Abstract
Endocrine disrupting chemicals (EDCs) are implicated in the developmental mis-programming of energy metabolism. This study examined the impact of combined gestational and lactational exposure to the fungicide tolylfluanid (TF) on metabolic physiology in adult offspring. C57BL/6J dams received standard rodent chow or the same diet containing 67 mg/kg TF. Offspring growth and metabolism were assessed up to 22 weeks of age. TF-exposed offspring exhibited reduced weaning weight. Body weight among female offspring remained low throughout the study, while male offspring matched controls by 17 weeks of age. Female offspring exhibited reduced glucose tolerance, markedly enhanced systemic insulin sensitivity, reduced adiposity, and normal gluconeogenic capacity during adulthood. In contrast, male offspring exhibited impaired glucose tolerance with unchanged insulin sensitivity, no differences in adiposity, and increased gluconeogenic capacity. These data indicate that developmental exposure to the TF induces sex-specific metabolic disruptions that recapitulate key aspects of other in utero growth restriction models.
Keywords: tolylfluanid, endocrine disruptor, insulin sensitivity, glucose tolerance, perinatal, sex differences, adipose, gluconeogenesis, glucocorticoid
1. Introduction
Sex differences in metabolic disease prevalence are characterized by higher diabetes rates in males and higher obesity rates in females that are attributed to differences in physiology and environmental interactions [1, 2]. An expanding body of epidemiological and animal studies suggests that developmental exposures to endocrine disrupting chemicals (EDCs) can lead to adverse effects on metabolic physiology that differ by sex [3, 4]. Sex-specific differences in metabolic outcomes are known to occur after developmental exposure to other environmental stressors, including both overnutrition and undernutrition [1]; however, the mechanisms responsible remain poorly understood. Assessing alterations in whole-body glucose-regulating physiological parameters is essential for subsequent mechanistic delineation of how EDC exposures misprogram metabolism and increase disease risk in a sex-specific manner. The urgency to address this data gap is heightened by the fact that nearly 10% of the U.S. population has diabetes [5], and an estimated 629 million individuals across the globe are projected to have the disease by 2045 [6].
While tens of thousands of chemicals lack basic endocrine toxicological screening [7], 800–1000 compounds have already been identified as putative EDCs [8]. Among these, EDCs that modulate glucocorticoid receptor (GR) signaling remain understudied, and little is known about the long-term consequences of early-life exposure to GR-modulating EDCs despite the critical role that maternal and fetal glucocorticoids play in the development of key metabolic tissues, including pancreatic β-cells [9], adipose tissue [10], and liver [11]. In humans, prenatal treatment with pharmacological glucocorticoids administered to accelerate lung maturation has been shown to decrease birth weight [12] and may lower insulin sensitivity during adulthood, potentially with more pronounced effects in women [13]. Multiple animal studies have shown that dexamethasone (DEX) treatment during the last week of gestation promotes later-life metabolic defects, including insulin resistance and the upregulation of the hepatic gluconeogenic machinery [14–16]. While the prenatal programming of metabolic health by pharmacological glucocorticoids has been extensively studied [17, 18], large data gaps exist regarding the later-life metabolic consequences of exposure to GR-active chemicals with lower GR affinity. With at least 34 putative human GR-modulating pesticides identified [19] and relatively high glucocorticoid receptor activity detected in U.S. surface waters [20], it is essential to understand the impacts of developmental exposure to GR-modulating EDCs on metabolic physiology and long-term disease risk.
Tolylfluanid (TF) is a phenylsulfamide fungicide used in agriculture and as a booster biocide in marine paints [21]. TF has been found on agricultural goods in Europe [22–24], where it has also been detected in groundwater in agricultural regions [25]. While not approved for use in the United States, TF is permitted on imported foods. Previous studies have shown that TF activates GR signaling in adipocytes, with consequential induction of cellular insulin resistance [26–28]. Adult mice exposed to TF near the maximum U.S. tolerance limit for imported foods exhibit weight gain, glucose intolerance, insulin resistance, and disrupted circadian rhythms [21]. While the impact of dietary TF on energy homeostasis remains controversial [29], data suggest that the precise physiological effects may be nutrient-dependent [30, 31]. The present study sought to expand upon these data to ascertain the sex-specific physiological effects of perinatal exposure to TF on later-life metabolic health.
2. Material and methods
2.1. Animals, TF exposure, and tissue processing
Eight-week old C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed at 22.2 ± 1.1°C under a 12:12-hour light-dark cycle. Mating cages were arranged with one male and two females per cage. Control mating cages received ad libitum access to a standard rodent chow (Teklad Global Diet 2918, Harlan Laboratories, Madison, WI), while TF cages received the identical diet supplemented with 100 mg/kg TF added at the time of manufacturing (Harlan Laboratories, Madison, WI); diet preparation led to a final TF concentration of 67 mg/kg that was stable for 6 months at −20°C. This dose was shown to increase adiposity and lower insulin sensitivity in adult male mice in previous studies [21]. Upon confirming pregnancy by vaginal plug formation, dams were housed singly and continued on their respective diet throughout gestation and lactation until postnatal day (PD) 21. Initial breeding was performed with a 1:1.5 control:TF ratio to account for potential unknown effects of TF on fertility. The offspring analyzed in this study came from four distinct cohorts that included 29 control pregnancies and 42 TF pregnancies. Litter size and litter sex ratios were assessed at weaning; none of the litters were culled. Metabolic phenotyping was performed in a randomly selected subset of the offspring (Supplemental Figure 1). Offspring were housed by treatment and sex in groups of 2–3 littermates per cage. Offspring were handled and weighed weekly after weaning, and food was weighed and replaced weekly. All animals were treated humanely in accordance with protocols approved by the Institutional Animal Care and Use Committees at the University of Chicago and the University of Illinois at Chicago. Dams were euthanized after weaning by CO2 asphyxiation followed by cervical dislocation. Offspring were euthanized by isoflurane intoxication followed by exsanguination via cardiac puncture. Relevant metabolic tissues were dissected, weighed, flash frozen in liquid nitrogen, and stored at −80°C prior to processing.
2.2. Intraperitoneal glucose tolerance test (IP-GTT)
IP-GTT was performed at postnatal week (PW) 10 after a 6-hour fast beginning at 7:30 am as previously described [21]. Fasting blood glucose was measured using a Freestyle Lite glucometer (Abbott Laboratories, Abbott Park, IL). Plasma insulin concentrations were determined using the ALPCO mouse insulin ELISA kit per the manufacturer’s instructions (ALPCO, Salem, NH). Homeostatic model assessment of insulin resistance (IR) and β-cell function (HOMA-IR and HOMA-β, respectively) were calculated using fasting blood glucose and fasting plasma insulin levels as previously described [32].
2.3. Intraperitoneal insulin tolerance test (IP-ITT)
IP-ITT was performed at postnatal Week (PW) 16 (PW16) as previously described [21] using Humalog insulin (Eli Lilly, Indianapolis, IN) (0.4 U/kg body weight for females, 0.5 U/kg body weight for males) after a 3-hour fast beginning at 9:00 am. Mice with blood glucose readings that dropped below the limits of detection for the glucometer (20 mg/dl per the manufacturer) were confirmed on repeat testing, and if confirmed, hypoglycemic mice immediately received a rescue IP injection of dextrose. Post-hypoglycemia data points were excluded from analysis.
2.4. Intraperitoneal pyruvate tolerance test (IP-PTT)
At PW 20–22, mice were fasted for 16 hours overnight from 5:00 pm to 9:00 am. This duration of fasting has been used previously to assess gluconeogenesis [33, 34]. Fasting blood glucose was measured, followed by IP injection of sodium pyruvate (Sigma, St. Louis, MO) (1 g/kg body weight), and blood glucose was measured serially by tail vein sampling using a Freestyle Lite glucometer.
2.5. Quantitative polymerase chain reaction
RNA extraction and quantitative RT-PCR were performed as previously described [27] from mice fasted overnight using the E.Z.N.A. Total RNA Kit II (Omega Bio-tek Inc., Norcross, GA). Primer sequences (Integrated DNA Technologies, Coralville, IA) can be found in Supplemental Table 1. Gene expression levels were evaluated by the ΔΔ-Ct method [35] with GAPDH used to control for total mRNA recovery; control values were normalized to a group mean of 1.0.
2.6. Serum collection and analysis.
At the time of terminal sacrifice, blood was collected by cardiac puncture. Whole blood was collected in microfuge tubes, allowed to clot at room temperature for 45 minutes, and then centrifuged at 1500 g for 15 minutes at 4°C to collect serum. Serum was aliquoted in separate tubes to minimize repeated freezing-thawing during later analyses; samples were stored at −80°C.
2.7. Adipose tissue insulin signaling immunoblotting
At the time of tissue harvest following a 3-hour fast, perigonadal fat was assessed for insulin sensitivity by quantifying the ratio of phosphorylated-to-total Akt at the serine 473 site (S473) at insulin concentrations of 0, 1, 5, and 10 nM as previously described [27]. Mouse monoclonal anti-total Akt (40D4) at 1:750 dilution and rabbit anti-phospho-S473 Akt (D9E) at 1:500 dilution (Cell Signaling Technology, Danvers, MA) were used as primary antibodies. Goat anti-rabbit IRDye® 680RD and goat anti-mouse IRDye® 800CW (LI-COR, Inc., Lincoln, NE) were used as secondary antibodies to simultaneously image Akt and phospho-Akt. Densitometry was performed using ImageStudioLite version 5.2.5 (LI-COR, Inc., Lincoln, NE).
2.8. Pancreatic histology and immunohistochemistry
At the time of tissue harvest, the pancreas was dissected, weighed, and fixed in 4% paraformaldehyde overnight followed by paraffin embedding. Pancreas sections (5 μm in thickness) were immunostained with the following primary antibodies at 1:500 dilution: polyclonal guinea pig anti-porcine insulin (DAKO, Carpinteria, CA), mouse monoclonal anti-human glucagon (Sigma-Aldrich), polyclonal goat anti-somatostatin (Santa Cruz Biotechnology, Santa Cruz, CA), and DAPI (Invitrogen, Carlsbad, CA). The primary antibodies were detected using a combination of DyLight 488-, 549-, and 649-conjugated secondary antibodies (1:200, Jackson Immuno Research Laboratory, West Grove, PA). Antibodies used in this study have been previously validated [36].
2.9. Image capture and endocrine cell quantification
As previously described [36], microscopic images of pancreatic sections were taken with an Olympus IX8 DSU spinning disk confocal microscope (Melville, NY) with Stereo Investigator imaging software (SI; Micro Bright Field, Williston, VT). A modified method of “virtual slice capture” was used. Quantification of cellular composition (i.e., each area of β-, α-, and δ-cell populations, or sum of endocrine cell populations per islet area) was carried out using custom-written scripts for Fiji/ImageJ (https://rsbweb.nih.gov/ij/). MATLAB (MathWorks, Natick, MA) was used for mathematical analyses.
2.10. Statistics
Relative to control mice, perinatal TF exposure consistently decreased weaning weight, our primary outcome measure, with no differences in the magnitude of decrease across the cohorts (data not shown); therefore, data were pooled from all studies. In collaboration with the Statistical Laboratory at the University of Illinois at Chicago, Analysis of Response Profile, which does not make a parametric assumption on the form of the mean trajectory, was used to analyze effects on glucose tolerance, insulin sensitivity, and pyruvate tolerance using R (R Foundation for Statistical Computing, Vienna, Austria). The main goal in the Analysis of Response Profiles is to characterize the patterns of change in the mean response over time in the two groups and to determine whether the shapes of the mean response profiles do or do not differ for the two groups. The model employed for these analyses used time and treatment as main effects, and treatment-by-time interaction effects. Post-hoc unpaired t tests without assumption of consistent standard deviations were performed on datasets with different Analysis of Response Profiles to ascertain time points at which blood glucose levels were significantly different. For non-time-dependent outcomes, control and TF treatment groups were compared by F-testing to determine differences in variance; for F <0.05, t tests were performed with Welch’s correction, whereas when F >0.05, standard Student’s t tests were performed. Log-Rank test was used to compare survival curves. Data are presented as mean ± standard error of the mean (SEM). A value of P <0.05 was considered statistically significant. GraphPad Prism version 7.0 (La Jolla, CA) was used for all other comparisons.
3. Theory:
Glucocorticoids play a central role in metabolic programming, and pharmacologic treatment with glucocorticoids during development alters metabolic outcomes. This study was designed to test the hypothesis that the environmental GR-modulating EDC TF disrupts later-life metabolic homeostasis.
4. Results
4.1. Perinatal TF exposure does not alter litter size or sex-ratio
Litter size and sex ratio were assessed at weaning as a crude measure for developmental toxicity. Mean litter size (Control = 6.2, TF = 5.6) was not significantly different between groups (p = 0.31) (Figure 1A). Similarly, litter male-to-female sex ratio was also not significantly different at weaning between groups (Control = 1.7 versus TF = 1.2; p = 0.20) (Figure 1B). These data suggest that developmental exposure to TF did not result in significant gestational toxicity.
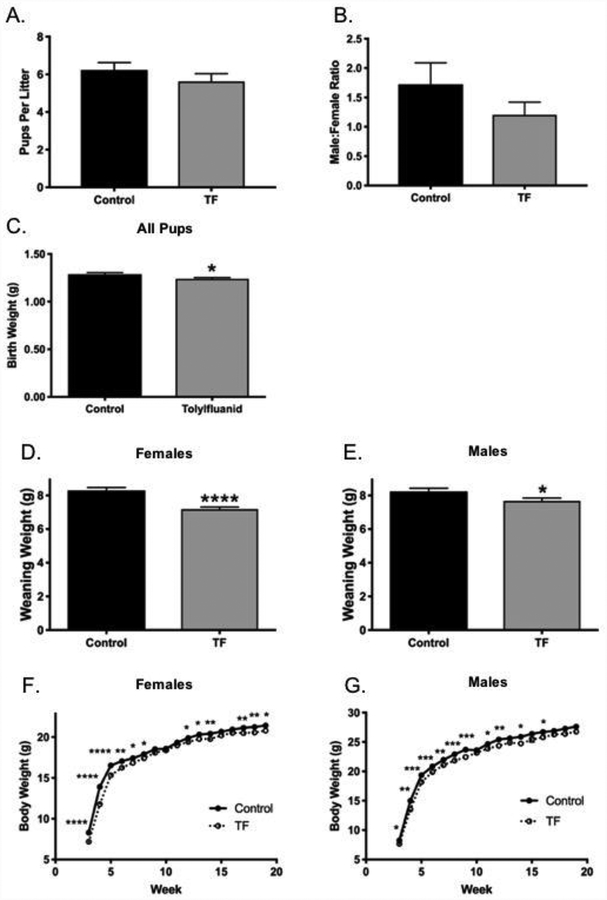
Figure 1. Litter and growth outcomes of perinatal TF exposure in female and male offspring.
Pregnant C57BL/6J mice were provided a standard chow with or without tolylfluanid (TF) at a concentration of 67 mg/kg throughout pregnancy and lactation. Litter size (Panel A) and offspring sex-ratio (Panel B) was assessed at weaning (PD21), Control n=17 litters, TF n=24 litters. Body weight was measured at birth (Panel C), Control n=30 pups, TF n=25 pups. Offspring were weaned at 3 weeks and weighed until they reached 19 weeks of age. Weaning weight for females (Panel D) and males (Panel E). Weekly body weight for females (Panel F) and males (Panel G). Control n=25, TF n=53 for female offspring weaning and weekly body weights. Control n=36, TF n=43 for male offspring weaning and weekly body weights. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
4.2. Perinatal TF exposure reduces birth weight, weaning weight, and long-term body weights in offspring
Offspring body weight was measured at birth, at weaning on PD21, and once weekly post-weaning. TF exposure lowered birthweights by 3.7%, and lowered weaning weights in females and males by 13.4% and 6.8%, respectively (Figures 1C, 1D, 1E). Female offspring from TF exposed dams had significantly lower body weight throughout the entirety of the study (Figure 1F), while male offspring from exposed dams achieved comparable weight to that of control males by 17 weeks of age (Figure 1G).
4.3. Perinatal TF exposure results in sex-specific patterns of mild glucose intolerance during acute glucose challenge
To assess whether perinatal TF exposure altered glucose homeostasis later in life, offspring underwent an IP-GTT at PW10. Perinatal TF exposure resulted in mildly impaired glucose tolerance in the offspring (Figure 2). Female offspring exposed to TF had higher blood glucose levels early after glucose challenge (i.e., 12.4% higher at 10 minutes and 12.7% higher at 20 minutes post-injection) (Figure 2A). In addition, insulin levels were 22.4% higher at 30 minutes post-glucose load compared to controls (Figure 2B). Male offspring exposed to TF had higher blood glucose levels compared to controls later during the course of the GTT (i.e. 20% higher at 40 minutes, 17.9% higher at 90 minutes, and 16.2% higher at 120 minutes post-glucose injection) (Figure 2C), without significant differences in circulating insulin levels over the first 60 minutes of the IP-GTT (Figure 2D).
Figure 2. Perinatal TF exposure impairs glucose clearance in a sex-specific manner during acute glucose challenge.
An IP-GTT was performed in the offspring at PW10 by IP injection of dextrose (2 g/kg). Serial blood glucose measurements were taken for 120 minutes in females (Panel A) and males (Panel C). Control n=20, TF n=39 for female offspring. Control n=27, TF n=33 for male offspring. Plasma insulin during IP-GTT was assessed at baseline and 10, 30, and 60 minutes after dextrose injection in females (Panel B) and males (Panel D). Control n=11, TF n=20 for female offspring; control n=20, TF n=20 for male offspring. GTT, glucose tolerance test; IP, intraperitoneal; *p<0.05; ***p<0.001.
4.4. Perinatal TF exposure increases whole-body insulin sensitivity in female offspring but not male offspring
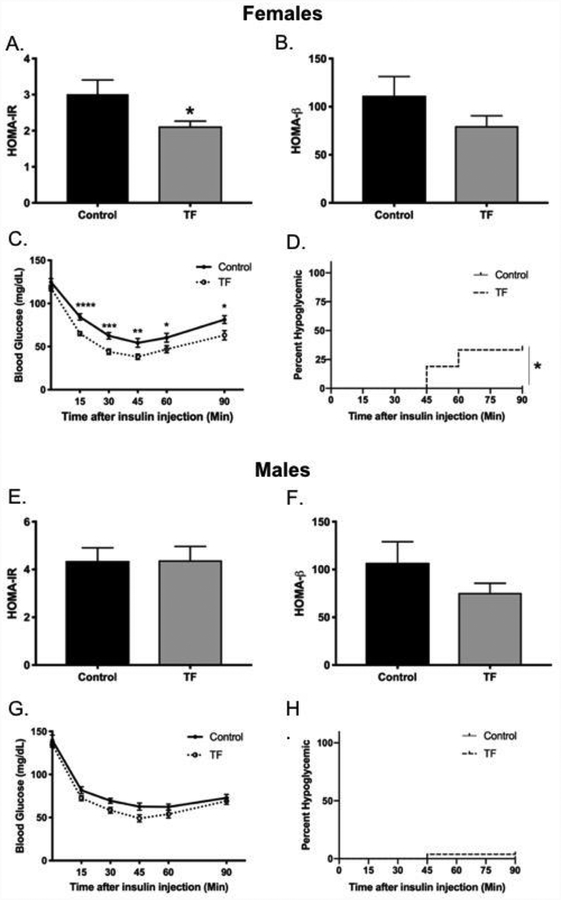
Global insulin sensitivity was assessed to determine whether the observed impairments in glucose tolerance in TF-exposed offspring were attributable to impairments in insulin action. Unexpectedly, TF-exposed female offspring showed evidence of markedly enhanced insulin sensitivity (Figure 3). At PW10, assessment of steady-state glucose-insulin homeostasis demonstrated that TF-exposed female offspring had 29.7% lower HOMA-IR (Figure 3A) without significant differences in β-cell function as assessed by HOMA-β (Figure 3B). At PW16 TF-exposed female offspring exhibited a markedly enhanced response to insulin during an IP-ITT with significantly lower blood glucose levels at every time point post insulin injection (Figure 3C). Among TF-exposed female offspring, 38% experienced severe hypoglycemia (<20 mg/dl) during the IP-ITT requiring rescue with dextrose, while no control females exhibited this trait (Control n=0; TF n=7 with 4 at 45 minutes, 3 at 60 minutes, 1 at 90 minutes) (Figure 3D). Among male offspring, there were no significant differences in HOMA-IR and HOMA-β at PW10 (Figures 3E and 3F), nor was there a difference in response to insulin during the IP-ITT at PW16 (Figure 3G). Only one exposed male offspring became hypoglycemic during the course of the IP-ITT (Control n=0; TF n=1 at 45 minutes) (Figure 3H).
Figure 3. Perinatal TF exposure results in sex-specific differences in insulin sensitivity.
HOMA-IR (Panel A) and HOMA-β (Panel B) were calculated for female offspring using fasting blood glucose and plasma insulin levels at PW10 after a 6-hour fast, (Control n=21, TF n=31). An IP-ITT was performed at PW16 by IP injection of insulin (0.4 U/kg), and serial blood glucose measured for 90 minutes in female offspring (Control n=12; TF n=21) (Panel C). HOMA-IR (Panel E) and HOMA-β (Panel F) were calculated for male offspring using fasting blood glucose and plasma insulin levels at PW 10 after a 6-hour fast (Control n=28; TF n=19). An IP-ITT was performed at PW16 of exposure by IP injection of insulin (0.5 U/kg for males), and serial blood glucose was measured for 90 minutes for male offspring (Control n=15; TF n=26) (Panel G). Survival curves for females (Panel D) and males (Panel H) show the percentage of mice that experienced severe hypoglycemia (<20 mg/dl) during IP-ITT at PW16. ITT, insulin tolerance test. *p<0.05; **p<0.01; ***p<0.001, ****p<0.0001.
4.5. Perinatal TF decreases adiposity and increases adipose tissue insulin sensitivity in female offspring but not male offspring
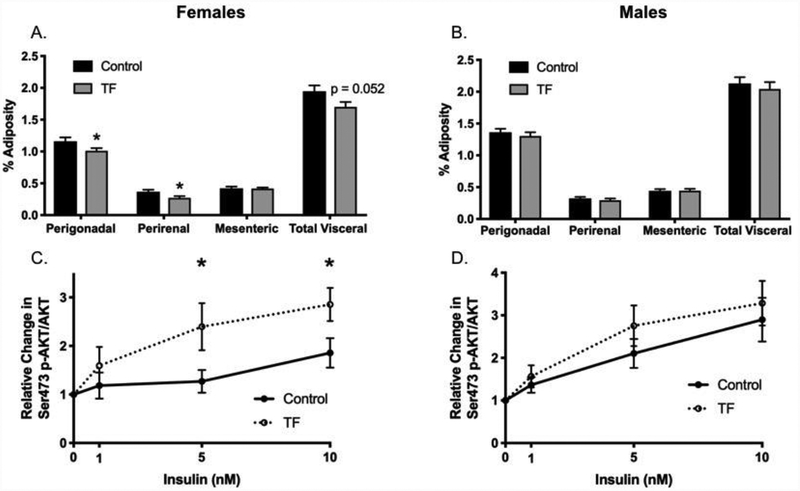
Adiposity and adipose tissue insulin sensitivity were assessed at the time of sacrifice in the offspring. Adiposity was defined as adipose tissue mass relative to total body weight for the perigonadal (epididymal or periuterine), perirenal, mesenteric, or total visceral (sum of perigonadal, perirenal, and mesenteric) depots. TF-exposed female offspring had 12.9% lower perigonadal adiposity and 26.4% lower perirenal adiposity; there were no differences in mesenteric adiposity (Figure 4A). Across all adipose depots analyzed, there was a trend toward lower total visceral adiposity at week 19 (p=0.052) (Figure 4A). Adipose insulin sensitivity was assessed ex vivo in perigonadal adipose tissue. Among TF-exposed female offspring, adipose tissue insulin sensitivity was enhanced relative to control mice with a 88.7% and 53.7% increase in Akt phosphorylation at 5 nM and 10 nM insulin, respectively (Figure 4B). To ascertain the molecular mechanism responsible for the observed enhancement in adipose insulin sensitivity, expression of insulin signaling intermediates was quantified; however, no differences were observed in the expression of the insulin receptor or insulin receptor substrate-1 (data not shown). Among male offspring, there were no differences in either depot-specific or total adiposity between control and TF-exposed mice (Figure 4C). Relative insulin-stimulated AKT phosphorylation was not significantly different between control and TF-exposed male offspring (Figure 4D).
Figure 4. Perinatal TF exposure results in sex-specific differences in adiposity and adipose insulin sensitivity.
Visceral adipose depots were collected and weighed at sacrifice (PW19). Perigonadal, perirenal, and mesenteric fat depot weight was normalized to total body weight. Total visceral adiposity was calculated by adding all of the individual depots and normalizing to body weight for female offspring (Panel A), Control n=25, TF n=50; and male offspring (Panel C), Control n=35, TF n=31. A subset of the perigonadal tissue was stimulated with different insulin concentrations for 10 minutes, and insulin sensitivity was assessed as the ratio of the band sizes of phosphorylated-to-total Akt at the S473 site for female offspring (Panel B) Control n=14, TF n=14 (except for 10 nM, n=13); and male offspring (Panel D), Control n=11, TF n=11 (except for 10 nM, n=10). *p<0.05.
4.6. Perinatal TF does not affect pancreatic endocrine cell area in exposed offspring
To ascertain whether alterations in glucose homeostasis, insulin sensitivity, and adiposity were attributable to developmental disruption of the endocrine pancreas, pancreatic α-cell, β-cell, and δ-cell areas were quantified using immunohistochemistry. There were no differences in α-cell, β-cell, δ-cell, or total islet area relative to the total pancreatic area analyzed between control and TF-exposed offspring Table 1 and Supplemental Figure 2).
Table 1. Perinatal TF exposure does not alter pancreatic endocrine cell area.
Pancreas was collected at sacrifice (PW 19). Histological slides were immunostained for insulin, glucagon, and somatostatin. β-cell, α-cell, and δ-cell areas were calculated as the percent cell area relative to the pancreatic area analyzed, and islet area was calculated as the sum of three endocrine cell types relative to analyzed area. For females, control n=9, TF n=8. For males n=9 per group.
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Control | TF | p value | Control | TF | p value | |
| β-cell area (%) | 0.39 ± 0.07 | 0.28 ± 0.05 | 0.23 | 0.22 ± 0.05 | 0.33 ± 0.04 | 0.11 |
| α-cell area (%) | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.67 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.35 |
| δ-cell area (%) | 0.15 ± 0.07 | 0.29 ± 0.22 | 0.57 | 0.09 ± 0.04 | 0.04 ± 0.02 | 0.34 |
| Islet area (%) | 0.61 ± 0.12 | 0.65 ± 0.21 | 0.87 | 0.36 ± 0.09 | 0.41 ± 0.07 | 0.64 |
4.7. Perinatal TF exposure results in sex-specific changes in hepatic gluconeogenic capacity in exposed offspring
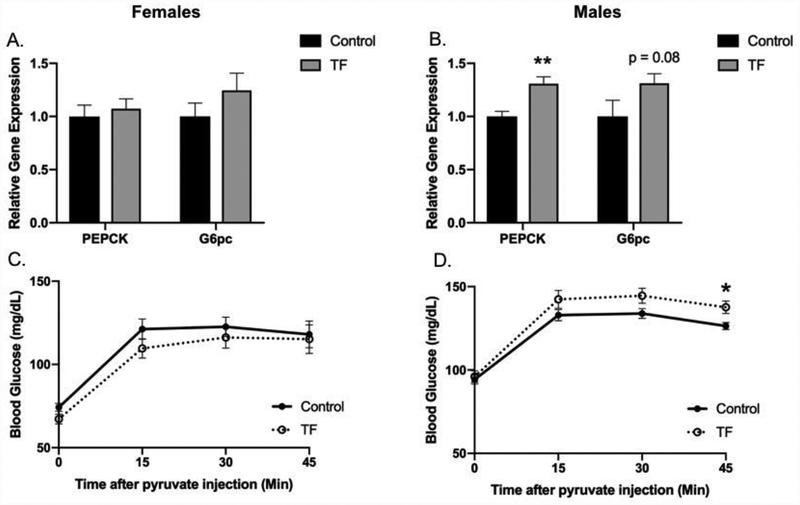
On PW4 offspring were fasted overnight and hepatic expression of genes regulating gluconeogenesis was measured. Compared to control male offspring, male offspring perinatally exposed to TF had 30.7% higher hepatic phosphoenolpyruvate carboxykinase (PEPCK) gene expression following overnight fasting with a trend toward higher expression of glucose-6-phosphatase (G6PC) (p=0.08) (Figure 5A). There were no differences in PEPCK or G6PC gene expression between female offspring (Figure 5B). Fasting circulating corticosterone, a key gluconeogenesis-stimulating hormone, was not different between groups in either sex (data not shown). Dynamic differences in gluconeogenic capacity among offspring were assessed by IP-PTT at PW20–22 to ascertain whether the observed differences in gene expression early in life had lasting physiological effects during adulthood. Male offspring perinatally exposed to TF showed higher blood glucose levels during the pyruvate challenge (p=0.047), (Figure 5C), while there were no differences in circulating glucose in female offspring during the pyruvate challenge (Figure 5D).
Figure 5. Perinatal TF exposure results in sex-specific differences in gluconeogenic capacity.
Hepatic gene expression was measured by qPCR at PW4 after an overnight fast (16 hours) for females (Panel A) (Control n=9–10, TF n=10) and males (Panel B) (control n=9–10, TF n=12) per group. An IP-PTT was performed at PW20–22 after an overnight fast by IP injection of sodium pyruvate (1 g/kg) and serial blood glucose measured for 75 minutes in female offspring (Panel C) (n=5 per group) and male offspring (Panel D) (n=10 per group). PTT, pyruvate tolerance test; PEPCK, phosphoenolpyruvate carboxykinase; G6pc, glucose-6-phosphatase; *p<0.05; **p<0.01.
5. Discussion
Informed by previous work demonstrating that dietary exposure to TF increased adiposity and decreased systemic and adipose-specific insulin sensitivity in adult male mice [21], the current study examined whether similar exposures during development elicited comparable metabolic derangements in offspring. Of note, the TF dose used herein did not alter litter size or sex ratio at the time of weaning, suggesting a lack of overt toxicity to developing fetuses. However, perinatal TF exposure lowered birthweight and reduced weaning weight in both female and male offspring, indicating that exposed offspring did not catch-up in growth by the time of weaning. Reduced birth weight results from impaired fetal growth that reflects an adverse intrauterine environment and is a known risk factor for later-life cardiometabolic disease [37]. Indeed, the present studies demonstrate sex-specific metabolic impairments in the adult offspring of TF-exposed dams, including mild impairments in glucose tolerance without disruptions in insulin secretion or endocrine pancreas morphology. In addition, female offspring exhibited decreased adiposity and markedly enhanced global insulin sensitivity, while gluconeogenic capacity was enhanced in TF-exposed male offspring. Collectively, these findings add to the growing body of research demonstrating sex-specific and long-term alterations in metabolic physiology caused by early-life exposures to EDCs [4].
Perinatal TF exposure resulted in modest, sexually-dimorphic impairments in whole-body glucose clearance during an acute glucose challenge. Despite relatively higher insulin sensitivity at steady-state (as assessed by HOMA-IR), exposed female offspring exhibited elevated blood glucose levels relative to controls during the early phase of the glucose tolerance test. Importantly, the observed glucose intolerance among TF-exposed females was not due to impairments in insulin action as TF-exposed females were markedly more insulin sensitive than control mice. In evaluating insulin-glucose dynamics in this study, the modest blood glucose elevation in females was noted to normalize after 30 minutes when circulating insulin was significantly higher for exposed females. This suggests that hyperglycemia may have arisen from relative reductions or delays in insulin release from pancreatic β-cells. Of note, however, TF exposure did not result in morphological changes in the endocrine pancreas, suggesting overt β-cell toxicity was not responsible for the observed findings.
In contrast to females, exposed male offspring experienced a more pronounced impairment in glucose clearance that was notable at later time points post-glucose challenge. Importantly, this hyperglycemia was not accompanied by compensatory β-cell insulin hypersecretion as seen in TF-exposed female offspring. Furthermore, the observed impairment in glucose clearance was not due to diminished systemic insulin action. Thus, we hypothesized that increased or sustained hepatic glucose output may have contributed to hyperglycemia in TF-exposed male offspring. Indeed, male TF-exposed offspring exhibited enhanced hepatic PEPCK gene expression at PW4, suggesting an upregulation of the gluconeogenic machinery early in life that may persist into later-life. Indeed, with provocative testing TF-exposed male offspring exhibited enhanced gluconeogenic capacity as evidenced by higher hepatic glucose production following pyruvate challenge. Importantly, this was a sex-dependent effect as exposed female offspring did not exhibit these changes. Taken together, these data indicate that the path to glucose intolerance among mice exposed perinatally to TF is sexually dimorphic.
The observed effects of developmental TF exposure on insulin sensitivity and adiposity in female offspring coincide with results from previous in utero growth restriction studies demonstrating that prenatal growth restriction that is not followed by catch-up growth is accompanied by increased insulin sensitivity and decreased adiposity [38–47]. This enhancement of systemic insulin sensitivity is mirrored at the tissue level by enhanced adipose insulin sensitivity resulting from molecular effects upstream of Akt; however, our studies were unable to identify changes in insulin signaling intermediates to explain this phenomenon (data not shown). One possibility is that insulin sensitivity is tuned to be more responsive through the differential phosphorylation of insulin receptor substrates [48]; however, further molecular analyses will be required to test this hypothesis. Interestingly, evidence from rat studies suggest that compensatory increases in global insulin sensitivity following developmental growth-inhibiting insults are followed by later-life onset of insulin resistance and metabolic deterioration [41, 49–51]. For example, in rat models of uteroplacental insufficiency, female offspring exhibited enhanced insulin sensitivity at up to 12 months of age [42]; however, age-related deterioration in metabolic health and the development of insulin resistance was observed at 21 months of age [51]. The current study followed the exposed offspring for up to 22 weeks of age, which is within the timeframe during which other rodent models of growth restriction have observed enhanced insulin sensitivity [38–43]. Whether mice developmentally exposed to TF ultimately go on to develop insulin resistance and worsened glucose intolerance with aging requires additional study.
Importantly, the current study adds to the growing body of evidence implicating hepatic gluconeogenesis as a sensitive metabolic endpoint for developmental misprogramming [14–16, 52, 53]. The upregulation of gluconeogenesis with antecedent increased PEPCK expression in TF-exposed male offspring is consistent with several studies in rats demonstrating that gluconeogenesis in male offspring is disrupted by either glucocorticoid exposure or protein restriction during development [14–16, 52, 53]. Importantly, in the present studies PEPCK upregulation was not due to enhanced corticosterone secretion (data not shown), suggesting that another mechanism is responsible, including potential epigenetic alterations; however, this hypothesis requires further study. Interestingly, our study suggests that gluconeogenic capacity in exposed female offspring trends lower and that these mice are more prone to hypoglycemia with insulin challenge. The reasons behind this sex-specific defect in counterregulation needs further study.
Critically, the sex-specific differences in effects on growth, insulin sensitivity, glucose tolerance, adiposity, and hepatic glucose output resulting from perinatal TF exposure highlights the importance of studying males and females in developmental studies that assess GR signaling modulation, especially since most of the prenatal studies published to date have focused on male offspring [14–16, 52, 53]. The current study adds to this area of study by demonstrating that perinatal exposure to a fungicide that alters GR signaling also has lasting effects on growth and metabolism. As evidence continues to emerge suggesting that a subset of EDCs modulate GR signaling, the current studies provide a useful framework for interrogated the long-term metabolic consequences of developmental exposure to these agents.
Despite the strengths of the current study, work presented herein has several limitations. These studies only examined one concentration of TF; thus, assessments of dose-response relationships are not possible. In addition, these studies used the inbred C57BL/6J mice strain commonly employed in metabolic disease research. While our previous work demonstrated similar effects of TF on adipocytic insulin signaling in four different rodent strains, including the C57BL/6J, as well as in human adipose tissue [54], developmental programming is complex, and the relative lack of genetic diversity in this strain may limit the generalizability of the findings. The present work only followed offspring for 20–22 weeks after birth; thus, it remains unknown whether further aging would unmask metabolic deterioration in exposed mice as in other growth restriction models. While this work found enhanced adipose-specific insulin sensitivity that could partly explain the increase in whole body insulin sensitivity in exposed female offspring, additional mechanistic studies are needed to identify the molecular bases for the physiological alterations identified herein. Finally, it is also possible that the observed effects of TF could be mediated by alterations in maternal metabolism. Based on data that exposure of adult male mice to TF did not alter adiposity, glucose tolerance, or insulin sensitivity until 8 weeks of exposure [21], it does not seem likely that this was a major driver of the observed effects; however, this requires formal assessment. Despite these limitations the observed phenotypic disruptions in body weight, adiposity, insulin sensitivity, and gluconeogenesis induced by TF reflect growth and metabolic changes observed in other developmental stress models including prenatal DEX exposure, in utero protein restriction, or uterine artery ligation studies [38–43]. Future studies are warranted to elucidate the common molecular mechanisms by which these diverse stressors impact long-term metabolic health.
6. Conclusion
The present study provides further evidence that sex-specific alterations in metabolic physiology can be programmed by environmental insults during periods of enhanced susceptibility, including in utero and early post-natal life. Furthermore, these results add to existing evidence that developmental stressors that induce growth restriction without catch-up growth increase insulin sensitivity. Finally, this study provides insights into how early life GR-modulating EDC exposure abnormally programs metabolic physiology later in life. These data underscore the importance of early developmental windows in long-term metabolic health and highlight the need to address maternal and early childhood exposure to metabolism-disrupting chemicals.
Supplementary Material
Highlights.
Tolylfluanid (TF) is a fungicide that disrupts glucocorticoid receptor signaling.
Gestational plus lactational exposure to TF disrupts metabolism in adult offspring.
The metabolic impact of developmental TF exposure is sex-specific.
Acknowledgments
The authors wish to thank Matthew J. Brady, PhD, and Rhonda Kineman, PhD for their feedback and support.
Funding
This work was supported by the National Institutes of Health [grant numbers K08 ES019176, R21 ES021354, R01 ES028879, T32 HD007009, P60-DK020595, and P30 ES027792].
Abbreviations:
- DEX
dexamethasone
- EDCs
endocrine-disrupting chemicals
- GR
glucocorticoid receptor
- IP-GTT
intraperitoneal glucose tolerance test
- IP-ITT
intraperitoneal insulin tolerance test
- IP-PTT
intraperitoneal pyruvate tolerance test
- PD
postnatal day
- PW
postnatal week
- TF
tolylfluanid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
RMS has received honoraria from CVS Health and American Medical Forum. These activities are not related to the studies presented herein.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Dearden L, Bouret SG, Ozanne SEJMm (2018) Sex and gender differences in developmental programming of metabolism. 15: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mauvais-Jarvis F, Arnold AP, Reue KJCm (2017) A guide for the design of pre-clinical studies on sex differences in metabolism. 25(6): 1216–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanson MA, Gluckman PD (2008) Developmental origins of health and disease: new insights. Basic & clinical pharmacology & toxicology 102(2): 90–93 [DOI] [PubMed] [Google Scholar]
- [4].Heindel JJ, Blumberg B, Cave M, et al. (2017) Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology 68: 3–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].CDC (2014) National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. In: Atlanta, GA: US Department of health and human services [Google Scholar]
- [6].International Diabetes Federation (2017) IDF Diabetes Atlas, 8th Edition Available from https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html. Accessed 01/15/2018 2018 [Google Scholar]
- [7].Judson R, Richard A, Dix DJ, et al. (2009) The toxicity data landscape for environmental chemicals. Environ Health Perspect 117(5): 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bergman A, Heindel JJ, Kasten T, et al. (2013) The impact of endocrine disruption a consensus statement on the state of the science. Environmental health perspectives 121(4): A104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gesina E, Tronche F, Herrera P, et al. (2004) Dissecting the role of glucocorticoids on pancreas development. Diabetes 53(9): 2322–2329 [DOI] [PubMed] [Google Scholar]
- [10].Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nature reviews Molecular cell biology 7(12): 885–896 [DOI] [PubMed] [Google Scholar]
- [11].Kamiya A, Kinoshita T, Ito Y, et al. (1999) Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. The EMBO journal 18(8): 2127–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bloom SL, Sheffield JS, McIntire DD, Leveno KJ (2001) Antenatal dexamethasone and decreased birth weight. Obstetrics & Gynecology 97(4): 485–490 [DOI] [PubMed] [Google Scholar]
- [13].Dalziel SR, Walker NK, Parag V, et al. (2005) Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. The Lancet 365(9474): 1856–1862 [DOI] [PubMed] [Google Scholar]
- [14].Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR (1998) Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. Journal of Clinical Investigation 101(10): 2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Somm E, Vauthay DM, Guérardel A, et al. (2012) Early metabolic defects in dexamethasone-exposed and undernourished intrauterine growth restricted rats. PloS one 7(11): e50131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].O’Regan D, Kenyon C, Seckl J, Holmes M (2004) Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. American Journal of Physiology-Endocrinology and Metabolism 287(5): E863–E870 [DOI] [PubMed] [Google Scholar]
- [17].Fowden AL, Forhead AJ (2015) Glucocorticoids as regulatory signals during intrauterine development. Experimental physiology 100(12): 1477–1487 [DOI] [PubMed] [Google Scholar]
- [18].Fowden A, Valenzuela O, Vaughan O, Jellyman J, Forhead A (2016) Glucocorticoid programming of intrauterine development. Domestic animal endocrinology 56: S121–S132 [DOI] [PubMed] [Google Scholar]
- [19].Zhang J, Zhang J, Liu R, Gan J, Liu J, Liu W (2015) Endocrine-Disrupting Effects of Pesticides through Interference with Human Glucocorticoid Receptor. Environmental science & technology 50(1): 435–443 [DOI] [PubMed] [Google Scholar]
- [20].Conley JM, Evans N, Cardon MC, et al. (2017) Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environmental science & technology 51(9): 4781–4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Regnier SM, Kirkley AG, Ye H, et al. (2014) Dietary Exposure to the Endocrine Disruptor Tolylfluanid Promotes Global Metabolic Dysfunction in Male Mice. Endocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Česnik HB, Gregorčič A, Bolta ŠV, Kmecl V (2006) Monitoring of pesticide residues in apples, lettuce and potato of the Slovene origin, 2001–04. Food additives and contaminants 23(2): 164–173 [DOI] [PubMed] [Google Scholar]
- [23].Stensvand A, Christiansen A (2000) Investigation on fungicide residues in greenhouse-grown strawberries. Journal of agricultural and food chemistry 48(3): 917–920 [DOI] [PubMed] [Google Scholar]
- [24].Sadło S, Szpyrka E, Jaźwa A, Zawiślak A (2007) Pesticide Residues in Fruit and Vegetables from Southeastern Poland, 2004–05. Polish Journal of Environmental Studies 16(2) [Google Scholar]
- [25].Reemtsma T, Alder L, Banasiak U (2013) Emerging pesticide metabolites in groundwater and surface water as determined by the application of a multimethod for 150 pesticide metabolites. Water research 47(15): 5535–5545 [DOI] [PubMed] [Google Scholar]
- [26].Neel BA, Brady MJ, Sargis RM (2013) The endocrine disrupting chemical tolylfluanid alters adipocyte metabolism via glucocorticoid receptor activation. Molecular endocrinology 27(3): 394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sargis RM, Neel BA, Brock CO, et al. (2012) The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1822(6): 952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sargis RM, Johnson DN, Choudhury RA, Brady MJ (2010) Environmental Endocrine Disruptors Promote Adipogenesis in the 3T3-L1 Cell Line through Glucocorticoid Receptor Activation. Obesity (Silver Spring, Md) 18(7): 1283–1288. 10.1038/oby.2009.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, McCommis KS, Ferguson D, Hall AM, Harris CA, Finck BNJE(2017) Inhibition of the mitochondrial pyruvate carrier by tolylfluanid. 159(2): 609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Regnier SM, Kirkley AG, Ruiz D, et al. (2018) Diet-dependence of metabolic perturbations mediated by the endocrine disruptor tolylfluanid. 7(1): 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].A’Lexxus FD, Thomas AA, Shorter KS, Brown SL, Baumgarner BLJB, communications br (2018) Cellular fatty acid level regulates the effect of tolylfluanid on mitochondrial dysfunction and insulin sensitivity in C2C12 skeletal myotubes. 505(2): 392–398 [DOI] [PubMed] [Google Scholar]
- [32].Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7): 412–419 [DOI] [PubMed] [Google Scholar]
- [33].McCommis KS, Chen Z, Fu X, et al. (2015) Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling. Cell metabolism 22(4): 682–694. 10.1016/j.cmet.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pusec CM, De Jesus A, Khan MW, et al. (2019) Hepatic HKDC1 Expression Contributes to Liver Metabolism. Endocrinology 160(2): 313–330. 10.1210/en.2018-00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nature protocols 3(6): 1101–1108 [DOI] [PubMed] [Google Scholar]
- [36].Poudel A, Fowler JL, Zielinski MC, Kilimnik G, Hara MJ Sr (2016) Stereological analyses of the whole human pancreas. 6: 34049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Braun T, Challis JR, Newnham JP, Sloboda DM (2013) Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocrine reviews 34(6): 885–916 [DOI] [PubMed] [Google Scholar]
- [38].Agnoux AM, Antignac J-P, Simard G, et al. (2014) Time-window dependent effect of perinatal maternal protein restriction on insulin sensitivity and energy substrate oxidation in adult male offspring. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology: ajpregu. 00015.02014 [DOI] [PubMed] [Google Scholar]
- [39].Ozanne S, Wang C, Coleman N, Smith G (1996) Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. American Journal of Physiology-Endocrinology And Metabolism 271(6): E1128–E1134 [DOI] [PubMed] [Google Scholar]
- [40].Shepherd P, Crowther N, Desai M, Hales C, Ozanne S (1997) Altered adipocyte properties in the offspring of protein malnourished rats. British Journal of Nutrition 78(1): 121–129 [DOI] [PubMed] [Google Scholar]
- [41].Hales C, Desai M, Ozanne S, Crowther N (1996) Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. In. Portland Press Limited; [DOI] [PubMed] [Google Scholar]
- [42].Tran M, Young ME, Jefferies AJ, et al. (2015) Uteroplacental insufficiency leads to hypertension, but not glucose intolerance or impaired skeletal muscle mitochondrial biogenesis, in 12‐month‐old rats. Physiological reports 3(9): e12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gallo LA, Tran M, Moritz KM, et al. (2012) Cardio‐renal and metabolic adaptations during pregnancy in female rats born small: implications for maternal health and second generation fetal growth. The Journal of physiology 590(3): 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Berends L, Fernandez-Twinn D, Martin-Gronert M, Cripps R, Ozanne S (2013) Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. International journal of obesity (2005) 37(8): 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mericq V, Ong K, Bazaes R, et al. (2005) Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small-and appropriate-for-gestational-age children. Diabetologia 48(12): 2609–2614 [DOI] [PubMed] [Google Scholar]
- [46].Soto N, Bazaes RA, Peña V, et al. (2003) Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. The Journal of Clinical Endocrinology & Metabolism 88(8): 3645–3650 [DOI] [PubMed] [Google Scholar]
- [47].Bazaes RA, Salazar TE, Pittaluga E, et al. (2003) Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics 111(4): 804–809 [DOI] [PubMed] [Google Scholar]
- [48].Sun XJ, Liu F (2009) Phosphorylation of IRS proteins Yin-Yang regulation of insulin signaling. Vitam Horm 80: 351–387. S0083–6729(08)00613–4 [pii] 10.1016/S0083-6729(08)00613-4 [DOI] [PubMed] [Google Scholar]
- [49].Ozanne S, Dorling M, Wang C, Nave B (2001) Impaired PI 3-kinase activation in adipocytes from early growth-restricted male rats. American Journal of Physiology-Endocrinology And Metabolism 280(3): E534–E539 [DOI] [PubMed] [Google Scholar]
- [50].Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN (2001) Diabetes in old male offspring of rat dams fed a reduced protein diet. Experimental Diabetes Research 2(2): 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fernandez-Twinn D, Wayman A, Ekizoglou S, Martin M, Hales C, Ozanne S (2005) Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 288(2): R368–R373 [DOI] [PubMed] [Google Scholar]
- [52].Franko KL, Forhead A, Fowden A (2010) Differential effects of prenatal stress and glucocorticoid administration on postnatal growth and glucose metabolism in rats. Journal of Endocrinology 204(3): 319–329 [DOI] [PubMed] [Google Scholar]
- [53].Pantaleão LC, Murata G, Teixeira CJ, et al. (2017) Prolonged fasting elicits increased hepatic triglyceride accumulation in rats born to dexamethasone-treated mothers. Scientific Reports 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sargis RM, Neel BA, Brock CO, et al. (2012) The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim Biophys Acta 1822: 952–960. S0925–4439(12)00043–9 [pii] 10.1016/j.bbadis.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.