nonselective blockade of both adenosine A1 and A2A receptors by caffeine. These actions effectively amplify dopamine neurotransmission in the dorsal nucleus accumbens shell and medial prefrontal cortex (Acquas et al., 2002; Borycz et al., 2007; De Luca et al., 2007; Solinas et al., 2002).
We demonstrate that chronic caffeine consumption during the adolescent period produces cross-sensitization to cocaine even when cocaine is administered in the absence of caffeine. Since these effects are observed following the removal of caffeine, it is possible that caffeine withdrawal may play a role in these effects. Physical withdrawal symptoms typically emerge and dissipate within the first 72 h of caffeine removal (Finn and Holtzman, 1986; Holtzman, 1983; Rhoads et al., 2011). Therefore, it seems unlikely that the effects on the acquisition of cocaine self-administration could be attributed to withdrawal symptoms since the behavioral procedures were conducted at least seven days following caffeine consumption. However, the results may reflect a caffeine-induced protracted withdrawal state existing within specific neurobiological systems that alter the sensitivity to subsequent cocaine administration.
The mesolimbic dopamine system matures during adolescence, allowing for the development of appropriate goal-directed behaviors in adulthood (Wahlstrom et al., 2010). For example, the adolescent period is associated with progressive increases in dopamine innervation (Berger et al., 1985; Giorgi et al., 1987; Kalsbeek et al., 1988; Nomura et al., 1976; Ungethüm et al., 1996), increases in dopamine D1 and D2 receptor densities (Andersen et al., 1997, 2000; Gelbard et al., 1989; Leslie et al., 1991; Tarazi and Baldessarini, 2000; Tarazi et al., 1999; Teicher et al., 1995), and refinements in dopamine receptor sensitivities (Andersen, 2002; Dwyer and Leslie, 2016). It is possible that caffeine exposure during adolescence alters the maturation of the mesolimbic dopamine system and disrupts the development of appropriate motivated behaviors in adulthood.
Previous work has demonstrated that adolescent caffeine exposure alters dopamine neurotransmission within the striatal areas of the mesolimbic dopamine system. Adolescent consumption of caffeine decreased basal extracellular levels of dopamine in the nucleus accumbens of outbred Sprague-Dawley rats (O’Neill et al., 2015). This study also observed increased expression of the dopamine transporter and the dopamine D2 receptor in the nucleus accumbens, both of which may contribute to reduced extracellular basal dopamine. Cocaine-induced dopamine release was significantly enhanced in rats that consumed caffeine during adolescence (O’Neill et al., 2015), although adolescent caffeine exposure failed to alter dopamine release resulting from the administration of another psychostimulant drug, 3,4-methylendioxymethamphetamine (MDMA), (Cadoni et al., 2017). Adolescent caffeine consumption also increases total dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) expression in the nucleus accumbens (Boeck et al., 2009; O’Neill et al., 2015). Increased DARPP-32 expression is associated with increased dopamine signaling and contributes to heightened cocaine sensitivity and reward (Fienberg et al., 1998; Zachariou et al., 2006). Together, these findings suggest that enhancements in cocaine reinforcement may be due to enhanced dopamine neurotransmission following adolescent consumption of caffeine.
It is noteworthy that rats exposed to caffeine as adults show no alteration in the acquisition of cocaine self-administration or performance on a PR schedule. This observation is incongruent with a previous finding demonstrating that pre-exposure to experimenter-delivered caffeine in adult rats increased cocaine self-administration (Horger et al., 1991). The reason for this discrepancy is unclear especially since dosing was similar between the studies. However, other procedural differences in the route of administration and the timing of behavioral testing may account for the different outcomes. While our study utilized an oral caffeine administration procedure, the Horger et al. (1991) study used experimenter-delivered administration. Thus, the combined effects of caffeine and the stress of the i.p. injection may produce greater effects on cocaine self-administration in adult rats. Our study also tested the acquisition of cocaine self-administration seven days after the removal of caffeine to minimize the contribution of physical withdrawal symptoms to our behavioral studies. The Horger et al. (1991) study began self-administration testing one day following the removal of caffeine when physical withdrawal symptoms are most robust (Finn and Holtzman, 1986; Holtzman, 1983). Together, it appears that both adult and adolescent caffeine exposure have the potential to enhance cocaine’s reinforcing properties depending on the experimental conditions.
The developmental nature of our findings suggests that caffeine consumption during adolescence, when the brain is undergoing rapid developmental changes (Arain et al., 2013; Wahlstrom et al., 2010), may be a vulnerable period leading to greater caffeine-induced neurobiological changes that correspond with enhanced cocaine reinforcement. However, rats that consumed caffeine as adolescents were tested at a different developmental period compared with rats that consumed caffeine as adults. Thus, adolescent caffeine-consuming rats were tested at an earlier age than rats consuming caffeine as adults. Despite the fact that cocaine self-administration between the adult and adolescent water-consuming control groups appears similar, this is a potential caveat to our findings. Perhaps more important, is the fact that adolescent rats consistently consumed more caffeine than adult rats during the consumption phase. While caffeine intake began to normalize between adolescent and adult rats during the third and fourth week, adolescent rats still had higher daily caffeine intake over the course of the procedure. It is possible that the higher caffeine consumption in adolescent rats may compound with the developmental exposure to drive the enhancements observed in cocaine self-administration. However, differential clearance rates of caffeine between adult and adolescent rats may partially mitigate the higher caffeine consumption in adolescent rats. Pharmacokinetic studies demonstrate that caffeine-clearance rates in adolescents are faster in early adolescence when the largest consumption differences were observed and become quite similar to the clearance rates of adults by late adolescence when consumption levels began to normalize (Bienvenu et al., 1990; Ginsberg et al., 2002; Latini et al., 1980). The differential effects of the consumption differences, pharmacokinetic differences, and developmental differences between adolescent and adult rats remains unclear as to how they may contribute to effects of caffeine on cocaine self-administration that we observe in adolescent rats that consumed caffeine.
Unlike the observations on cocaine self-administration, adolescent caffeine consumption had no effect on subsequent cocaine-seeking behavior. These tests were conducted after the acquisition, maintenance and extinction of cocaine self-administration and it is possible that the neurobiological changes that contributed to enhanced cocaine reinforcement subsided during this time. This seems unlikely since we observed enhanced performance on both the PR and within-session procedures, both of which were conducted after similar periods of time following caffeine exposure. Alternatively, it is possible that cocaine self-administration and/or extinction training produced neurobiological changes that influence the consequences of adolescent caffeine consumption, effectively “normalizing” the two groups. Previous work has demonstrated that differences in the initial acquisition of self-administration can be eliminated with extended drug administration (Piazza et al., 1989). There have also been clear demonstrations of extinction training producing neurobiological changes following cocaine self-administration (Schmidt et al., 2001; Self et al., 2004; Sutton et al., 2003). Therefore, it is possible that either cocaine self-administration and/or extinction training may have ameliorated or reversed caffeine-induced changes and prevented any changes in cocaine seeking from being observed (Millan et al., 2011). Given this possibility, it would be valuable to measure drug seeking in an abstinence paradigm without the use of extinction training to more accurately mimic the human condition (Peck and Ranaldi, 2014). Nonetheless, our data indicate that cocaine seeking induced by either a conditioned stimulus or a cocaine prime are similar in both caffeine- and water-consuming groups. Thus, adolescent exposure to caffeine may lead to enhanced cocaine reinforcement, but may not affect relapse-like behavior during withdrawal.
A final consideration is how the caffeine consumption achieved in our model corresponds with consumption in humans. Studies examining caffeine consumption in children and adolescents have reported average caffeine intake between 100–400 mg of caffeine per day (Frary et al., 2005; Rudolph et al., 2014; Temple, 2009). Although these amounts are typically not adjusted for body weight, estimated caffeine doses would range between 1–10 mg/kg depending on the consumption and bodyweight measures throughout adolescence. We find that adolescent rats consumed considerably higher amounts of caffeine (~30 mg/kg/day) comparatively, although pharmacokinetic differences between the two species make accurate comparisons difficult. The half-life of caffeine in adult rats is far shorter (t1/2≈1 h) than that of adult humans (t1/2≈5 h), suggesting that a lower mg/kg intake in humans could potentially show similar effects (Arnaud, 1987; Bonati et al., 1982). In addition, studies demonstrate that caffeine clearance rates are extremely slow in neonates with a half-life greater than 40 h (Aranda et al., 1979), and become progressively faster through early development until late adolescence when the clearance rates are similar to adults (Bienvenu et al., 1990; Ginsberg et al., 2002; Latini et al., 1980).
The data presented here indicate that caffeine consumption during adolescence enhances the acquisition of cocaine self-administration and promotes increased cocaine reinforcement. These changes persist into adulthood in the absence of continued caffeine consumption. These data suggest that caffeine consumption during adolescent development may increase vulnerability to the development of cocaine-use disorder or other addictions. Given the increasing prevalence of caffeine consumption among children and adolescents (Ahluwalia and Herrick, 2015; Frary et al., 2005; Temple, 2009), it is important to enhance awareness of the potentially deleterious long-term effects of caffeine consumption during adolescent development.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by National Institutes of Health (Grant DA033358).
Footnotes
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Acquas E, Tanda G and Di Chiara G (2002) Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 27: 182–193. [DOI] [PubMed] [Google Scholar]
- Ahluwalia N and Herrick K (2015) Caffeine intake from food and beverage sources and trends among children and adolescents in the United States: Review of national quantitative studies from 1999 to 2011. Adv Nutr 6: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andén NE and Jackson DM (1975) Locomotor activity stimulation in rats produced by dopamine in the nucleus accumbens: Potentiation by caffeine. J Pharm Pharmacol 27: 666–670. [DOI] [PubMed] [Google Scholar]
- Andersen SL (2002) Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD). Behav Brain Res 130: 197–201. [DOI] [PubMed] [Google Scholar]
- Andersen SL (2003) Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27: 3–18. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, et al. (1997) Sex differences in dopamine receptor overproduction and elimination. Neuroreport 8: 1495–1498. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, et al. (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37: 167–169. [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, et al. (2013) Maturation of the adolescent brain. Neuropsychiatr Dis Treat 9: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda JV, Collinge JM, Zinman R, et al. (1979) Maturation of caffeine elimination in infancy. Arch Dis Child 54: 946–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud MJ (1987) The pharmacology of caffeine. Prog Drug Res 31: 273–313. [DOI] [PubMed] [Google Scholar]
- Arria AM, Caldeira KM, Bugbee BA, et al. (2017) Trajectories of energy drink consumption and subsequent drug use during young adulthood. Drug Alcohol Depend 179: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Verney C, Febvret A, et al. (1985) Postnatal ontogenesis of the dopaminergic innervation in the rat anterior cingulate cortex (area 24). Immunocytochemical and catecholamine fluorescence histochemical analysis. Brain Res 353: 31–47. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Pons G, Rey E, et al. (1990) Effect of hypophysectomy on caffeine elimination in rats. Fundam Clin Pharmacol 4: 393–399. [DOI] [PubMed] [Google Scholar]
- Boeck CR, Marques VB, Valvassori SS, et al. (2009) Early long-term exposure with caffeine induces cross-sensitization to methylphenidate with involvement of DARPP-32 in adulthood of rats. Neurochem Int 55: 318–322. [DOI] [PubMed] [Google Scholar]
- Bonati M, Latini R, Galletti F, et al. (1982) Caffeine disposition after oral doses. Clin Pharmacol Ther 32: 98–106. [DOI] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, et al. (2007) Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem 101: 355–363. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Simola N, et al. (2017) Widespread reduction of dopamine cell bodies and terminals in adult rats exposed to a low dose regimen of MDMA during adolescence. Neuropharmacology 123: 385–394. [DOI] [PubMed] [Google Scholar]
- Carlezon WA and Konradi C (2004) Understanding the neurobiological consequences of early exposure to psychotropic drugs: Linking behavior with molecules. Neuropharmacology 47 (Suppl 1): 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME and Meisch RA (2011) Acquisition of drug self-administration In: Olmstead MC(ed) Animal Models of Drug Addiction. New York: Humana Press, pp. 237–265. [Google Scholar]
- De Luca MA, Bassareo V, Bauer A, et al. (2007) Caffeine and accumbens shell dopamine. J Neurochem 103: 157–163. [DOI] [PubMed] [Google Scholar]
- Dwyer JB and Leslie FM (2016) Adolescent maturation of dopamine D1 and D2 receptor function and interactions in rodents. PLoS One 11: e0146966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, et al. (1998) DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science 281: 838–842. [DOI] [PubMed] [Google Scholar]
- Finn IB and Holtzman SG (1986) Tolerance to caffeine-induced stimulation of locomotor activity in rats. J Pharmacol Exp Ther 238: 542–546. [PubMed] [Google Scholar]
- Franklin JL, Wearne TA, Homewood J, et al. (2017) The behavioral effects of chronic sugar and/or caffeine consumption in adult and adolescent rats. Behav Neurosci 131: 348–358. [DOI] [PubMed] [Google Scholar]
- Frary CD, Johnson RK and Wang MQ (2005) Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 105: 110–113. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, et al. (1999) Actions of caffeine in the brain with special reference to factors that contribute to its wide-spread use. Pharmacol Rev 51: 83–133. [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, et al. (2000) Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: Effect of caffeine dose. J Pharmacol Exp Ther 295: 1101–1111. [PubMed] [Google Scholar]
- Gauvin DV, Criado JR, Moore KR, et al. (1990) Potentiation of cocaine’s discriminative effects by caffeine: A time-effect analysis. Pharmacol Biochem Behav 36: 195–197. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Harland RD, Michaelis RC, et al. (1989) Caffeine-phenylethylamine combinations mimic the cocaine discriminative cue. Life Sci 44: 67–73. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, et al. (1989) Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Res Dev Brain Res 49: 123–130. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Hattis D, Sonawane B, et al. (2002) Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 66: 185–200. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, et al. (1987) Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Brain Res 432: 283–290. [DOI] [PubMed] [Google Scholar]
- Harland RD, Gauvin DV, Michaelis RC, et al. (1989) Behavioral interaction between cocaine and caffeine: A drug discrimination analysis in rats. Pharmacol Biochem Behav 32: 1017–1023. [DOI] [PubMed] [Google Scholar]
- Harvey RC, Sen S, Deaciuc A, et al. (2011) Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: Cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology 36: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman MA, Weil J and Gonzalez de Mejia E (2010) Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75: 77–87. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Tuttle TD and Higgins CL (2010) Energy beverages: Content and safety. Mayo Clinic Proceedings 85: 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman SG (1983) Complete, reversible, drug-specific tolerance to stimulation of locomotor activity by caffeine. Life Sci 33: 779–787. [DOI] [PubMed] [Google Scholar]
- Horger BA, Wellman PJ, Morien A, et al. (1991) Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. Neuroreport 2: 53–56. [DOI] [PubMed] [Google Scholar]
- Jaszyna M, Gasior M, Shoaib M, et al. (1998) Behavioral effects of nicotine, amphetamine and cocaine under a fixed-interval schedule of food reinforcement in rats chronically exposed to caffeine. Psychopharmacology (Berl) 140: 257–271. [DOI] [PubMed] [Google Scholar]
- Jordan CJ, Lemay C, Dwoskin LP, et al. (2016) Adolescent d-amphetamine treatment in a rodent model of attention deficit/hyperactivity disorder: Impact on cocaine abuse vulnerability in adulthood. Psychopharmacology 233: 3891–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Barnes C, et al. (2009) Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology (Berl) 203: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, et al. (1988) Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol 269: 58–72. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J and O Gardner C (2006) Caffeine intake, toxicity and dependence and lifetime risk for psychiatric and substance use disorders: An epidemiologic and co-twin control analysis. Psychol Med 36: 1717–1725. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Schorsch HK and Austin BP (2018) Adolescent d-amphetamine exposure enhances the acquisition of cocaine self-administration in male and female rats. Exp Clin Psychopharmacol 26: 18–28. [DOI] [PubMed] [Google Scholar]
- Latini R, Bonati M, Marzi E, et al. (1980) Caffeine disposition and effects in young and one-year old rats. J Pharm Pharmacol 32: 596–599. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, et al. (1991) Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: A quantitative autoradiographic analysis. Brain Res Dev Brain Res 62: 109–114. [DOI] [PubMed] [Google Scholar]
- Leviton A (1992) Behavioral correlates of caffeine consumption by children. Clin Pediatr 31: 742–750. [DOI] [PubMed] [Google Scholar]
- Marin MT, Zancheta R, Paro AH, et al. (2011) Comparison of caffeine-induced locomotor activity between adolescent and adult rats. Eur J Pharmacol 660: 363–367. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ and McNally GP (2011) Extinction of drug seeking. Behav Brain Res 217: 454–462. [DOI] [PubMed] [Google Scholar]
- Miller KE (2008) Energy drinks, race, and problem behaviors among college students. J Adolesc Health 43: 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra AL, Vadlamani NL and Pontani RB (1986) Effect of caffeine on cocaine locomotor stimulant activity in rats. Pharmacol Biochem Behav 24: 761–764. [DOI] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, et al. (2002) Adenosinergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology 161: 348–355. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL and Debry G (1992) Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17: 139–170. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Naitoh F and Segawa T (1976) Regional changes in monoamine content and uptake of the rat brain during postnatal development. Brain Res 101: 305–315. [DOI] [PubMed] [Google Scholar]
- O’Neill CE, LeTendre ML and Bachtell RK (2012) Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology 37: 1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill CE, Levis SC, Schreiner DC, et al. (2015) Effects of adolescent caffeine consumption on cocaine sensitivity. Neuropsychopharmacology 40: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill CE, Newsom RJ, Stafford J, et al. (2016) Adolescent caffeine consumption increases adulthood anxiety-related behavior and modifies neuroendocrine signaling. Psychoneuroendocrinology 67: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Ferris J, et al. (2017) Energy drink use frequency among an international sample of people who use drugs: Associations with other substance use and well-being. Drug Alcohol Depend 174: 70–79. [DOI] [PubMed] [Google Scholar]
- Peck JA and Ranaldi R (2014) Drug abstinence: Exploring animal models and behavioral treatment strategies. Psychopharmacology 231: 2045–2058. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, et al. (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245: 1511–1513. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, et al. (2000) Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20: 4226–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto JP, Galvalisi M, López-Hill X, et al. (2015) Caffeine enhances and accelerates the expression of sensitization induced by coca paste indicating its relevance as a main adulterant. Am J Addict 24: 475–481. [DOI] [PubMed] [Google Scholar]
- Prieto JP, Scorza C, Serra GP, et al. (2016) Caffeine, a common active adulterant of cocaine, enhances the reinforcing effect of cocaine and its motivational value. Psychopharmacology 233: 2879–2889. [DOI] [PubMed] [Google Scholar]
- Rath M (2012) Energy drinks: What is all the hype? The dangers of energy drink consumption. J Am Acad Nurse Pract 24: 70–76. [DOI] [PubMed] [Google Scholar]
- Rhoads DE, Huggler AL and Rhoads LJ (2011) Acute and adaptive motor responses to caffeine in adolescent and adult rats. Pharmacol Biochem Behav 99: 81–86. [DOI] [PubMed] [Google Scholar]
- Rudolph E, Faerbinger A and Koenig J (2014) Caffeine intake from all sources in adolescents and young adults in Austria. Eur J Clin Nutr 68: 793–798. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger B and Snow S (1990) Caffeine preexposure sensitizes rats to the motor activating effects of cocaine. Behav Pharmacol 1: 447–451. [PubMed] [Google Scholar]
- Schenk S, Valadez A, Horger BA, et al. (1994) Interactions between caffeine and cocaine in tests of self-administration. Behav Pharmacol 5: 153–158. [DOI] [PubMed] [Google Scholar]
- Schenk S, Worley CM, McNamara C, et al. (1996) Acute and repeated exposure to caffeine: Effects on reinstatement of extinguished cocaine-taking behavior in rats. Psychopharmacology (Berl) 126: 17–23. [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, et al. (2001) Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci 21: RC137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, et al. (2004) Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem 11: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Ferre S, You ZB, et al. (2002) Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 22: 6321–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Jordan CJ, Kantak KM, et al. (2013) Adolescent atomoxetine treatment in a rodent model of ADHD: Effects on cocaine self-administration and dopamine transporters in frontostriatal regions. Neuropsychopharmacology 38: 2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, et al. (2003) Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421: 70–75. [DOI] [PubMed] [Google Scholar]
- Tarazi FI and Baldessarini RJ (2000) Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 18: 29–37. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC and Baldessarini RJ (1999) Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci 21: 43–49. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL and Hostetter JC (1995) Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89: 167–172. [DOI] [PubMed] [Google Scholar]
- Temple JL (2009) Caffeine use in children: What we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev 33: 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungethüm U, Chen Y, Gross J, et al. (1996) Effects of perinatal asphyxia on the mesostriatal/mesolimbic dopamine system of neonatal and 4-week-old male rats. Exp Brain Res 112: 403–410. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T and Luciana M (2010) Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev 34: 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzak WJ, Evans S, Floress MT, et al. (2011) Caffeine consumption in young children. J Pediatr 158: 508–509. [DOI] [PubMed] [Google Scholar]
- White BC and Keller GE (1984) Caffeine pretreatment: Enhancement and attenuation of d-amphetamine-induced activity. Pharmacol Biochem Behav 20: 383–386. [DOI] [PubMed] [Google Scholar]
- Worley CM, Valadez A and Schenk S (1994) Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav 48: 217–221. [DOI] [PubMed] [Google Scholar]
- Young R, Gabryszuk M and Glennon RA (1998) (−)Ephedrine and caffeine mutually potentiate one another’s amphetamine-like stimulus effects. Pharmacol Biochem Behav 61: 169–173. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, et al. (2006) Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology 31: 555–562. [DOI] [PubMed] [Google Scholar]