Abstract
In spite of tremendous research advancements in nonalcoholic fatty liver disease (NAFLD), our understanding of sex-differences in NAFLD remains insufficient. This review summarizes current knowledge on sex differences in NAFLD, identifies current gaps, and discusses important considerations for future research. The prevalence and severity of NAFLD are higher in men than in women during the reproductive age. However, after menopause, NAFLD occurs at a higher rate in women suggesting that estrogen is protective. Sex differences also exist for the major risk factors of NAFLD. In general, animal models of NAFLD recapitulate sex differences observed in patients with more severe steatosis and steatohepatitis, more pro-inflammatory/pro-fibrotic cytokines, and a higher incidence of hepatic tumors in males than females. Based on computer modeling, female and male livers are metabolically distinct with unique regulators modulating sex-specific metabolic outcomes. Analysis of the literature reveals that most published clinical and epidemiological studies fail to examine sex differences appropriately. Considering the paucity of data on sex differences and the knowledge that regulators of pathways relevant to current therapeutic targets for NAFLD differ by sex, clinical trials should be designed to test drug efficacy and safety according to sex, age, reproductive stage (i.e., menopause) and synthetic hormone use.
Conclusion:
Sex differences do exist in the prevalence, risk factors, fibrosis, and clinical outcomes of NAFLD suggesting that, while not yet incorporated, sex will probably be considered in future practice guidelines. Adequate consideration of sex differences, sex hormones/menopause status, age, and other reproductive information in clinical investigation and gene association studies of NAFLD are needed to fill current gaps and implement precision medicine for patients with NAFLD.
Keywords: gender difference, fibrosis, hepatocellular tumors, metabolic syndrome, menopause, molecular pathogenesis, nonalcoholic steatohepatitis
BACKGROUND
The nonalcoholic fatty liver disease (NAFLD) epidemic is a global public health concern with a heavy healthcare burden.(1) NAFLD is the fastest growing cause for orthotopic liver transplantation due to end-stage liver disease (ESLD) (2) and hepatocellular carcinoma (HCC).(3) Heterogeneity in NAFLD risk profiles and treatment responsiveness challenges accurate identification of high-risk individuals and personalized preventive/therapeutic strategies thus hampering attempts to decelerate this ever-increasing health threat.
The study of sex differences is a rapidly growing area of medicine. The 2014 NIH announcement prompting researchers to assess sex differences in preclinical NIH-funded studies facilitated a steady increase in the number of publications on sex differences.(4) (Figure 1) Biological sex differences in normal physiology and disease arise principally from sex chromosomes and sex hormones. Physiological levels of sex hormones vary significantly throughout the reproductive and menstrual cycle in premenopausal women and influence physiological functions and disease susceptibility. Thus, without considering sex and age, clinical and animal studies may fail to identify the influence of biological sex on study outcomes or arrive at erroneous conclusions.
Figure. 1. Number of annual publications on sex differences in NAFLD and related fields.
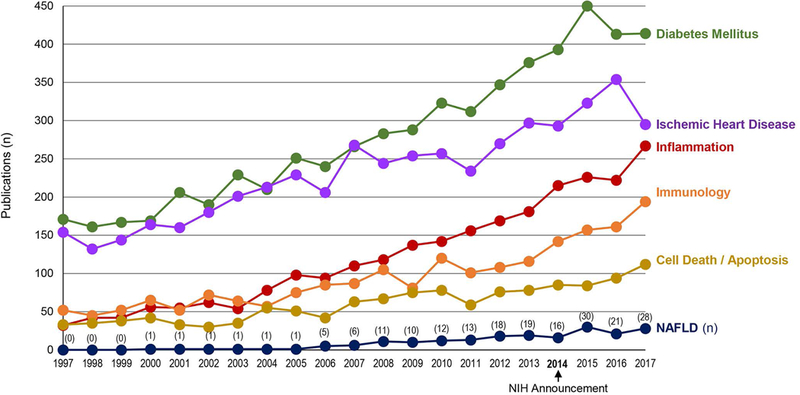
Data were obtained from PubMed using the keyword ‘sex difference’ combined with ‘inflammation’, ‘immunology’, ‘cell death or apoptosis’, ‘diabetes mellitus’, or ‘ischemic heart disease’. Research on sex differences in NAFLD has apparently lagged behind other areas.
Differences in men and women that are influenced by socio-cultural factors are termed gender differences, which should be distinguished from biological sex differences. Sex and gender differences undergird fundamental biological variation in disease as well as its progression. For this reason consideration of sex and age (e.g., puberty, menopause) is crucial in determining risk assessment, disease prevention, and treatment.(5) Most precision medicine approaches typically omit discussion of sex differences as they relate to disease susceptibility, phenotypes, and outcomes.(6) Sex differences have been extensively studied in recent years in fields relevant to NAFLD and its pathogenesis (Figure 1). Compared to other areas of study, fewer publications describe sex differences in NAFLD even though major risk factors for NAFLD (i.e., metabolic syndrome (MetS), regional adiposity, type 2 diabetes (T2D)) are known to display profound sex differences.
Differences in socio-cultural characteristics (i.e., gender differences), such as dietary patterns, exercise, and quality of life (7, 8), are as equally important to consider in NAFLD as sex differences. Due to space constraints, gender differences will not be discussed here. This review will rather focus on biological sex differences in primary NAFLD in adults and, to a lesser extent, children, identify important gaps in knowledge, and address basic and clinical unanswered research questions.
OVERVIEW OF NAFLD PATHOBIOLOGYAND SEX DIFFERENCES
The pathobiology of NAFLD is complex and multi-phasic (Figure 2). Positive energy balance, dysfunctional adipose tissue, systemic inflammation, insulin resistance (IR), and hepatic lipid accumulation are all fundamental drivers of liver injury, while intestinal microbiota and bile acids (BA) interact with key players in the pathogenesis of NAFLD in a multiphasic manner. Nonalcoholic steatohepatitis (NASH) increases the risk for hepatic fibrosis, cirrhosis, ESLD and HCC.(9, 10) Known sex differences in key mechanisms are depicted in Figure 2.
Figure. 2. Overview of NAFLD pathogenesis and sex differences.
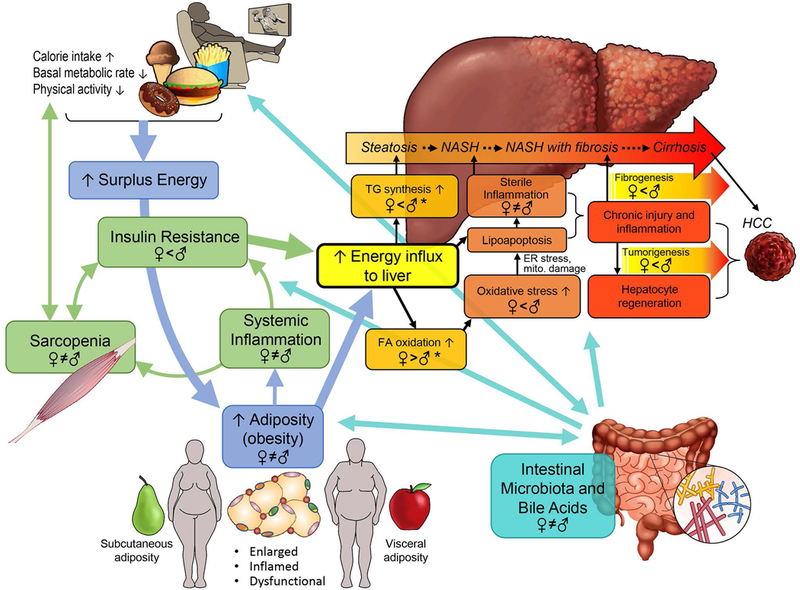
TG: triglycerides. Women and men store surplus calories differently: gluteo-femoral subcutaneous in women vs. visceral adiposity in men. Enlarged, dysfunctional adipose tissue, especially visceral adiposity, leads to systemic inflammation and insulin resistance, which facilitates energy influx to the liver and increases metabolic stress in hepatocytes. Sarcopenia exacerbates these changes, by generating a vicious cycle. When hepatocytes fail to adapt, the increased metabolic stress triggers oxidative stress or direct toxic effect of free FA on hepatocytes, and induces lipoapoptosis, which, in turn, leads to sterile inflammation. Chronic inflammation promotes fibrosis, cirrhosis, and tumorigenesis. Intestinal microbiota and BA play pivotal roles in regulating NAFLD pathogenesis in a multi-phasic manner while interacting with key players. Known sex differences and hormonal effects are depicted in a mechanism-specific way for further discussion in this review. Sex differences in TG synthesis, FA oxidation, and oxidative stress are not covered in this review. Sex difference in oxidative stress is well accepted. * see reference (85)
SEX DIFFERENCES IN CLINICAL NAFLD
Disease Risk
Prevalence of NAFLD is globally 25.24% and varies among countries.(9) In general adult populations, overall NAFLD prevalence is higher in men than in women (Table 1). Menopause or age-specific sex difference was infrequently considered in published studies, but when examined, NAFLD prevalence and incidence are higher in men than in premenopausal women (or ≤ age 50–60 years) while they tend to become more common in women after menopause (or ≥ age 50–60 years) (11–15)(Table 1). Postmenopausal women on hormone replacement therapy (HRT) had a lower prevalence of NAFLD compared to postmenopausal women not on HRT (16). In a randomized clinical trial, combined HRT significantly decreased aminotransferase levels in postmenopausal women with T2D and presumed NAFLD compared to placebo controls.(17) Collectively, current evidence suggests that estrogen protects from NAFLD.
Table 1.
Population-based studies: sex-differences in frequency of NAFLD, fatty liver, and NAFLD fibrosis
| Author, year (Ref) | Country | M (n)/ F (n) | Age, yrs | Consideration of Menopausal status | Ethnicity | Main findings |
|---|---|---|---|---|---|---|
| Prevalence and incidence of NAFLD in adults | ||||||
| Zelber-Sagi S et al., 2006(86) | Israel | 174/152 | 50.5 ± 10.3 | No | Not specified | Overall NAFLDUS prevalence higher in men than in women (38% vs 21%). Male gender and metabolic factors independently predicted NAFLD. |
| Park et al., 2006(11) | Korea | 3,530/3,118 | 48.0 ± 11.8 | 1273 post-menopausal women | Asian | Menopause was an independent risk factor for prevalent NAFLDUS (OR 1.71) among women. |
| Caballeria et al., 2010(87) | Spain | 323/443 | 53±14 | No | Not specified | NAFLDUS prevalence significantly higher in men (33%) than women (20%). Male sex, age, MetS, IR and alanine aminotransferase independently predicted NAFLD. |
| Wong VW et al., 2012(12) | Hong Kong | 389/533 | 48 ± 11 | Age 50 years cut-off | Asian | NAFLDMRS prevalence higher in men than in women (37% vs 23%) overall. It peaked at 40 years and remained constant after in men while it increased steadily after 50 years in women. |
| Eguchi Y et al., 2012(13) | Japan | 2,627/2,448 | 50.0 ± 9.5 | No | Asian | NAFLDUS prevalence higher in men than in women at all ages (overall: 41% vs 18%) and increases with age in women (31% after 60 years). |
| Lazo M et al., 2013(88) | USA | Total 12,454 | 20–74 | No | European descent | Computed NAFLDUS prevalence after adjustment for other confounders was higher in men (20.2%) than in women (15.8%). |
| Li Z et al., 2014(89) | China | 201,481/ 152,124 from 48 Chinese studies | Weighted average: 40.32 for men, 34.8 for women | No | Asian | This meta-analysis of Chinese studies found a higher prevalence of NAFLDUS in men (24.8%) than in women (13.2%). |
| Wang Z et al., 2014(14) | China | 15,551/9,481 | 44.1 ± 15.0 | Age 60 years cut-off | Asian | NAFLDUS prevalence was higher in men than women (32% vs 13%) but comparable in women > 60 years (27.6%). |
| van den Berg et al., 2017(90) | Netherlands | 14,226/23,270 | 44 (36–51)$ | No | European | Male sex was independently associated with NAFLD prevalence (FLI ≥ 60). |
| Long MT et al., 2018(15) | USA | 364/321 | 45.0 ± 6.2 | Yes (N not reported) | Largely European descent | Incidence of fatty liver CT was similar between postmenopausal women and men (19% vs 22%) and was lower in premenopausal women (9%). |
| Prevalence of fatty liver# in adults | ||||||
| Browning JD et al., 2004(91) | USA | 2240/1194 | 46 ± 9 | No | Whites 38%, Blacks 43%, Hispanics 17.5% | Fatty liver MRS prevalence was higher in men than in women (42% vs 24%) among whites, but not in other ethnic groups. |
| Zhou YJ et al., 2007(92) | China | 1311/2232 | 7– 70+ | No | Asian | Fatty liverUS prevalence significantly higher in men (13.8%) than women (7.1%) before 50 years; the opposite after 50 years. |
| Prevalence of NAFLD and fatty liver in children | ||||||
| Anderson et al., 2015(18) | Europe, America, Asia | Total 23,208 from 20 general populations and 23,892 from 56 obese clinical populations | 2−19 | - | European, American, Asian | This meta-analysis of children found NAFLD US, LBx, MRI prevalence higher in male than female adolescents both in general (9% vs 6%, based on 15 studies) and obese populations (30% vs 22%, based on 27 studies). |
| Prevalence of NAFLD fibrosis | ||||||
| Kim et al., 2013(93) | USA | 5,296/5,858 | 20−74 | No | Non-Hispanic white, non-Hispanic black, Mexican American | 4,083 of 11,154 had NAFLDUS, of them 173 (3.2%) had elevated NFS (>0.676) with no sex difference. |
| Hartleb et al., 2017(94) | Poland | 1405/1598 | 79.6±8.3 | No | European | NAFLD (FLI ≥ 60) with fibrosis was more prevalent in males when defined by Fib-4 (≥ 1.45) but not NFS (>0.676). |
| Caballeria et al., 2018(19) | Spain | 1289/1725 | 54±12 | No | European | Higher liver stiffness (TE) was independently associated with male sex. |
| Tanaka et al. 2018(20) | Japan | 3316/3611 | 50 (42−57) $ | No | Asian | Among 1935 patients with NAFLDUS, 0.9% men and 1.0% women had advanced fibrosis (FIB-4 ≥ 2.67). |
: more than minimal alcohol use and/or viral hepatitis infection were not excluded.
: median and interquartile range
NAFLD definition - US: ultrasonography; MRS: Magnetic resonance spectroscopy; CT: computerized tomography; MRI: Magnetic resonance imaging; LBx: liver biopsy
Abbreviations: ALP, alkaline phosphatase; AST, aspartate aminotransferases; AUC, area under the curve; FL, fatty liver; FLI: fatty liver index; HDL-C, high density lipoprotein cholesterol; IR,insulin resistance; hsCRP, high sensitivity C reactive protein; LDL-C, low density lipoprotein cholesterol; MetS, metabolic syndrome; MVA, multivariable analysis; NA, not assessed; NASH, nonalcoholic steatohepatitis; NFS: NAFLD fibrosis score; SUA, serum uric acid; T2D, type 2 diabetes; TC, total cholesterol; TE: transient elastography; USA, United States of America; VAT, visceral adipose tissue; VFA, visceral fat area; WC, waist circumference
In pediatric populations, a meta-analytic study showed that the pooled NAFLD prevalence is higher in boys than in girls in general populations and obese clinical cohorts (18). The study also revealed significant variance across the published reports, which is partly explained by the technique used to diagnose NAFLD (ultrasound vs. aminotransferases) (18) and by failure to consider pubertal stages when sex hormone levels change dramatically in a sex-specific manner.
Several studies have non-invasively evaluated the risk of fibrosis/mortality in general populations, but the results are inconsistent in terms of sex differences (Table 1). An increased liver stiffness measured by transient elastography occurred more often in men and was associated with MetS features in a cohort without known liver conditions or other severe comorbidities.(19) The risk of liver fibrosis (Fibrosis-4 ≥ 2.67) in patients with NAFLD was lower in men than women after adjusting for other metabolic variables.(20) The risk of fibrosis progression assessed by the AST to Platelet Ratio Index in a NAFLD population was associated with obesity and weight gain, but not sex.(21) These studies did not consider menopause status or age-specific sex-difference, which may have confounded the results (Table 1). Lastly, a multi-national study with biopsy-confirmed NAFLD and advanced hepatic fibrosis showed that older age and male sex were associated with worse survival and greater incidence of HCC.(22)
Regional Adiposity and MetS
The risk of developing NAFLD increases after menopause owing to body fat distribution shifting to the abdominal position.(11, 23) Men and postmenopausal women are at a greater risk of MetS compared to premenopausal women.(24) Of note, premature ovarian insufficiency carries a higher risk of MetS and IR.(25) Menstrual irregularities in premenopausal women are a risk factor for developing T2D.(26) Sleep deprivation/disorders, which are associated with obesity and MetS,(27) exert worse metabolic consequences in women compared to men.(28) Serum uric acid levels are closely linked to sex and menopause status (29) and are associated with NAFLD, but only in men with T2D.(30) These findings suggest that sex modulates the association between hyperuricemia and NAFLD. Sex differences in adiposity and other metabolic risk factors are likely to contribute to sex differences in the driving force of disease (Figure 2).
Histologic Features
Current evidence suggests that sex, puberty, and menopause significantly affect NAFLD histology. In a NAFLD registry study, Mallory-Denk bodies appeared only after puberty while severe portal inflammation was more prevalent before puberty.(31) In another NAFLD registry study of adults, premenopausal women had more severe lobular inflammation, hepatocyte ballooning and Mallory-Denk bodies than men or postmenopausal women after adjusting for variables defining hepatic metabolic stress.(32) This suggests that sex hormones modulate hepatic injury/inflammation for any given level of metabolic stress. Estrogen is known to increase T and T-regulatory cell numbers,(33) which may either enhance or reduce inflammation in the liver. Immune cells are well known to differ by sex(33). However, sex hormone effects on immune phenotypes (pro-inflammatory vs. regulatory) in NASH remain to be investigated.
Although premenopausal women have more severe hepatocyte injury and inflammation, they have less hepatic fibrosis compared to men and postmenopausal women,(32) suggesting multi-phasic effects of sex and hormones on NAFLD pathogenesis (Figure 2). The specific mechanisms underlying the incongruous data are currently unknown. Future studies characterizing the immune infiltrate in NASH human liver are needed to explore sex differences in inflammation and cytokine profiles which may promote fibrosis. Among postmenopausal women with NAFLD, premature menopause and a longer duration of estrogen deficiency are associated with more severe liver fibrosis.(34) Collectively, data indicate that estrogen protects the liver from fibrosis in NAFLD. Features of hepatic injury and inflammation in response to metabolic stress appear to be diverse and depend on sex, pubertal stage, and sex hormone levels. This area requires further research.
Hepatocellular Tumors
Irrespective of its etiology, HCC is more common in men than women.(35) Hepatocellular adenoma (HCA) predominantly occurs in women, but men have a 10-fold increased risk of HCC.(36) Large cohort studies of patients with NASH-cirrhosis found that men had a 2- to 7-fold higher risk of developing HCC than women.(10, 22) Women have higher survival rates from HCC than men before age 55, but this advantage is reversed after this age.(37) Chronic injury and inflammation are well-established prerequisites for tumorigenesis, and, in NASH, HCC risk parallels increasing hepatic fibrosis (Figure 2).(38) A cross-sectional study of 87 patients with NASH found that men developed HCC at earlier liver fibrosis stages than women.(38) Overall, data indicate that men are at a higher risk of NAFLD-HCC than women.
SEX DIFFERENCES IN EXPERIMENTAL NAFLD
Computational models
A recent mice computational model concluded that female and male livers are metabolically distinct organs.(39) Simulating transcriptional regulation of estradiol, androgen, and sex-specific patterns of growth hormone secretion (40), the modeling approach identified genes which regulate sex-specific effects on metabolism pertaining to hepatic triglyceride accumulation (e.g., triglyceride export, fatty acid (FA) oxidation): peroxisome proliferator-activated receptor (PPPAR)- γ coactivator 1-α coPGC1a), farnesoid X receptor (FXR), liver X receptor (LXR), and PPAR-α.(39) These regulators are currently being investigated as novel therapeutic targets in NASH (41) stressing the importance of examining sex differences in the efficacy and safety of drugs that target these genes.
Animal models
Most experimental NAFLD studies using genetically engineered mice and mice fed altered diets find that disease is more severe in males, recapitulating the main feature of clinical NAFLD. However, sex differences differ by model, mouse strain, and/or outcome criteria. FXR deficient male mice fed a Western diet had more severe steatohepatitis than females.(42) Another study examining the effect of a high-fat, high-fructose diet found that males had higher hepatic triglyceride levels and developed more severe steatosis compared to females for most (but not all) of the more than 100 inbred mouse strains used.(43) Studies with high-fat diets (HFD) found more severe liver histology changes in males than females.(44) Methionine-choline deficient diet-induced steatosis is more severe in male than female mice.(45) Contrarily, female C57BL/6 mice fed a high-fructose diet had similar liver steatosis to males but greater hepatic inflammation and decreased adiponectin in the visceral adipose tissue despite higher absolute weight gain in males.(46)
Sex hormones function as effect modifiers. Female rodents are protected from the adverse metabolic consequences of a high-fructose diet, but this protection is lost with ovariectomy.(47, 48) Importantly, this sex difference in the metabolic effects of fructose overfeeding has also been observed in humans, with triglyceride and alanine aminotransferase rising significantly in men but not women (49) although sex differences in histologic features remain unknown.
SEX DIFFERENCES IN NAFLD PATHOBIOLOGY
Adipose Tissue, Skeletal Muscle and Metabolism
Regional fat distribution is directly associated with the risk of metabolic disorders and NAFLD, with a lower risk resulting from gynoid gluteo-femoral subcutaneous distribution and a higher risk with android visceral adiposity.(50) Compared to abdominal adipose tissue, gluteo-femoral adipoctyes have a lower lipolytic response to epinephrine and norepinephrine (51) and release fewer FA. Estradiol lowers lipolysis and improves adipose tissue insulin sensitivity (52, 53) which, in turn, reduces excess delivery of FA to the liver. Regardless of menopausal status, serum adiponectin is higher in women than men.(54) Collectively, women, especially premenopausal women, are protected from the adverse consequence of excess fat storage. Higher androgen levels in women increase abdominal adiposity and the risk of metabolic disorders while in men androgen reduces abdominal adiposity. (55)
Skeletal muscle, one of the major organs responsible for peripheral glucose disposal, is generally more insulin sensitive in women than men.(56) Sarcopenia, which is associated with IR, reduced physical activity, pro-inflammatory cytokines and the lack of anabolic hormones (57), has been associated with NAFLD independent of MetS features.(58) Sex-differences in muscle physiology, sarcopenia, and response to treatment have been extensively reviewed elsewhere (59). Skeletal muscle expression of estrogen receptor-α is markedly reduced in women with MetS (60) and, compared to placebo controls, HRT increases lean body mass while reducing fat mass in postmenopausal women.(61) Hepatic insulin sensitivity is also lower in obese men compared to BMI-matched women.(62)
Intestinal Microbiome and Bile Acids
Dysbiosis and BA play pivotal roles in the pathogenesis of NAFLD, NASH, and HCC. Recent advancements are summarized elsewhere.(63, 64) Gut microbiota modulates the ‘gut-liver axis’ via FXR signaling in the intestine, releasing fibroblast growth factor-15/19 (FGF-15/19) which regulates BA synthesis and lipid/glucose metabolism. Serum FGF-19 is decreased in patients with NASH (65) but increases after gastric bypass surgery.(66) A non-tumorigenic analogue of FGF-19 demonstrated a significant reduction in steatosis, serum aminotransferases, and fibrosis markers in patients with NASH, regardless of patient sex.(67) Various factors (e.g., age, sex, diet, physical activity)(63) influence gut microbiota composition and diversity. Recent clinical studies demonstrate that BMI-specific sex differences and variation by menopausal status occur in gut microbiota, (68, 69) possibly explaining sex differences observed in the adiposity and metabolism of NAFLD patients.(70) Serum and hepatic BA profiles in female mice are different from males in an age-specific manner due to sex-divergent expression of BA transporters Ntcp and Oatp1b2 and BA synthetic enzyme CYP7a1.(71) How sex differences in BA affect NAFLD risk and treatment response in humans remains to be investigated.
Innate Immune Response
The innate immune response to damaged hepatocytes is a key component of the pathogenesis of NASH, given that the damaged hepatocyte recruits inflammation which promotes remodeling and fibrosis. A HFD induces steatohepatitis and inflammasome activation only in male mice.(44) Key cells involved in the innate immune response in the liver are Kupffer cells and neutrophils. Most immune cells express multiple sex hormone receptors which drive immune responses in a sex-specific manner (reviewed in (33)). Briefly, Kupffer cells from female mice express higher levels of the TLR-associated transcription factor MyD88, greater p38 MAP kinase phosphorylation and, therefore, greater activation following lipopolysaccharide challenge than macrophages from male mice.(72) Macrophages from males express higher levels of TLR4 than females. TLR4 increases the pro-inflammatory and pro-fibrotic cytokine IL-1β, which leads to increased IL-6. Inflammatory chemokine/cytokine production also differs by sex in NAFLD animal models with higher production of the chemokine CXCL10 and the pro-inflammatory cytokines, TNFα, IL-1β, and IL-6, in macrophages from males while macrophages from females produce higher anti-inflammatory prostanoids.(72) Thus, animal models demonstrate that innate immune cells from male mice are activated in such a manner as to promote liver inflammation and fibrosis, while macrophages from female mice display either a regulatory or a more protective, anti-fibrotic phenotype.
Remodeling and Fibrosis
Remodeling is a dynamic process cycling between the breakdown of extracellular matrix components and the build-up of scar tissue. Matrix remodeling is driven by matrix metalloproteinases (MMPs) that differ by sex.(73)
Hepatocyte apoptosis, activation of Kupffer and other innate immune cells, and pro-fibrotic cytokines are necessary for fibrogenesis. The most characteristic fibrosis pattern in the liver from patients with NASH is pericellular fibrosis in zone 3 (i.e. chicken-wire appearance). Animal models have shown that estradiol inhibits stellate cell activation and liver fibrosis via estrogen receptor-β in both sexes.(74, 75) Stellate cells express progesterone receptors in both sexes and, in culture, progesterone activates stellate cells by inducing ROS generation, MAPK pathway activation, and TGFβ1 expression, all of which are inhibited by estrogen.(76) Whether synthetic progesterone use in oral contraceptives, which alters the estrogen to progesterone ratio, is detrimental to NAFLD progression in premenopausal women remains to be investigated.
Another fibrosis pattern seen in the liver of patients with NASH is periportal/portal fibrosis, which is associated with activated hepatic progenitor cells and portal inflammation.(77) Since different mechanisms are involved in these fibrosis patterns, whether or not estrogen and progesterone have similar effects on periportal/portal fibrosis vs. pericellular fibrosis remains unknown.
Tumorigenesis
Hepatic tumorigenesis is driven by chronic liver inflammation, persistent tissue injury, and subsequent compensatory hepatocyte proliferation. Male animals develop hepatic tumors more often than females, which is consistent with the clinical notion of increased NAFLD-HCC in men. This is explained, at least in part, by a higher production of IL-6 by Kupffer cells in males and inhibitory effects of estrogen on IL-6 production in females, which reduces hepatic injury and compensatory proliferation of hepatocytes.(78) Sex difference in IL-6 levels has been attributed to TLR4-induced MyD88 activation.(78) Western diet-fed FXR deficient mice develop fatty adenoma only in males.(42) Phosphatase and tensin homolog (PTEN) deficient mice develop steatosis, NASH, and HCC in a male-dominant manner.(79) Transgenic Mito-Ob obese mice that over-express prohibitin in adipocytes develop IR, NASH, and HCC with age in a male-specific manner.(80)
Men are over-represented in the molecular subgroup of HCA harboring mutations of beta-catenin in exon 3, which is strongly associated with androgen exposure and HCC development. This subgroup of HCA is also found more often in women who have a low lifetime estrogen exposure.(81) Estrogen protects from liver tumorigenesis (78) and, theoretically, prolonged estrogen depletion (i.e., premature menopause) may lead to increased HCC risk, which needs to be investigated in women with NAFLD.
CONSIDERATION OF SEX DIFFERENCES IN NAFLD RESEARCH
Our review has critically discussed sex differences in clinical and experimental NAFLD and has highlighted that many clinical/epidemiological publications on NAFLD do not properly analyze such sex differences. Indeed most studies ‘assume’ sex and age as independent variables, without assessing their interaction and without considering menopause status in the study design/analysis. Sex differences affect various physiological functions and act as effect modifiers (Figure 3). Thus, analyzing a population without considering potential sex-specific effects or hormonal effects, probably masks important observations. How reproductive status and synthetic hormone use impact disease risk in women with NAFLD deserves further investigation.
Figure. 3. Sex difference considerations in NAFLD research.
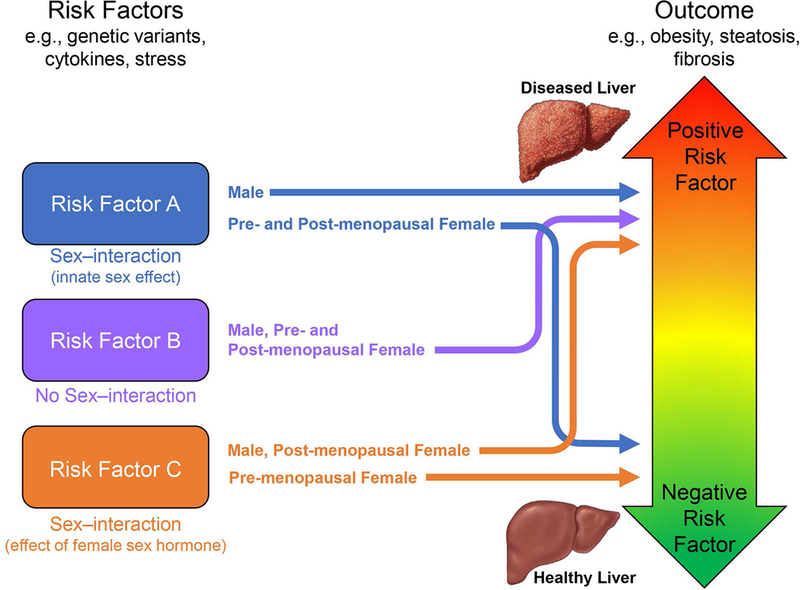
Risk factor A- A factor whose effect is significantly modified by sex or innate sex-related attributes (i.e., chromosomes) so that the outcome will significantly differ between women and men. Risk factor B- A factor whose effect is modified by neither sex nor sex hormones, resulting in an outcome which is invariably consistent among premenopausal women, postmenopausal women and men. Risk factor C- A factor whose effect is significantly modified by female sex hormones. In this case, the association between risk factor C and the outcome will significantly differ among premenopausal, postmenopausal women, and men. In the analysis of risk factors A and C, the lack of proper cohort classification (i.e., subgroup analysis) would either result in a spurious conclusion or mask important sex-specific effects. Both sex and sex hormones interact with numerous NAFLD risk factors and alter the risk profiles and phenotypes of NAFLD in individuals.
In genome-wide association studies (GWAS) on NAFLD, sex is generally considered only as a co-variate, but sex-gene and gene-sex hormone interactions are neglected despite the fact that both experimental and clinical studies have documented the importance of sex as an effect modifier in gene-association studies.(43, 82) Immune-regulating genes on X-chromosomes (83) are typically excluded from GWAS studies owing to their complex regulation. A new mathematical model should promote research in this area.(84)
Although sex differences do exist in NASH mechanisms, current evidence is insufficient to allow sex-specific personalized therapies. Further investigations are needed with proper consideration of sex and reproductive status in the study design, from pre-clinical to epidemiological studies, and clinical trials. Currently, there is no specific regulatory guideline for sex/gender consideration by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. Global regulatory recommendations should be enacted to institutionalize sex/gender consideration in future drug development.
Summary
Given the heterogeneity in NAFLD, evidence-based, tailored clinical care is crucial to reduce the burden of the NAFLD epidemic. Sex and sex hormones are one of the largest influencers of biological variance in human diseases. A proper consideration of sex, age, hormonal status, and socio-cultural gender differences will lead to a better understanding of sex and gender differences in NAFLD risk, therapeutic targets, and treatment responses and aids to achieve precision medicine.
Acknowledgements
The authors would like to thank Dr. Christine M. Hunt for her input and Ms. Jacqueline Mole for her editorial assistance. This work was partly supported by NIH R01 HL111938 (DF), NIH R21 ES024414 (DF), NIH 5U01DK061713-17 (M.A.), AHA 16GRNT30950007 (DF), the Durham VA Medical Center and the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT), (CIN 13–410) at the Durham VA Health Care System.
ABBREVIATIONS
- BA
bile acids
- BMI
body mass index
- CXCL10
C-X-C motif chemokine 10
- ESLD
end-stage liver disease
- FA
fatty acids
- FGF
fibroblast growth factor
- FIB-4
fibrosis-4 index
- FXR
farnesoid X receptor
- GWAS
genome-wide association mapping studies
- HCA
hepatocellular adenoma
- HCC
hepatocellular carcinoma
- HFD
high-fat diet
- HRT
hormone replacement therapy
- IL
interleukin
- IR
insulin resistance
- LXR
liver X receptor
- MetS
metabolic syndrome
- MMPs
metalloproteineases
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PGC1
peroxisome proliferator-activated receptor-γ coactivator 1
- PPAR
peroxisome proliferator-activated receptor
- PTEN
phosphatase and tensin homolog
- TGF
transforming growth factor
- TLR
Toll-like receptor
- TN
tumor necrosis factor
- T2D
type 2 diabetes
Footnotes
Conflict of interest: none
Contributor Information
Amedeo Lonardo, Email: a.lonardo@libero.it, lonardo.amedeo@aou.mo.it.
Fabio Nascimbeni, Email: fabio.nascimbeni@libero.it.
Stefano Ballestri, Email: stefanoballestri78@gmail.com, s.ballestri@ausl.mo.it.
DeLisa Fairweather, Email: Fairweather.DeLisa@mayo.edu.
Sanda Win, Email: swin@usc.edu.
Tin A. Than, Email: tthan@usc.edu.
Manal F. Abdelmalek, Email: manal.abdelmalek@duke.edu.
Ayako Suzuki, Email: ayako.suzuki@duke.edu.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol 2018. [DOI] [PMC free article] [PubMed]
- 3.Zobair Younossi MS, Ong Janus P., Jacobson Ira M., Elisabetta, Bugianesi AD, Yuichiro Eguchi, Wong Vincent W., Francesco Negro,, Yusuf Yilmaz MR-G, Jacob George, Aijaz Ahmed, Robert Wong,, Issah Younossi MZ, Arian Afendy. Non-alcoholic Steatohepatitis is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2018;In Press. [DOI] [PubMed]
- 4.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller VM, Rocca WA, Faubion SS. Sex Differences Research, Precision Medicine, and the Future of Women’s Health. J Womens Health (Larchmt) 2015;24:969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229–2234. [DOI] [PubMed] [Google Scholar]
- 7.Craft BB, Carroll HA, Lustyk MK. Gender Differences in Exercise Habits and Quality of Life Reports: Assessing the Moderating Effects of Reasons for Exercise. Int J Lib Arts Soc Sci 2014;2:65–76. [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci G, Canducci E, Guida A, Frascari A, Rossi A, Bersani G, Ravani B, et al. The gender-related differences of nutrient intakes in a group of Italian obese patients display the ongoing transition from Mediterranean to western dietary patterns. Obes Surg 2014;24:965–967. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 10.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–1978. [DOI] [PubMed] [Google Scholar]
- 11.Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 2006;21:138–143. [DOI] [PubMed] [Google Scholar]
- 12.Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409–415. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol 2012;47:586–595. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Xu M, Hu Z, Hultstrom M, Lai E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur J Gastroenterol Hepatol 2014;26:1015–1021. [DOI] [PubMed] [Google Scholar]
- 15.Long MT, Pedley A, Massaro JM, Hoffmann U, Ma J, Loomba R, Chung RT, et al. A simple clinical model predicts incident hepatic steatosis in a community-based cohort: The Framingham Heart Study. Liver Int 2018. [DOI] [PMC free article] [PubMed]
- 16.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 2002;122:1649–1657. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40–44. [DOI] [PubMed] [Google Scholar]
- 18.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caballeria L, Pera G, Arteaga I, Rodriguez L, Aluma A, Morillas RM, de la Ossa N, et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin Gastroenterol Hepatol 2018;16:1138–1145 e1135. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Takahashi H, Hyogo H, Ono M, Oza N, Kitajima Y, Kawanaka M, et al. Epidemiological survey of hemoglobin A1c and liver fibrosis in a general population with non-alcoholic fatty liver disease. Hepatol Res 2018. [DOI] [PubMed]
- 21.Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Obesity and Weight Gain Are Associated With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;17:543–550 e542. [DOI] [PubMed] [Google Scholar]
- 22.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155:443–457 e417. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link JC, Reue K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu Rev Nutr 2017;37:225–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrigan EC, Nelson LM, Bakalov VK, Yanovski JA, Vanderhoof VH, Yanoff LB, Bondy CA. Effects of ovarian failure and X-chromosome deletion on body composition and insulin sensitivity in young women. Menopause 2006;13:911–916. [DOI] [PubMed] [Google Scholar]
- 26.Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S, Azizi F. Menstrual Cycle Irregularity and Metabolic Disorders: A Population-Based Prospective Study. PLoS One 2016;11:e0168402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 2014;14:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CW, Yun KE, Jung HS, Chang Y, Choi ES, Kwon MJ, Lee EH, et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol 2013;59:351–357. [DOI] [PubMed] [Google Scholar]
- 29.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2008;10:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan N, Zhang L, Xia Z, Peng L, Wang Y, Peng Y. Sex-Specific Association between Serum Uric Acid and Nonalcoholic Fatty Liver Disease in Type 2 Diabetic Patients. J Diabetes Res 2016;2016:3805372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki A, Abdelmalek MF, Schwimmer JB, Lavine JE, Scheimann AO, Unalp-Arida A, Yates KP, et al. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2012;10:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang JD, Abdelmalek MF, Guy CD, Gill RM, Lavine JE, Yates K, Klair J, et al. Patient Sex, Reproductive Status, and Synthetic Hormone Use Associate With Histologic Severity of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2017;15:127–131 e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buskiewicz IA, Huber SA, Fairweather D: Chapter 4 - Sex Hormone Receptor Expression in the Immune System. In: Neigh GN, Mitzelfelt MM, eds. Sex Differences in Physiology Boston: Academic Press, 2016; 45–60. [Google Scholar]
- 34.Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, Unalp-Arida A, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016;64:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villa E Role of estrogen in liver cancer. Womens Health (Lond) 2008;4:41–50. [DOI] [PubMed] [Google Scholar]
- 36.Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut 2011;60:85–89. [DOI] [PubMed] [Google Scholar]
- 37.Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, Setiawan VW, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer 2014;120:3707–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:428–433; quiz e450. [DOI] [PubMed] [Google Scholar]
- 39.Cvitanovic Tomas T, Urlep Z, Moskon M, Mraz M, Rozman D. LiverSex Computational Model: Sexual Aspects in Hepatic Metabolism and Abnormalities. Front Physiol 2018;9:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 2006;20:2613–2629. [DOI] [PubMed] [Google Scholar]
- 41.Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018;68:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jena PK, Sheng L, Liu HX, Kalanetra KM, Mirsoian A, Murphy WJ, French SW, et al. Western Diet-Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment. Am J Pathol 2017;187:1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norheim F, Hui ST, Kulahcioglu E, Mehrabian M, Cantor RM, Pan C, Parks BW, et al. Genetic and hormonal control of hepatic steatosis in female and male mice. J Lipid Res 2017;58:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol 2014;20:8525–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, et al. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 2003;18:1272–1282. [DOI] [PubMed] [Google Scholar]
- 46.Spruss A, Henkel J, Kanuri G, Blank D, Puschel GP, Bischoff SC, Bergheim I. Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol Med 2012;18:1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol 2002;283:H2478–2484. [DOI] [PubMed] [Google Scholar]
- 48.Conti FF, Brito Jde O, Bernardes N, Dias Dda S, Sanches IC, Malfitano C, Llesuy SF, et al. Cardiovascular autonomic dysfunction and oxidative stress induced by fructose overload in an experimental model of hypertension and menopause. BMC Cardiovasc Disord 2014;14:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, Schneiter P, et al. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 2008;31:1254–1256. [DOI] [PubMed] [Google Scholar]
- 50.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leibel RL, Edens NK, Fried SK. Physiologic basis for the control of body fat distribution in humans. Annu Rev Nutr 1989;9:417–443. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab 2004;89:1869–1878. [DOI] [PubMed] [Google Scholar]
- 53.Park YM, Pereira RI, Erickson CB, Swibas TA, Cox-York KA, Van Pelt RE. Estradiol-mediated improvements in adipose tissue insulin sensitivity are related to the balance of adipose tissue estrogen receptor alpha and beta in postmenopausal women. PLoS One 2017;12:e0176446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henneman P, Janssens AC, Zillikens MC, Frolich M, Frants RR, Oostra BA, van Duijn CM, et al. Menopause impacts the relation of plasma adiponectin levels with the metabolic syndrome. J Intern Med 2010;267:402–409. [DOI] [PubMed] [Google Scholar]
- 55.Escobar-Morreale HF, Alvarez-Blasco F, Botella-Carretero JI, Luque-Ramirez M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod 2014;29:2083–2091. [DOI] [PubMed] [Google Scholar]
- 56.Hoeg LD, Sjoberg KA, Lundsgaard AM, Jordy AB, Hiscock N, Wojtaszewski JF, Richter EA, et al. Adiponectin concentration is associated with muscle insulin sensitivity, AMPK phosphorylation, and ceramide content in skeletal muscles of men but not women. J Appl Physiol (1985) 2013;114:592–601. [DOI] [PubMed] [Google Scholar]
- 57.Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055–2065. [DOI] [PubMed] [Google Scholar]
- 58.Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J Hepatol 2015;63:486–493. [DOI] [PubMed] [Google Scholar]
- 59.Anderson LJ, Liu H, Garcia JM. Sex Differences in Muscle Wasting. Adv Exp Med Biol 2017;1043:153–197. [DOI] [PubMed] [Google Scholar]
- 60.Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One 2010;5:e10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res 2001;9:622–626. [DOI] [PubMed] [Google Scholar]
- 62.Ter Horst KW, Gilijamse PW, de Weijer BA, Kilicarslan M, Ackermans MT, Nederveen AJ, Nieuwdorp M, et al. Sexual Dimorphism in Hepatic, Adipose Tissue, and Peripheral Tissue Insulin Sensitivity in Obese Humans. Front Endocrinol (Lausanne) 2015;6:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharpton SR, Ajmera V, Loomba R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin Gastroenterol Hepatol 2019;17:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;152:1679–1694 e1673. [DOI] [PubMed] [Google Scholar]
- 65.Alisi A, Ceccarelli S, Panera N, Prono F, Petrini S, De Stefanis C, Pezzullo M, et al. Association between Serum Atypical Fibroblast Growth Factors 21 and 19 and Pediatric Nonalcoholic Fatty Liver Disease. PLoS One 2013;8:e67160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012;153:3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, Kugelmas M, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391:1174–1185. [DOI] [PubMed] [Google Scholar]
- 68.Santos-Marcos JA, Rangel-Zuniga OA, Jimenez-Lucena R, Quintana-Navarro GM, Garcia-Carpintero S, Malagon MM, Landa BB, et al. Influence of gender and menopausal status on gut microbiota. Maturitas 2018;116:43–53. [DOI] [PubMed] [Google Scholar]
- 69.Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, Delgado-Lista J, Quintana-Navarro GM, et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS One 2016;11:e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheng L, Jena PK, Liu HX, Kalanetra KM, Gonzalez FJ, French SW, Krishnan VV, et al. Gender Differences in Bile Acids and Microbiota in Relationship with Gender Dissimilarity in Steatosis Induced by Diet and FXR Inactivation. Sci Rep 2017;7:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu ZD, Csanaky IL, Klaassen CD. Gender-divergent profile of bile acid homeostasis during aging of mice. PLoS One 2012;7:e32551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015;109:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murawaki Y, Ikuta Y, Okamoto K, Koda M, Kawasaki H. Serum matrix metalloproteinase-3 (stromelysin-1) concentration in patients with chronic liver disease. J Hepatol 1999;31:474–481. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, Honda H, et al. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun 2001;286:1059–1065. [DOI] [PubMed] [Google Scholar]
- 75.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology 1999;29:719–727. [DOI] [PubMed] [Google Scholar]
- 76.Itagaki T, Shimizu I, Cheng X, Yuan Y, Oshio A, Tamaki K, Fukuno H, et al. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut 2005;54:1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014;59:1393–1405. [DOI] [PubMed] [Google Scholar]
- 78.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317:121–124. [DOI] [PubMed] [Google Scholar]
- 79.Anezaki Y, Ohshima S, Ishii H, Kinoshita N, Dohmen T, Kataoka E, Sato W, et al. Sex difference in the liver of hepatocyte-specific Pten-deficient mice: A model of nonalcoholic steatohepatitis. Hepatol Res 2009;39:609–618. [DOI] [PubMed] [Google Scholar]
- 80.Ande SR, Nguyen KH, Gregoire Nyomba BL, Mishra S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes NASH and HCC. Sci Rep 2016;6:23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nault JC, Paradis V, Cherqui D, Vilgrain V, Zucman-Rossi J. Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol 2017;67:1074–1083. [DOI] [PubMed] [Google Scholar]
- 82.Kara Wegermann CAM, Jiayin Zheng, Manal F. Abdelmalek, Shein-Chung Chow, Cynthia D. Guy, Anna Mae Diehl, Ayako Suzuki. Gender and menopause significantly modify effects of metabolism-related SNPs on fibrosis stage in adult patients with nonalcoholic fatty liver disease (NAFLD). Hepatology 2017;66:55A. [Google Scholar]
- 83.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010;10:594–604. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Yu R, Shete S. X-chromosome genetic association test accounting for X-inactivation, skewed X-inactivation, and escape from X-inactivation. Genet Epidemiol 2014;38:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pramfalk C, Pavlides M, Banerjee R, McNeil CA, Neubauer S, Karpe F, Hodson L. Sex-Specific Differences in Hepatic Fat Oxidation and Synthesis May Explain the Higher Propensity for NAFLD in Men. J Clin Endocrinol Metab 2015;100:4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int 2006;26:856–863. [DOI] [PubMed] [Google Scholar]
- 87.Caballeria L, Pera G, Auladell MA, Toran P, Munoz L, Miranda D, Aluma A, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol 2010;22:24–32. [DOI] [PubMed] [Google Scholar]
- 88.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Z, Xue J, Chen P, Chen L, Yan S, Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol 2014;29:42–51. [DOI] [PubMed] [Google Scholar]
- 90.van den Berg EH, Amini M, Schreuder TC, Dullaart RP, Faber KN, Alizadeh BZ, Blokzijl H. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS One 2017;12:e0171502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 92.Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol 2007;13:6419–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hartleb M, Baranski K, Zejda J, Chudek J, Wiecek A. Non-alcoholic fatty liver and advanced fibrosis in the elderly: Results from a community-based Polish survey. Liver Int 2017;37:1706–1714. [DOI] [PubMed] [Google Scholar]


