Abstract
Stressors experienced during adolescence have been demonstrated to have a long-lasting influence on affective behavior in adulthood. Notably, most studies to date have found these outcomes after chronic stress during adolescence. In the present study we tested how exposure to a single episode of acute footshock during early adolescence would modify subsequent adult anxiety- and depressive-like behaviors in male and female Sprague-Dawley rats. Adolescent rats were exposed to inescapable footshock (80 shocks, 5 s, 1.0 mA, 90 sec variable inter-trial interval (ITI)) at Post-natal day (PND) 29-30 and remained undisturbed until adulthood where they were evaluated with several behavioral assays for anxiety as well as depressive-like behavior via forced swim. In addition, gene expression changes were assessed immediately after a 30 min forced swim challenge in adulthood among several stress-related brain regions including the Central Amygdala (CeA), Medial Amygdala (MeA), ventral Hippocampus (vHPC), and Paraventricular Nucleus (PVN). Studies used real-time RT-PCR to examine the cytokines Interleukin-1β (IL-1β) and Interleukin-6 (IL-6), corticotropin-releasing hormone (CRH), the immediate early genes c-Fos, c-Jun, Egr1 and Arc, and several genes relating to corticosteroid receptor function (glucocorticoid and mineralocorticoid receptor (GR and MR, respectively), Gilz (glucocorticoid-induced leucine zipper), Sgk1 (Serum and Glucocorticoid regulated Kinase 1)). Behaviorally, males displayed signs of increased anxiety, most notably in the light-dark box, whereas females did not. No notable depressive-like behavior was observed in forced swim as a result of adolescent stress history, but adolescent footshock exacerbated the c-Fos response in the MeA produced by swim in both sexes. Forced swim led to increased IL-1β expression in the PVN regardless of adolescent stress history, whereas most HPA (hypothalamic-pituitary-adrenal) axis-related genes were largely unaffected in the vHPC. To determine the potential for β-adrenergic receptors to contribute to the male-specific anxiety-like behavior, two further studies applied a β-adrenergic agonist (isoproterenol) or antagonist (propranolol) in male rats. These studies found that propranolol administered 2h after footshock led to a reduction in some anxiety-like behaviors as compared to controls. Overall, these findings suggest that exposure to a single, intense stress challenge imposed during adolescence may have sex-specific consequences across the lifespan and may implicate the MeA in developmental plasticity.
Keywords: adolescence, stress, rat, anxiety, corticosterone, sex differences, Sprague Dawley, HPA-axis
Introduction
Adolescence is a period of transition marked by behavioral change and brain maturation, including structural changes in the HPA axis, limbic, and cortical regions (Brenhouse & Andersen, 2011; Russell D Romeo, 2017; L P Spear, 2000). Indeed, psychological disorders often emerge during this period (Patton & Viner, 2007; Romeo & McEwen, 2006), and adolescents are at elevated risk for development of disorders such as Post-Traumatic Stress Disorder (PTSD) (Zhang, Liu, Jiang, Wu, & Tian, 2014). Consistent with this, adolescents are differentially responsive to stress challenges relative to adult counterparts (Klein & Romeo, 2013). The adolescent HPA axis response to stress was slower to resolve, presumably due to the immature state of negative feedback systems (Cheryl M. McCormick & Mathews, 2007). Furthermore, adolescence marks a period of transition since repeated stress during pre-adolescence in neonates (PND 2-9) sensitized the HPA-axis (Knuth & Etgen, 2005; McCormick, Kehoe, & Kovacs, 1998) whereas in adulthood habituation was observed (Barnum, Blandino, & Deak, 2007; Hauger, Lorang, Irwin, & Aguilera, 1990). When repeated stress was applied in adolescence, corticosterone (CORT) and Adrenocorticotrophic Hormone (ACTH) habituation to stressors was attenuated relative to adults (Romeo & McEwen, 2006).
Importantly, many of the developmental changes occurring during adolescence are sex-specific, which may be in part due to the effects of gonadal hormones (for review, see Brenhouse & Andersen, 2011). Furthermore, stressors in adolescence can lead to sex-specific alterations in adulthood (Green & McCormick, 2013). In females, adolescent stress manifested as HPA-axis dysfunction (Bourke & Neigh, 2011; Wulsin, Wick-Carlson, Packard, Morano, & Herman, 2016), including increased basal concentrations of CORT that persisted into adulthood (Barha, Brummelte, Lieblich, & Galea, 2011), whereas males exhibited increased anxiety across a multitude of testing paradigms (L.E. Chaby, Cavigelli, Hirrlinger, Caruso, & Braithwaite, 2015; Cheryl M. McCormick, Mathews, Thomas, & Waters, 2010). There is evidence that these effects are specific to the adolescent period, as mice exposed to 12 days of chronic unpredictable stress (CUS) during adolescence exhibited increased anxiety-like behavior in both the marble burying task and the elevated zero maze 30 days later. Importantly, this study showed that adults exposed to the identical stressor had no effect on subsequent marble burying, suggesting adolescents may be uniquely vulnerable to long-lasting effects of stress experienced during this critical developmental period (Yohn & Blendy, 2017). This suggests that exposure to stress challenges during adolescence modifies the developmental trajectory of neural circuitry underlying affective responses and represents a window of vulnerability toward future affective dysfunction in a sex-specific manner.
The remodeling that occurs throughout adolescence includes synaptogenesis and pruning, which is primarily conducted by microglia, the innate immune cells of the brain (Selemon & Zecevic, 2015). As these cells are part of the innate immune response, stressful events that activate neuroimmune responses can alter their behavior and potentially affect the trajectory of brain development. Stressors, such as footshock, have been shown to induce expression of pro-inflammatory cytokines such as IL-1β through effects that likely involve microglia (Blandino et al., 2009). Expression of IL-1β modulates behavioral responses to stress (Arakawa, Blandino, & Deak, 2009), drives the HPA-axis by stimulating the release of hypothalamic CRH (Berkenbosch, van Oers, del Rey, Tilders, & Besedovsky, 1987; Ericsson & Sawchenko, 1993), and can modify neural plasticity (Goshen & Yirmiya, 2009). Furthermore, early life expression of pro-inflammatory cytokines has been shown to inhibit the development of cortical dendrites and may be a risk factor for the development of mental disorders (Gilmore, Fredrik Jarskog, Vadlamudi, & Lauder, 2004; Zubareva & Klimenko, 2013).
The central noradrenergic system orchestrates many features of the initial stress response (Morilak et al., 2005), with ample evidence supporting the role of ascending noradrenergic pathways as critical for subsequent cytokine expression (Blandino, Hueston, Barnum, Bishop, & Deak, 2013; J.D. Johnson et al., 2005). Pharmacological studies support these conclusions, and have delineated a unique role for α1-adrenergic receptors in the expression and release of blood-borne cytokines, whereas expression of cytokines within the parenchyma of the central nervous system (CNS) appears to be driven exclusively by β-adrenergic receptors (Blandino et al., 2009; Blandino, Barnum, & Deak, 2006; J.D. Johnson et al., 2005). Thus, the noradrenergic system is well-positioned to induce neuroimmune responses in brain regions undergoing developmental changes in adolescence as it projects from brainstem nuclei to the amygdala, hippocampus, prefrontal cortex, and hypothalamus, and ultimately contributes to adolescent stress-related dysfunction that emerges later in life.
Our goal was to assess adult HPA axis and behavioral outcomes after exposure to a single, intense stress challenge imposed during adolescence. In doing so, we utilized a footshock model, which we have used frequently in adult rats and is well characterized to induce central inflammatory cytokine expression and strong HPA axis activation (Blandino et al., 2009, 2006, 2013; Catanzaro, Hueston, Deak, & Deak, 2014; Deak, Bellamy, & D’Agostino, 2003; Lovelock & Deak, 2018). To assess anxiety-like behavior, we utilized the light-dark box, capitalizing on the natural inclination of rats towards exploratory behavior (Crawley & Goodwin, 1980) along with tests of social investigation and social interaction. Forced swim was used as a test for depressive-like behavior and also as a stressor (Deak et al., 2003; Hueston & Deak, 2014) to probe for changes in HPA axis reactivity. Additionally, three families of genes were assessed immediately after adult stress exposure to assess genes with established roles in stress reactivity. These included two neuroimmune genes (IL-1β, IL-6) known to be regulated by footshock, several Immediate Early Genes (IEGs) that effectively report cellular activation and relate to subsequent neuroplasticity under various conditions (c-Fos, c-Jun, Erg1, and Arc). These genes were examined in key limbic sites (central and medial nuclei of the amygdala, ventral hippocampus) and the paraventricular nucleus of the hypothalamus as a first step toward identifying neural pathways that might be responsible for the observed behavioral changes. Finally, several genes in the ventral hippocampus relating to corticosteroid regulation were evaluated, including both GR and MR expression, which often show dynamic modulation in response to stress exposure (eg., Xu et al., 2019); as well as two genes downstream of corticosteroid receptor activation: Gilz, a regulatory gene known to mediate the interaction between glucocorticoids and cytokines (Ayroldi & Riccardi, 2009), and Sgk1, a gene encoding a protein kinase involved in cellular stress responses (Hinds et al., 2017). We expected that adolescent footshock exposure would manifest in adulthood as increased anxiety, altered social interaction, and depressive-like behavior, and that in parallel we would detect increased signs of inflammation and neuronal activation in anxiety and stress-related brain regions. Furthermore, we used a β-adrenergic agonist (isoproterenol) and antagonist (propranolol) to assess the potential for the lasting anxiety-like outcomes to be mediated by β-adrenergic receptors, predicting that β-adrenergic receptor activation during adolescence would recapitulate the adult anxiety, whereas blockade of β-adrenergic receptors would block the effects of adolescent footshock. Propranolol was delivered before and after the footshock to test the importance of the timing of the pharmacological intervention as there is precedence in clinical research applying it after traumatic stress as a treatment for potential PTSD.
Results
Experiment 1
Light-dark box
A 2×2 ANOVA found a main effect of adolescent footshock on total time spent in the light [F(1, 26)=11.63, p<0.01] with shocked rats spending less time in the light, indicating increased anxiety (Figure 1D). Similarly, in latency to reenter the light side of the box there were main effects of adolescent footshock [F(1, 26)=9.48, p<0.01] and sex [F(1, 26)=4.70, p<0.05], with rats shocked in adolescence taking longer to re-enter the light side of the apparatus, and males taking longer than females (Figure 1C). There was also a main effect of the total number of transitions between the two chambers [F(1, 26)=14.78, p<0.05] with shocked rats showing a reduced number of crosses through the center aperture (Figure 1E). Shocked rats also showed less cautious exploration of the light side of the chamber as measured by number of times the rat’s head passed through the aperture past the ears but the rat did not make a transition, which was defined as head pokes [F(1, 26)=4.99, p<0.05]. A significant interaction [F(1, 26)=10.65, p<0.01] revealed that this effect was driven by the male shocked rats, which engaged in head poking less than any other group. In males, follow up t-tests found that adolescent shock increased latency into light [t(13)=4.50, p<0.001] and decreased time spent in light [t(13)=−4.52, p<0.001], number of entries into the light [t(13)=−4.93, p<0.001], and number of head pokes through the aperture [t(13)=−3.31, p<0.01], all of which demonstrate an increase in anxiety-like behavior. T-tests found no effect on any measure in females. All behavioral data can be found in Table 1.
Figure 1.
A. Experimental design. Male and female subjects were either exposed to footshock in adolescence or remained in the homecage, then were allowed to grow to adulthood. Then, independent cohorts of rats were exposed to one of two, 2-day behavioral testing procedures (the social interaction procedure, or Light-Dark box and activity testing). Two days later, subjects were exposed to forced swim or remained in the homecage, and brains and blood were collected for analysis. B-F. Graphs depicting results from anxiety testing in the light-dark box. G, H. Corticosterone and progesterone levels after the forced swim stressor. For all graphs, significant main effects of Sex are indicated with an asterisk (*). Main effects of Adolescent footshock are indicated by a dollar sign ($). Significant interactions terms are indicated by distinct letters (i.e., different letters represent statistically different).
Table 1 –
Behavioral results from Experiment 1
| Behavior | Male Homecage |
Male Adolescent Shock |
Female Homecage |
Female Adolescent Shock |
|---|---|---|---|---|
| Light-Dark Box | ||||
| Latency into dark (s) | 23.00 ± 10.84 | 16.43 ± 7.09 | 18.13 ± 7.46 | 10.00 ± 2.58 |
| Latency into light (s) | 280.25 ± 27.46* | 528.57 ± 40.64* | 234.88 ± 58.61* | 331.14 ± 79.86* |
| Time in light (s) | 131.91 ± 19.21 | 23.74 ± 34.55 | 139.58 ± 26.46 | 88.31 ± 30.50 |
| Transitions | 5.00 ± 0.63 | 1.00 ± 0.49 | 4.63 ± 0.80 | 3.00 ± 0.93 |
| Head pokes | 4.03 ± 1.42a | 1.29 ± 0.42c | 8.13 ± 0.77bc | 9.43 ± 1.23ab |
| Locomotor Activity | ||||
| Total distance traveled (cm) | 2200.25 ± 351.82 | 2621.00 ± 314.69 | 2111.75 ± 343.11 | 2773.00 ± 355.07 |
| Time spent moving (s) | 263.00 ± 38.88 | 319.49 ± 32.04 | 225.03 ± 31.63 | 280.76 ± 31.64 |
| Discrete horizontal movements | 192.75 ± 23.61* | 230.88 ± 15.02* | 169.13 ± 18.49* | 240.13 ± 22.42* |
| Vertical beam interruptions | 1376.43 ± 222.08* | 1679.71 ± 221.98* | 876.14 ± 221.77* | 1118.43 ± 225.99* |
| Number of Rears | 65.43 ± 14.71* | 64.71 ± 27.86* | 118.14 ± 27.41* | 158.43 ± 27.83* |
| Time reared (s) | 1452.66 ± 92.68* | 1525.54 ± 191.27* | 506.84 ± 155.01* | 621.50 ± 221.54* |
| Social Interaction Procedure | ||||
| Pre-Exposure | ||||
| 1st 10 minutes (pre-hut) | ||||
| Time on hut side (s) | 299.15 ± 10.07 | 341.24 ± 18.50 | 329.89 ± 16.10 | 306.02 ± 23.90 |
| Crosses | 31.50 ± 2.93 | 23.88 ± 2.57 | 31.75 ± 1.68 | 29.88 ± 2.73 |
| 2nd 10 minutes (hut introduced) | ||||
| Time on hut side (s) | 226.79 ± 19.17* | 155.27 ± 29.84* | 287.41 ± 41.58* | 349.58 ± 45.80* |
| Crosses | 32.25 ± 2.34 | 18.75 ± 1.39 | 32.88 ± 3.57 | 26.88 ± 2.64 |
| 3rd 10 minutes (hut present) | ||||
| Time on hut side (s) | 258.54 ± 38.19* | 201.41 ± 52.17* | 349.19 ± 63.66* | 360.00 ± 49.40* |
| Crosses | 18.13 ± 1.36a | 12.50 ± 1.98b | 14.13 ± 1.99ab | 16.75 ± 1.98ab |
| Test Day | ||||
| 1st 10 minutes (hut present) | ||||
| Crosses | 32.13 ± 3.29 | 27.88 ± 4.24 | 26.13 ± 3.82 | 26.38 ± 2.79 |
| Time on hut side (s) | 307.07 ± 34.21 | 215.25 ± 52.27 | 283.70 ± 56.16 | 362.35 ± 34.84 |
| 2nd 10 minutes (partner in hut) | ||||
| Crosses | 24.625 ± 4.90 | 27.38 ± 3.84 | 26.38 ± 4.80 | 22.25 ± 2.85 |
| Time on hut side (s) | 434.02 ± 28.58 | 404.34 ± 31.43 | 419.77 ± 49.46 | 426.32 ± 30.68 |
| Change in hut preference (s) | 126.95 ± 40.36 | 189.09 ± 24.82 | 136.07 ± 35.74 | 119.48 ± 42.24 |
| Social Interaction (hut removed) | ||||
| Sniffing | 30.25 ± 2.56 | 30.63 ± 3.55 | 29.38 ± 3.70 | 23.63 ± 3.14 |
| Climbing Over/Under | 2.5 ± 0.60 | 2.75 ± 0.94 | 3.25 ± 0.92 | 2.13 ± 0.52 |
| Grooming | 0.38 ± 0.18 | 0.38 ± 0.18 | 0.63 ± 0.26 | 0.13 ± 0.13 |
| Play (wrestling/boxing) | 3.13 ± 1.61 | 1.25 ± 0.84 | 3.88 ± 1.79 | 3.38 ± 1.40 |
| Dorsal contacts | 8.13 ±1.80 | 7.25 ± 2.17 | 9.88 ± 1.62 | 5.13 ± 1.26 |
| Total Social Interaction | 44.38 ± 4.21 | 42.25 ± 6.58 | 47.00 ± 5.92 | 34.38 ± 4.31 |
| Crosses towards partner | 20.38 ±2.52 | 22.00 ± 1.86 | 18.75 ± 2.23 | 18.25 ± 1.83 |
| Crosses away from partner | 14.38 ± 1.19 | 11.50 ± 1.63 | 17.13 ± 2.64 | 14.63 ± 1.35 |
| Total Crosses | 34.75 ± 3.05 | 33.50 ± 2.73 | 35.88 ± 4.29 | 32.88 ± 1.23 |
| Toward:away ratio | 1.48 ± 0.21* | 2.13 ± 0.28* | 1.25 ± 0.17* | 1.37 ± 0.21* |
| Forced Swim | ||||
| Latency to immobility | 108.75 ± 16.22 | 136.43 ± 6.88 | 118..13 ± 14.30 | 111.25 ± 13.12 |
| First 5 minute bin | ||||
| Climbing | 22.13 ± 2.17 | 23.13 ± 2.50 | 25.75 ± 1.62 | 21.50 ± 2.15 |
| Swimming | 17.00 ± 1.92* | 16.50 ± 2.07* | 19.63 ± 1.71* | 22.50 ± 2.41* |
| Floating | 20.88 ± 2.71* | 20.38 ± 1.53* | 14.63 ± 1.38* | 16.00 ± 2.27* |
Note. Means and SEM for locomotor activity across 30 minutes. Distances are measured in cm, time in seconds, and movements/beam interruptions are measured in counts. Bold indicates a main effect of adolescent footshock,
indicates a main effect of sex, and letters indicate a significant overall interaction, with individual letters indicating groups that are statistically different (i.e., different letters = statistical difference).
Locomotor Chamber
Locomotor chamber data were analyzed with 2×2 ANOVAs performed on the 30 minute session and t-tests were run for each sex individually. Separate repeated measures analyses were performed on 5 minute epochs, but those results are not reported as they did not substantively change the interpretation. Main effects of sex were found in vertical beam breaks [F(1, 24)= 12.00, p<0.01], number of rears [F(1, 24)=47.48, p<0.001], and time spent reared [F(1, 24)=112.99, p<0.001]. Females showed increases in horizontal [t(14)=2.44, p<0.05] and vertical [t(14)=3.18, p<0.01] movements.
Social Interaction
All data from the two day social interaction procedure were analyzed in 10 minute bins that correspond with built-in transition points (e.g. introduction of the hut). On pre-exposure day, when the hut was introduced in the second time bin, male rats spent less time on the side of the chamber with the hut [F(1, 28)=12.78, p<0.01], and rats that experienced adolescent footshock crossed the center of the chamber less often [F(1, 28=14.02, p<0.001]. In the third time bin, males continued to spend less time on the hut side [F(1, 28)=5.54, p<0.05] and there was a significant interaction in the number of crosses [F(1, 28)=1.95, p<0.05]. Post-hoc analysis found that males that were shocked crossed less than male home cage controls (p<0.05). Follow up t-tests found significant decreases in crosses by shocked males during the second [t(14)=4.96, p<0.001] and third [t(14)=2.34, p<0.05], pre-exposure time bins, potentially indicating neophobia in response to the introduction of the hut. There was also a trend (p=0.063) during the second time bin for shocked males to spend less time on the hut side of the cage than their non-shocked counterparts. On test day, no differences were seen in the first two bins, but a trend for an interaction was found in the first 10 minutes [F(1, 28)=3.51, p=0.07]. During the free social interaction portion of the test, no significant differences were found between groups except for in the crosses towards:away ratio [F(1, 28)=5.19, p<0.05] where males showed a tendency to move towards the partner more than females.
Forced Swim
To assess the influence of adolescent stress exposure on depressive-like tendencies, rats were exposed to a 30 min session of forced swim and behavior was assessed in the first 5 min. Swimming behavior was higher in females [F(1, 28)=4.45, p<0.05], and floating behavior was greater in males in the first 5 minutes [F(1, 28)=6.75, p<0.05], however no differences were seen in either sex due to adolescent stress. Notably, latency to show immobilization did not differ among groups.
Plasma measures
Plasma concentrations of CORT and PROG (progesterone) were assessed in trunk blood samples collected immediately after cessation of the 30 min swim session. As expected, there were main effects of sex [F(1, 54)=434.081, p<0.0001] and swim [F(1, 54)=1546.34, p<0.0001] on CORT levels, and a significant interaction between sex and swim [F(1, 54)=314.57, p<0.001]. 2×2 ANOVAs analyzing each sex separately found a main effect of swim in males [F(1, 28)=1178.35, p<0.001] and females [F(1, 26)=843.78, p<0.001] but no effect of adolescent shock. Similarly, there were main effects of sex [F(1, 56)=106.59, p<0.001] and swim [F(1, 56)=80.10, p<0.001] on PROG levels and an interaction between sex and swim [F(1, 56)=8.92, p<0.01]. See Figure 1G and H.
Gene Expression
There were no group differences in expression of the housekeeping gene cyclophilin. In the PVN, we found a main effect of swim, increasing expression of both c-Fos [F(1, 55)=29.94, p<0.001] and IL-1β [F(1, 54)=23.21, p<0.001] (Table 2). In the MeA, there were main effects of swim [F(1, 56)=406.08, p<0.001] and adolescent shock [F(1, 56)=406.08, p<0.05] on immediate early gene c-Fos with a significant interaction between swim and adolescent shock [F(1, 56)=6.73, p<0.05]. There were main effects of both sex [F(1, 56)=9.02, p<0.01] and swim [F(1, 56)=9.29, p<0.01] on CRH expression, with higher expression in females and in rats exposed to swim. Swim also mildly but significantly increased IL-1β [F(1, 55)=7.59, p<0.01] and reduced Tumor necrosis factor alpha (TNF-α) [F(1, 56)=5.15, p<0.05] expression in the MeA. In the CeA, swim increased c-Fos [F(1, 56)=163.44, p<0.001], Arc [F(1, 56)=14.30, p<0.001], and IL-1β [F(1, 55) = 11.37, p<0.01]. In the vHPC, swim increased c-Fos expression [F(1, 53)=67.36, p<0.001]. Further, there were no changes in expression of GR or MR receptors, but swim increased Gilz [F(1, 53)=13.41, p<0.001].
Table 2 –
Gene expression data from Experiment 1
| Target | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Adol shock only |
Acute Adult Swim |
Adol shock/ Adult swim |
Control | Adol shock only |
Acute Adult Swim |
Adol shock/ Adult swim |
|
| PVN | ||||||||
| c-Fos | 114.8 ± 25.6 | 125.3 ± 23.9 | 552.9 ± 87.4^ | 472.4 ± 28.8^ | 100.3 ± 6.2 | 138.7 ± 23.6 | 518.2 ± 62.2^ | 490.7 ± 86.1^ |
| IL-1β | 107.2 ± 14.5 | 97.1 ± 14.8 | 204.6 ± 22.8^ | 215.1 ± 19.1^ | 87.5 ± 6.1 | 67.3 ± 10.8 | 170.7 ± 27.3^ | 193.0 ± 18.1^ |
| IL-6 | 139.4 ± 45.0 | 217.3 ± 27.1 | 184.3 ± 57.5 | 141.1 ± 33.4 | 194.4 ± 21.7 | 210.2 ± 43.2 | 205.3 ± 43.2 | 215.4 ± 29.5 |
| CRH | 129.2 ± 29.6 | 188.9 ± 36.5 | 215.9 ± 36.7^ | 244.0 ± 42.6^ | 149.7 ± 33.7 | 183.6 ± 30.3 | 219.1 ± 47.2 ^ | 215.1 ± 40.2^ |
| Medial Amygdala | ||||||||
| c-Fos | 109.0 ± 15.0 | 90.2 ± 12.7 | 401.3 ± 52.9^ | 491.5 ± 52.9^ | 98.1 ± 5.4 | 103.9 ± 13.2 | 399.1 ± 18.3^ | 471.3 ± 18.9^ |
| Arc | 102.5±8.7 | 112.7±8.2 | 154.2±17.7^ | 191.4±28.3^ | 96.7±9.4 | 116.6±11.9 | 168.6±20.0^ | 190.6±20.6^ |
| Erg1 | 103.5±11.0 | 97.5±2.7 | 175.2±17.1^ | 216.5±12.8^ | 96.4±9.7 | 107.4±9.2 | 202.4±17.6^ | 219.1±18.8^ |
| c-Jun | 104.5±10.6 | 101.2±10.8 | 140.5±11.1^ | 138.1±8.3^ | 104.9±11.2 | 101.9±7.8 | 157.3±16.7^ | 141.6±8.7^ |
| IL-1β | 108.3 ± 17.6 | 91.2 ± 19.9 | 145.5 ± 20.6^ | 129.4 ± 12.4^ | 119.7 ± 31.0 | 76.0 ± 14.9 | 125.4 ± 19.5 ^ | 157.8 ± 22.8^ |
| IL-6 | 103.0 ± 8.4 | 105.3 ± 17.1 | 92.6 ± 10.6 | 76.6 ± 8.5 | 76.1 ± 15.1* | 102.1 ± 17.5* | 122.1 ± 24.0* | 115.2 ± 16.4* |
| CRH | 105.1 ± 12.3 | 117.2 ± 12.6 | 170.3 ± 30.0^ | 186.8 ± 19.6^ | 176.1 ± 32.1* | 179.6 ± 30.8* | 211.4 ± 16.4^* | 207.5 ± 23.1^* |
| Central Amygdala | ||||||||
| c-Fos | 104.8 ± 11.3 | 98.9 ± 15.1 | 435.5 ± 66.5^ A | 431.3 ± 42.1^ A | 89.1 ± 9.6 | 118.7 ± 16.9 | 319.7 ± 26.0^ | 393.0 ± 46.0^ |
| Arc | 105.4±14.0 | 88.5±17.8 | 141.9±18.4^ | 148.1±22.4^ | 81.2±14.6 | 97.3±13.0 | 130.2±21.6^ | 157.8±27.5^ |
| IL-1β | 101.8 ± 7.1 | 117.4 ± 9.6 | 174.6 ± 19.2 | 161.5 ± 20.3 | 111.8 ± 22.3 | 108.1 ± 20.1 | 125.5 ± 11.7 | 145.9 ± 21.7 |
| IL-6 | 105.1 ± 12.0 | 111.7 ± 14.9 | 104.4 ± 12.9^ | 120.2 ± 17.2^ | 118.2 ± 17.3* | 151.9 ± 15.6* | 178.9 ± 19.9^* | 179.0 ± 18.4^* |
| CRH | 104.0 ± 10.4 | 115.1 ± 16.8 | 130.7 ± 11.4 | 113.9 ± 14.9 | 106.5 ± 20.5 | 110.2 ± 16.6 | 111.2 ± 14.8 | 139.7 ± 26.5 |
| Ventral Hippocampus | ||||||||
| c-Fos | 104.2 ± 13.1 | 77.6 ± 9.0 | 395.6 ± 47..7 | 389.6 ± 31.7 | 100.7 ± 13.5 | 76.9 ± 7.6 | 256.4 ± 40.5 | 327.0 ± 67.4 |
| IL-6 | 107.8 ± 14.5ac | 91.9 ± 19.6ac | 82.1 ± 8.6a | 122.7 ± 17.8ac | 124.6 ± 18.8ac | 117.7 ± 13.9ac | 205.0 ± 21.7b | 132.2 ± 10.7c |
| GR | 100.9 ± 5.0 | 96.00 ± 5.5 | 95.1 ± 2.7 | 105.4 ± 6.5 | 107.2 ± 6.8 | 94.1 ± 3.9 | 102.7 ± 3.9 | 100.8 ± 4.3 |
| MR | 100.6 ± 4.2 | 80.4 ± 9.0 | 89.7 ± 7.7 | 91.8 ± 6.7 | 86.7 ± 9.4 | 85.5 ± 7.3 | 90.6 ± 6.8 | 90.4 ± 4.3 |
| Gilz | 101.3 ± 6.1 | 90.6 ± 7.0 | 108.1 ± 9.7^ | 114.5 ± 12.4^ | 93.6 ± 8.4 | 97.1 ± 10.3 | 131.9 ± 12.6^ | 122.5 ± 4.1^ |
| Sgk1 | 102.6±9.0 | 82.9±12.2 | 100.7±13.6 | 116.6±11.0 | 103.1±15.8 | 82.5±8.5 | 118.4±14.3 | 93.5±9.3 |
Note. Gene expression results from real-time RT-PCR after 30 min forced swim in adulthood. Bold indicates a main effect of adolescent footshock,
indicates a main effect of sex,
indicates a main effect of forced swim, and letters indicate a significant overall interaction, with individual letters indicating groups that are statistically different (i.e., different letters = statistical difference).
Experiment 2:
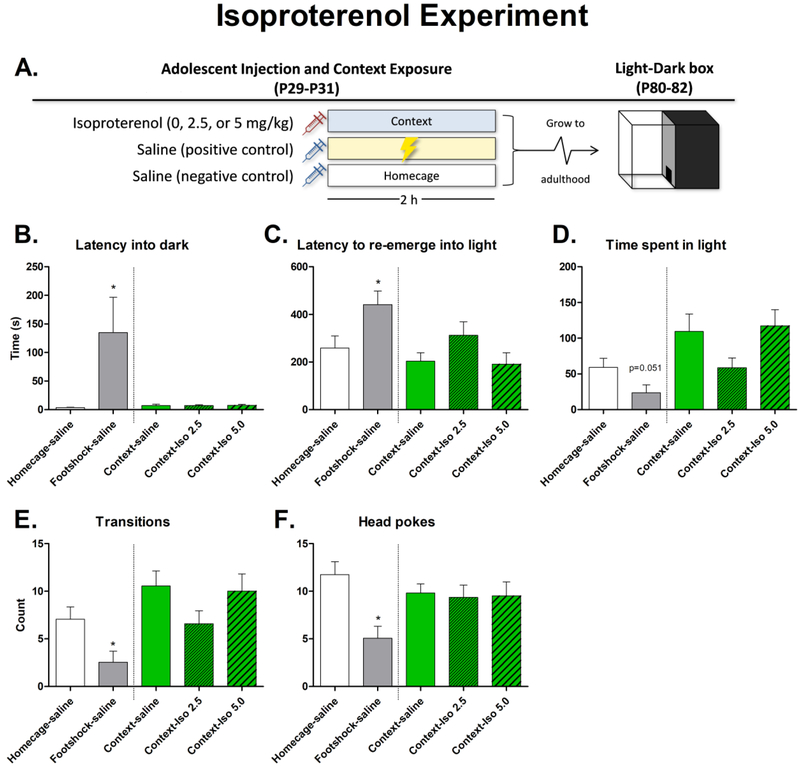
An omnibus one-way ANOVA found an effect of isoproterenol on each dependent measure: latency into dark [F(4, 45)=4.27, p<0.01], latency to re-emerge into light [F(4, 45)=4.04, p<0.01], time spent in light [F(4, 45)=4.85, p<0.01], number of transitions [F(4, 45)=4.49, p<0.01], and head pokes [F(4, 45)=3.69, p<0.05]. Additional analyses were performed to more precisely examine the results: T-tests were used to compare the homecage control group to the footshock positive control group to confirm replication of the result from experiment 1 (planned a priori), and a one-way ANOVA comparing the three groups that were exposed to the shock context (but not shock itself) in order to determine the effects of isoproterenol treatment. The t-test found that latency into dark [t(18)=2.12, p<0.05] and latency to re-emerge into light [t(18)=2.37, p<0.05] were higher in the footshock group as compared to controls; transitions [t(18)=2.58, p<0.05] and head pokes [t(18)=3.61, p<0.01] were lower in the footshock group, and there was a strong trend for the footshocked group to spend less time in the light (p=0.051). The one-way ANOVA between the context exposed groups did not find significant differences due to isoproterenol injection in any measure. See Figure 2.
Figure 2.
A. Experimental design. Subjects (males) were injected with isoproterenol or saline, then were either placed in an operant chamber, received footshock in an operant chamber, or remained in the homecage. They were allowed to grow to adulthood and were tested in the light-dark box for anxiety-like behavior. B-F. Graphs depicting results from anxiety testing in the light-dark box. Significant main effects of footshock are indicated with an asterisk (*).
Experiment 3:
A one-way ANOVA found an effect of treatment on latency to re-enter the light portion of the chamber [F(5, 42)=2.69, p<0.05]. Post-hoc analysis revealed rats that received propranolol 2 hours post-shock took less time to re-enter the light as compared to the footshock control group (p<0.05), propranolol before footshock (p<0.05), and the propranolol immediately after footshock (p<0.01) groups (Figure 3C). Though not statistically significant, there was a trend for the footshock saline-injected control group to take more time to enter the light as compared to the non-shocked saline control (p=0.099). See Figure 3.
Figure 3.
A. Experimental design. Subjects (males) were injected with propranolol immediately before, immediately after, or 2 h after footshock. They were allowed to grow to adulthood and were tested in the light-dark box for anxiety-like behavior. B-F. Graphs depicting results from anxiety testing in the light-dark box. Significant differences in Panel C are indicated by distinct letters (i.e., different letters represent statistically different). In Panel C, a main effect of experimental condition was observed, and significant differences determined by post-hoc comparisons are indicated by distinct letters (i.e., different letters represent statistically different).
Discussion
The primary goal of these experiments was to examine the impact of a single, intensely stressful experience imposed during adolescence on anxiety and depression-like tendencies later in adulthood. We found some outcomes in adulthood were sex-specific, with males displaying an anxiety-like phenotype evidenced by altered exploration of the light-dark box and signs of neophobia when a novel object was introduced in the social interaction procedure. In contrast, female rats did not display compelling evidence of persistent anxiety. Notably, neither depressive-like behavior in the forced swim test (FST) nor altered social investigation/interaction were observed in either sex. Importantly, the lasting male-specific anxiety-like effect of adolescent footshock was replicated in experiment 2, yet no significant effects were seen in adult anxiety-like behavior in the light-dark box as a result of isoproterenol administration. In experiment 3, when the β-adrenergic antagonist propranolol was injected 2 h after adolescent footshock, latency to re-emerge into the light was reduced relative to saline injected controls.
In experiment 1, the anxiety-like effects in males were most prevalent in the light-dark box (see Figure 1). When males and females were examined independently (as planned comparisons), these effects were significant in males but not females. In the social interaction procedure, when the hut was introduced on the pre-exposure day, male rats spent less time on the hut side (Table 1), and there was a trend for shocked males to avoid the hut. As neophobia can be used as an index of anxiety (Greggor, Thornton, & Clayton, 2015), these findings are suggestive of a male-specific neophobic response to the hut, corroborating the effects seen in the light-dark box. Interestingly, when a partner rat was introduced under the hut and when free social interaction was permitted, no effects were noted and social interaction was not impaired. It is possible that the presence of a conspecific attenuated the anxiety-like response similar to how social buffering is able to alleviate fear response and anxiety (Hennessy, Kaiser, & Sachser, 2009; Kikusui, Winslow, & Mori, 2006; Takahashi et al., 2013), or it may be that the adolescent footshock does not manifest as social anxiety later in life. Importantly, in the locomotor chambers there were no effects of adolescent footshock (Table 1) suggesting that the anxiety-like changes above were not due to general activity level alterations. Curiously, these findings contrast with a recent study showing that chronic unpredictable stress during adolescence led to increased exploration of a novel object 15 days later, an effect that was interpreted as increased “boldness” (Lauren E. Chaby, Cavigelli, White, Wang, & Braithwaite, 2013). Whether these disparate outcomes reflect the timing and nature of the adolescent stress procedure, or intrinsic differences in the subsequent tests of exploration remains to be determined.
Much of the literature examining outcomes after stressors in early adolescence have found evidence for increased anxiety, though sex differences have not been entirely clear or consistent across experiments (for review, see McCormick & Green, 2013). For example, chronic social instability stress in Long-Evans rats from PND 30-PND 45 led to increased anxiety-like behavior (reduced time spent in the open arm) in the elevated plus maze at PND 70 in males, and in females the effects were estrous cycle-dependent (McCormick, Smith, & Mathews, 2008). Thus, it is possible that the variability due to the estrous cycle made it difficult to detect anxiety-like effects in females in our studies. Controlling for this in future studies may reveal an interaction between ovarian hormones and adolescent stress history, though careful consideration of vaginal lavage affecting stress-related outcomes will be necessary. Another study applying a social stressor, social defeat stress, from PND 28-PND 41 found increased anxiety in both males and females in the elevated plus maze (Weathington, Arnold, & Cooke, 2012), whereas an anxiogenic effect (novelty-suppressed feeding) was observed in males after chronic variable stress starting on PND 30 and extending until PND 78 (L.E. Chaby et al., 2015). These studies, and indeed the majority of experiments in the broader literature, differ from the present experiment in that they applied stressors over the course of many days as opposed to a single episode of footshock. Also, few prior studies have used the light-dark box as the primary measure of anxiety after adolescent stress. Regardless, as a whole our findings support and expand on the preponderance of studies demonstrating lasting increases in anxiety-like behaviors in males after adolescent stress, showing lasting effects after a single stressor exposure.
Contrary to our expectations, forced swim did not reveal any depressive-like behavioral effects after shock in adolescence (Table 1), suggesting that adolescent shock produced a specific rather than generalized form of affective dysfunction later in life. Chronic variable stress in mid-adolescence has previously been shown to increase immobility in the forced swim test at PND 73 (Wilkin, Waters, McCormick, & Menard, 2012) and PND 101 (Wulsin et al., 2016), though these experiments differ from Experiment 1 in both timing (early vs mid adolescence) and nature of the stressor (single event vs multiple days). In addition, no changes were seen in the plasma CORT response to forced swim in either sex. This is in contrast to other recent findings from our lab where we found females (but not males) exposed to adolescent intermittent alcohol displayed enhanced HPA axis reactivity when challenged as adults (Vore, Doremus-Fitzwater, Gano, & Deak, 2017), and broader evidence suggesting that exposing females to stress during adolescence can produce long-term changes in HPA axis function (Bale & Epperson, 2015; Barha et al., 2011). Further studies determining why these female-specific HPA axis programming effects differ between adolescent interventions are warranted and are the subject of forthcoming studies from our lab.
Although the mechanisms by which adolescent footshock increased adult anxiety are at present unclear, we utilized gene expression analysis as a first test of several potential mechanisms. First, because footshock is known to increase IL-1β gene expression, we examined two neuroimmune genes, IL-1β and IL-6. Although both of these genes were increased in select regions after forced swim (replicating our prior results), neither gene displayed a sensitized response due to adolescent stress history. Second, we assessed several IEGs as both a reporter of neuronal activity and for their relation to neuroplasticity. Since most of these genes displayed relatively weak induction after forced swim exposure, we focused predominantly on c-Fos. While forced swim increased c-Fos in all brain regions examined (Table 2), the MeA displayed enhanced c-Fos in both males and females due to footshock in adolescence. This suggests that shock induced adaptations that increased inducible activity within neurons in the MeA, which has previously been implicated in anxiety-related behavior (Ebner, Rupniak, Saria, & Singewald, 2004). Though the basolateral amygdala (BLA) is the focus of a majority of studies on consequences of stress exposure and subsequent anxiety, both are modulated by stress, show alterations under experimental conditions of anxiety. Importantly, the MeA projects to the hypothalamus (for review, see Roozendaal, McEwen, & Chattarji, 2009) and direct MeA stimulation drives HPA axis activation (Dunn & Whitener, 1986). Further, norepinephrine (NE) levels were increased in the MeA in response to immobilization stress, and blockade of noradrenergic receptors reduced the subsequent activation of the HPA-axis (Ma & Morilak, 2005). Additional examination of changes in the MeA using IHC or in situ hybridization will be necessary to identify specific neuronal populations in which the adaptations are occurring. Finally, our gene expression analyses included several genes relating to HPA axis regulation, including CRH, both corticosteroid receptors (MR and GR), and two genes down-stream of corticosteroid receptor activation (Gilz and Sgk1). CRH, thought to be a mediator of anxiety-like behaviors in the amygdala (Liebsch et al., 1995), was increased specifically in the MeA. This effect appears to be primarily driven by males, whereas females displayed higher baseline levels of CRH even in controls. However, like with IL-1β, shock in adolescence did not affect the response to forced swim. In the vHPC, which is also implicated in the expression of anxiety (Adhikari, Topiwala, & Gordon, 2010; Bannerman et al., 2003), neither GR nor MR expression were modulated by shock experienced in adolescence nor by the acute experience of swim (Table 2). The apparent lack of changes in corticosteroid receptors and IL-1β suggest that these targets are not likely mechanisms to explain the outcomes of adolescent footshock observed here. Similar null effects were observed in Gilz and Sgk1, further suggesting that HPA axis regulation was largely unaffected by adolescent footshock exposure. Overall, while there were a number of effects of swim and sex on stress-related targets, c-Fos in the MeA was the only target affected by adolescent stress. The amygdala is a key modulator of emotional memory consolidation (Roozendaal et al., 2009), and thus this finding suggests this region may serve as a target for future development of therapeutic agents.
Despite providing an important replication of the adolescent footshock effect on anxiety expressed in adulthood, Experiment 2 suggested that activation of β-adrenergic receptors via isoproterenol injection did not effectively recapitulate the influence of adolescent footshock among male rats, at least at the 2 doses of isoproterenol tested here. Although Experiment 3 yielded a weaker replication of the original effect of adolescent footshock on adult anxiety, it is important to note that the directionality of the effects was still the same and approached significance. Nevertheless, administration of propranolol 2 hours after the footshock (but not immediately before or after footshock) reduced the latency for rats to re-emerge into the light side of the light-dark box in adulthood as compared to controls that received saline injections (Figure 3C). These findings are consistent with a present clinical treatment for PTSD, where propranolol injections within 6 hours of a traumatic event has been shown to reduce PTSD symptoms in emergency room patients (Pitman et al., 2002). However, how this treatment may interact with ongoing maturation is not yet known. Our hypothesis was that stress-dependent release of NE and subsequent activation of β-adrenergic receptors may be responsible for programming changes that led to later anxiety, suggesting that propranolol administration might disrupt memory consolidation and prevent the intense emotional association that can lead to later affective dysfunction (Przybyslawski, Roullet, & Sara, 1999). The 2 h post-shock effect of propranolol fits with this hypothesis and highlights the importance of careful consideration of the timing of pharmacotherapeutic interventions (before or after precipitating stress challenge) for determining the ultimate outcome. Similarly, the lack of effect of propranolol administered prior to footshock is consistent with the outcome of Experiment 2 suggesting that NE release and subsequent activation of β-adrenergic receptors during footshock is not critical to the developmental programming effects on anxiety.
An important consideration for future studies may be the influence of pubertal stage and/or pubertal hormones in defining the window of vulnerability to stress in adolescence (Vetter-O’Hagen & Spear, 2012). It should be noted that in the present studies stress was imposed prior to puberty, corresponding to early adolescence (Spear, 2015). Indeed, exposure to stress prior to puberty can have transgenerational effects that persist across at least 2 generations, further supporting the vulnerability of the early adolescent period to early life adversity (Zaidan & Gaisler-Salomon, 2015). Interestingly, recent studies in which peri-pubertal rats (5-6 weeks of age) were exposed to restraint plus predator odor led to enhanced anxiety in the elevated plus maze and spontaneous discharge of neurons in the locus coeruleus (LC), a key site controlling forebrain release of NE, for at least a week after stress cessation (Borodovitsyna, Flamini, & Chandler, 2018). It is also possible that pre- or peri-pubertal stress may alter the surge in pubertal hormones and/or the timing of pubertal onset. However, due to concerns about potential handling effects, puberty onset was not examined in the present studies. A separate but related matter is that our studies did not control for estrous stage in adult females nor examine how adolescent footshock might alter estrous cyclicity later in life. Finally, it may be important to include additional control groups, such as rats exposed to the shock context (but without actual shock) to better understand the nature of the stress challenge that is necessary to produce long-lasting changes in anxiety. These issues will be the subject of future studies and are beyond the scope of the present work.
Overall, the present results indicate that a single session of footshock imposed in early adolescence led to increased anxiety in male rats in adulthood. Injection of a β-adrenergic agonist in lieu of the footshock did not recapitulate the effect, indicating that the mechanism is unlikely to arise solely from a downstream effect of norepinephrine release during footshock. However, β-adrenergic receptor antagonism 2 hours post-shock significantly reduced later-life anxiety, commensurate with approaches for treating PTSD (Pitman et al., 2002). Based on the preliminary gene analyses conducted here, neither of the neuroimmune genes examined displayed altered induction patterns in rats with a history of adolescent stress. Similarly, none of the HPA axis-related genes displayed expression patterns that would be suggestive of HPA axis alterations due to adolescent stress. In contrast, the IEG c-Fos displayed enhanced expression in the MeA (but not CeA), suggesting MeA sensitization may be related to long-lasting anxiety incurred by adolescent footshock. As a key feature of adolescence is the maturation of brain regions implicated in affective disorders, it is likely a critical period in which stress challenges lead to long-term changes. Indeed, many pathophysiological processes ranging from addiction vulnerability, to affective disorders, and even acceleration of natural aging can begin with stress during adolescence (Chambers, Taylor, & Potenza, 2003). Thus, it is important that researchers pursue the mechanisms underlying developmental programming effects of early life stress in order to develop effective clinical treatments.
Methods
Subjects
All experiments used Sprague-Dawley rats bred and born in our colony from breeders originally acquired from Envigo/Harlan. Litters were culled to a total of 10 pups and no litter had less than 3 pups of either sex. Juveniles were pair-housed with non-littermates at PND 21-22. Rats had access to food and water ad libitum and were provided wooden chew sticks for enrichment. Colony conditions were maintained at 22±1°C with 12:12 light–dark cycle and experiments were conducted during the light phase of the circadian cycle. Rats were handled for 3-5 min on each of two days before experimentation as adolescents, and again one day prior to adult testing. Rats in the Home Cage Control condition were treated identically to subjects in the stress condition, with the exception that they remained in their home cages undisturbed (i.e., home cage controls were not transferred to the shock apparatus). In all experiments, experimental groups contained no more than 1 −2 pups from the same litter to avoid litter effects. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Binghamton University and animals were treated in accordance with Public Health Service (PHS) policy.
Experiment 1 procedure
The purpose of experiment 1 was to assess adult behavioral outcomes after adolescent footshock. Adolescent male and female rats from our colony (N = 64; n = 8 per group) were either exposed to footshock in early adolescence (PND 29-PND 30) or remained in their home cages undisturbed (i.e., home cage controls were not transferred to the shock apparatus). After cessation of footshock, all rats remained undisturbed in the colony until adulthood (PND 69-PND 71). In adulthood, two independent cohorts of rats were randomly assigned to either (a) a two-day social interaction procedure, or (b) light-dark box testing on day 1 and locomotor chamber testing on day 2. Two days later, half of the rats from each cohort were exposed to 30 min of forced swim while the other half remained in their home cages (see Figure 1A). Thus, the order of behavioral testing was scripted (not counter-balanced) within each cohort of rats. Brains and plasma were harvested immediately after the cessation of swim. Corticosterone and progesterone levels were measured in blood. Stress and anxiety-related brain regions of interest (vHPC, CeA, MeA, PVN) were punched as previously described (Lovelock & Deak, 2017) and mRNA expression was analyzed using real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) to assess neuroimmune genes (IL-1β, IL-6), immediate early genes (c-Fos, c-Jun, Erg1 and Arc), and genes relating to HPA function (CRH, MR, GR, Gilz, Sgk1) to probe for alterations in each of these systems that may underlie behavioral changes. Because c-Fos displayed the greatest sensitivity in the MeA, other limited probing of other IEGs was performed in other brain regions.
Footshock
Rats were exposed to 80 inescapable footshocks (1.0 mA, 5 s each, 90 s variable ITI, approximately 120 min) as previously described (Hueston et al., 2014). Briefly, footshock was administered in a chamber measuring 30.5 (L) × 26.5 (W) × 33 (H) cm (Habitest Chamber, Model H10-11R-TC-SF, Coulbourn Instruments, Allentown, PA, USA). The side walls of the chamber were constructed of stainless steel except the front doors that were constructed of clear Plexiglas. The floor consisted of steel rods through which a scrambled shock from a shock generator (LABLINC Model H01-01, and Precision Animal Shocker Model H13-15, Coulbourn Instruments, Allentown, PA, USA) could be delivered. The sound-attenuating chambers were illuminated by a 20-W white light bulb and background noise was provided by individual ventilation fans. Each shock chamber and waste collection tray was cleaned after each session.
Light-Dark Box
The test apparatus (30 × 30 × 20 cm) consisted of two adjacent chambers with an open 9 × 7 cm door between them. One chamber was covered and lined with opaque black material (dark side), and the other was uncovered with white walls (light side) and lights directly overhead. Light measured 250 lux in the center of the open chamber, and the dark chamber measured no more than 2 lux. Rats were placed in the open chamber facing away from the door and remained in the apparatus for 30 min. The session was recorded with an overhead camera and behavior was scored by a blind observer. The length of the test (30 min) was longer than the timespan in which behavioral measures were compared (10 min) in order to keep the length of procedure equivalent to that of the social interaction procedure, and the following behavioral measures were recorded: latency into dark, latency to return into the light, time spent in light, entrances into light, and head pokes (defined as both ears passing through the door followed by withdrawal back into the dark side).
Locomotor Activity Chamber
Locomotor activity testing was conducted in six identical acrylic chambers (40 × 40 × 30 cm; Accuscan Instruments, Columbus, OH, USA). Each chamber was surrounded by a 15 × 15 infrared photocell array interfaced with a computer using Versamax and Versadat programs (Accuscan Instruments). Rats were placed in the chambers for 30 minutes, and data were aggregated across the test period. The following behavioral measures were analyzed: total distance traveled, time spent moving, number of discrete horizontal movements, number of vertical beam interruptions, number of rears, time spent reared, distance traveled in the center of the chamber, and time spent in the center of the chamber.
Social Interaction Procedure
Social interaction testing took place during the light cycle, and measurements were recorded and scored by a blind observer. Weight differences between test subjects and partners were minimized, and social partners always weighed less than experimental animals to avoid aggression on the part of the subject. On day 1 of testing, subjects were placed in the test chamber (73.3 × 48.6 × 53.3 cm) for a total of 30 minutes. A line on the floor of the chamber defined the two halves, and in time spent on each side of the chamber was recorded for all 10 min time bins to ensure that individual subjects did not display a side preference/bias prior to introduction of the hut. Subjects first habituated for 10 min after which a novel weighted-down white steel mesh hut (15 × 15 × 15 cm) was introduced. The next day, rats were again placed in the chamber for 30 minutes. The mesh hut was present for the first 10 minutes, and in the second 10 minute time bin a novel same-sex rat was placed under the hut which allowed the test subjects to see and smell the partner but prevented interaction. In the last 10 minutes, the hut was removed and the rats were allowed to interact freely. The following social behaviors were scored: sniffing, crawling over/under the partner, grooming, play, dorsal contacts, and crosses over the center line of the chamber towards and away from the partner. This multi-phase test of social investigation and social interaction was adapted from our prior work (Arakawa, Arakawa, Blandino, & Deak, 2011; Lord et al., 2014; Perkins et al., 2016)
Forced Swim
Rats were transported to a dedicated procedural room and immediately placed in a cylinder (45 cm high, 20 cm diameter) filled 30 cm high with clean water that was maintained at 25°C as previously described (Deak et al., 2003). Rats were forced to swim for 30 min, after which brains and blood were collected. The purpose of the FST here was two-fold. First, by coding behavioral responses in the first 5 min, we expected to capture depressive-like tendencies for which the FST has been well-validated. Second, a more extended swim session of 30 min has been shown to serve as a potent stressor evidenced by induction of certain neuroimmune genes (including IL-1β), activation of IEGs, and alterations in HPA axis activity (Hueston & Deak, 2014). Sessions were recorded using a video camera at a downward angle and recordings were scored by an observer blind to experimental conditions. Scoring was done on an interval based system (5 sec each), whereby intervals were classified as climbing (characterized by vertical motion of the front paws, often directed at the walls), swimming (predominantly horizontal movement and swimming in circles), or immobility (absence of movement except that which was necessary to keep the rat’s head above water).
Tissue Collection and Processing
All tissue collection, processing, and real-time RT-PCR was conducted using procedures described in previous work (Lovelock & Deak, 2017). Specifically, tissue was harvested after rapid decapitation and trunk blood was collected in EDTA-coated vacutainers. Plasma was separated in a refrigerated centrifuge and frozen at −20°C until time of assay. Brains were removed immediately after decapitation and whole brains were flash frozen in 2-methylbutane (EMD Millipore, cat. No. MX0760-1, Billerica, MA) and stored at −80°C. Brains were sectioned coronally (60 μm) on a cryostat (Leica Model CM1850, Wetzlar, Germany) and bilateral tissue punches were taken from structures of interest, according to the atlas of Paxinos and Watson (2005). For schematic illustrations of tissue punches, please see Lovelock & Deak, 2017.
For RNA extractions, each tissue sample was placed in a 2.0 ml Eppendorf tube with 500 μL Trizol® RNA reagent and a 5 mm stainless steel bead. Tissue was then homogenized using a Qiagen TissueLyser II™ (Qiagen, Valencia, CA) for 2-4 min at 20 Hz to ensure thorough homogenization of samples. Total cellular RNA was extracted from tissue using Qiagen RNeasy Mini kits according to the manufacturer’s instructions. RNA was separated from the supernatant through chloroform extraction performed at 12,000 g for 15 min at 4°C. Equal volume of 70% ethanol was added to the collected RNA and purified through RNeasy mini columns. Columns were washed and eluted with 30 μL of RNase-free water (65°C). RNA yield and purity was determined using the NanoDrop 2000 spectrophotometer (NanoDrop, Wilmington, DE). RNA was stored at −80°C prior to cDNA synthesis.
Real Time RT-PCR
Synthesis of cDNA was performed on 0.3-1.0 μg of normalized total RNA from each sample using the QuantiTect Reverse Transcription kit (Cat No. 205313, Qiagen, Valencia, CA) which included a DNase treatment step. All cDNA was stored at −20°C until further processing. Probed cDNA amplification was performed in a 20 μL reaction consisting of 10 μL IQ SYBR Green supermix (Bio-Rad Laboratories), 0.1 μL forward and reverse primer, 2 μL cDNA template, and 8.8 μL ribonuclease-free water run in triplicate in a 384-well plate (BioRad Laboratories) using the BioRad CFX 384 Real Time System C1000 Thermal Cycler (BioRad Laboratories). Relative gene expression was quantified using the delta- delta (2-ΔΔCT) method relative to the stable housekeeping gene β-actin (Livak & Schmittgen, 2001). Housekeeping genes were analyzed separately to ensure stability across treatment groups prior to use as a reference. See Supplementary Table 1 for primer sequences. All primers were designed using the NIH Primer BLAST website with an optimal annealing temperature at 60 ± 0.2°C.
Measurement of Hormones
Total serum CORT and progesterone levels were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Enzo Life Sciences; Farmingdale, NY) as previously described (D. F. Lovelock & Deak, 2017), with one exception. Samples were heat-inactivated to denature endogenous corticosteroid binding globulin (CBG) via immersion in a 75°C water bath for 60 min. Prior assays show this procedure produces superior denaturation of CBG than the enzyme cleavage step provided in the kit (see Spencer & Deak, 2017 for discussion). Inter-assay coefficients of variation were 3.67% for CORT and 8.45% for progesterone.
Experiment 2 procedure
This study hypothesized that activation of β-adrenergic receptors during the normal course of footshock might be causally related to the subsequent behavioral changes observed during adulthood. Thus, our approach was to inject isoproterenol during adolescence and expose rats to the shock context (with no actual shock being delivered) to recapitulate the effects of adolescent footshock and subsequently measure anxiety-like behavior in the light-dark box in adulthood. In this sense, the goal was to test whether activation of β-adrenergic receptors (by isoproterenol) during adolescence was sufficient to produce long-lasting effects on anxiety. To test this, adolescent (PND 29-PND 30) male rats (N = 50, n = 10/group) were given intraperitoneal injections of 0, 2.5, or 5 mg/kg isoproterenol with saline as a vehicle and exposed to the shock chamber for 2 h (i.e., without shock) in order to provide a context for the physiological changes induced by the drug. The doses were chosen based on (Johnson et al., 2008) which found that IL-1β protein expression in the hypothalamus, pituitary, amygdala, and hippocampus peaked 60-120 min later depending on structure. A negative control group that received a saline injection was left in the home cage and a positive control group exposed to footshock (and saline) were also incorporated into the study design to replicate the findings from Exp 1. As adults (PND 80-PND 82), all subjects were tested in the light-dark box for 30 minutes.
Experiment 3 procedure
To further test the role of β-adrenergic receptors during adolescence as a potential driver of adult anxiety-like behavior, rats were injected with propranolol during or after the adolescent footshock exposure as an intervention that could potentially prevent increased anxiety-like behavior in the light-dark box later in adulthood. Adolescent (PND 29-PND 30) male rats from our colony (N = 48, n = 8/group) were given intraperitoneal injections of 20 mg/kg propranolol (dose based on Blandino et al., 2006, 2009) or saline (vehicle) and were either exposed to 2 hours of footshock in early adolescence or remained in the homecage. Injections occurred at one of three times: immediately before, immediately after, or 2 hours after footshock to examine the timing of drug delivery relative to the stressor. A single group of saline injected rats exposed to footshock were utilized, with saline injections being distributed across the 3 injection times (i.e., one injection per rat). Rats that received saline injections and were exposed to footshock were collapsed into a single saline-footshock group (n=8). Subsequently, subjects remained undisturbed in the colony until adulthood. As adults (PND 80-PND 82) all subjects were tested in the light-dark box for 30 minutes.
Statistics
All analyses were conducted in Statistica with ANOVAs appropriate for the particular design, as noted in the individual studies below. Post hoc analyses were performed using the Fisher’s LSD method when a significant interaction was found. In the data sets where ANOVAs were necessary to clarify sex differences, data were first analyzed with an omnibus ANOVA using sex as a variable, then planned follow up ANOVAs or t-tests were used to analyze each sex separately. Findings of these follow-up tests are only reported in the text and only when significant, and they are not reported for gene expression results as their inclusion did not affect interpretation. Criterion for rejection of the null hypothesis was always p < 0.05.
Supplementary Material
Research Highlights:
Exposure to brief stress occurred during early adolescence (PND 29-30) in rats of both sexes
Stressed males but not females showed increased anxiety in the light-dark box as adults
These effects were specific to anxiety, with few other behavioral changes observed
Propranolol attenuated long-lasting effects of adolescent stress
Adolescent footshock led to enhanced c-fos induction in the MeA of both sexes as adults
Acknowledgements:
Research reported in this publication was supported in part by the National Institute of Health Grants P50AA017823 and RO1AG043467 to T. Deak, and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
Abbreviations:
- (MeA)
Medial Amygdala
- (CeA)
Central Amygdala
- (PVN)
Paraventricular Nucleus of the Hypothalamus
- (PND)
Post-natal Day
- (MR)
Mineralocorticoid Receptor
- (GR)
Glucocorticoid Receptor
- (GILZ)
Glucocorticoid-Induced Leucine Zipper
- (Sgk1)
Serum and Glucocorticoid regulated Kinase 1
- (IL-1β)
Interleukin-1β
- (IL-6)
Interleukin-6
- (PTSD)
Post-traumatic Stress Disorder
- (HPA)
Hypothalamic-Pituitary-Adrenal
- (CRH)
Corticotropin-Releasing Hormone
- (RT-PCR)
Reverse Transcription-Polymerase Chain Reaction
- (vHPC)
Ventral Hippocampus
- (CUS)
Chronic Unpredictable Stress
- (CORT)
Corticosterone
- (PROG)
Progesterone
- (ACTH)
Adrenocorticotrophic Hormone
- (IEG)
Immediate Early Genes
- (TNFα)
Tumor Necrosis Factor alpha
- (CNS)
Central Nervous System
- (BLA)
Basolateral Amygdala
- (NE)
Norepinephrine
- (IHC)
Immunohistochemistry
- (ELISA)
Enzyme-Linked Immunosorbent Assay
- (ANOVA)
Analysis of Variance
- (FST)
Forced Swim Test
- (CBG)
Corticosteroid Binding Globulin
- (LC)
Locus Coeruleus
- (ITI)
Inter-Trial Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Topiwala MA, & Gordon JA (2010). Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron, 65(2), 257–269. 10.1016/j.neuron.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blandino P, & Deak T (2011). The role of neuroinflammation in the release of aversive odor cues from footshock-stressed rats: Implications for the neural mechanism of alarm pheromone. Psychoneuroendocrinology, 36(4), 557–568. 10.1016/j.psyneuen.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blandino P, & Deak T (2009). Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiology & Behavior, 98(1–2), 139–146. 10.1016/j.physbeh.2009.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroldi E, & Riccardi C (2009). Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. The FASEB Journal, 23(11), 3649–3658. 10.1096/fj.09-134684 [DOI] [PubMed] [Google Scholar]
- Bale TL, & Epperson CN (2015). Sex differences and stress across the lifespan. Nature Neuroscience, 18(10), 1413–1420. 10.1038/nn.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, & Rawlins JNP (2003). Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research, 139(1–2), 197–213. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12642189 [DOI] [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, & Galea LAM (2011). Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus, 21(11), 1216–1227. 10.1002/hipo.20829 [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Blandino P, & Deak T (2007). Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. Journal of Neuroendocrinology, 19(8), 632–642. 10.1111/j.1365-2826.2007.01571.x [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, & Besedovsky H (1987). Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1 Science (New York, N.Y.), 238(4826), 524–526. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2443979 [DOI] [PubMed] [Google Scholar]
- Blandino P, Barnum CJ, & Deak T (2006). The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1β responses to stress. Journal of Neuroimmunology, 173(1–2), 87–95. 10.1016/j.jneuroim.2005.11.021 [DOI] [PubMed] [Google Scholar]
- Blandino P, Barnum CJ, Solomon LG, Larish Y, Lankow BS, & Deak T (2009). Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain, Behavior, and Immunity, 23(7), 958–968. 10.1016/j.bbi.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Blandino P, Hueston CM, Barnum CJ, Bishop C, & Deak T (2013). The Impact of Ventral Noradrenergic Bundle Lesions on Increased IL-1 in the PVN and Hormonal Responses to Stress in Male Sprague Dawley Rats. Endocrinology, 154(7), 2489–2500. 10.1210/en.2013-1075 [DOI] [PubMed] [Google Scholar]
- Borodovitsyna O, Flamini MD, & Chandler DJ (2018). Acute Stress Persistently Alters Locus Coeruleus Function and Anxiety-like Behavior in Adolescent Rats. Neuroscience, 373, 7–19. 10.1016/j.neuroscience.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Bourke CH, & Neigh GN (2011). Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior, 60(1), 112–120. 10.1016/j.yhbeh.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, & Andersen SL (2011). Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience and Biobehavioral Reviews, 35(8), 1687–1703. 10.1016/j.neubiorev.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro JM, Hueston CM, Deak MM, & Deak T (2014). The impact of the P2X7 receptor antagonist A-804598 on neuroimmune and behavioral consequences of stress. Behavioural Pharmacology, 25(5–6), 582–598. 10.1097/FBP.0000000000000072 [DOI] [PubMed] [Google Scholar]
- Chaby LE, Cavigelli SA, Hirrlinger AM, Caruso MJ, & Braithwaite VA (2015). Chronic unpredictable stress during adolescence causes long-term anxiety. Behavioural Brain Research, 278, 492–495. 10.1016/j.bbr.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Chaby LE, Cavigelli SA, White A, Wang K, & Braithwaite VA (2013). Long-term changes in cognitive bias and coping response as a result of chronic unpredictable stress during adolescence. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, & Potenza MN (2003). Developmental Neurocircuitry of Motivation in Adolescence: A Critical Period of Addiction Vulnerability. American Journal of Psychiatry, 160(6), 1041–1052. 10.1176/appi.ajp.160.6.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, & Goodwin FK (1980). Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, Biochemistry, and Behavior, 13(2), 167–170. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6106204 [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, & D’Agostino LG (2003). Exposure to forced swim stress does not alter central production of IL-1. Brain Research, 972(1–2), 53–63. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12711078 [DOI] [PubMed] [Google Scholar]
- Dunn JD, & Whitener J (1986). Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology, 42(3), 211–217. 10.1159/000124442 [DOI] [PubMed] [Google Scholar]
- Ebner K, Rupniak NM, Saria A, & Singewald N (2004). Substance P in the medial amygdala: Emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proceedings of the National Academy of Sciences, 101(12), 4280–4285. 10.1073/pnas.0400794101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, & Sawchenko PE (1993). c-fos-Based Functional Mapping of Central Pathways Subserving Effects of Interleukin 1 on the Hypothalamo–Pituitary–Adrenal Axis. Methods in Neurosciences, 16, 155–172. 10.1016/B978-0-12-185281-8.50015-6 [DOI] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, & Lauder JM (2004). Prenatal Infection and Risk for Schizophrenia: IL-1β, IL-6, and TNFα Inhibit Cortical Neuron Dendrite Development. Neuropsychopharmacology, 29(7), 1221–1229. 10.1038/sj.npp.1300446 [DOI] [PubMed] [Google Scholar]
- Goshen I, & Yirmiya R (2009). Interleukin-1 (IL-1): A central regulator of stress responses. Frontiers in Neuroendocrinology, 30(1), 30–45. 10.1016/j.yfrne.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Green MR, & McCormick CM (2013). Effects of stressors in adolescence on learning and memory in rodent models. Hormones and Behavior, 64(2), 364–379. 10.1016/j.yhbeh.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Greggor AL, Thornton A, & Clayton NS (2015). Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Current Opinion in Behavioral Sciences, 6, 82–89. 10.1016/J.COBEHA.2015.10.007 [DOI] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, & Aguilera G (1990). CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Research, 532(1–2), 34–40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2178035 [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, & Sachser N (2009). Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology, 30(4), 470–482. 10.1016/j.yfrne.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Hinds LR, Chun LE, Woodruff ER, Christensen JA, Hartsock MJ, & Spencer RL (2017). Dynamic glucocorticoid-dependent regulation of Sgk1 expression in oligodendrocytes of adult male rat brain by acute stress and time of day. PLOS ONE, 12(4), e0175075 10.1371/journal.pone.0175075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueston CM, & Deak T (2014). The inflamed axis: The interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic–pituitary–adrenal axis. Physiology & Behavior, 124, 77–91. 10.1016/j.physbeh.2013.10.035 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, & Fleshner M (2005). Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience, 135(4), 1295–1307. 10.1016/j.neuroscience.2005.06.090 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, & Fleshner M (2008). Role of central β-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain, Behavior, and Immunity, 22(7), 1078–1086. 10.1016/j.bbi.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: relief from stress and anxiety. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361(1476), 2215–2228. 10.1098/rstb.2006.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ZA, & Romeo RD (2013). Changes in hypothalamic–pituitary–adrenal stress responsiveness before and after puberty in rats. Hormones and Behavior, 64(2), 357–363. 10.1016/j.yhbeh.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Knuth ED, & Etgen AM (2005). Corticosterone secretion induced by chronic isolation in neonatal rats is sexually dimorphic and accompanied by elevated ACTH. Hormones and Behavior, 47(1), 65–75. 10.1016/j.yhbeh.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, … Montkowski A. (1995). Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regulatory Peptides, 59(2), 229–239. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8584759 [DOI] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2–ΔΔCT Method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lord B, Aluisio L, Shoblock JR, Neff RA, Varlinskaya EI, Ceusters M, …Bhattacharya A. (2014). Pharmacology of a Novel Central Nervous System-Penetrant P2X7 Antagonist JNJ-42253432. Journal of Pharmacology and Experimental Therapeutics, 351(3), 628–641. 10.1124/jpet.114.218487 [DOI] [PubMed] [Google Scholar]
- Lovelock DF, & Deak T (2017). Repeated exposure to two stressors in sequence demonstrates that corticosterone and paraventricular nucleus of the hypothalamus interleukin-1β responses habituate independently. Journal of Neuroendocrinology, 29(9), e12514 10.1111/jne.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelock DF, & Deak T (2018). Neuroendocrine and neuroimmune adaptation to Chronic Escalating Distress (CED): A novel model of chronic stress. Neurobiology of Stress, 9(June), 74–83. 10.1016/j.ynstr.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, & Morilak DA (2005). Norepinephrine Release in Medial Amygdala Facilitates Activation of the Hypothalamic-Pituitary-Adrenal Axis in Response to Acute Immobilisation Stress. Journal of Neuroendocrinology, 17(1), 22–28. 10.1111/j.1365-2826.2005.01279.x [DOI] [PubMed] [Google Scholar]
- McCormick CM, & Green MR (2013). From the stressed adolescent to the anxious and depressed adult: Investigations in rodent models. Neuroscience, 249, 242–257. 10.1016/j.neuroscience.2012.08.063 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, & Kovacs S (1998). Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. International Journal of Developmental Neuroscience : The Official Journal of the International Society for Developmental Neuroscience, 16(3–4), 175–185. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9785114 [DOI] [PubMed] [Google Scholar]
- McCormick CM, & Mathews IZ (2007). HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology Biochemistry and Behavior, 86(2), 220–233. 10.1016/j.pbb.2006.07.012 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, & Waters P (2010). Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition, 72(1), 73–85. 10.1016/j.bandc.2009.06.003 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, & Mathews IZ (2008). Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural Brain Research, 187(2), 228–238. 10.1016/j.bbr.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, & Petre CO (2005). Role of brain norepinephrine in the behavioral response to stress. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29(8), 1214–1224. 10.1016/j.pnpbp.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Patton GC, & Viner R (2007). Pubertal transitions in health. The Lancet, 369(9567), 1130–1139. 10.1016/S0140-6736(07)60366-3 [DOI] [PubMed] [Google Scholar]
- Perkins AE, Doremus-Fitzwater TL, Spencer RL, Varlinskaya EI, Conti MM, Bishop C, & Deak T (2016). A working model for the assessment of disruptions in social behavior among aged rats: The role of sex differences, social recognition, and sensorimotor processes. Experimental Gerontology, 76, 46–57. 10.1016/j.exger.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, … Orr SP (2002). Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry, 51(2), 189–192. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11822998 [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, & Sara SJ (1999). Attenuation of Emotional and Nonemotional Memories after their Reactivation: Role of Adrenergic Receptors. Retrieved from http://www.jneurosci.org/content/jneuro/19/15/6623.full.pdf [DOI] [PMC free article] [PubMed]
- Romeo RD (2017). The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Research, 1654(Pt B), 185–191. 10.1016/j.brainres.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Romeo RD, & McEwen BS (2006). Stress and the Adolescent Brain. Annals of the New York Academy of Sciences, 1094(1), 202–214. 10.1196/annals.1376.022 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, & Chattarji S (2009). Stress, memory and the amygdala. Nature Reviews Neuroscience, 10(6), 423–433. 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- Selemon LD, & Zecevic N (2015). Schizophrenia: a tale of two critical periods for prefrontal cortical development. Translational Psychiatry, 5(8), e623 10.1038/tp.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24(4), 417–463. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10817843 [DOI] [PubMed] [Google Scholar]
- Spear LP (2015). Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiology & Behavior, 148, 122–130. 10.1016/j.physbeh.2015.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, & Deak T (2017). A users guide to HPA axis research. Physiology & Behavior,178, 43–65. 10.1016/j.physbeh.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, & Mori Y (2013). Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behavioural Brain Research, 240, 46–51. 10.1016/j.bbr.2012.11.017 [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, & Spear LP (2012). Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental Psychobiology, 54(5), 523–535. 10.1002/dev.20610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vore AS, Doremus-Fitzwater T, Gano A, & Deak T (2017). Adolescent Ethanol Exposure Leads to Stimulus-Specific Changes in Cytokine Reactivity and Hypothalamic-Pituitary-Adrenal Axis Sensitivity in Adulthood. Frontiers in Behavioral Neuroscience, 11(May), 1–15. 10.3389/fnbeh.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Arnold AR, & Cooke BM (2012). Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Hormones and Behavior, 61(1), 91–99. 10.1016/j.yhbeh.2011.10.008 [DOI] [PubMed] [Google Scholar]
- Wilkin MM, Waters P, McCormick CM, & Menard JL (2012). Intermittent physical stress during early- and mid-adolescence differentially alters rats’ anxiety- and depression-like behaviors in adulthood. Behavioral Neuroscience, 126(2), 344–360. 10.1037/a0027258 [DOI] [PubMed] [Google Scholar]
- Wulsin AC, Wick-Carlson D, Packard BA, Morano R, & Herman JP (2016). Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology, 65, 109–117. 10.1016/j.psyneuen.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang R, Liu Y, Wang W, Liu D, Jiang H, & Pan F (2019). Short- and long-term alterations of FKBP5-GR and specific microRNAs in the prefrontal cortex and hippocampus of male rats induced by adolescent stress contribute to depression susceptibility. Psychoneuroendocrinology, 101, 204–215. 10.1016/j.psyneuen.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Yohn NL, & Blendy JA (2017). Adolescent Chronic Unpredictable Stress Exposure Is a Sensitive Window for Long-Term Changes in Adult Behavior in Mice. Neuropsychopharmacology, 42(8), 1670–1678. 10.1038/npp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidan H, & Gaisler-Salomon I (2015). Prereproductive stress in adolescent female rats affects behavior and corticosterone levels in second-generation offspring. Psychoneuroendocrinology, 58, 120–129. 10.1016/j.psyneuen.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu H, Jiang X, Wu D, & Tian Y (2014). A longitudinal study of posttraumatic stress disorder symptoms and its relationship with coping skill and locus of control in adolescents after an earthquake in China. PloS One, 9(2), e88263 10.1371/journal.pone.0088263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubareva OE, & Klimenko VM (2013). Increases in Proinflammatory Cytokine Levels at Early Ages as a Risk Factor for the Development of Nervous and Mental Pathology. Neuroscience and Behavioral Physiology, 43(4), 535–541. 10.1007/s11055-013-9767-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.