Abstract
Background:
Postpartum recovery from pelvic floor trauma associated with vaginal delivery may be impaired by the transient hypoestrogenic state associated with breastfeeding.
Objective:
The aim of our study was to examine the association between exclusive breastfeeding and pelvic floor disorders one to two decades after the first vaginal delivery. We hypothesize that compared to women who did not breastfeed following vaginal delivery, women who breastfeed would have a higher proportion of PFDs, and those women who practiced sustained exclusive/unsupplemented breastfeeding would have the highest proportion.
Study Design:
This is a secondary analysis of the Mothers’ Outcomes After Delivery study, a prospective cohort study of pelvic floor disorders after childbirth. Participants were recruited 5–10 years after their first delivery and followed annually for up to 9 years. This analysis focused on participants who experienced at least one vaginal delivery. Each participant completed a self-administered questionnaire regarding breastfeeding. Based on questionnaire responses, breastfeeding status was classified into three ordinal categories: “Unexposed” (did not breastfeed or breastfed <1 week), “Limited Exclusive Breastfeeding” (breastfed without supplementation for ≥1 week but <12 weeks), and “Sustained Exclusive Breastfeeding” (unsupplemented breastfeeding ≥ 12 weeks). Our primary outcomes of interest were the proportions of stress urinary incontinence, anal incontinence, and pelvic organ prolapse. The outcomes of interest were defined using the Epidemiology of Prolapse and Incontinence Questionnaire and the Pelvic Organ Prolapse Quantification Examination at enrollment and annually for up to 9 years thereafter. Additionally, a sub-analysis examined the relationship between breastfeeding and anal incontinence in an obstetric anal sphincter injury specific population. Generalized estimating equations were utilized to determine the relationship between breastfeeding and the outcomes of interest.
Results:
Among 705 women, 189 (27%) were classified as “Unexposed,” 145 (20%) were categorized as “Limited Exclusive Breastfeeding,” and the remaining 371 (53%) women met our definition of “Sustained Exclusive Breastfeeding.” Median follow-up was 5 years contributing to a total of 3079 person years.
The proportion of each pelvic floor disorder, based upon 3079 person years of follow-up was: stress urinary incontinence (27%), pelvic organ prolapse (20%), or anal incontinence (25%). Using generalized estimating equations adjusting for race, education, parity, and body mass index, Sustained Exclusive Breastfeeding was not significantly associated with stress urinary incontinence (adjusted odds ratio: 0.82, 95% confidence interval 0.55, 1.23), pelvic organ prolapse (adjusted odds ratio: 0.78, 95% confidence interval 0.49, 1.26), and anal incontinence (adjusted odds ratio: 0.67, 95% confidence interval 0.44, 1.00).
Regarding our obstetric anal sphincter injury sub-analysis, 123 women within our cohort experienced obstetric anal sphincter injuries at delivery. Anal incontinence was reported in 32% of these women. However, there was no observed relationship between breastfeeding and the development of anal incontinence during study follow-up in this population.
Conclusion:
Breastfeeding after vaginal childbirth was not associated with the development of stress urinary incontinence, pelvic organ prolapse, or anal incontinence 1 to 2 decades after the first vaginal delivery.
Keywords: Anal Incontinence, Breastfeeding, Pelvic Floor Disorders, Pelvic Organ Prolapse, Stress Urinary Incontinence
Introduction
Breastfeeding is associated with several known maternal and newborn benefits, and is a prevalent and appropriately encouraged practice. However, one potentially negative aspect of breastfeeding is its transient impact on the pelvic floor. Studies have demonstrated a statistically significant relationship between breastfeeding duration and urinary incontinence up to two years following childbirth. The Eden Cohort observed a relationship between breastfeeding ≥3 months and persistent postpartum incontinence at 24 months (odds ratio (OR) 1.54, 95% Confidence Interval (CI) 1.08 – 2.19), while KL Burgio et al., reported an association between the “duration of breastfeeding” and urinary incontinence at 12 months postpartum (OR 1.17; p=0.023).1,2
The association between breastfeeding and stress incontinence has been attributed to hypoestrogenism during lactation. Indeed, Estrogen receptors (ER) are found throughout the urogenital tract and obstetric and endocrine literature demonstrates breastfeeding mothers may experience estrogen depression, vaginal atrophy/dyspareunia, and prolonged postpartum amenorrhea.3–6 In postmenopausal women, the absence of estrogen is associated with decreased ratios of type I collagen and the impairment of connective tissue tensile strength.7 Additionally, animal studies suggest that estrogen deprivation following injury, impairs the process of muscle regeneration and repair, leading to increased scaring through fibrosis. Scar tissue can’t contract, hence significantly damaged muscle loses strength, endurance, and is at increased risk of re-injury.8–11 Considering the potential for pelvic floor trauma at the time of vaginal delivery,12 it is plausible that the unique hormonal milieu of the breastfeeding mother may lead to fibrosis and a higher incidence of pelvic floor disorders (PFDs) remote from delivery.
Little is known regarding the long term consequences of breastfeeding on the pelvic floor. Our primary aim was to examine the association between breastfeeding and PFDs remote from vaginal childbirth. Our secondary aim specifically targeted women with obstetric anal sphincter injuries (OASIS), to examine the relationship between breastfeeding and anal incontinence in the setting of confirmed delivery related trauma. In both populations we hypothesize that compared to women who did not breastfeed following vaginal delivery, women who breastfeed would have a higher proportion of PFDs, and women who practiced sustained unsupplemented/exclusive breastfeeding would have the highest proportion.
Materials and Methods
Subjects
This is a secondary data analysis of the Mothers’ Outcomes After Delivery (MOAD) study, a prospective cohort study examining the incidence of pelvic floor disorders in parous women. This study was coordinated by investigators from Johns Hopkins University and the Greater Baltimore Medical Center. Recruitment methods have been previously described.13 In brief, women were recruited from a large community hospital 5–10 years after their first delivery. Cesarean and vaginal delivery groups were matched to ensure similar age and time intervals from delivery to enrollment. All participants in the MOAD study completed an informed consent and institutional review board approval was obtained.
MOAD participants were seen for an initial visit and then annually thereafter up to nine years. At each visit, questionnaires were completed and a structured physical exam was performed. Study personnel were blinded to both the obstetric history and current symptoms. This analysis focused on MOAD participants who experienced at least one vaginal delivery. Among multiparous participants, we considered total parity but only the obstetric characteristics associated with the participant’s index vaginal delivery.
Exposures
Breastfeeding following vaginal delivery was the primary exposure of interest. Breastfeeding data were collected via self-administered questionnaire completed by each participant at the time of MOAD study enrollment. The breastfeeding questions were adopted from the National Immunization Survey.13,14 For each delivery, the participant was asked whether she breastfed. Women who reported breastfeeding were then prompted to answer two follow-up questions regarding duration (“How old was this child when he/she completely stopped breastfeeding?”) and exclusivity (“How old was this child when you first fed him/her something OTHER THAN breastmilk?”). Exclusive/unsupplemented breastfeeding is defined as the duration for which the child’s solitary source of nutrition came from breastmilk.
As previously reported by Handa et al, the first delivery was the birth most likely to cause harm to the pelvic floor.11 Hence, among multiparous women within this cohort, we focused on breastfeeding and obstetric exposures relevant to the first vaginal birth. For the purpose of this analysis, three ordinal breastfeeding categories were described; “Unexposed” (did not breastfeed or breastfed <1 week), “Limited Exclusive Breastfeeding” (Limited ExBF) (women who breastfed “exclusively” ≥1 week but <12 weeks), and “Sustained Exclusive Breastfeeding” (Sustained ExBF) (women who breastfed “exclusively” for ≥ 12 weeks) as reported on the study questionnaire. Women were assigned to breastfeeding categories based on the duration of breastfeeding exclusivity alone, once supplementation was introduced and exclusivity was broken, concomitant breastfeeding did not further affect assignment. The breastfeeding categories were designed considering the available literature reporting a return of menses in non-breastfeeding women between 8–11 weeks postpartum and prolonged postpartum amenorrhea in those individuals who continued to breastfeed exclusively.4,5
Obstetric exposures were obtained from review of hospital delivery records and included: maternal age at first vaginal delivery, prolonged second stage of labor (>120 min), macrosomia (neonatal weight ≥4000g), years between delivery and MOAD enrollment, and operative vaginal delivery (forceps or vacuum assisted vaginal delivery). OASIS were defined as vaginal delivery associated 3rd and 4th degree perineal lacerations. For the small percentage of subsequent births (3.5%) occurring at outside hospitals, maternal recall of delivery events was substituted for a formal review of obstetric records.
Potential demographic confounders were considered including; parity (described as ≥3 vaginal deliveries versus <3 vaginal deliveries), race (self-reported and classified as black or non-black), education, body mass index (measured each study visit and calculated kg/m2), cigarette smoking (described as never or ever, based on smoking ≥100 cigarettes in her lifetime), and age at MOAD enrollment.
Outcomes
The pelvic floor outcomes of interest were stress urinary incontinence (SUI), pelvic organ prolapse (POP), and anal incontinence (AI). SUI and AI were determined using the Epidemiology of Prolapse and Incontinence Questionnaire, a validated self-administered questionnaire.15 Scores greater than previously validated threshold values have been shown to correspond to clinically significant pelvic floor symptoms (SUI: score > 47mm, AI: score >23mm).
POP was evaluated using the Pelvic Organ Prolapse Quantification examination.16 The examination was performed by physicians and a research nurse, each of whom demonstrated competency in performing the examination prior to study initiation, with competency periodically reconfirmed throughout the study. While there is no universally accepted definition for pelvic organ prolapse, the consensus recognizes both subjective and objective findings must be considered.17 For this research, POP was defined as descent or prolapse of the vaginal walls and/or cervix beyond the hymen.
At each study visit, subjects were questioned about PFDs and interventions. Positive responses would lead to a follow-up question regarding specific PFD treatment modalities, including physical therapy, medications, pessary, and surgery. Participants who confirmed treatment for a PFD were considered to have the condition, regardless of current symptoms. If a participant met the previously defined criteria for SUI, POP, or AI at any visit during her longitudinal follow-up, she was categorized as ever having the disorder. In this way, we were able to assess the proportion of women who had each PFD throughout the entire study.
Statistical Analysis
We described maternal and obstetric characteristics by the three exposure categories (“Unexposed,” “Limited ExBF,” and “Sustained ExBF”) and tested associations utilizing Pearson χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. In these analyses, each of the 3 PFDs of interest (SUI, POP, and AI), were considered separately.
Due to the longitudinal nature of the data, each participant had a variable number of follow-up visits (one to nine), therefore, study visits were described in “person-years” for each exposure group. Our analysis accounted for the possibility that outcomes measured at each follow-up visit may be related, hence subsequent visits for a single participant could not be viewed as independent measures. The described correlation prevents the use of standard logistic regression while adjusting for potential confounders. To account for the loss of independence associated with within woman longitudinal measures, and to analyze the longitudinal measures collectively, generalized estimating equations (GEE) were utilized to determine the relationship between breastfeeding and the PFDs of interest. Multivariable models were used to control for potential confounders including race, education, parity, and body mass index, and to adjust for baseline imbalances between the previously defined exposure groups. Unadjusted and adjusted odds ratios (ORs) are presented as measures of associations between the PFDs of interest and the three breastfeeding categories.
We also performed a sub-analysis of our study population to examine the association between breastfeeding and anal incontinence (AI) specific to OASIS. . Only women who experienced OASIS during their first vaginal delivery were included in this analysis. GEE were similarly used to account for longitudinal data collection and to measure the relationship between breastfeeding and AI in the OASIS population. All statistical analysis was completed using STATA (StataCorp 2015, version 14SE, College Station, Tx). Statistical significance was defined at the 5% significance level.
Results
Of the 1529 participants who enrolled in the MOAD study, 750 experienced at least one vaginal delivery. MOAD participants who did not enter a self-reported breastfeeding status for the first vaginal delivery (n=1) and those who reported breastfeeding without providing a definitive duration (n=44) were not included in the analysis. Thus, a total of 705 women are included in our longitudinal study. (Figure 1)
Figure 1.
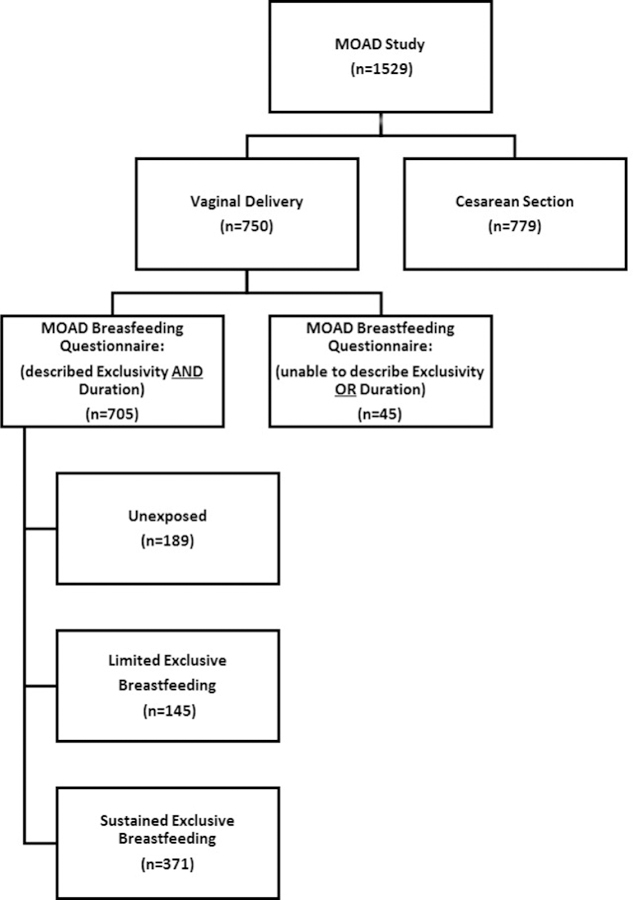
Flow diagram of study population.
Of 705 women, 371 (53%) participants met the definition of “Sustained ExBF” while 334 (47%) participants did not. Of those who did not breastfed exclusively for at least 12 weeks, 189 (27%) were categorized as “Unexposed” and 145 (20%) were categorized as “Limited ExBF.” Breastfeeding data was obtained from the self-assessment questionnaire a median of 6.2 [IQR 5.5, 7.7] years after primary vaginal delivery. Women in the Limited ExBF group, exclusively breastfed for a median of 4 [interquartile range (IQR) 2, 8] weeks compared to 20 [IQR 17, 26] weeks among the women in the Sustained ExBF group. In this study population, the median age of participants at time of vaginal delivery was 32 years and median age of participants at the time of study enrollment was 38 years. Characteristics of the study population did not differ significantly by breastfeeding category, with the exception of Education, body mass index (BMI), and Parity, as described in Table 1. Study participants who did not breastfeed were more likely to be obese at enrollment than those who practiced Sustained ExBF (25% compared to 15%; P<0.01). Alternatively, women who breastfed were more likely have a graduate degree (Sustained ExBF 44%, Limited ExBF 43%, Unexposed 31%; P=0.03) and be multiparous (Sustained ExBF 24%, Limited ExBF 16%, Unexposed 11%; p<0.01).
Table 1.
Obstetric and demographic characteristics of 705 vaginally parous women, by breastfeeding category.
| Characteristics α | Unexposed (n=189, PY=769) | Limited Exclusive Breastfeeding (n=145, PY=607) | Sustained Exclusive Breastfeeding (n=371, PY=1703) | p |
|---|---|---|---|---|
| Black Race | 26 (14%) | 22 (15%) | 37 (10%) | 0.19 |
| Age at first vaginal delivery, years | 0.11 | |||
| <30 years | 57 (30%) | 30 (21%) | 107 (29%) | |
| 30 to <35 years | 77 (41%) | 74 (51%) | 178 (48%) | |
| ≥ 35 years | 55 (29%) | 41 (28%) | 86 (23%) | |
| Macrosomia (≥4 kg) β | 10 (5%) | 16 (11%) | 28 (8%) | 0.15 |
| Episiotomy β | 102 (54%) | 68 (48%) | 204 (55%) | 0.30 |
| OASIs (3rd/4th Degree) β | 33 (18%) | 23 (16%) | 67 (18%) | 0.86 |
| Prolonged second stage of labor (>2 hours) β | 52 (28%) | 37 (26%) | 101 (28%) | 0.85 |
| Vacuum-assisted vaginal delivery β | 20 (11%) | 17 (12%) | 37 (10%) | 0.83 |
| Forceps-assisted vaginal delivery β | 28 (15%) | 18 (13%) | 43 (12%) | 0.53 |
| Age at study entry, years | 0.64 | |||
| <40 years | 112 (59%) | 89 (62%) | 235 (63%) | |
| 40 to <45 years | 56 (30%) | 44 (30%) | 109 (29%) | |
| ≥ 45 years | 21 (11%) | 12 (8%) | 27 (7%) | |
| Education level at study entry | 0.03 | |||
| ≤ High School graduate | 8 (4%) | 3 (2%) | 11 (3%) | |
| College degree | 123 (65%) | 79 (54%) | 197 (53%) | |
| Graduate degree | 58 (31%) | 63 (43%) | 163 (44%) | |
| BMI at study entry, kg/m2 | <0.01 | |||
| Normal (18.5 to <25 kg/m2) | 84 (44%) | 75 (52%) | 211 (57%) | |
| Overweight (25 to <30 kg/m2) | 58 (31%) | 51 (35%) | 106 (29%) | |
| Obese (≥30 kg/m2) | 47 (25%) | 19 (13%) | 54 (15%) | |
| Tobacco use (≥100 cigarettes in lifetime) at study entry | 49 (26%) | 50 (35%) | 107 (29%) | 0.244 |
| Parity (≥3 vaginal deliveries) at study entry | 22 (11%) | 23 (16%) | 89 (24%) | <0.01 |
| Longitudinal follow-up, years | 5.0 [1.6, 6.1] | 4.4 [2.6, 6.1] | 5.1 [3.0, 7.0] | 0.032 |
| Stress Urinary Incontinenceµ N=188/705 (27%) | 53 (28%) | 43 (30%) | 92 (25%) | 0.47 |
| Pelvic Organ Prolapseµ N=143/705 (20%) | 36 (19%) | 29 (20%) | 78 (21%) | 0.86 |
| Anal Incontinenceµ N=173/705 (25%) | 52 (28%) | 34 (23%) | 87 (23%) | 0.54 |
Abbreviations: PY, person years; BMI, body mass index
Variables reported as n (percent) for categorical and median [interquartile range] for continuous; missing data includes episiotomy: n=3; OASIS: n=16; prolonged second stage: n=20; vacuum-assisted delivery: n=1; forceps-assisted delivery: n=1
Characteristics reported at the first vaginal delivery
Proportion of PFDs, Criteria for PFD at some point during follow-up
The longitudinal nature of our cohort captured a variable number of annual follow-up visits (between one and nine) for each individual participant. Median follow-up was 5 years, and the 705 study participants contributed to a total of 3079 person-years of follow-up. The percentage of women who met the criteria of each PFD at any point during follow-up was (N=188/705, 27%) SUI, (N=143/705, 20%) POP, and (N=173/705, 25%) AI (Table 1). Within the cohort, 7% (14 out of 188) of SUI cases, 6% (9 out of 143) of POP cases, and only 2% (3 out of 173) of AI cases, were diagnosed based on reported prior PFD treatment alone. Using GEE, unadjusted and adjusted odds ratios (ORs) based on breastfeeding exposures were reported in Table 2. There was no statistically significant relationship between breastfeeding and the development of SUI, POP, and AI in the unadjusted modes. In models adjusting for potential confounders including race, education, body mass index, and parity, the lack of association persisted between breastfeeding and SUI (Limited ExBF adjusted odds ratio (aOR)=1.29, 95% CI 0.81, 2.08 and Sustained ExBF aOR=0.82, 95% CI 0.54, 1.23), POP (Limited ExBF aOR=0.91, 95% CI 0.51, 1.61 and Sustained ExBF aOR=0.77, 95% CI 0.47, 1.23), and AI (Limited BF aOR=0.78, 95% CI 0.47, 1.30 and Sustained ExBF aOR=0.66, 95% CI 0.44, 1.01).
Table 2.
Associationsa between breastfeeding categories and pelvic floor disorders.
| Pelvic Floor Disorders | Unexposed | Limited Exclusive BF | Prolonged Exclusive BF | |
|---|---|---|---|---|
| Stress Urinary Incontinence | Odds Ratio | 1 | 1.10 [0.69, 1.77] | 0.75 [0.50, 1.12] |
| aOR | 1 | 1.29 [0.81, 2.08] | 0.82 [0.54, 1.23] | |
| Pelvic Organ Prolapse | Odds Ratio | 1 | 0.84 [0.47, 1.50] | 0.73 [0.46, 1.17] |
| aOR | 1 | 0.91 [0.51, 1.61] | 0.77 [0.47, 1.23] | |
| Anal Incontinence | Odds Ratio | 1 | 0.75 [0.45, 1.25] | 0.70 [0.47, 1.05] |
| aOR | 1 | 0.78 [0.47, 1.30] | 0.66 [0.44, 1.01] | |
Unadjusted odds ratios (95% CI) from univariable logistic regression models using generalized estimating equations and adjusted odds ratios (95% CI) from multivariable models adjusting for race, education, body mass index at study entry, and parity (≥3) at study entry.
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval
Within our cohort, 123 women experienced OASIS during the first delivery; these women contributed 528 person-years of follow-up. Number (Thirty-two percent) of these women had AI during study participation. Median age of this population was 39 years at enrollment. Of these women, 33 (27%) were categorized as “Unexposed,” 23 (19%) were categorized as “Limited ExBF,” and the remaining 67 (54%) met the study definition of “Sustained ExBF.” Among women who sustained OASIS, there was no statistically significant relationship between the development of AI and Sustained ExBF (OR 0.65, 95% CI 0.29, 1.47). However our results suggest that Limited ExBF (OR 0.25, 95% CI 0.06, 0.99) may be associated with reduced odds for AI within the OASIS population. Additionally, there was no statistically significant relationship between breastfeeding and the development of SUI (Limited ExBF; OR=0.60, 95% CI 0.15, 2.33 and Sustained ExBF; OR=0.80, 95% CI 0.31, 2.11) or POP (Limited ExBF; OR=0.31, 95% CI 0.06, 1.53 and Sustained ExBF; OR=0.39, 95% CI 0.14, 1.10) in the OASIS sub-analysis population.
Discussion/Comment
Principal Findings:
The most important observation in our study was that there was no association between breastfeeding and SUI, POP, and AI one to two decades after vaginal delivery.
Results:
We are unaware of any previous studies exploring the relationship between breastfeeding and pelvic floor disorders remote from delivery for comparison. However, there are studies that demonstrate an association between persistent postpartum urinary incontinence and prolonged duration of breastfeeding. Quiboeuf et al. demonstrated a statistically significant relationship between mothers who breastfed ≥ 3 months and persistent urinary incontinence at 24 months postpartum (OR 1.54, 95% CI 1.08, 2.19). Burgio et al. also reported a relationship between the length/duration of breastfeeding and urinary incontinence at 12 months postpartum (OR 1.17, p=0.023).1,2 We believed Sustained ExBF would contribute to the cumulative development of PFDs and potentially account for the described persistence of de novo postpartum PFDs. However these relationships were not observed.
We further found that breastfeeding women who experienced a known pelvic floor trauma in the form of OASIS, did not demonstrate an increased proportion of AI when compared to non-breastfeeding women, suggesting breastfeeding does not cause long term consequences secondary to impaired anal sphincter recovery. Of note, an isolated observation appears to suggests that Limited ExBF (OR 0.25, 95% CI 0.06, 0.99) may be associated with reduced odds for AI within the OASIS population. However, we exercise caution in the interpretation of this finding, as it remains the lone outlier of significance within our statistical analysis and an appropriate “dose response” is not observed within the “Sustained ExBF group.”
Clinical Implications:
Despite the growing body of evidence describing estrogen’s role in facilitating regeneration and repair of skeletal muscle, one must consider that all musculoskeletal traumas are not created equal. More severe injuries may require months to heal, but some may be so severe that recovery simply is not possible (ie. levator avulsion). Alternatively, less severe injuries may complete the healing process in days to weeks. Considering that the hormonal differences assumed present between the exposed and the unexposed populations are thought to not be physiologically observed until after 8–11 weeks postpartum,4,5 any injury that heals prior to that critical juncture would avoid the hormonal influence of our study’s intended exposure.
Ripperda et al. using a rodent model, demonstrated that the application of vaginal estrogen after trauma results in divergent effects on the vaginal stromal and epithelial compartments. It was observed, that while intravaginal estrogen promotes vaginal epithelial wound healing, in the stroma (fibromuscular tissue), estrogen resulted in decreased collagen content and decreased expression of transcripts for matrix components. These results suggest that vaginal estrogen may actually have adverse effects on the early phases of stromal healing.18 Ripperda’s surprising findings were specific to the epithelial and stromal compartments of the rat vagina and can’t be generalized to the other structures, however his observations highlight the complex relationship between estrogen and the pelvic floor, and provide alternative mechanisms to consider as we evaluate our own results.
It is also worth noting the relatively young age of our study population. Of the 705 women in our analysis, only 49 women (7%) were categorized as postmenopausal prior to the conclusion of the study. Our study hypothesized that sustained breastfeeding was but one potential contributing factor, among several, in the cumulative development of PFDs. There is consensus that PFDs are likely multifactorial rather than the result of a singular catastrophic event and that the prevalence of PFDs significantly increases with age,19 hence it is reasonable to consider how our results may change as the cohort ages and inadequately healed pelvic floor musculature is further burdened by the eventual impact of the menopausal transition.
Lastly, it is possible that breastfeeding behaviors are representative of a woman’s health literacy and focus on general wellness. In fact, breastfeeding women in our cohort were less likely to be obese and more likely to have completed a higher level of education. Population based studies demonstrate an association between education and improved nutrition, greater access to healthcare resources, and a more expansive support network.20 These factors support consideration that non-breastfeeding mothers may be exposed to a greater number of potential risk factors for PFDs than their breastfeeding counterparts.
Research Implications:
We have a poor understanding of the healing and repair mechanisms of the pelvic floor following obstetrical trauma. Future research evaluating these processes on a cellular level is essential to improve our understanding of PFDs as well as estrogen’s role in this complex recovery process. A more robust understanding of pelvic floor regeneration and recovery could direct future prophylactic interventions and assisted recovery strategies.
Strengths and Limitations:
The strengths of this study include the large study population, longitudinal study design, and observation one to two decades after vaginal delivery. Obstetric exposures of the index delivery were determined by hospital records rather than maternal recall, and SUI, AI, and POP were defined by validated questionnaires or examination.
Despite its strengths, our study was limited by our inability to confirm delivery trauma to the pelvic floor. Additionally we were unable to measure post-partum estrogen levels. While exclusive breastfeeding acted as a proxy for hypoestrogenism, we are unable to say with certainty that the “Sustained ExBF” group indeed experienced a hypoestrognic state, or that the hypoestrogenic state resolved promptly in women not breastfeeding ≥12 weeks. Similarly, no data was collected regarding hormonal contraception use and its potential influence upon the breastfeeding mother’s hormonal milieu.
Seventeen percent of vaginal deliveries in our cohort were diagnosed with OASIS. While our rate is high, it is similar to the 17.7% rate reported by Fenner et al (2003).22 In 1998, the rate of OASIS in the US was estimated at 5.7% but rates vary substantially between hospitals, influenced by patient and hospital characteristics.23 Since 1998–2008, when this population delivered, OASIS in the US has declined by almost 50%.23 The increased OASIS rate in this population provided a relatively large sample to address AI after OASIS at a single institution. However, this also raises the question of whether the findings from this study, particularly regarding anal incontinence (but also SUI and POP), are generalizable to other vaginal delivery populations.
A post-hoc power calculation demonstrated our ability to detect clinically meaningful differences in PFDs between Exposure groups (OR > 1.65). However, statistical power was more limited for the OASIS sub-analysis, as our sample size limited our detection to only larger differences (OR at 3.10 at 80% power).
Finally breastfeeding data obtained from the self-assessment questionnaire was entirely reliant on participant recall 5–10 years after delivery (median 6.2 [IQR 5.5, 7.7] years), and is susceptible to response bias, differential misclassification, and sampling bias. Additionally, as with all observational studies, we cannot exclude the possibility that unmeasured characteristics/variables were relevant to our statistical findings and outcomes.
Conclusion
In conclusion, in both the vaginal delivery and the OASIS specific populations, there was no observed relationship between breastfeeding and SUI, POP, and AI regardless of exclusivity or duration of breastfeeding exposure, one to two decades after the first vaginal delivery. Despite reports of prolonged postpartum incontinence and dyspareunia associated with lactation, our findings should offer reassurance regarding potential long term consequences of breastfeeding on the pelvic floor.
Implications and Contributions:
A. Why was this study conducted?
To determine if post-partum hypoestrogenism associated with breast feeding impairs recovery from vaginal childbirth and therefore increase pelvic floor disorders later in life.
B. What are the key findings?
In this cohort of parous women, there was no significant relationship between breastfeeding and pelvic floor disorders one to two decades after vaginal delivery, regardless of breastfeeding duration or exclusivity.
C. What does this study add to what is already known?
While prior studies have demonstrated a short term increase in urinary incontinence during breastfeeding, these results suggest there is no long term consequences or increase in pelvic floor disorders, among women who breastfeed after vaginal delivery.
Acknowledgments
Funding support provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD056275, R01HD082070) for the conduct of research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Presented at the American Urogynecologic Society (AUGS) Pelvic Floor Disorders Week, October 9–13th, 2018, Chicago, Illinois.
This study was performed in the following locations: The Department of Gynecology and Obstetrics, Division of Female Pelvic Medicine and Reconstructive Surgery, John Hopkins University School of Medicine, Baltimore, MD.
Condensation: Pelvic floor disorders are not associated with breastfeeding in women who have had at least one vaginal delivery.
Contributor Information
David A. Lovejoy, Johns Hopkins School of Medicine, Department of Gynecology and Obstetrics, Division of Female Pelvic Medicine and Reconstructive Surgery.
Jennifer L. Roem, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology.
Joan L. Blomquist, Greater Baltimore Medical Center, Department of Gynecology, Division of Urogynecology.
References
- 1.Quiboeuf E, Saurel-Cubizolles MJ, Fritel X. Trends in urinary incontinence in women between 4 and 24 months postpartum in the EDEN cohort. BJOG 2016; 123(7): 1222–8 [DOI] [PubMed] [Google Scholar]
- 2.Burgio KL, Zyczynski H, Locher JL, Richter HE, Redden DT, Wright KC. Urinary Incontinence in the 12-month postpartum period. Obstet Gynecol 2003; 102(6): 1291–8 [DOI] [PubMed] [Google Scholar]
- 3.Alligood-Percoco NR, Klerulff KH, Repke JT. Risk Factors for Dyspareunia After First Childbirth. Obstet Gynecol 2016. September;128(3)512–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell OM, Gray RH. Characteristics and determinants of postpartum ovarian function in women in the United States. Am J Obstet Gynecol 1993; 169(1): 55–60 [DOI] [PubMed] [Google Scholar]
- 5.Heinig MJ, Nommsen-Rivers LA, Peerson JM, Dewey KG. Factors related to duration of postpartum amenorrhea among USA women with prolonged lactation. J Bioscoc Sci 1994; 26(4):517–27 [DOI] [PubMed] [Google Scholar]
- 6.Bonnar J, Franklin M, Nott PN, McNeilly AS. Effect of Breastfeeding on pituitary ovarian function after childbirth. Cr Med J 1975; 4(5988): 82–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, Watkins SC. Impact of menopause on collage subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol 2004. August;190: 620–7 [DOI] [PubMed] [Google Scholar]
- 8.Kitajima Y, Ono Y. Estrogens maintain skeletal muscle and satellite cell functions. J of Endocrinology 2016; 229(3): 267–75 [DOI] [PubMed] [Google Scholar]
- 9.Ahmed Y, Lin DL, Ferguson C, Esparza N, Damaser MS. Effect of Estrogen on Urethral Function and Nerve Regeneration following pudendal nerve crush injury in the female rat. J of Urology 2006; 175(5): 1948–52 [DOI] [PubMed] [Google Scholar]
- 10.Laumonier T, Menetrey J. Muscle injuries and strategies for improving their repair. J Exp Orthop 2016; 3(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber RL and Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol 2013. August 1;305(3):C241–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delancey JOL, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 2003; 101(1): 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic Floor Disorders 5–10 Years After Vaginal or Cesarean Childbirth. Obstet Gynecol 2011; 118(4): 777–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forste R, Hoffmann JP. Are US mothers meeting the healthy people 2010 breastfeeding targets for initiation, duration, and exclusivity? The 2003 and 2004 National Immunization Surveys. J Hum Lact 2008. August; 24(3): 278–88 [DOI] [PubMed] [Google Scholar]
- 15.Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapsed and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct 2005. Jul-Aug; 16(4): 272–84 [DOI] [PubMed] [Google Scholar]
- 16.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolpase and pelvic floor dysfunction. Am J Obstet Gynecol 1996. July; 175(1): 10–7 [DOI] [PubMed] [Google Scholar]
- 17.Gutman RE, Ford DE, Quiroz LH, Shippey SH, Handa VL. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J Obstet Gynecol 2008. [DOI] [PMC free article] [PubMed]
- 18.Ripperda CM, Maldonado PA, Acevedo JF, Keller PW, Akgul Y, Shelton JM, Word RA. Vaginal estrogen: a dual-edged sword in postoperative healing of the vaginal wall. Menopause 2017. July; 24(7): 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JM, Vaughan CP, Goode PS, et al. Prevalence and Trends of Symptomatic Pelvic Flor Disorders in US Women. Obstet Gynecol 2014. January; 123(1): 141–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieterich CM, Felice JP, O’Sullivan E, Rasmussen KM. Breastfeeding and Health Outcomes for the Mother-Infant Dyad. Pediatr Clin North Am 2013. February; 60(1): 31–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenner DE, Genberg B, Brahma P, Marek L, DeLancey JO. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol 2003. December 189(6):1543–9 [DOI] [PubMed] [Google Scholar]
- 22.Friedman AM, Ananth CV, Prendergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol 2015. April;125(4):927–37 [DOI] [PubMed] [Google Scholar]


