Abstract
Human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND) persist despite effective antiretroviral therapies (ART). Evidence suggests that modern HAND is driven by subtle synaptodendritic damage in select brain regions, as ART-treated patients do not display overt neuronal death in postmortem brain studies. HAND symptoms are also aggravated by drug abuse, particularly with injection opioids. Opioid use produces region-specific synaptodendritic damage in similar brain regions, suggesting a convergent mechanism that may enhance HAND progression in opioid-using patients. Importantly, studies indicate that synaptodendritic damage and cognitive impairment in HAND may be reversible. Activation of the homeostatic chemokine receptor CXCR4 by its natural ligand CXCL12 positively regulates neuronal survival and dendritic spine density in cortical neurons, reducing functional deficits. However, the molecular mechanisms that underlie CXCR4, as well as opioid-mediated regulation of dendritic spines are not completely defined. Here, we will consolidate studies that describe the region-specific synaptodendritic damage in the cerebral cortex of patients and animal models of HAND, describe the pathways by which opioids may contribute to cortical synaptodendritic damage, and discuss the prospects of using the CXCR4 signaling pathway to identify new approaches to reverse dendritic spine deficits. Additionally, we will discuss novel research questions that have emerged from recent studies of CXCR4 and μ-opioid actions in the cortex. Understanding the pathways that underlie synaptodendritic damage and rescue are necessary for developing novel, effective therapeutics for this growing patient population.
Keywords: HAND, neuroHIV, dendritic spines, opioids, CXCR4, CXCL12
1. Introduction
Human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND) are a group of neurological disorders resulting from HIV infection of select central nervous system (CNS) cells and chronic low-level CNS inflammation, often in combination with other comorbid factors (Saylor et al., 2016). Progression to the most severe forms of HAND has been sharply decreased by antiretroviral therapies (ART) (Heaton et al., 2010), which transformed HIV infection into a chronic illness by effectively controlling viral replication. However, the overall prevalence of HAND in patients on ART has remained roughly similar to that of the pre-ART era, and HAND prevalence is expected to rise as ART-treated patients age (Tozzi et al., 2007; Heaton et al., 2011; Saylor et al., 2016). The majority of ART-treated patients with HAND present with asymptomatic or mild symptoms, showing deficits in cognitive domains including working memory, executive function, learning, information processing speed, and fine motor coordination (Eggers et al., 2017; Sacktor, 2018). Asymptomatic forms of HAND, which are determined by neurocognitive testing, remain important as they increase the risk of cognitive decline and disease progression in aging patients (Grant et al., 2014). Unfortunately, all clinical trials testing adjuvant therapies for HAND have not shown clinical efficacy (McGuire et al., 2014), leaving HAND as a major therapeutic hurdle in the ART era. Failure of these trials suggests that our understanding of HAND is insufficient, and continued research is necessary to uncover the pathophysiological mechanisms underlying HAND, and to identify novel molecular targets for future drug development.
ART-treated patients with HAND generally do not display overt neuronal death or neurodegeneration (Gelman, 2015). Instead, subtle synaptodendritic injury in specific brain areas more likely underlies the neurocognitive symptoms of HAND in treated patients (Masliah et al., 1997; Everall et al., 1999; Ellis et al., 2007). Although most studies examined brain tissue from patients with poorly controlled infection, advanced neuroimaging studies in virally suppressed patients suggest that brain inflammation and synaptic deficits persist (Harezlak et al., 2011; Ances and Hammoud, 2014; Strain et al., 2017; Sanford et al., 2018). Nevertheless, it is crucial that future studies examine brain tissue from ART-treated patients with well-controlled infection to examine region-specific and subtle synaptodendritic injury. Synaptic injury involves dendritic spines, which are protrusions that often host the postsynaptic compartment of an excitatory synapse (Nimchinsky et al., 2002). Dendritic spines are key mediators of learning and memory processes, and dendritic spine deficits in brain regions including the prefrontal cortex (PFC) often predict impairment in specific cognitive domains (Morrison and Baxter, 2012). In patients with HAND, dendritic spine deficits in critical brain regions may be driven by multiple factors, including chronic neuroinflammation (Spudich, 2016), low-level expression of neurotoxic HIV proteins (Ellis et al., 2007; Hu, 2016; Smith et al., 2018), aging of ART-treated patients (Levine et al., 2016; Cole et al., 2017; Saloner et al., 2019), and even neurotoxic side-effects of some ART (Dalwadi et al., 2018; Stern et al., 2018). Cognitive symptoms are also exacerbated by illicit drug abuse, including injection opioids (Byrd et al., 2011). Sharing injection equipment is a major vector for the spread of HIV, and infected individuals are much more likely than the general population to inject opioid drugs (Degenhardt et al., 2013; Reddon et al., 2019). In line with the hypothesis that HAND manifests due to subtle damage to the dendritic arbor, opioids also aggravate synaptodendritic injury from HIV proteins or inflammatory molecules in vivo and in vitro (Fitting et al., 2010; Fitting et al., 2014). Therefore, understanding how opioid use contributes to synaptodendritic injury in HAND is a necessary step in the process of developing effective therapies for these patients.
Recent work from our lab and others suggests that cortical dendritic spine deficits and cognitive impairment are reversible (Nightingale et al., 2014; Saylor et al., 2016), likely through activation of the chemokine receptor CXCR4 by its natural ligand CXCL12 (Pitcher et al., 2014; Capsoni et al., 2017; Festa et al., 2018). Chemokine signaling is often considered for its roles in the immune system and cellular chemotaxis, but CXCR4 signaling additionally promotes neuroprotective and homeostatic processes in the CNS (Li and Ransohoff, 2008). These processes include survival signaling (Meucci et al., 1998; Khan et al., 2008; Nicolai et al., 2010), neuron-glia communication (Guyon, 2014), modulation of neurotransmission (Guyon and Nahon, 2007), inhibitory synapse formation (Wu et al., 2017), and regulation of cortical dendritic spines (Pitcher et al., 2014). Several studies show that CXCR4 signaling in the CNS is disrupted by HIV infection and μ-opioid agonists, which may partially contribute to synaptodendritic injury (Festa and Meucci, 2012; Festa et al., 2015). Here, we will discuss published and emerging evidence of region-specific synaptodendritic damage in HAND patients and animal models, as well as how opioid and CXCR4 signaling affects dendritic spines in specific cortical regions.
2. Dendritic Spines
Dendritic spines are characterized by their morphology (Figure 1), which reflects the maturity of the spine, and its integration into the local neuronal circuit (Nimchinsky et al., 2002; Berry and Nedivi, 2017). Spines may arise directly from the dendritic shaft (Lai and Ip, 2013) or from long and thin processes called filopodia (Yuste and Bonhoeffer, 2004). Filopodia lack much of the actin cytoskeleton and protein expression needed to become a stable, mature spine, but they may form synapses with other neurons and stabilize over time (Zuo et al., 2005). After a filopodia forms a synapse, it may develop into a thin spine, which is the first mature type of spine. Thin spines are characterized by a long neck region and a small head region with a post-synaptic density containing NMDA receptors and few AMPA receptors (Ganeshina et al., 2004). Although thin spines are more stable than filopodia, they remain somewhat transient and may retract in the absence of neuronal activity or in response to other stimuli (Holtmaat et al., 2005). Thin spines are thought to be involved in short-term cognitive processing and flexibility, as learning tasks often remodel thin spine organization and density in various brain areas (Dumitriu et al., 2010; Motley et al., 2018), and thin spine density correlates with synaptic plasticity and learning, particularly in the aged brain (Hao et al., 2006). A thin spine may further mature into a mushroom spine, which is the most stable type of dendritic spine (Zuo et al., 2005). Mushroom spines have a smaller neck region than a thin spine but have a larger head region and post-synaptic density containing AMPA receptors (Matsuzaki et al., 2001). The long-term stability of mushroom spines suggests that they are involved in long-term cognitive processing, including memory and recall (Bourne and Harris, 2007). Lastly, stubby spines are considered immature spines that lack a neck region (Harris et al., 1992). As the neck of a dendritic spine effectively isolates post-synaptic calcium influx from the dendritic shaft (Noguchi et al., 2005), stubby spines may lack the calcium buffering capacity of other mature spine types. Therefore, any disease state that increases stubby spine density may result in excessive calcium influx into the dendrite, inappropriate activation of dendritic calcium signaling pathways, and potentially excitotoxicity.
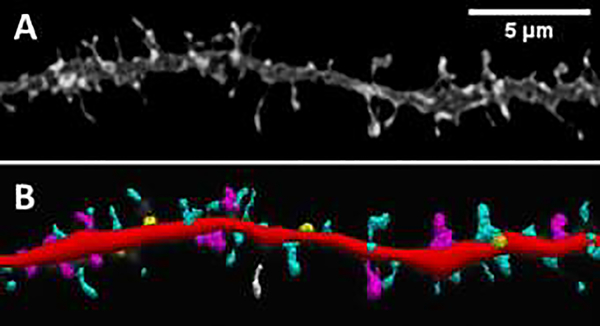
Figure 1.
Dendritic spine morphologies
A. Grayscale micrograph of a dendrite from the rat layer 2/3 medial prefrontal cortex that was stained with DiI, a lipophilic fluorescent dye that labels dendritic spines (Seabold et al., 2010). B. The spines on this dendrite can be characterized into different morphological classes using Neurolucida 360 software (Rodriguez et al., 2008): Dendrite (red), filopodia (white), thin spines (cyan), mushroom spines (magenta), and stubby spines (yellow).
3. Region-specific synaptic injury in HAND patients and animal models
Evidence suggests that the PFC, as well as other frontal cortex areas are altered in patients with HAND (Guha et al., 2016; Sanford et al., 2017). The PFC integrates signaling from many different brain areas and plays important roles in cognitive processes including learning and memory, executive function, cognitive flexibility, and selective attention (Euston et al., 2012; Lew et al., 2018). HAND patients may display decreased resting state functional connectivity (Ann et al., 2016; McIntosh et al., 2017), thinning of PFC areas (Thompson et al., 2005), increased expression of interferon and proinflammatory cytokine genes (Sanna et al., 2017), and abnormalities in neurotransmitter systems (Gelman et al., 2012; Buzhdygan et al., 2016). All of these may correlate with synaptodendritic injury in the PFC and HAND progression. Imaging studies of postmortem brain tissue from HIV-infected patients have also shown synaptic injury in other brain regions that underlie learning and memory processes, including the striatum and hippocampus (Sa et al., 2004; Moore et al., 2006), suggesting that specific areas of the brain are more vulnerable to injury during HIV infection. In both HAND patients and animal models of HAND, dendritic spine density in frontal cortex regions is often significantly reduced (Everall et al., 1999; Festa et al., 2015; McLaurin et al., 2019).
Much of the work on understanding how HIV infection contributes to dendritic spine deficits has utilized in vitro or animal models, including rodent models. Among these, the HIV-transgenic rat (HIV-Tg) has recently received increased attention as it recapitulates pathological features of virally suppressed patients (Li et al., 2013; Repunte-Canonigo et al., 2014; Festa et al., 2015; Vigorito et al., 2015). The HIV-Tg rat constitutively expresses all HIV proteins except gag and pol, which are required to produce infectious viral particles (Reid et al., 2001). Therefore, the non-infectious HIV-Tg rat somewhat models the pathology of an ART-treated patient, where viral replication is not detectable. Our group and others have shown that HIV-Tg rats have reduced dendritic spine density in layer 2/3 neurons of the medial PFC (mPFC) (Festa et al., 2015; McLaurin et al., 2019). Spine deficits mostly occur on proximal dendrites and sex differences in learning tasks were reported (McLaurin et al., 2019). Other studies demonstrated similar alterations in specific brain regions and associated behavioral outcomes (Wayman et al., 2016; Casas et al., 2018; Ohene-Nyako et al., 2018). Interestingly, our group has also observed changes in the morphology of mPFC dendritic spines, which underlie cognitive impairment (Festa et al., 2018; Festa et al., manuscript under revision). Increased density of immature spines in HIV-Tg rats suggests that their PFC neurons are more vulnerable to excessive calcium signaling and excitotoxicity. In line with this, controlling calcium flux in HIV-Tg rat mPFC neurons through modulation of NMDA and L-type calcium channels can be neuroprotective (Khodr et al., 2018). Additionally, dendritic spine changes in HIV-Tg rats are correlated with nitrosative stress and increased phosphorylated tau, suggesting that microtubule dysfunction may also affect spine density (Cho et al., 2017).
Since dendritic spine alterations in mPFC neurons are associated with measurable decline of cognitive flexibility, spine deficits in other brain areas associated with specific behaviors may also produce corresponding behavioral impairment. The motor symptoms of HAND have also been reduced by ART (Heaton et al., 2011; DeVaughn et al., 2015), but patients may still display subtle impairment including reduced psychomotor speed, fine motor control (Wilson et al., 2013), and mild extrapyramidal symptoms (Tierney et al., 2018). Dopaminergic dysfunction is likely an aspect of motor impairment in HAND (Gelman et al., 2012), but it is possible that spine deficits in motor areas of the cortex also contribute to motor impairment (Kida and Mitsushima, 2018) or are a consequence of dopamine dysfunction. Indeed, imaging studies in human patients have shown that cortical areas that control motor function have reduced grey matter area and reduced overall tissue volume, suggesting that these regions are altered or damaged during HIV infection (Thompson et al., 2005; Wilson et al., 2015). Our studies show that two distinct animal models of HAND present with dendritic spine deficits in layer 2/3 neurons of the motor cortex (Figure 2). In HIV-Tg rats, motor cortex overall dendritic spine density was decreased, and the percentage of immature filopodia spines was increased (Figure 2A). Sholl analysis of these neurons showed fewer intersections at 100 μM from the soma (Figure 2A), suggesting that subtle dendritic simplification also occurs in the HIV-Tg rat motor cortex. These results were almost mirrored in rats treated with the HIV envelope protein gp120IIIB by intracerebroventricular injection, which were previously examined in (Festa et al., 2015). Rats exposed to gp120IIIB showed decreased overall spine density, reduced percentage of thin spines, and increased percentage of filopodia (Figure 2C). Sholl analysis of these neurons showed fewer intersections at 80, 100, and 120 μM from the soma (Figure 2C), again suggesting dendritic simplification. Moreover, HIV-Tg rats did not show any differences in dendritic spine density, morphology, or Sholl analysis in layer 2/3 neurons of the somatosensory cortex (Figure 2B), and gp-120 injected rats only showed a reduced percentage of mushroom spines in this region (Figure 2D). These results provide further evidence that specific areas of the cortex are more vulnerable to HIV-induced synaptic damage, which may underlie specific cognitive or behavioral symptoms.
Figure 2. Synaptodendritic damage in motor and somatosensory cortices in animal models of HAND.
Studies in A and B were completed using adult (4–5 months-old) male F344 HIV-1 transgenic rats and wild-type F344 controls (n=4 rats per group). The preparation of brain tissue, dendritic spine staining (Seabold et al., 2010), and the analysis of layer 2/3 cortical neurons (Festa et al., 2015) were performed as previously described. A. HIV-Tg rat motor cortex. The overall dendritic spine density in motor cortex layer 2/3 neurons of HIV-Tg rats was significantly reduced compared to wild-type controls (t[14] = 4.183, p=0.0009). Dendritic spine morphology analysis showed that the percentage of immature filopodia are significantly increased in these same neurons (filopodia t[434] = 6.094, p<0.0001). Further, Sholl analysis showed the HIV-Tg rats have significantly fewer intersections at 100 μM (p=0.0172) from the soma in this region (distance F[9,100]= 41.98, p<0.0001; group F[1,100]= 7.590, p=0.007). B. HIV-Tg rat somatosensory cortex. There were no significant changes in dendritic spine density (t[14] = 1.044, p=0.3142), morphology, or Sholl analysis (distance F[9,100] = 66.68, p<0.0001; group F[1, 100] = 2.93, p=0.0901) in HIV-Tg rats compared to wild-type.
Studies in C and D were completed using adult (4–5 months-old) male Sprague Dawley rats. Each rat was stereotaxically implanted with a cannula targeting the lateral ventricle, infused with either gp120IIIB (50 ng/μL in 0.1% BSA, n=5) or vehicle (n=6) once daily for 7 days, and sacrificed 28 days after the final infusion, as previously reported (Festa et al., 2015). The preparation of brain tissue, dendritic spine staining, and analysis of layer 2/3 cortical neurons were also performed as previously reported (Festa et al., 2015). C. gp120-infused rat motor cortex. The overall dendritic spine density in motor cortex layer 2/3 neurons of gp-120 infused rats was significantly decreased (t[20] = 4.896, p<0.0001). Dendritic spine morphology analysis showed that the percentage of thin spines was significantly decreased (thin t[84] = 4.027, p=0.0009), and the percentage of filopodia was significantly increased in these same neurons (filopodia t[84] = 3.856, p=0.0013). Further, Sholl analysis showed that gp-120 infused rats had significantly fewer dendritic intersections at 80 μm (p=0.0006), 100 μm (p=0.0029), and 120 μm (p=0.0197) from the soma in this region (distance F[9, 200] = 23.98, p<0.0001; group F[1, 200] = 50.64, p<0.0001). D. gp120 infused rat somatosensory cortex. There were no significant differences in overall dendritic spine density in layer 2/3 somatosensory cortex neurons of either group (t[20] = 1.046, p=0.3082), but spine morphology analysis showed that the percentage of mushroom spines were significantly decreased in gp-120 infused rats (mushroom t[126] = 4.059, p=0.0006). Further, Sholl analysis did not show any changes of dendritic intersections in this region (distance F[9, 200] = 66.38, p<0.0001; group F[1, 200] = 3.978, p= 0.0475).
Data in all figures represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Spine density data was analyzed with two-tailed Students t-test; each point in HIV-Tg studies is one dendrite, and each point in gp120 studies is the average spine density of one neuron (two points per animal). Spine morphology data was analyzed with multiple t-tests and Holm Sidak correction. Sholl analysis was analyzed with two-way ANOVA and Sidak’s multiple comparisons test. All animals in these studies were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities in accordance with the National Institutes of Health guidelines and institutional approval by the Drexel University Institutional Animal Care and Use Committee.
4. Opioids contributions to cortical synaptodendritic damage
Synaptodendritic injury and dendritic spine deficits in HAND may be aggravated by comorbid use and abuse of opioid drugs. This can result in further progression of HAND symptoms, which was observed in a study of opioid-using HIV patients from the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) group (Byrd et al., 2011). However, the mechanisms by which opioids contribute to synaptic damage in HAND are not clearly defined, and more work is required to elucidate opioids’ actions on individual neurons, as well as on neuronal circuit activity and connectivity. Since the CHARTER study, several groups have identified potential mechanisms by which μ-opioid agonists contribute to synaptic damage of various brain regions using in vivo models, as well as primary culture models. These mechanisms range from direct effects of opioid signaling in specific neuronal populations to downstream effects of indirect opioid signaling in glial cells (Dutta and Roy, 2012). Since the expression of μ-opioid receptors (μORs) varies in different brain regions (Mansour et al., 1994; Ruzicka et al., 1995), μ-opioid agonists may also potentiate the region-specific synaptodendritic damage observed in HAND. This section will highlight recent studies of the functions of μORs in cortical tissue and how these findings may relate to HAND pathology.
Expression of μORs in cortical neurons may differ depending on the cortical region and species examined. Studies of human PFC tissue suggest that μOR transcripts and protein are expressed in pyramidal neurons and non-pyramidal neurons in layers 2–5 (Peckys and Landwehrmeyer, 1999; Schmidt et al., 2003), although neuronal subtype distinctions were made by morphological examination and not by immunostaining against common neuronal markers. However, studies from rat cortex suggest that μORs are mainly expressed in GABAergic inhibitory neurons, as two separate groups did not observe μOR staining or transcript expression in excitatory neurons (Taki et al., 2000; Ferezou et al., 2007). Another study from a different group suggested that μORs are expressed in excitatory synapses of mouse cortical neurons and rat hippocampal neurons, as μOR staining co-localized with NMDA and AMPA receptor staining (Liao et al., 2005). Therefore, GABAergic interneurons may be the major target of μ-opioids in the rat cortex, but excitatory neurons may also contribute to opioid signaling pathways if μORs are expressed on excitatory synapses. The different results of these studies may be due to several factors, including differences between primary cultured neurons and cortical tissue, antibody quality and specificity between species, examination of different cortical regions, and incomplete identification of the multitude of μOR splice variants (Pasternak, 2014; Xu et al., 2017). Of note, μORs are also expressed by rodent cortical astrocytes (El-Hage et al., 2005; Burbassi et al., 2010) and microglia (Maduna et al., 2018), suggesting that μ-opioids have the capacity to modulate several cortical cell types. Furthermore, astrocyte μOR expression varies depending on cortical area or brain region examined (Ruzicka et al., 1995).
Just as HIV infection may produce brain region-specific synaptodendritic damage, evidence suggests that morphine, a prototypical μ-opioid agonist, also affects selected brain regions. One group found that daily, long-term morphine self-administration or experimenter-administration in rats reduced dendritic spine density in various brain regions out to at least one month after morphine cessation (Robinson et al., 2002). Both treatment paradigms reduced spine density in the mPFC, the primary somatosensory cortex, and the visual cortex, while only self-administration reduced spine density in the hippocampal CA1 and dentate gyrus. Notably, self-administration produced more pronounced spine deficits in all examined regions except for the somatosensory cortex, which showed less pronounced spine deficits (Robinson et al., 2002). Morphine can also have long-term effects on dendritic spine density in primary neurons. Using mouse cortical and rat hippocampal neurons, one group showed that morphine activation of μORs destabilized existing spines, resulting in reduced dendritic spine density (Liao et al., 2005). This occurred after long-term morphine treatment over 3–6 days, was rescued by the μOR antagonist CTOP, and was not observed in μOR knockout animals. Interestingly, blocking neuronal activity with tetrodotoxin did not prevent morphine’s ability to reduce spine numbers, suggesting that μOR signaling at the cellular level regulates dendritic spine stability (Liao et al., 2005). Our group has shown similar results in vivo and in vitro, where morphine caused a large reduction of dendritic spine density in primary rat cortical neurons after 24 hour exposure, and morphine similarly reduced dendritic spine density in layer 2/3 PFC neurons of rats implanted with morphine pellets for 96 hours (Pitcher et al., 2014; Nash et al., 2019).
5. μOR-dependent pathways that regulate dendritic spine density
As dendritic spines are activity-dependent structures, one may expect that the well documented activity-dampening effects of μ-opioid agonists are involved in the reduction of dendritic spines. However, recent research suggests that several interesting and perhaps unexpected pathways downstream of μORs in cortical neurons modulate dendritic spines. These recently discovered pathways, which will be described in the following section, may function in combination with altered neuronal activity to modulate dendritic spines in the cortex. Additionally, morphine’s actions on dendritic spines are reported to be enhanced in the presence of HIV proteins, suggesting a potential synergy of HIV infection and opioid use (Fitting et al., 2014). Below, we discuss recent work describing studies that illuminate morphine, or μOR-mediated pathways involved in modulating cortical dendritic spine density and stability.
5.1. Molecular mediators
A group of studies has demonstrated that morphine and other μ-opioid agonists modulate dendritic spines through aberrant calcium signaling. Although these studies are mostly in hippocampal neurons, the pathways that they identify may also apply to cortical neurons and therefore should be carefully considered. One study demonstrated that prolonged morphine exposure decreased the density and stability of hippocampal dendritic spines, which was dependent on the activity of Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) and calcineurin (Miller et al., 2012). Blocking the morphine-mediated activation of calcineurin preserved AMPA receptor expression in dendritic spines and AMPA receptor-mediated miniature excitatory postsynaptic currents (Miller et al., 2012). As AMPA receptors are highly expressed in mature spines (Matsuzaki et al., 2001), the loss of these receptors suggests that mature spines are affected by prolonged morphine use. Furthermore, long-term morphine exposure inhibited CaMKIIα activity and caused its translocation out of dendritic spines. This was associated with dendritic spine loss, as CaMKIIα could no longer activate synaptically localized Rac1, a small guanosine triphosphatase and regulator of dendritic spine structure (Xie et al., 2007). Further, neurons transfected with a dominant negative Rac1, as well as a constitutively active Rac1, blocked morphine’s ability to reduce dendritic spine density (Miller et al., 2012), highlighting the importance of Rac1 signaling to dendritic spine homeostasis.
Morphine also activates an additional signaling pathway that reduces cortical dendritic spine density that may not be directly associated with dampened neuronal activity. Our group has shown that cortical neurons exposed to morphine for 6–24 hours upregulated ferritin heavy chain (FHC), a subunit of the iron storage protein ferritin (Sengupta et al., 2009). Interestingly, FHC inhibits activation of the homeostatic chemokine receptor CXCR4 by its natural ligand CXCL12, as well as activation of its downstream effectors including ERK1/2 and Akt (Li et al., 2006; Sengupta et al., 2009; Aversa et al., 2017). As CXCL12/CXCR4 signaling increases dendritic spine density in primary cortical neurons, spine deficits occur when this pathway is blocked by morphine-mediated upregulation of FHC (Sengupta et al., 2009; Pitcher et al., 2014). FHC was previously known for its role in iron storage and oxidation (Arosio et al., 2015), but the novel interaction with the CXCR4 signaling complex suggests that the functional roles of FHC may not be restricted to cellular iron metabolism. Our mechanistic studies showed that the μOR antagonist CTAP and FHC knockdown both prevented morphine mediated CXCR4 inhibition (Sengupta et al., 2009), demonstrating morphine was not acting through other opioid receptors or toll-like receptor 4. Additionally, neuronal FHC is upregulated by interleukin-1β (IL-1β) secreted from glial cells exposed to HIV proteins such as gp120 (Festa et al., 2015), demonstrating morphine and HIV neurotoxins may inhibit CXCR4 signaling through a convergent mechanism. Furthermore, our evidence suggests that these pathways are present in vivo, as clinical samples of HAND patients with and without poly-drug use (including opioids), as well as simian immunodeficiency virus infected macaques treated with morphine, showed increased expression of FHC and reduced phosphorylation of the CXCR4 c-terminus in PFC neurons (Pitcher et al., 2014). Strikingly, neuronal FHC expression levels correlated with the severity of HAND symptoms in human patients (Pitcher et al., 2014), suggesting that morphine regulation of CXCR4 contributed to cognitive deficits via a reduction of cortical dendritic spine density.
Interestingly, we have shown that morphine post-transcriptionally regulates FHC levels in cortical neurons by modulating neuronal iron metabolism. Briefly, morphine causes iron efflux from endolysosomes to the cytoplasm prior to FHC upregulation, and chelation of endolysosomal iron with deferoxamine blocks morphine mediated FHC upregulation (Nash et al., 2019). Chelation of extracellular iron did not prevent morphine induced FHC upregulation, suggesting that morphine only modulates intracellular iron stores to upregulate FHC. The mechanism by which cytoplasmic labile iron upregulates neuronal FHC could involve the post-transcriptional iron regulatory protein (IRP) system (Wilkinson and Pantopoulos, 2014). The IRP system controls translation of mRNAs involved in iron metabolism based on free iron levels. On 5’ untranslated regions of FHC transcripts, IRPs tightly bind to iron response elements (IREs) in conditions of low labile iron and prevent ribosomal translation. When labile iron levels increase, free iron interacts with IRPs and releases them from IREs on the FHC transcript, resulting in translation of FHC. If this pathway is activated, it may also impact the expression of other proteins that are regulated by iron.
5.2. Neuronal networks
Cortical γ-aminobutyric acid (GABA)ergic interneurons appear as major targets of μ-opioid agonists, but it is unclear if μOR activation specifically on interneurons contributes to dendritic spine deficits on local excitatory neurons. However, recent studies show that μOR signaling in the PFC dysregulates circuits involved in motivational behaviors, which may result from a network-level effect mediated by altered GABAergic transmission (Baldo, 2016). Therefore, it is possible that the actions of μ-opioid agonists on cortical interneurons may contribute to HAND through network dysregulation in addition to synaptodendritic damage (Figure 3). As morphine inhibits the release of GABA (Qu et al., 2015; Yokota et al., 2016) this can lead to an increased activity of excitatory neurons driving spine compensatory changes or sublethal damage. GABAergic tone may also be affected by μOR activation on interneurons that upregulates FHC and inhibits CXCR4 function in the same cell. GABAergic interneuron CXCR4 signaling plays important roles in regulation of inhibitory neurotransmission and synapse formation in several regions of the brain. In the mouse mPFC, CXCR4 is expressed on inhibitory synapses from layer 5 parvalbumin interneurons, which form inhibitory synapses onto layer 5 pyramidal neurons expressing CXCL12 on their cell bodies (Wu et al., 2017). Additionally, CXCR4 signaling on interneurons from distinct subcortical areas promotes synaptic release of GABA (Guyon et al., 2006; Heinisch and Kirby, 2010). If CXCR4 signaling also promotes GABA release from cortical interneurons, then μ-opioid agonist induced inhibition of CXCR4 in these cells may disrupt GABAergic transmission in addition to inhibitory synapse formation in the cortex. Furthermore, several GABAB agonists and antagonists, including GABA itself, act allosterically on CXCR4 and alter normal responses to CXCL12 signaling (Guyon et al., 2013), and this also may be disrupted by μ-opioids.
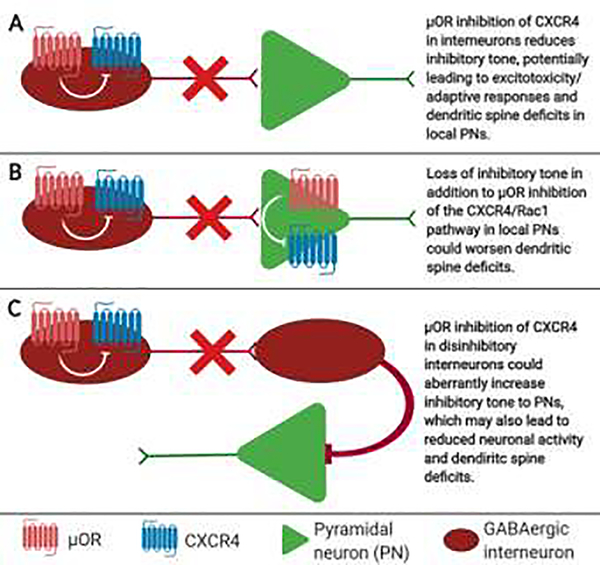
Figure 3. Potential network-level interactions of μOR and CXCR4 leading to dendritic spine deficits.
Although μOR activation could lead to CXCR4 inhibition and dendritic spine deficits all in the same excitatory pyramidal neuron (PN), μOR regulation of CXCR4 in GABAergic interneurons may also regulate dendritic spine density in PNs by altering the excitatory/inhibitory balance of the local circuit through distinct mechanisms. Some possibilities include A. Reduced GABAergic innervation of PNs; B. an overall decrease in neuronal activity of GABAergic interneurons and PNs; or C. Reduced activity of GABAergic disinhibition circuits.
Although cortical interneurons only make up roughly 15% of the total cortical neuron population in rats (Meyer et al., 2011), they are organized as subpopulations that perform diverse and specialized functions (Tremblay et al., 2016; Lim et al., 2018). These subpopulations are reported to express μORs at different levels and may therefore be more or less susceptible to exogenous μ-opioid agonist exposure. One study shows that cortical interneurons expressing vasoactive intestinal peptide (VIP), corticotropin releasing factor, and choline acetyltransferase showed the highest μOR expression, while inhibitory neurons expressing parvalbumin (PV), somatostatin (SST) and neuropeptide Y (NPY) displayed lower expression levels (Taki et al., 2000). In another study, μOR transcripts were mostly found in cortical interneurons that expressed VIP, cholecystokinin (CKK), and calretinin (CR), in addition to layer I interneurons that expressed NPY (Ferezou et al., 2007). As these studies suggest that all the major cortical interneuron subpopulations express μORs to some extent, μ-opioid agonists may produce a broad suppression of GABAergic tone in the cortex. Alternatively, μ-opioid agonists could dose-dependently inhibit GABA release from interneuron subpopulations with higher μOR expression. VIP expressing interneurons showed high expression of μOR transcript and protein in both studies, suggesting that these neurons are major targets of μ-opioid agonists. VIP interneurons are involved in disinhibition circuits, as they form synapses on nearby interneurons that innervate excitatory neurons (Pi et al., 2013; Karnani et al., 2016). Therefore, inhibiting VIP interneurons with μ-opioid agonists may result in increased GABAergic tone on local excitatory neurons. However, μ-opioid agonist induced inhibition of PV, SST, CCK, and other interneurons that directly synapse onto local pyramidal neurons may decrease overall GABAergic tone in the cortex. In line with this, one study showed that the specific μOR agonist DAMGO inhibited sodium currents in non-pyramidal PFC neurons, and had no effect on sodium currents in pyramidal excitatory neurons (Witkowski and Szulczyk, 2006). This reduction of GABAergic tone may contribute to excitotoxicity and synaptodendritic damage, especially in patients with HAND that may be more vulnerable to μ-opioid agonist induced synaptic damage (Byrd et al., 2011). Several recent studies have examined the contributions of interneuron subpopulations to cognitive function, demonstrating the importance of these neurons in learning and memory processes (Batista-Brito et al., 2017; Ognjanovski et al., 2017; Eavri et al., 2018; Lucas and Clem, 2018). As the consequences of CXCR4 signaling vary between different brain areas and types of neurons, region-specific and focused studies are necessary to determine how μ-opioid agonists regulate GABAergic transmission, if μ-opioid agonist induced inhibition of CXCR4 signaling is involved, and if the actions of μ-opioid agonists on interneurons contribute to cognitive impairment in HAND.
6. CXCL12/CXCR4 regulation of cortical dendritic spines and synapses
Although chemokine receptor signaling is typically associated with functional responses and chemotaxis of immune cells, CXCL12/CXCR4 signaling has additional functions in the CNS, as reviewed in (Nash and Meucci, 2014). These include homeostatic and neuromodulatory functions, including regulation of axonal pathfinding and outgrowth (Ohshima et al., 2008), neuronal branching (Pujol et al., 2005), neurogenesis and neuronal precursor differentiation (Peng et al., 2007; Hattori and Miyata, 2018), neuron-glia communication (Guyon, 2014), and neuronal excitability (Guyon and Nahon, 2007). In addition to these functions, our group has shown that CXCL12/CXCR4 signaling increases dendritic spine density of cortical neurons in vitro and in vivo (Pitcher et al., 2014). In cultured cortical neurons, CXCL12 treatment for 1 hour increased spine density in the absence of glia, suggesting that direct activation of CXCR4 on cortical neurons regulates dendritic spines.
Several reports have shown that CXCR4 and its natural ligand CXCL12 are expressed in various cells of the cortex during development and adulthood. CXCR4 is expressed in primary cortical neurons from several species and coupled to functional outcomes, including calcium signaling and activation of cellular kinases (Meucci et al., 1998; Bajetto et al., 1999; Klein et al., 1999). In the adult brain, CXCR4 and CXCL12 are constitutively expressed in several regions, including the cerebral cortex and hippocampus (Meucci et al., 1998; Banisadr et al., 2002; Pitcher et al., 2014). Although some information regarding CXCR4 expression on neuronal subpopulations has been reported (Banisadr et al., 2002; Wu et al., 2017), a complete characterization of CXCR4 expression on subpopulations of cortical neurons has not been published. ACKR3, another chemokine receptor that binds CXCL12, is also expressed in adult neurons of the cortex (Shimizu et al., 2011; Banisadr et al., 2016)
Our previous studies suggest that CXCL12/CXCR4 signaling increases spine density in vitro and in rat layer 2/3 mPFC neurons (Pitcher et al., 2014; Festa et al., 2015), but CXCL12’s effects on dendritic spines are not limited to mPFC layer 2/3 neurons. We also observed similar trends in rat layer 5 mPFC neurons, including increased overall dendritic spine density and a specific increase in thin spine density in the prelimbic cortex (unpublished observations). This finding is of added importance because layer 5 neuronal connectivity between prelimbic and infralimbic cortices of rat mPFC primes the brain to set up new associations, which are critical for set-shifting tasks and general working memory (Mukherjee and Caroni, 2018). Reciprocal connectivity between layer 5 neurons of the prelimbic and infralimbic cortices is also involved in similar adaptive behaviors, including conditioned place preference (Pena-Bravo et al., 2017) and extinction of fear memory (Marek et al., 2018). Fear memory extinction also increases layer 5 dendritic spine density (Lai et al., 2012). Generally, layer 5 prelimbic cortex activity is involved in applying previously learned associations (Hok et al., 2005), while layer 5 infralimbic cortex activity is involved in setting up alternative associations (Suto et al., 2016). Therefore, it is possible that dendritic alterations in these sub-regions, which may reduce their activity and connectivity, may drive cognitive deficits in working memory tasks. Furthermore, a CXCL12-mediated rescue of thin dendritic spines in layer 5 neurons of these regions in HIV-Tg rats may underlie improved performance in the extradimensional shift portion of set shifting studies (Festa et al., 2015; Festa et al., 2018).
CXCL12 also regulates inhibitory synapse formation on layer 5 pyramidal neurons of the mPFC. A report demonstrated that CXCL12 is expressed on the cell bodies of mouse layer 5 PFC neurons and promotes somatic synapse formation from PV interneurons, as PV synapses express CXCR4 and ACKR3 (Wu et al., 2017). Conditional knockout of CXCL12 from layer 5 mPFC neurons in this system resulted in reduced PV interneuron terminal innervation and reduced inhibitory post-synaptic currents on these neurons. This could be due to reduced Rac1 activation in PV interneurons, as another group showed that Rac1 conditional knockout in mouse hippocampal neurons reduced spontaneous inhibitory postsynaptic currents and PV terminals in the CA1 pyramidal layer (Pennucci et al., 2016). Taken together, the evidence suggests that CXCL12/CXCR4 signaling promotes inhibition of layer 5 pyramidal neurons of the mPFC while simultaneously increasing dendritic spine density in this area. Therefore, CXCL12 may also improve cognitive function through regulation of the excitatory/inhibitory balance of mPFC neurons (Ferguson and Gao, 2018).
7. CXCR4 signaling pathways that regulate dendritic spine density
As cognitive impairment and dendritic spine deficits in HAND may be reversible through CXCL12/CXCR4 signaling, it is critical to understand the underlying signaling pathways that produce these outcomes (Figure 4). Dendritic spine homeostasis is influenced by neuronal activity, actin stabilization, and expression of synaptic proteins (Bertling and Hotulainen, 2017), and evidence suggests that all of these processes are regulated, at least in part, by the CXCL12/CXCR4 signaling axis. Although more studies are needed to fully elucidate the signaling pathways downstream of CXCR4 that regulate dendritic spines, the most current knowledge is discussed in this section. The pathways discussed here may also be therapeutically useful for neurological disorders beyond HAND, as dendritic spine deficits drive cognitive impairment in Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and other dementias (Herms and Dorostkar, 2016), as well as schizophrenia (Moyer et al., 2015).
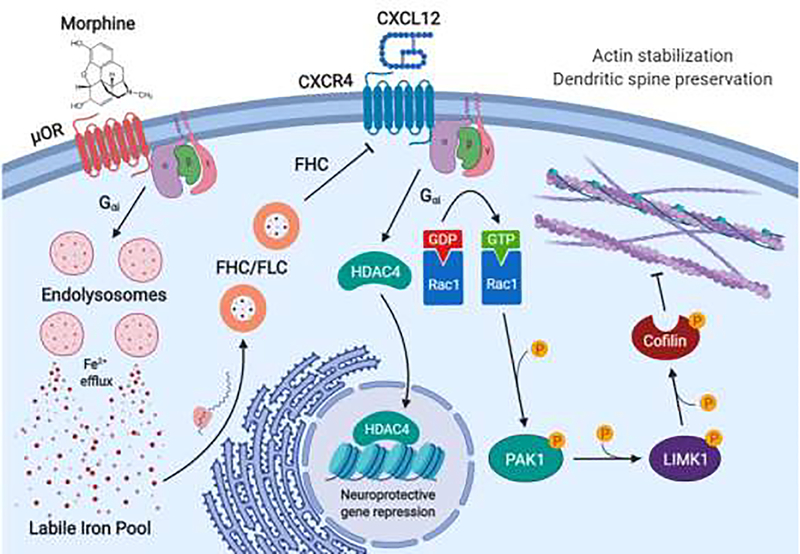
Figure 4.
μOR regulation of CXCR4 working model
Morphine or other μ-opioids binding to μORs on cortical neurons activates a Gαi-protein pathway that leads to de-acidification of endolysosomes, and efflux of endolysosomal iron to the cytoplasm. This increases labile iron levels in the neuron, resulting in increased production of the iron storage proteins ferritin heavy chain (FHC) and ferritin light chain (FLC) through a post-transcriptional mechanism. In addition to its iron storage functions, FHC also associates with the homeostatic chemokine receptor CXCR4 and blocks its downstream signaling. Normally, CXCR4 activation by its natural ligand CXCL12 increases dendritic spine density in cortical neurons, which may occur through activation of several pathways. First, CXCR4 activation leads to HDAC4 translocation to the nucleus, which regulates several genes involved in synaptic activity. Specific knockdown of HDAC4 prevents CXCL12 from increasing dendritic spine density, suggesting that CXCL12/CXCR4 signaling regulates synaptic protein expression. Second, CXCL12 may activate the Rac1/PAK pathway in cortical neurons, resulting in phosphorylation of the actin severing protein cofilin. Phosphorylated cofilin is unable to break down the actin cytoskeleton, which may result in stabilization of actin-rich dendritic spines.
7.1. Molecular mediators
The Rac1/PAK pathway regulates the stability of actin, which is the major cytoskeletal component of dendritic spines (Hotulainen and Hoogenraad, 2010; Basu and Lamprecht, 2018). Rac1 is activated by CXCR4 signaling (Lopez-Haber et al., 2016; Mills et al., 2018; Bajanca et al., 2019), which begins a phosphorylation cascade of second messengers, including p21-activated kinase (PAK), LIM kinase 1 (LIMK1), and the actin severing protein cofilin (Delorme et al., 2007). Phosphorylation of cofilin on S3 blocks its ability to disassemble actin filaments (Arber et al., 1998; Yang et al., 1998), which may preserve the structural integrity of dendritic spines. If Rac1 or any of its downstream mediators emerge as suitable drug targets for HAND, modulation of these proteins should be careful and deliberate. Two reports show that over-activation of Rac1 impaired neuritogenesis and synaptic maturation in cortical neurons, which contributed to cognitive impairment (Pyronneau et al., 2017; Zamboni et al., 2018). Therefore, a balance between activation and inhibition of the Rac1 pathway may be necessary to achieve an optimal therapeutic effect.
Dendritic spine maturation depends on synaptic activity and protein expression, which may both be regulated by CXCL12/CXCR4 signaling. Our studies in cultured cortical neurons showed that CXCL12 promoted synaptic activation of NMDA receptors while inhibiting extrasynaptic NMDA activation (Nicolai et al., 2010). This process occurred through a CXCR4 mediated repression of the NMDA NR2B subunit, which is highly expressed in extrasynaptic NMDA receptors (Tovar and Westbrook, 2002; Miwa et al., 2008; Yang et al., 2017). Our work suggests that repression of NR2B may be due to translocation of the class IIa histone deacetylase 4 (HDAC4) to the nucleus (Nicolai et al., 2010; Pitcher et al., 2014). HDAC4 alone has little to no catalytic activity (Lahm et al., 2007) but binds select transcription factors, including MEF2, and recruits other HDACs that deacetylate histones and repress gene expression (Wu et al., 2016). In neurons, HDAC4 translocates between the cytoplasm and nucleus in response to various stimuli and is hypothesized to have specific functions in each subcellular compartment (Fitzsimons, 2015). After nuclear translocation, HDAC4 suppresses a set of genes that is involved in synaptic activation (Sando et al., 2012). A mutated HDAC4 (S3A) with constitutive nuclear translocation severely reduces dendritic branching and complexity while preserving dendritic spines of hippocampal neurons (Litke et al., 2018). CXCL12 induces nuclear translocation of HDAC4 for at least 1 hour after treatment (Pitcher et al., 2014), but beyond this acute period, HDAC nuclear localization is unclear. It is possible that this pathway is a transient, neuroprotective response that represses synaptic activity in response to excitotoxicity. Additionally, HDAC4-mediated repression of MEF2 may enhance dendritic spine density and memory formation, as MEF2 restricts dendritic spine growth in primary hippocampal neurons and disrupts learning and memory processes (Cole et al., 2012). In line with this study, HDAC4 knockdown also produces memory deficits, suggesting that its role in the nuclear compartment is neuroprotective under certain circumstances and/or that it has additional memory-promoting functions in the cytoplasm (Kim et al., 2012). HDAC4 phosphorylation and nuclear export depends on neuronal activity and calcium influx, and cytoplasmic HDAC4 is expressed in some dendritic spines (Darcy et al., 2010). Cytoplasmic HDAC4 could contribute to memory formation and stabilization of dendritic spines through small ubiquitin-like modifier (SUMO)ylation of synaptic proteins (Craig and Henley, 2012). Several studies have reported the involvement of HDAC4 in SUMOylation of the transcription factor MEF2 (Gregoire and Yang, 2005; Zhao et al., 2005), suggesting that synaptic proteins may also be targeted. Synaptically localized HDAC4 may SUMOylate Rac1, which is typically required for Rac1 activation and proper cell motility (Castillo-Lluva et al., 2010). As CXCR4 increases dendritic spine density, HDAC4 may also be involved in Rac1 activation.
7.2. Neuronal networks
CXCR4 signaling may also produce circuit-level effects that alter cortical dendritic spine density by differential modulation of inhibitory and excitatory neurons. For example, CXCR4 activation on nearby interneurons may enhance GABA release, as reported in non-cortical areas (Heinisch and Kirby, 2010; Guyon et al., 2013), acutely dampening excitatory neuronal activity. At the same time, CXCR4 activation of the Rac1/PAK/cofilin pathway on excitatory neurons may stabilize transient dendritic spines that would normally retract in response to reduced neuronal activity. Interestingly, both of these pathways may also be inhibited by GABA spillover from the synapse, as GABA and other compounds that interact with GABA receptors are reported to be negative allosteric modulators of CXCR4 (Guyon et al., 2013). For interneurons, GABA spillover may begin a feedback mechanism that prevents further GABA release by inhibiting interneuron CXCR4 signaling. If this is the case, then CXCR4 activation status may follow the oscillatory patterns that are driven by cortical interneuron activity (Cardin, 2018). On excitatory neurons, CXCR4 signaling may stabilize dendritic spines and counteract neuronal activity-dependent spine retraction. During periods of increased neuronal activity, CXCR4 stabilized dendritic spines may then integrate into the synaptic circuit and mature. The long-term dysregulation of this hypothesized feedback mechanism by opioids or HIV neurotoxins may also explain some of the reported neurocognitive symptoms in HAND with opioid use. In HIV infected patients, the continued presence of even low levels of X4 gp120 may dysregulate this normal feedback between CXCR4 and GABA systems through altered CXCR4 activation and membrane trafficking. Indeed, one report shows that gp120 increases GABAergic currents in hippocampal slices by modulating α5-containing GABAA receptors (Green and Thayer, 2019). The same group also reported that IL-1β release in response to gp120 enhances the same tonic GABA current, suggesting that sustained low-level inflammation may also enhance GABA release through this pathway. As removal of microglia from the culture blocked these effects, it is possible that the chronic inflammatory environment is a pre-requisite for gp120-induced dysregulation of CXCR4 signals, dendritic spine deficits, and cognitive deficits. Furthermore, inhibition of CXCR4 by μ-opioid agonists is likely to worsen dendritic spine deficits and potentially dysregulate the functions and/or inhibitory oscillations of cortical interneurons. For excitatory neurons, morphine inhibits the CXCR4-mediated stabilization of transient dendritic spines, suggesting that fewer dendritic spines will be preserved during periods of enhanced GABAergic tone. However, morphine-mediated inhibition of CXCR4 on inhibitory neurons may also disrupt their activity and subsequent GABA neurotransmission. Therefore, the pathological outcomes of μ-opioid agonist use are likely affected by many factors, including the doses and duration of use, receptor expression on neuronal subpopulations, and the stage of impairment in a patient with HAND.
8. Conclusions and future research
HIV infection and opioid use may converge to produce sub-lethal synaptic injury and cognitive impairment in HAND patients, and studies that exploit the dendritic spine promoting CXCL12/CXCR4 signaling axis show that this synaptic injury may be reversible. As reversing dendritic spine deficits in specific brain regions, including the PFC, results in improved cognitive performance in HIV-Tg rats, novel therapeutics that protect against the reduction of dendritic spines or restore spines that have been lost may lead to functional recovery of patients with HAND, as well as other neurocognitive disorders. However, direct pharmacological targeting of CXCR4 may not be a realistic strategy due to the widespread expression and functions of the receptor. Therefore, future research must aim to more fully understand the signaling pathways downstream of CXCR4 that are involved in the regulation of dendritic spines and how these pathways vary depending on neuronal sub-type and brain region. Furthermore, additional research must dissect the mechanism of CXCR4 regulation by μ-opioid receptors in a cell-type and region-specific manner because preventing this pathway may be another approach to enhance CXCR4 signaling and rescue dendritic spine deficits in opioid using HAND patients. In order to identify new drug targets and develop effective therapeutics, future work must identify the mechanisms driving synaptic injury and whether they exist as complex cellular signaling cascades or as region-specific dysregulation of neuronal network activity.
Region-specific dendritic spine deficits may drive impairment in HAND
CXCL12/CXCR4 signaling stabilizes dendritic spines
μ-opioid use contributes to spine loss in various ways, including CXCR4 inhibition
Acknowledgements
This work was supported by the National Institutes of Health grants DA015014, DA032444, DA040519, and T32-MH078795.
Funding: This work was supported by National Institutes of Health [grant numbers DA015014, DA032444, and DA040519] to Olimpia Meucci. Lindsay Festa was a fellow of the “Interdisciplinary and Translational Research Training in neuroAIDS” National Institutes of Health [grant number T32-MH078795]. The funding sources had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Abbreviations
- HAND
HIV-associated neurocognitive disorders
- ART
antiretroviral therapy
- μOR
μ opioid receptor
- PFC
prefrontal cortex
- HIV-Tg
HIV-transgenic rat
- FHC
ferritin heavy chain
- FLC
ferritin light chain
- IRP
iron regulatory protein
- IRE
iron responsive element
- VIP
vasoactive intestinal peptide
- PV
parvalbumin
- HDAC4
histone deacetylase 4
- MEF2
myocyte enhancer factor-2
Footnotes
Declarations of interest: none.
Data statement: The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Approval: All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ances BM, Hammoud DA, 2014. Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS. 9, 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann HW, Jun S, Shin NY, Han S, Ahn JY, Ahn MY, Jeon YD, Jung IY, Kim MH, Jeong WY, Ku NS, Kim JM, Smith DM, Choi JY, 2016. Characteristics of Resting-State Functional Connectivity in HIV-Associated Neurocognitive Disorder. PLoS One. 11, e0153493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P, 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 393, 805–9. [DOI] [PubMed] [Google Scholar]
- Arosio P, Carmona F, Gozzelino R, Maccarinelli F, Poli M, 2015. The importance of eukaryotic ferritins in iron handling and cytoprotection. Biochem J. 472, 1–15. [DOI] [PubMed] [Google Scholar]
- Aversa I, Zolea F, Ierano C, Bulotta S, Trotta AM, Faniello MC, De Marco C, Malanga D, Biamonte F, Viglietto G, Cuda G, Scala S, Costanzo F, 2017. Epithelial-to-mesenchymal transition in FHC-silenced cells: the role of CXCR4/CXCL12 axis. J Exp Clin Cancer Res. 36, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajanca F, Gouignard N, Colle C, Parsons M, Mayor R, Theveneau E, 2019. In vivo topology converts competition for cell-matrix adhesion into directional migration. Nat Commun. 10, 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G, 1999. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 73, 2348–57. [DOI] [PubMed] [Google Scholar]
- Baldo BA, 2016. Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends Neurosci. 39, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S, 2002. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 16, 1661–71. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Podojil JR, Miller SD, Miller RJ, 2016. Pattern of CXCR7 Gene Expression in Mouse Brain Under Normal and Inflammatory Conditions. J Neuroimmune Pharmacol. 11, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Lamprecht R, 2018. The Role of Actin Cytoskeleton in Dendritic Spines in the Maintenance of Long-Term Memory. Front Mol Neurosci. 11, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Vinck M, Ferguson KA, Chang JT, Laubender D, Lur G, Mossner JM, Hernandez VG, Ramakrishnan C, Deisseroth K, Higley MJ, Cardin JA, 2017. Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits. Neuron. 95, 884–895 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KP, Nedivi E, 2017. Spine Dynamics: Are They All the Same? Neuron. 96, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertling E, Hotulainen P, 2017. New waves in dendritic spine actin cytoskeleton: From branches and bundles to rings, from actin binding proteins to post-translational modifications. Mol Cell Neurosci. 84, 77–84. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM, 2007. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 17, 381–6. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Sengupta R, Meucci O, 2010. Alterations of CXCR4 function in mu-opioid receptor-deficient glia. Eur J Neurosci. 32, 1278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan T, Lisinicchia J, Patel V, Johnson K, Neugebauer V, Paessler S, Jennings K, Gelman B, 2016. Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection. J Neuroimmune Pharmacol. 11, 279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur J, Grant I, Group C, 2011. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 58, 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Malerba F, Carucci NM, Rizzi C, Criscuolo C, Origlia N, Calvello M, Viegi A, Meli G, Cattaneo A, 2017. The chemokine CXCL12 mediates the anti-amyloidogenic action of painless human nerve growth factor. Brain. 140, 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, 2018. Inhibitory Interneurons Regulate Temporal Precision and Correlations in Cortical Circuits. Trends Neurosci. 41, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas R, Muthusamy S, Wakim PG, Sinharay S, Lentz MR, Reid WC, Hammoud DA, 2018. MR brain volumetric measurements are predictive of neurobehavioral impairment in the HIV-1 transgenic rat. Neuroimage Clin. 17, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Lluva S, Tatham MH, Jones RC, Jaffray EG, Edmondson RD, Hay RT, Malliri A, 2010. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat Cell Biol. 12, 1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YE, Lee MH, Song BJ, 2017. Neuronal Cell Death and Degeneration through Increased Nitroxidative Stress and Tau Phosphorylation in HIV-1 Transgenic Rats. PLoS One. 12, e0169945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CJ, Mercaldo V, Restivo L, Yiu AP, Sekeres MJ, Han JH, Vetere G, Pekar T, Ross PJ, Neve RL, Frankland PW, Josselyn SA, 2012. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat Neurosci. 15, 1255–64. [DOI] [PubMed] [Google Scholar]
- Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, Wit FW, Portegies P, Geurtsen GJ, Schmand BA, Schim van der Loeff, MF, Franceschi C, Sabin CA, Majoie CB, Winston A, Reiss P, Sharp DJ, collaboration, C., 2017. Increased brain-predicted aging in treated HIV disease. Neurology. 88, 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TJ, Henley JM, 2012. Protein SUMOylation in spine structure and function. Curr Opin Neurobiol. 22, 480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwadi DA, Ozuna L, Harvey BH, Viljoen M, Schetz JA, 2018. Adverse Neuropsychiatric Events and Recreational Use of Efavirenz and Other HIV-1 Antiretroviral Drugs. Pharmacol Rev. 70, 684–711. [DOI] [PubMed] [Google Scholar]
- Darcy MJ, Calvin K, Cavnar K, Ouimet CC, 2010. Regional and subcellular distribution of HDAC4 in mouse brain. J Comp Neurol. 518, 722–40. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJ, Vos T, 2013. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 382, 1564–74. [DOI] [PubMed] [Google Scholar]
- Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM, 2007. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 13, 646–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVaughn S, Muller-Oehring EM, Markey B, Bronte-Stewart HM, Schulte T, 2015. Aging with HIV-1 Infection: Motor Functions, Cognition, and Attention--A Comparison with Parkinson’s Disease. Neuropsychol Rev. 25, 424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH, 2010. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 30, 7507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Roy S, 2012. Mechanism(s) involved in opioid drug abuse modulation of HAND. Curr HIV Res. 10, 469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eavri R, Shepherd J, Welsh CA, Flanders GH, Bear MF, Nedivi E, 2018. Interneuron Simplification and Loss of Structural Plasticity As Markers of Aging-Related Functional Decline. J Neurosci. 38, 8421–8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, Obermann M, Rosenkranz T, Schielke E, Straube E, German Association of Neuro, A.u.N.-I., 2017. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol. 264, 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF, 2005. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 50, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E, 2007. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 8, 33–44. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL, 2012. The role of medial prefrontal cortex in memory and decision making. Neuron. 76, 1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E, 1999. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 9, 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, Lambolez B, 2007. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex. 17, 1948–57. [DOI] [PubMed] [Google Scholar]
- Ferguson BR, Gao WJ, 2018. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front Neural Circuits. 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Meucci O, 2012. Effects of opiates and HIV proteins on neurons: the role of ferritin heavy chain and a potential for synergism. Curr HIV Res. 10, 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Gutoskey CJ, Graziano A, Waterhouse BD, Meucci O, 2015. Induction of Interleukin-1beta by Human Immunodeficiency Virus-1 Viral Proteins Leads to Increased Levels of Neuronal Ferritin Heavy Chain, Synaptic Injury, and Deficits in Flexible Attention. J Neurosci. 35, 10550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Platt B, Tian Y, Floresco S, Meucci O, 2018. Abstracts from the Joint Meeting of the International Society for NeuroVirology (ISNV) and the Society on NeuroImmune Pharmacology (SNIP) April 10–14, 2018, Chicago, Illinois, USA. J Neuroimmune Pharmacol. 13, 1–102. [DOI] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF, 2010. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 177, 1397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF, 2014. Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na(+) influx, mitochondrial instability, and Ca(2)(+) overload. J Neurosci. 34, 12850–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons HL, 2015. The Class IIa histone deacetylase HDAC4 and neuronal function: Nuclear nuisance and cytoplasmic stalwart? Neurobiol Learn Mem. 123, 149–58. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y, 2004. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 125, 615–23. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH Jr., Soukup VM, 2012. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol. 7, 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, 2015. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr HIV/AIDS Rep. 12, 272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Franklin DR Jr., Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK, Group C, 2014. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 82, 2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MV, Thayer SA, 2019. HIV gp120 upregulates tonic inhibition through alpha5-containing GABAARs. Neuropharmacology. 149, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire S, Yang XJ, 2005. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol Cell Biol. 25, 2273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, Brier MR, Ortega M, Westerhaus E, Nelson B, Ances BM, 2016. Topographies of Cortical and Subcortical Volume Loss in HIV and Aging in the cART Era. J Acquir Immune Defic Syndr. 73, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelsi D, Rovere C, Rostene W, Parsadaniantz SM, Nahon JL, 2006. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem. 96, 1540–50. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL, 2007. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 38, 365–76. [DOI] [PubMed] [Google Scholar]
- Guyon A, Kussrow A, Olmsted IR, Sandoz G, Bornhop DJ, Nahon JL, 2013. Baclofen and other GABAB receptor agents are allosteric modulators of the CXCL12 chemokine receptor CXCR4. J Neurosci. 33, 11643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, 2014. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci. 8, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH, 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 26, 2571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, Consortium, H.I.V.N., 2011. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 25, 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B, 1992. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 12, 2685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Miyata T, 2018. Microglia extensively survey the developing cortex via the CXCL12/CXCR4 system to help neural progenitors to acquire differentiated properties. Genes Cells. 23, 915–922. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 75, 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H, 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch S, Kirby LG, 2010. SDF-1alpha/CXCL12 enhances GABA and glutamate synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuropharmacology. 58, 501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms J, Dorostkar MM, 2016. Dendritic Spine Pathology in Neurodegenerative Diseases. Annu Rev Pathol. 11, 221–50. [DOI] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B, 2005. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A. 102, 4602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K, 2005. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 45, 279–91. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC, 2010. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 189, 619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, 2016. HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr Drug Targets. 17, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Jackson J, Ayzenshtat I, Hamzehei Sichani A, Manoocheri K, Kim S, Yuste R, 2016. Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J Neurosci. 36, 3471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, Brandimarti R, Shimizu S, Nicolai J, Crowe E, Meucci O, 2008. The chemokine CXCL12 promotes survival of postmitotic neurons by regulating Rb protein. Cell Death Differ. 15, 1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodr CE, Chen L, Al-Harthi L, Hu XT, 2018. Aging alters voltage-gated calcium channels in prefrontal cortex pyramidal neurons in the HIV brain. J Neurovirol. 24, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida H, Mitsushima D, 2018. Mechanisms of motor learning mediated by synaptic plasticity in rat primary motor cortex. Neurosci Res. 128, 14–18. [DOI] [PubMed] [Google Scholar]
- Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, Olson EN, Monteggia LM, 2012. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 32, 10879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD, 1999. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 163, 1636–46. [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P, 2007. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 104, 17335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB, 2012. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 483, 87–91. [DOI] [PubMed] [Google Scholar]
- Lai KO, Ip NY, 2013. Structural plasticity of dendritic spines: the underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta. 1832, 2257–63. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Quach A, Moore DJ, Achim CL, Soontornniyomkij V, Masliah E, Singer EJ, Gelman B, Nemanim N, Horvath S, 2016. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 22, 366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, McDermott TJ, Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology. 91, e1860–e1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ransohoff RM, 2008. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 84, 116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Cao J, Wang S, Wang J, Sarkar S, Vigorito M, Ma JZ, Chang SL, 2013. Transcriptome sequencing of gene expression in the brain of the HIV-1 transgenic rat. PLoS One. 8, e59582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Luo C, Mines M, Zhang J, Fan GH, 2006. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 281, 37616–27. [DOI] [PubMed] [Google Scholar]
- Liao D, Lin H, Law PY, Loh HH, 2005. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci U S A. 102, 1725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Mi D, Llorca A, Marin O, 2018. Development and Functional Diversification of Cortical Interneurons. Neuron. 100, 294–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litke C, Bading H, Mauceri D, 2018. Histone deacetylase 4 shapes neuronal morphology via a mechanism involving regulation of expression of vascular endothelial growth factor D. J Biol Chem. 293, 8196–8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Haber C, Barrio-Real L, Casado-Medrano V, Kazanietz MG, 2016. Heregulin/ErbB3 Signaling Enhances CXCR4-Driven Rac1 Activation and Breast Cancer Cell Motility via Hypoxia-Inducible Factor 1alpha. Mol Cell Biol. 36, 2011–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Clem RL, 2018. GABAergic interneurons: The orchestra or the conductor in fear learning and memory? Brain Res Bull. 141, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduna T, Audouard E, Dembele D, Mouzaoui N, Reiss D, Massotte D, Gaveriaux-Ruff C, 2018. Microglia Express Mu Opioid Receptor: Insights From Transcriptomics and Fluorescent Reporter Mice. Front Psychiatry. 9, 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ, 1994. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 643, 245–65. [DOI] [PubMed] [Google Scholar]
- Marek R, Xu L, Sullivan RKP, Sah P, 2018. Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nat Neurosci. 21, 654–658. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I, 1997. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 42, 963–72. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H, 2001. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 4, 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JL, Barrett JS, Vezina HE, Spitsin S, Douglas SD, 2014. Adjuvant therapies for HIV-associated neurocognitive disorders. Ann Clin Transl Neurol. 1, 938–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RC, Chow DC, Lum CJ, Hidalgo M, Shikuma CM, Kallianpur KJ, 2017. Reduced functional connectivity between ventromedial prefrontal cortex and insula relates to longer corrected QT interval in HIV+ and HIV- individuals. Clin Neurophysiol. 128, 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Booze RM, Mactutus CF, 2019. Disruption of Timing: NeuroHIV Progression in the Post-cART Era. Sci Rep. 9, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ, 1998. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 95, 14500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HS, Schwarz D, Wimmer VC, Schmitt AC, Kerr JN, Sakmann B, Helmstaedter M, 2011. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A. Proc Natl Acad Sci U S A. 108, 16807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC, Zhang L, Dummer BW, Cariveau DR, Loh H, Law PY, Liao D, 2012. Differential modulation of drug-induced structural and functional plasticity of dendritic spines. Mol Pharmacol. 82, 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SC, Howell L, Beekman A, Stokes L, Mueller A, 2018. Rac1 plays a role in CXCL12 but not CCL3-induced chemotaxis and Rac1 GEF inhibitor NSC23766 has off target effects on CXCR4. Cell Signal. 42, 88–96. [DOI] [PubMed] [Google Scholar]
- Miwa H, Fukaya M, Watabe AM, Watanabe M, Manabe T, 2008. Functional contributions of synaptically localized NR2B subunits of the NMDA receptor to synaptic transmission and long-term potentiation in the adult mouse CNS. J Physiol. 586, 2539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I, Group, H., 2006. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 20, 879–87. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG, 2012. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 13, 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley SE, Grossman YS, Janssen WGM, Baxter MG, Rapp PR, Dumitriu D, Morrison JH, 2018. Selective Loss of Thin Spines in Area 7a of the Primate Intraparietal Sulcus Predicts Age-Related Working Memory Impairment. J Neurosci. 38, 10467–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CE, Shelton MA, Sweet RA, 2015. Dendritic spine alterations in schizophrenia. Neurosci Lett. 601, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Caroni P, 2018. Infralimbic cortex is required for learning alternatives to prelimbic promoted associations through reciprocal connectivity. Nat Commun. 9, 2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash B, Meucci O, 2014. Functions of the chemokine receptor CXCR4 in the central nervous system and its regulation by mu-opioid receptors. Int Rev Neurobiol. 118, 105–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash B, Tarn K, Irollo E, Luchetta J, Festa L, Halcrow P, Datta G, Geiger JD, Meucci O, 2019. Morphine-Induced Modulation of Endolysosomal Iron Mediates Upregulation of Ferritin Heavy Chain in Cortical Neurons. eNeuro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai J, Burbassi S, Rubin J, Meucci O, 2010. CXCL12 inhibits expression of the NMDA receptor’s NR2B subunit through a histone deacetylase-dependent pathway contributing to neuronal survival. Cell Death Dis. 1, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, Solomon T, 2014. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 13, 1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K, 2002. Structure and function of dendritic spines. Annu Rev Physiol. 64, 313–53. [DOI] [PubMed] [Google Scholar]
- Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H, 2005. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 46, 609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognjanovski N, Schaeffer S, Wu J, Mofakham S, Maruyama D, Zochowski M, Aton SJ, 2017. Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required formemory consolidation. Nat Commun. 8, 15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohene-Nyako M, Persons AL, Napier TC, 2018. Region-specific changes in markers of neuroplasticity revealed in HIV-1 transgenic rats by low-dose methamphetamine. Brain Struct Funct. 223, 3503–3513. [DOI] [PubMed] [Google Scholar]
- Ohshima Y, Kubo T, Koyama R, Ueno M, Nakagawa M, Yamashita T, 2008. Regulation of axonal elongation and pathfinding from the entorhinal cortex to the dentate gyrus in the hippocampus by the chemokine stromal cell-derived factor 1 alpha. J Neurosci. 28, 8344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW, 2014. Opioids and their receptors: Are we there yet? Neuropharmacology. 76 Pt B, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB, 1999. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 88, 1093–135. [DOI] [PubMed] [Google Scholar]
- Pena-Bravo JI, Reichel CM, Lavin A, 2017. Abstinence from Cocaine-Induced Conditioned Place Preference Produces Discrete Changes in Glutamatergic Synapses onto Deep Layer 5/6 Neurons from Prelimbic and Infralimbic Cortices. eNeuro. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Kolb R, Kennedy JE, Zheng J, 2007. Differential expression of CXCL12 and CXCR4 during human fetal neural progenitor cell differentiation. J Neuroimmune Pharmacol. 2, 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennucci R, Talpo F, Astro V, Montinaro V, More L, Cursi M, Castoldi V, Chiaretti S, Bianchi V, Marenna S, Cambiaghi M, Tonoli D, Leocani L, Biella G, D’Adamo P, de Curtis I, 2016. Loss of Either Rac1 or Rac3 GTPase Differentially Affects the Behavior of Mutant Mice and the Development of Functional GABAergic Networks. Cereb Cortex. 26, 873–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A, 2013. Cortical interneurons that specialize in disinhibitory control. Nature. 503, 521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J, Abt A, Myers J, Han R, Snyder M, Graziano A, Festa L, Kutzler M, Garcia F, Gao WJ, Fischer-Smith T, Rappaport J, Meucci O, 2014. Neuronal ferritin heavy chain and drug abuse affect HIV-associated cognitive dysfunction. J Clin Invest. 124, 656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol F, Kitabgi P, Boudin H, 2005. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J Cell Sci. 118, 1071–80. [DOI] [PubMed] [Google Scholar]
- Pyronneau A, He Q, Hwang JY, Porch M, Contractor A, Zukin RS, 2017. Aberrant Rac1-cofilin signaling mediates defects in dendritic spines, synaptic function, and sensory perception in fragile X syndrome. Sci Signal. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu CL, Huo FQ, Huang FS, Tang JS, 2015. Activation of mu-opioid receptors in the ventrolateral orbital cortex inhibits the GABAergic miniature inhibitory postsynaptic currents in rats. Neurosci Lett. 592, 64–9. [DOI] [PubMed] [Google Scholar]
- Reddon H, Marshall BDL, Milloy MJ, 2019. Elimination of HIV transmission through novel and established prevention strategies among people who inject drugs. Lancet HIV. 6, e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr., Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J, 2001. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 98, 9271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, Califano A, Masliah E, Sanna PP, 2014. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 9, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B, 2002. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 46, 271–9. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL, 2008. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 3, e1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H, 1995. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 34, 209–20. [DOI] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM, 2004. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol. 107, 97–110. [DOI] [PubMed] [Google Scholar]
- Sacktor N, 2018. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol. 24, 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, Heaton RK, Campbell LM, Chen A, Franklin D Jr., Ellis RJ, Collier AC, Marra C, Clifford DB, Gelman B, Sacktor N, Morgello S, McCutchan JA, Letendre S, Grant I, Fennema-Notestine C, Group CS, 2019. Effects of comorbidity burden and age on brain integrity in HIV. AIDS. 33, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando R 3rd, Gounko N, Pieraut S, Liao L, Yates J 3rd, Maximov A, 2012. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell. 151, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, Collins DL, 2017. Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. J Acquir Immune Defic Syndr. 74, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fellows LK, Ances BM, Collins DL, 2018. Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol. 75, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna PP, Repunte-Canonigo V, Masliah E, Lefebvre C, 2017. Gene expression patterns associated with neurological disease in human HIV infection. PLoS One. 12, e0175316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC, 2016. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol. 12, 234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Schmolke C, Musshoff F, Menzen M, Prohaska C, Madea B, 2003. Area-specific increased density of mu-opioid receptor immunoreactive neurons in the cerebral cortex of drug-related fatalities. Forensic Sci Int. 133, 204–11. [DOI] [PubMed] [Google Scholar]
- Seabold GK, Daunais JB, Rau A, Grant KA, Alvarez VA, 2010. DiOLISTIC labeling of neurons from rodent and non-human primate brain slices. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, Rubin JB, Meucci O, 2009. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 29, 2534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Brown M, Sengupta R, Penfold ME, Meucci O, 2011. CXCR7 protein expression in human adult brain and differentiated neurons. PLoS One. 6, e20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, Kuhn TB, Chen J, Bamburg JR, 2018. HIV Associated Neurodegenerative Disorders: A New Perspective on the Role of Lipid Rafts in Gp120-Mediated Neurotoxicity. Curr HIV Res. 16, 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich SS, 2016. Immune activation in the central nervous system throughout the course of HIV infection. Curr Opin HIV AIDS. 11, 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AL, Lee RN, Panvelker N, Li J, Harowitz J, Jordan-Sciutto KL, Akay-Espinoza C, 2018. Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response. J Neuroimmune Pharmacol. 13, 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JF, Burdo TH, Song SK, Sun P, El-Ghazzawy O, Nelson B, Westerhaus E, Baker L, Vaida F, Ances BM, 2017. Diffusion Basis Spectral Imaging Detects Ongoing Brain Inflammation in Virologically Well-Controlled HIV+ Patients. J Acquir Immune Defic Syndr. 76, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T, Koya E, Mayford MR, Hope BT, Weiss F, 2016. Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki K, Kaneko T, Mizuno N, 2000. A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience. 98, 221–31. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT, 2005. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 102, 15647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney SM, Woods SP, Sheppard D, Ellis RJ, 2018. Extrapyramidal motor signs in older adults with HIV disease: frequency, 1-year course, and associations with activities of daily living and quality of life. J Neurovirol. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL, 2002. Mobile NMDA receptors at hippocampal synapses. Neuron. 34, 255–64. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P, 2007. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 45, 174–82. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B, 2016. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron. 91, 260–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, Connaghan KP, Chang SL, 2015. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 48, 336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Hu XT, Napier TC, 2016. HIV-1 Transgenic Rat Prefrontal Cortex Hyper-Excitability is Enhanced by Cocaine Self-Administration. Neuropsychopharmacology. 41, 1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson N, Pantopoulos K, 2014. The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol. 5, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O’Neill J, Knott NL, Fox HS, Swindells S, 2013. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J Neuroimmune Pharmacol. 8, 965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM, Aloi J, Robertson KR, Sandkovsky U, White ML, O’Neill J, Knott NL, Fox HS, Swindells S, 2015. Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum Brain Mapp. 36, 897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski G, Szulczyk P, 2006. Opioid mu receptor activation inhibits sodium currents in prefrontal cortical neurons via a protein kinase A- and C-dependent mechanism. Brain Res. 1094, 92–106. [DOI] [PubMed] [Google Scholar]
- Wu PR, Cho KKA, Vogt D, Sohal VS, Rubenstein JLR, 2017. The Cytokine CXCL12 Promotes Basket Interneuron Inhibitory Synapses in the Medial Prefrontal Cortex. Cereb Cortex. 27, 4303–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Hou F, Wang X, Kong Q, Han X, Bai B, 2016. Aberrant Expression of Histone Deacetylases 4 in Cognitive Disorders: Molecular Mechanisms and a Potential Target. Front Mol Neurosci. 9, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P, 2007. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 56, 640–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lu Z, Narayan A, Le Rouzic VP, Xu M, Hunkele A, Brown TG, Hoefer WF, Rossi GC, Rice RC, Martinez-Rivera A, Rajadhyaksha AM, Cartegni L, Bassoni DL, Pasternak GW, Pan YX, 2017. Alternatively spliced mu opioid receptor C termini impact the diverse actions of morphine. J Clin Invest. 127, 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K, 1998. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 393, 809–12. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zhu G, Liu D, Ju JG, Liao ZH, Xiao YX, Zhang Y, Chao N, Wang J, Li W, Luo JH, Li ST, 2017. Extrasynaptic NMDA receptor dependent long-term potentiation of hippocampal CA1 pyramidal neurons. Sci Rep. 7, 3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Koyanagi Y, Yamamoto K, Oi Y, Koshikawa N, Kobayashi M, 2016. Opioid subtype- and cell-type-dependent regulation of inhibitory synaptic transmission in the rat insular cortex. Neuroscience. 339, 478–490. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T, 2004. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 5, 24–34. [DOI] [PubMed] [Google Scholar]
- Zamboni V, Armentano M, Berto G, Ciraolo E, Ghigo A, Garzotto D, Umbach A, DiCunto F, Parmigiani E, Boido M, Vercelli A, El-Assawy N, Mauro A, Priano L, Ponzoni L, Murru L, Passafaro M, Hirsch E, Merlo GR, 2018. Hyperactivity of Rac1-GTPase pathway impairs neuritogenesis of cortical neurons by altering actin dynamics. Sci Rep. 8, 7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP, 2005. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol Cell Biol. 25, 8456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB, 2005. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 46, 181–9. [DOI] [PubMed] [Google Scholar]