Abstract
Purpose:
Lower urinary tract symptoms (LUTS) are prevalent and burdensome, yet methods to enhance diagnosis and appropriately guide therapies are lacking. We systematically reviewed the literature for human studies of biomarkers associated with LUTS.
Materials and Methods:
PUBMED, EMBASE, and Web of Science were searched from inception to February 13, 2018. Articles were included if they were in English, performed in benign urologic populations without neurologic disorders or interstitial cystitis/bladder pain syndrome, and assessed a biomarker’s association with or ability to predict specific LUTS or urologic conditions. Bioinformatic pathway analyses were conducted to determine whether individual biomarkers associated with symptoms are present in unifying pathways.
Results:
Of 6,150 citations identified, 125 met inclusion criteria. Most studies (93.6%) assessed biomarkers at one time point and were cross-sectional in nature. Few studies adjusted for potentially confounding clinical variables or assessed biomarkers in an individual over time. No individual biomarkers are currently validated as diagnostic tools for LUTS. When compared to controls, pathway analyses identified multiple immune response pathways that were enriched in overactive bladder syndrome, and cell migration/cytoskeleton remodeling pathways that were enriched in female stress incontinence.
Conclusions:
Major deficiencies in existing biomarker literature include poor reproducibility of laboratory data, unclear classification of LUTS patients, and lack of adjustment for clinical covariates. Despite limitations, we identified multiple putative pathways where panels of biologic markers need further research.
Keywords: Overactive bladder, urgency urinary incontinence, voiding dysfunction, nocturia, stress urinary incontinence, biomarkers
Introduction:
Lower urinary tract symptoms (LUTS) encompass a broad range of symptoms in men and women. These include: storage symptoms like overactive bladder (OAB) and urgency urinary incontinence (UUI); nocturia; stress urinary incontinence (SUI); and obstructive voiding symptoms like incomplete bladder emptying, hesitancy, or post-void symptoms. LUTS are highly prevalent1 and impact quality of life and emotional well-being2, but we still have a poor understanding of LUTS pathophysiology. New information linking LUTS to other inflammatory and metabolic disorders3–5 may provide a deeper understanding of LUTS and improve our ability to diagnose treat these symptoms.
The Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) was established with the goal of identifying and explaining clinically relevant subtypes of LUTS patients6. We aimed to systematically review the literature for biomarkers that are associated with LUTS, primarily storage and voiding symptoms in the absence of bladder pain or neurologic diagnoses. We also used bioinformatic pathway analyses to combine biomarker data from individual studies to assess if certain biological pathways are enhanced in specific LUTS subtypes.
Materials and Methods:
Data Sources
An experienced reference librarian searched PubMed/MEDLINE, EMBASE, and Web of Science databases between inception and February 13, 2018 using a combination of medical subheadings (MeSH), keywords, text words related to LUTS (including both voiding and storage symptoms), and biomarkers. The search was limited to English language original research publications in humans. The full search strategy is detailed in Supplemental Table 1.
Eligibility and Study Selection
We defined eligibility prior to literature searches using the PICOS (participants, interventions, comparators, outcomes, and study design) framework7. As detailed in Table 1, we limited our review to those studies performed in populations with benign urologic conditions without neurologic disorders, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), or interstitial cystitis/bladder pain syndrome (IC/BPS). In some studies, those with cancer or IC/BPS were compared to groups of patients with LUTS. In these instances, only the biomarker information associated with LUTS and control patients was included for this review. We excluded articles that examined associations between prostate specific antigen (PSA) and prostate cancer since this literature review was not intended to examine oncologic biomarkers. We also excluded articles that only reported on LUTS as a whole or used total AUA symptom index (AUA-SI) if data were not available for us to examine the specific symptom (e.g., urgency, voiding symptom, etc.) that contributed to the score. After the initial search, we first identified those articles where “prostatic neoplasm” was identified as a major subheading. Since oncologic studies could have theoretically included benign LUTS patients for comparison groups, one reviewer screened these articles based on title alone, and retained any potentially relevant studies for further review. Next, two independent reviewers screened all remaining articles based on title and abstract, and further excluded duplicates, reviews, and conference abstracts without full publications. To resolve discrepancies, two separate reviewers further assessed any citations where there was disagreement.
Table 1:
PICOS Criteria for Studies Included in Systematic Review
| Population of Interest | Inclusions: Adult men and women with lower urinary tract symptoms. Exclusions: Studies in animals, children, adults with neurologic diagnoses, genitourinary malignancy, post-surgical LUTS, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), or interstitial cystitis/bladder pain syndrome (IC/BPS) |
| Intervention of Interest | Diagnostic biomarkers that differentiate specific urinary symptoms (i.e. overactive bladder, nocturia, stress urinary incontinence, or voiding symptoms) |
| Comparison | Any comparison group including control group, historical control, and pre-post designs with subject as own control |
| Outcomes | Inclusions: Blood, urine, tissue, or salivary biochemical markers associated with specific urinary symptoms. Exclusions: studies where biochemical markers are studied in context with “all LUTS” without differentiation of specific symptoms |
| Study Design | Cross-sectional, observational, and randomized trial designs |
Data Abstraction & Analysis
Full text articles were reviewed using a standardized data abstraction form. We collected study characteristics, patient characteristics, intervention (type of biomarker and comparator groups), outcome definitions, and study results. Data were abstracted by one reviewer, and a second reviewer confirmed accuracy. Discrepancies were resolved by discussion or by referral to a third reviewer. For each article, the risk of bias was determined based on the study design and methodologic characteristics. All symptoms were collated under the following four symptom categories: 1) OAB; 2) nocturia; 3) SUI; and 4) voiding symptoms.
Pathway Analysis
The gene identifiers (IDs) from biomarker, proteomic, and gene expression studies associated with specific LUTS were imported into MetaCore™ (Clarivate Analytics; https://portal.genego.com). Due to limitations in combining gene expression and metabolite data in the MetaCore™ platform, we could not incorporate metabolite data with other biomarkers. Within established biological pathways, enrichment analyses were conducted to test whether specific biomarkers tended to show either up- or down-regulation in participants with LUTS relative to controls. Briefly, we first extracted the mean protein or expression abundances in LUTS participants and controls from relevant papers. The LUTS participant/control ratio of mean abundances provides an estimate of up- or down-regulation. Next, we imported these data into MetaCore™ to examine the intersection between the affected gene products (i.e., proteins and/or transcripts), and existing Gene Ontology (GO) networks. The enrichment analysis algorithm utilizes statistics (such as the hypergeometric mean) to take into account the number of affected proteins in a dataset, the total number of proteins submitted in a dataset, and the total number of proteins in the intersecting network. This analysis returns a p-value that specifies the likelihood that the intersection between the list of affected proteins and a particular network is obtained purely by chance. Furthermore, since we were testing multiple objects at once, we adjusted for multiple testing using false discovery rate (FDR)-adjusted p-values.
We decided a priori that meta-analyses would only be performed if at least three or more studies assessed the same biomarkers with sufficiently similar outcome definitions and with minimal risk of bias. All data are reported in accordance with the PRISMA statement8.
Results:
After excluding duplicates, we identified 6,150 citations through initial database searches. Of these, 207 were selected for full text review, and 125 met inclusion criteria for this systematic review (Figure 1, full reference list included in Supplemental Tables). In general, studies were cross-sectional and only indicated associations with urologic symptoms. Eight studies (6.4%) included repeat sampling on a cohort over time. Furthermore, few studies utilized regression analysis or modeling to adjust for potential confounders such as age, body mass index (BMI), or other inflammatory conditions. Meta-analyses already exist for one biomarker (nerve growth factor, NGF), as described below. Due to the overall poor quality of the literature, additional meta-analyses were not performed. Details about individual biomarkers evaluated in each study and biomarkers positively and negatively associated with individual symptoms are listed in Supplemental Tables 2–6.
Figure 1:
Literature searches were conducted with an experienced reference librarian. One reviewer first screened articles categorized under the “Prostatic Neoplasm” subheading to identify oncologic studies that included benign comparison groups, and retained any potentially relevant studies for further review. Next, two independent reviewers screened all remaining articles based on title and abstract. Two separate reviewers performed full text review and data abstraction.
Biomarkers of Overactive Bladder
OAB is typically defined as urgency, with or without urgency incontinence, usually with frequency and nocturia9. For this portion of the review, we considered biomarkers associated with OAB in general, UUI (“wet-OAB” in some studies), or urinary urgency/frequency (“dry-OAB” in some studies). Since there could be slightly different pathophysiology, studies that only assessed nighttime storage symptoms were considered in the nocturia studies below.
Multiple types of biomarkers were assessed in association with OAB (Table 2, Supplemental Tables 2 & 6). The largest body of literature exists for NGF, where 40 studies evaluated associations between NGF and OAB. These included 37 studies evaluating urinary NGF, 3 studies evaluating serum NGF, and 2 studies evaluating bladder urothelial tissue. One prior systematic review and two meta-analyses collectively summarized much of the published literature through 201610–12. These publications included 23 studies that were also included in this review. A majority of studies assessing urinary NGF (32/37) showed that urinary NGF is elevated in OAB compared to controls (Supplemental Table 6, A1). However, 31/37 studies used an enzyme-linked immunosorbent assay (ELISA) from Promega Corporation; the technical validity of this test antibody has been challenged due to non-specific binding13–15. This Promega ELISA kit was withdrawn from the market in 2014. Furthermore, most of these studies evaluated NGF at one time point and looked for associations with crude clinical phenotypes without controlling for potential clinical confounders.
Table 2:
Summary of Biomarker Studies for Overactive Bladder (OAB)
| Biomarker | # Studies | Summary of Effect |
|---|---|---|
| NGF | 40 | Multiple poorer quality studies showing associations between urinary NGF and OAB; higher quality studies do not show differences between NGF and controls when controlling for covariates, especially age. Limited data showing that serum NGF may be elevated in a subset of patients with OAB and metabolic syndrome, but not in controls or other types of OAB. Two studies in bladder tissue fail to demonstrate differences in urothelial NGF between OAB and controls. |
| BDNF | 7 | Multiple poorer quality studies showing associations between BDNF and OAB; higher quality studies do not show differences after controlling for potentially confounding factors |
| PGE2 | 7 | Mixture of effect; 4 poor quality studies showing an association while 3 poor quality studies do not |
| ATP | 6 | Variable results in 3 studies; 3 additional studies performed using urodynamic fluid (not solely in urine) |
| CRP | 13 | Conflicting results are noted in multiple analyses using regression to control for potentially confounding variables. |
| Other inflammatory | 17 | Overall poor quality studies that do not control for confounders and often do not adjust for multiple testing; variable associations noted (see Supplementary Tables 2,5) |
| Receptors/Channels | 11 | Multiple studies showing higher M3 muscarinic receptor expression/density and lower β−3 adrenergic receptor expression in OAB; no differences in M2 receptors between OAB and controls. More TRPV1 receptors in OAB; variable results with regards to purinergic receptors and gene for beta 3 adrenoreceptor (ADR3B) |
| Gene expression/proteomic | 2 | Small sample sizes, lack of adjustment for confounders, and lack of adjustment for false-discovery rate limit conclusive results |
| Metabolomic | 2 | Metabolites may be useful in phenotyping (understanding subtypes of OAB) |
| Microbiome | 9 | Mostly association studies; require further investigation with longitudinal sampling and studies that control for potentially confounding factors |
NGF: nerve growth factor, BDNF: brain derived neurotrophic factor; PGE2: prostaglandin E2; ATP: adenoside triphosphate; CRP: C-reactive protein
Individual study details are listed in Supplemental Table 2
Recently, more rigorous studies were performed using a different ELISA and also incorporating multivariable analyses. After controlling for age, BMI, and other relevant covariates, no differences in urinary NGF remained between OAB and control participants in three studies14, 16, 17. Only Pennycuff et al.16 was included in prior meta-analyses. A separate study looking at serum markers, also not included in prior meta-analyses, shows further evidence of the importance of potential confounders. Investigators found that individuals with OAB and metabolic syndrome had higher serum NGF when compared to both those with OAB without metabolic syndrome, and also when compared to healthy controls18. However, the relationships between systemic NGF and urinary NGF are not entirely clear. In a prior study performed with the Promega ELISA kit, Liu et al. demonstrated that serum and urine NGF are highly correlated and are both elevated in OAB patients compared to controls19. The same group studied urine versus urothelium, and found that despite higher urinary NGF in OAB participants over controls, there were no differences in NGF in urothelial tissue in the same subjects20. These findings corroborated those by Birder, et al. who also did not identify differences in NGF between OAB and controls in urothelial tissue biopsies21. Taken together these data suggest that if NGF is indeed elevated in urine, it may be filtered from blood and excreted, or alternatively that urinary NGF originates separately than serum NGF and is actively trafficked to the urine without a reservoir in the urothelium.
In multiple studies, urinary NGF decreases after successful treatment for OAB. However, these studies were also performed using the Promega ELISA kit with non-specific binding and were also conducted without addressing clinical confounders. Thus, it is not entirely clear if NGF is responding to changes in OAB status, or other physiologic indicators. NGF is a non-specific biomarker that also appears to change in other bladder conditions like IC/BPS and bladder outlet obstruction. Therefore, we can conclude that higher urinary and serum NGF may indeed be associated with OAB, but these associations appear to be mechanistically complex and could be due to a confounding factor.
The literature regarding brain derived neurotrophic factor (BDNF) and prostaglandin E2 (PGE2) parallels the findings concerning NGF. Though studies report associations, they either fail to control for potential confounders, or when investigators adjust for potentially confounding variables, they fail to confirm the initially proposed associations. Adenosine trisphosphate (ATP) has been studied in urine and voided urodynamic fluid with conflicting results; purinergic (P2X) receptors in bladder tissue also show conflicting results.
C-reactive protein (CRP) has been assessed in multiple studies that include regression analyses, also with conflicting results. This includes four studies in 4,019 participants where there were no differences in serum CRP22–25, and four separate studies in 5,926 men where CRP remained associated with OAB symptoms after adjusting for confounding factors26–29. Using participants from the Boston Area Community Health (BACH) study, Kupelian et al. initially did not see differences in CRP among those with OAB compared to controls30. However, they repeated their analyses after redefining OAB symptoms, and later found that higher CRP was associated with urinary urgency and frequency in both men and women31. Multivariable regression modeling was used in both of these studies.
Finally, there has been a significant increase in the study of the urinary microbiome and OAB. The microbiome appears to differ in certain clinical scenarios, one of which is OAB. Although there are differences in the abundance of sequence-identified microbes, we currently lack longitudinal data, and many existing studies do not control for potentially confounding variables such as age, hormonal status, body mass index, and presence of diabetes. Therefore, the implications of these initial association studies and how they relate to symptom development require further investigation.
Biomarkers of Nocturia
Eight studies assessed biomarkers specifically associated with nocturia, defined as two or more voids per night (Supplemental Tables 3 & 6). The five largest studies utilized multivariable regression modeling to control for potentially confounding factors and found that higher serum beta natriuretic peptide (BNP), lower urinary 6-sulfaoxymelatonin (urinary metabolite of melatonin), and higher serum CRP were associated with nocturia. In one study that performed serial blood sampling over 48 hours, older adults with nocturia had higher nighttime neurotransmitters associated with hypertension (noradrenaline and dopamine), higher BNP, lower melatonin, and lower vasopressin compared to control groups. Detailed references are listed in the supplemental tables.
Biomarkers of Stress Urinary Incontinence
Twenty-three studies assessed biomarkers associated with female SUI (Supplemental Tables 4 & 6). Two of these studies queried biomarkers predictive of favorable outcomes after surgery while controlling for covariates with regression analyses. Participants in the Value of Urodynamic (ValUE) trial32 underwent biomarker assessment before and after mid-urethral sling surgery. One year after surgery, urinary interleukin (IL) 12p70 decreased, while urinary NGF increased in a manner that was independent of any covariates, including urgency symptoms. Investigators also found that higher baseline N-telopeptide of crosslinked type I collagen (NTx) in urine was predictive of surgical failure one year later. NTx is a marker of bone resorption and remodeling, so the mechanistic implications of this association are not clear. Urine samples from ValUE trial participants were also subjected to urinary microbiome analysis. There were no associations between the urinary microbiome and SUI symptoms. Rather, microbial diversity was associated with concomitant UUI, hormone status, and BMI.
A number of investigators studied tissue from periurethral vaginal wall biopsies in women with SUI compared to controls. Associations between sex hormones and SUI were identified. Lower serum estradiol and lower estrogen and progesterone receptors were found in pre-menopausal women with SUI. However, protein levels and potential confounders were not thoroughly assessed, and there are risks of bias in these studies. It was suggested by weak evidence from other studies that collagen breakdown and decreased collagen turnover is associated with SUI. Though there are suggestions of hormonal influences on collagen turnover in periurethral tissue, these associations have not been rigorously studied in humans.
Among studies comparing the extracellular matrix (ECM) of women with SUI and controls, there were conflicting results. It is unclear whether confounding or bias contributed to these differences, as investigators did not systematically control for age, menopausal status, phase of the menstrual cycle, and/or the presence of concomitant prolapse. There was stronger evidence that calpain-2, a proteolytic enzyme involved in cellular function, degradation of myofibrils, and myoblast cell fusion is implicated in SUI33, 34.
Biomarkers of Voiding Symptoms
Voiding symptoms include straining, intermittent or weak urinary stream, incomplete bladder emptying, and post-void symptoms. We identified 11 studies that specifically assessed biomarkers in the context of voiding symptoms (Supplemental Tables 5 & 6). Individual voiding symptoms in these studies were generally not well specified.
The highest level of evidence comes from a population-based cohort study of 730 men in Australia. These men were sampled twice over a 5-year period, and investigators did not find any serum inflammatory biomarkers that were associated with incident voiding symptoms29.
Multiple population-based cross-sectional studies have been performed in other populations from the United States, China, and Korea. Two studies including ~2,300 men found inconsistent associations, while five other studies in over 8,700 men did not find associations between voiding symptoms and CRP. One study included approximately 1,850 women. In this study, incomplete emptying and weak stream were associated with higher CRP in women only.
Integrated Pathway Analyses
Biomarkers positively and negatively associated with each of the four symptom categories (Supplemental Table 6) were incorporated into pathway enrichment analyses. There were no significantly enriched pathways regarding biomarkers for nocturia or voiding symptoms.
For OAB, multiple enriched pathways were identified. The top ten pathways, based on FDR-adjusted significance, are displayed in Table 3. The majority of these affected pathways involve immune responses that are typically seen with an allergen, chemical irritant, or microbe. Enriched pathways are those involved in immune cell migration, adhesion, and inflammatory responses. Detailed maps of the top three enriched pathways with up- and down-regulated signals are displayed in Supplemental Figure 1. The GO cellular processes that correspond with the enriched pathways are depicted in Figure 2.
Table 3:
OAB Pathway Enrichment Analysis
| # | Pathway | Total Proteins in Pathway | Proteins from Dataset | p-value | FDR p-value |
Affected Proteins from Dataset |
|---|---|---|---|---|---|---|
| 1 | Maturation and migration of dendritic cells in skin sensitization | 41 | 12 | 7.143E-15 | 6.895E-12 | MHC class II beta chain, MHC class II, IL-1 beta, IL-6, IL-8, E-cadherin, CD83, HLA-DRB4, HLA-DRB, TNF-alpha, MHC class II alpha chain, IL-12 beta |
| 2 | NF-kB-, AP-1- and MAPKs-mediated proinflammatory cytokine production by eosinophils in asthma | 43 | 12 | 1.363E-14 | 6.895E-12 | IL-1 beta, IL-6, IL-4, GRO-1, IL-8, GM-CSF, CCL17, MIP-1-alpha, IL-5, ENA-78, TNF-alpha, MGF |
| 3 | PDE4 regulation of cyto/chemokine expression in arthritis | 49 | 12 | 7.771E-14 | 2.621E-11 | IL-1 beta, IL-6, IL-10, IL-8, GM-CSF, MIP-1-alpha, IP10, ENA-78, PKA-reg (cAMP-dependent), TNF-alpha, IL-12 beta, PI3K cat class IA |
| 4 | PDE4 regulation of cyto/chemokine expression in inflammatory skin diseases | 50 | 11 | 3.197E-12 | 8.088E-10 | IL-1 beta, IL-13, IL-6, IL-4, G-protein alpha-i family, IL-10, IL-8, IP10, PKA-reg (cAMP-dependent), TNF-alpha, IL-12 beta |
| 5 | TNF-alpha-induced inflammatory signaling in normal and asthmatic airway epithelium | 38 | 10 | 4.577E-12 | 9.263E-10 | IL-13, IL-6, IL-4, GRO-1, IL-8, GM-CSF, CCL17, IP10, IL-5, TNF-alpha |
| 6 | Inter-cellular relations in COPD (general schema) | 30 | 9 | 1.499E-11 | 2.529E-09 | IL-1 beta, IL-6, GRO-1, Granzyme B, IL-8, GM-CSF, IP10, TNF-alpha, EGF |
| 7 | Proinflammatory cytokine release from eosinophils in asthma | 34 | 9 | 5.305E-11 | 7.669E-09 | IL-1 beta, IL-13, IL-6, NGF, IL-4, IL-8, GM-CSF, IL-5, TNF-alpha |
| 8 | Immune response_T cell subsets: secreted signals | 25 | 8 | 1.185E-10 | 1.498E-08 | IL-13, IL-6, IL-4, IL-10, GM-CSF, MIP-1-alpha, IL-5, TNF-alpha |
| 9 | Proinflammatory cytokine production by Th17 cells in asthma | 53 | 10 | 1.652E-10 | 1.857E-08 | MHC class II, IL-1 beta, IL-13, IL-6, C3aR, IL-4, IL-10, IL-8, IL-5, TNF-alpha |
| 10 | Glomerular injury in Lupus Nephritis | 92 | 12 | 2.085E-10 | 2.110E-08 | IL-1 beta, IL-6, GRO-1, OX40L(TNFSF4), IFI56, IL-8, GM-CSF, GRO-2, MIP-1-alpha, IP10, NGAL, TNF-alpha |
The top 10 enriched molecular pathways that mapped to the dataset of biomarkers associated with OAB. Enriched pathways are identified using MetaCore™. P-value specifies the likelihood that the intersection between the list of affected proteins and a particular network is obtained purely by chance. False discovery rate (FDR)-adjusted p-values are also shown.
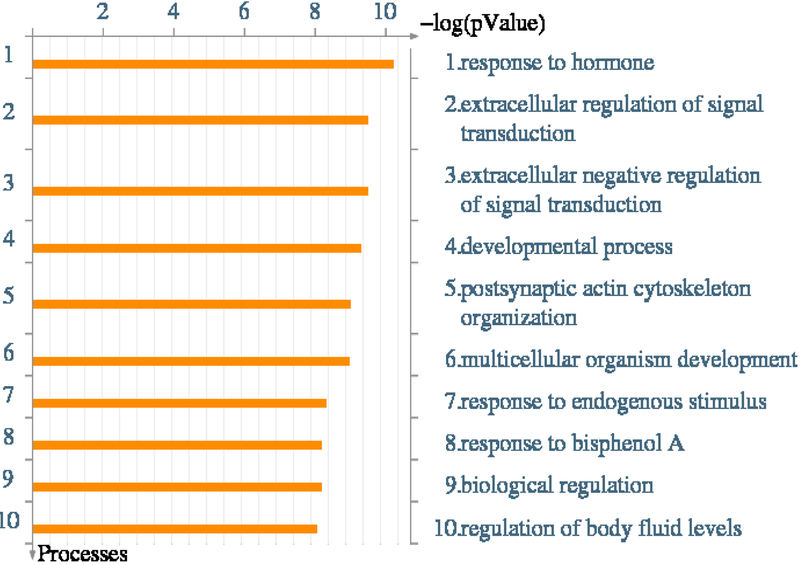
Figure 2:
Gene identifiers (IDs) from biomarker, proteomic, and gene expression studies associated with OAB were imported into MetaCore™ for pathway analysis. This analysis examines the intersection between the affected gene products (i.e. proteins and/or transcripts), and existing Gene Ontology networks. The figure depicts the top 10 cellular processes that were enriched in the biomarkers associated with OAB.
For female SUI, multiple enriched pathways were also identified (Table 4). The most enriched pathway was the Slit-ROBO cell-signaling pathway. This pathway is often a repulsive cue for axon guidance, but has also been implicated in angiogenesis and cell migration35. The second most enriched pathway was the cytoskeleton remodeling (keratin filaments) pathway that is involved in cell shape control and provides intracellular mechanical strength36, 37. Detailed maps of the top three enriched pathways with up- and down-regulated signals are displayed in Supplemental Figure 2. The GO cellular processes that correspond with the enriched pathways are depicted in Figure 3.
Table 4:
SUI Pathway Enrichment Analysis
| # | Pathway | Total Proteins in Pathway | Proteins from Dataset | p-value | FDR p-value |
Affected Proteins from Dataset |
|---|---|---|---|---|---|---|
| 1 | Development_Slit-Robo signaling | 30 | 4 | 3.782E-05 | 0.010 | ROBO4, Actin cytoskeletal, Actin, ACTB |
| 2 | Cytoskeleton remodeling_Keratin filaments | 36 | 4 | 7.896E-05 | 0.010 | Keratin 1, Epiplakin, Actin cytoskeletal, GRB2 |
| 3 | Immune response_Oncostatin M signaling via MAPK | 37 | 4 | 8.810E-05 | 0.010 | MMP-13, TIMP1, GRB2, MMP-1 |
| 4 | Blood coagulation_Blood coagulation | 39 | 4 | 1.087E-04 | 0.010 | Coagulation factor IX, Protein C inhibitor, Coagulation factor X, CPB2 |
| 5 | Immune response_Oncostatin M signaling via JAK-Stat | 22 | 3 | 3.594E-04 | 0.026 | TIMP1, STAT3, MMP-1 |
| 6 | Cell adhesion_ECM remodeling | 55 | 4 | 4.171E-04 | 0.026 | MMP-13, Actin cytoskeletal, TIMP1, MMP-1 |
| 7 | Development_Leptin signaling via JAK/STAT and MAPK cascades | 25 | 3 | 5.295E-04 | 0.029 | TIMP1, GRB2, STAT3 |
| 8 | Signal transduction_mTORC1 downstream signaling | 61 | 4 | 6.201E-04 | 0.029 | p70 S6 kinases, p70 S6 kinase2, Rictor, STAT3 |
| 9 | Role of Tissue factor-induced Thrombin signaling in cancerogenesis | 65 | 4 | 7.892E-04 | 0.033 | MMP-13, MRLC, Actin cytoskeletal, Coagulation factor X |
| 10 | Development_S1P1 receptor signaling via beta-arrestin | 34 | 3 | 1.322E-03 | 0.045 | p70 S6 kinases, p70 S6 kinase2, GRB2 |
The top 10 enriched molecular pathways that mapped to the dataset of biomarkers associated with female SUI. Enriched pathways are identified using MetaCore™. P-value specifies the likelihood that the intersection between the list of affected proteins and a particular network is obtained purely by chance. False discovery rate (FDR)-adjusted p-values are also shown.
Figure 3:
Gene identifiers (IDs) from biomarker, proteomic, and gene expression studies associated with SUI were imported into MetaCore™ for pathway analysis. The figure depicts the top 10 cellular processes that were enriched in the biomarkers associated with female SUI.
Discussion:
We have performed a comprehensive systematic review and pathway analyses of biomarkers associated with LUTS in humans. Unfortunately, there are no individual biomarkers that are strongly associated with specific LUTS. However, by integrating individual studies into pathway analyses, we have identified multiple candidate biologic pathways that could be considered for future research.
This study is strengthened by the coordinated search strategy, pre-defined review criteria, and strict methodology that we employed. Using these methods, we reviewed and summarized a large body of seemingly disparate literature. By incorporating pathway analyses, we were able to integrate this literature to identify potential targets for future studies. However, our study is limited in that much of the data that we entered into pathway analyses comes from individual studies where there was a moderate risk of bias. Most studies were cross-sectional, with biomarkers only being assessed at one time point, which limits causal inferences. Many studies lacked adjustment for confounding factors. Our pathway analyses indicate that for OAB in particular, multiple inflammatory and immune response pathways could be important. Immune response pathways can also be influenced by other covariates such as age and obesity, which are frequently encountered in patients with OAB but were not always adjusted for in primary studies. Therefore, our results should be interpreted with caution. An additional challenge is that due to technological limitations with available software, metabolite data could not be combined with proteomic data for pathway analyses.
Our review demonstrates that multiple investigators have assessed biomarkers to better understand LUTS, but there are ongoing issues with how investigators define and classify LUTS. For example, there are many instances where investigators publish biomarkers associated with LUTS as a whole, or biomarkers associated with a total score on a multifactorial symptom index. This may partially occur because patients rarely present with only one or very few LUTS38, 39. However, in these instances, imprecise classification of LUTS limits the conclusions that we can gain, particularly when it comes to pathophysiology. Future studies assessing whether biomarkers are associated with “symptom clusters”40 rather than traditional diagnostic groupings may be useful.
Our search identified a number of individual biomarkers that have been studied. However, thus far, single biomarkers have not proven sufficient for classifying and phenotyping individuals. This is partially due to a lack of consistency but also because many individual biomarkers fail to discriminate between LUTS and other bladder conditions. Some researchers have proposed that future research should focus on “fingerprints” or panels of multiple molecules to better understand, diagnose, and treat LUTS41, 42. Our pathway analyses provide some direction for this type of future research. For OAB in particular, our results indicate multiple immune response pathways that were highly significantly enriched, even after FDR adjustment. On the surface, pathway names like “Maturation and migration of dendritic cells in skin sensitization” may not carry much meaning. However, evidence suggests that this pathway involves cellular signaling in response to a “danger signal”43. This signaling response is heavily dependent on inflammasomes carrying multiple cytokines. Inflammasomes have recently been reported as important mediators of bladder pathology44 and may prove to be a useful target for future biomarker-based research. Similarly, the most enriched pathway in our SUI pathway analysis, the “Development_Slit-robo signaling” pathway, prevents axons from migrating to inappropriate locations. An interesting feature is that in reproductive tissues, this pathway is further regulated by sex hormones35, which have also been implicated in SUI. Careful study of these and other proposed pathways using panels of biomarkers may provide breakthroughs in understanding pathophysiologic mechanisms behind bladder symptoms.
Conclusions:
Without understanding the pathophysiology behind ill-defined symptomatic benign urologic conditions, precision medicine and improvements in patient care remain elusive. To advance the field and gain meaningful information about biomarkers and LUTS, we recommend that future studies use clear definitions of specific LUTS. Furthermore, studies that sample multiple time points and utilize rigorous adjustment for potentially confounding variables are needed. Ideally, individual biomarkers should also be explored in the context of biomarker panels that can better distinguish different LUTS based on proposed pathophysiologic mechanisms. These studies will likely require collaboration between clinician scientists who understand clinical phenotyping and potentially confounding clinical variables, basic scientists who understand the strengths and pitfalls of different molecular biology and biochemical techniques, and quantitative scientists who can incorporate clinical variables, metadata, and other relevant variables into robust statistical models.
Supplementary Material
Acknowledgments and Funding
This is publication number 17 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK097776, DK099879).
Research reported in this publication was supported at Northwestern University, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Siddiqui is supported by grant K23-DK110417 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PI: Cindy Amundsen, MD, Kevin Weinfurt, PhD; Co-Is: Kathryn Flynn, PhD, Matthew O. Fraser, PhD, Todd Harshbarger, PhD, Eric Jelovsek, MD, Aaron Lentz, MD, Drew Peterson, MD, Nazema Siddiqui, MD, Alison Weidner, MD; Study Coordinators: Carrie Dombeck, MA, Robin Gilliam, MSW, Akira Hayes, Shantae McLean, MPH
University of Iowa, Iowa City, IA (DK097772): PI: Karl Kreder, MD, MBA, Catherine S Bradley, MD, MSCE, Co-Is: Bradley A. Erickson, MD, MS, Susan K. Lutgendorf, PhD, Vince Magnotta, PhD, Michael A. O’Donnell, MD, Vivian Sung, MD; Study Coordinator: Ahmad Alzubaidi
Northwestern University, Chicago, IL (DK097779): PIs: David Cella, Brian Helfand, MD, PhD; Co-Is: James W Griffith, PhD, Kimberly Kenton, MD, MS, Christina Lewicky-Gaupp, MD, Todd Parrish, PhD, Jennie Yufen Chen, PhD, Margaret Mueller, MD; Study Coordinators: Sarah Buono, Maria Corona, Beatriz Menendez, Alexis Siurek, Meera Tavathia, Veronica Venezuela, Azra Muftic, Pooja Talaty, Jasmine Nero. Dr. Helfand, Ms. Talaty, and Ms. Nero are at NorthShore University HealthSystem.
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: Mitch Berger, MD, PhD, John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne P. Cameron, MD, John Wei, MD; Study Coordinators: Morgen Barroso, Linda Drnek, Greg Mowatt, Julie Tumbarello
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: John L. Gore, MD, MS; Study Coordinators: Alice Liu, MPH, Brenda Vicars, RN
Washington University in St. Louis, St. Louis Missouri (DK100017): PI: Gerald L. Andriole, MD, H. Henry Lai; Co-I: Joshua Shimony, MD, PhD; Study Coordinators: Susan Mueller, RN, BSN, Heather Wilson, LPN, Deborah Ksiazek, BS, Aleksandra Klim, RN, MHS, CCRC
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urology, and Hematology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: John Kusek, PhD; NIH Personnel: Tamara Bavendam, MD, Robert Star, MD, Jenna Norton
Arbor Research Collaborative for Health, Data Coordinating Center (DK097776 and DK099879): PI: Robert Merion, MD, FACS; Co-Is: Victor Andreev, PhD, DSc, Brenda Gillespie, PhD, Gang Liu, PhD, Abigail Smith, PhD; Project Manager: Melissa Fava, MPA, PMP; Clinical Study Process Manager: Peg Hill-Callahan, BS, LSW; Clinical Monitor: Timothy Buck, BS, CCRP; Research Analysts: Margaret Helmuth, MA, Jon Wiseman, MS; Project Associate: Julieanne Lock, MLitt
Robin Gilliam, MSW, Duke University, research coordinator, provided assistance with obtaining full text references. Megan von Isenburg, MSLS, Duke University, reference librarian, provided assistance with literature searches. Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance.
REFERENCES
- 1.Irwin DE, Milsom I, Hunskaar S et al. : Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol, 50: 1306, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Sexton CC, Irwin DE et al. : The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int, 101: 1388, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bunn F, Kirby M, Pinkney E et al. : Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int J Clin Pract, 69: 199, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Sebastianelli A, Gacci M: Current Status of the Relationship Between Metabolic Syndrome and Lower Urinary Tract Symptoms. Eur Urol Focus, 4: 25, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Denys MA, Anding R, Tubaro A et al. : Lower urinary tract symptoms and metabolic disorders: ICI-RS 2014. Neurourol Urodyn, 35: 278, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Yang CC, Weinfurt KP, Merion RM et al. : Symptoms of Lower Urinary Tract Dysfunction Research Network. J Urol, 196: 146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rios LP, Ye C, Thabane L: Association between framing of the research question using the PICOT format and reporting quality of randomized controlled trials. BMC Med Res Methodol, 10: 11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Altman DG, Liberati A et al. : PRISMA statement. Epidemiology, 22: 128; author reply 128, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Abrams P, Cardozo L, Fall M et al. : The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn, 21: 167, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Qu HC, Yan S, Zhang XL et al. : Urinary nerve growth factor levels could be a biomarker for overactive bladder symptom: a meta-analysis. Genet Mol Res, 13: 8609, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Seth JH, Sahai A, Khan MS et al. : Nerve growth factor (NGF): a potential urinary biomarker for overactive bladder syndrome (OAB)? BJU Int, 111: 372, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Sheng W, Zhang H, Ruth KH: Could urinary nerve growth factor be a biomarker for overactive bladder? A meta-analysis. Neurourol Urodyn, 36: 1703, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Malerba F, Paoletti F, Cattaneo A: NGF and proNGF Reciprocal Interference in Immunoassays: Open Questions, Criticalities, and Ways Forward. Front Mol Neurosci, 9: 63, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter HE, Moalli P, Amundsen CL et al. : Urinary Biomarkers in Women with Refractory Urgency Urinary Incontinence Randomized to Sacral Neuromodulation versus OnabotulinumtoxinA Compared to Controls. J Urol, 197: 1487, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamper M, Moser R, Viereck V: Have we been led astray by the NGF biomarker data? Neurourol Urodyn, 36: 203, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Pennycuff JF, Schutte SC, Hudson CO et al. : Urinary neurotrophic peptides in postmenopausal women with and without overactive bladder. Neurourol Urodyn, 36: 740, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Tyagi P, Tyagi V, Qu X et al. : Elevated CXC chemokines in urine noninvasively discriminate OAB from UTI. Am J Physiol Renal Physiol, 311: F548, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Dagdeviren H, Cengiz H: Association between Metabolic Syndrome and Serum Nerve Growth Factor Levels in Women with Overactive Bladder. Gynecol Obstet Invest, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Liu HT, Lin H, Kuo HC: Increased serum nerve growth factor levels in patients with overactive bladder syndrome refractory to antimuscarinic therapy. Neurourol Urodyn, 30: 1525, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Liu HT, Wang YS, Kuo HC et al. : Nerve Growth Factor Levels are Increased in Urine but Not Urothelium in Patients With Detrusor Overactivity. Tzu Chi Medical Journal, 22: 165, 2010 [Google Scholar]
- 21.Birder LA, Wolf-Johnston A, Griffiths D et al. : Role of urothelial nerve growth factor in human bladder function. Neurourol Urodyn, 26: 405, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi WS, Lee WK, Lee SH et al. : Is high-sensitivity c-reactive protein associated with lower urinary tract symptoms in aging men? Results from the Hallym aging study. Korean Journal of Urology, 53: 335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsiao SM, Lin HH, Kuo HC: The role of serum C-reactive protein in women with lower urinary tract symptoms. Int Urogynecol J, 23: 935, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Liu RT, Chung MS, Lee WC et al. : Prevalence of overactive bladder and associated risk factors in 1359 patients with type 2 diabetes. Urology, 78: 1040, 2011 [DOI] [PubMed] [Google Scholar]
- 25.St Sauver JL, Sarma AV, Jacobson DJ et al. : Associations between C-reactive protein and benign prostatic hyperplasia/lower urinary tract symptom outcomes in a population-based cohort. Am J Epidemiol, 169: 1281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Doo SW, Yang WJ et al. : Association Between High-sensitivity C-reactive Protein and Lower Urinary Tract Symptoms in Healthy Korean Populations. Urology, 86: 139, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Liao CH, Chung SD, Kuo HC: Serum C-reactive protein levels are associated with residual urgency symptoms in patients with benign prostatic hyperplasia after medical treatment. Urology, 78: 1373, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Lu Z, Gao Y, Tan A et al. : Increased high-sensitivity C-reactive protein predicts a high risk of lower urinary tract symptoms in Chinese male: Results from the Fangchenggang Area Male Health and Examination Survey. Prostate, 72: 193, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Martin S, Vincent A, Taylor AW et al. : Lower urinary tract symptoms, depression, anxiety and systemic inflammatory factors in men: A population-based cohort study. PLoS ONE, 10:10 Article Number: e0137903., 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kupelian V, McVary KT, Barry MJ et al. : Association of C-reactive protein and lower urinary tract symptoms in men and women: results from Boston Area Community Health survey. Urology, 73: 950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupelian V, Rosen RC, Roehrborn CG et al. : Association of overactive bladder and C-reactive protein levels. Results from the Boston Area Community Health (BACH) Survey. BJU Int, 110: 401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nager CW, Brubaker L, Litman HJ et al. : A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med, 366: 1987, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Zhang L, Jin H et al. : The role of calpain-calpastatin system in the development of stress urinary incontinence. Int Urogynecol J, 21: 63, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Yang SH, Wang J, Xu J et al. : MiR-93-mediated collagen expression in stress urinary incontinence via calpain-2. Molecular Medicine Reports, 17: 624, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Dickinson RE, Duncan WC: The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction, 139: 697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang SI, Kalinin A, Takahashi K et al. : Characterization of human epiplakin: RNAi-mediated epiplakin depletion leads to the disruption of keratin and vimentin IF networks. J Cell Sci, 118: 781, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Leung CL, Green KJ, Liem RK: Plakins: a family of versatile cytolinker proteins. Trends Cell Biol, 12: 37, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Cameron AP, Lewicky-Gaupp C, Smith AR et al. : Baseline Lower Urinary Tract Symptoms in Patients Enrolled in LURN: A Prospective, Observational Cohort Study. J Urol, 199: 1023, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helfand BT, Smith AR, Lai HH et al. : Prevalence and Characteristics of Urinary Incontinence in a Treatment Seeking Male Prospective Cohort: Results from the LURN Study. J Urol, 200: 397, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreev VP, Liu G, Yang CC et al. : Symptom Based Clustering of Women in the LURN Observational Cohort Study. J Urol, doi: 10.1016/j.juro.2018.06.068. [Epub ahead of print], 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pennycuff JF, Northington GM, Author A et al. : Current Concepts in Urinary Biomarkers for Overactive Bladder: What Is the Evidence? Current Bladder Dysfunction Reports, 12: 260, 2017 [Google Scholar]
- 42.Wrobel AF, Kluz T, Surkont G et al. : Novel biomarkers of overactive bladder syndrome. Ginekol Pol, 88: 568, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Ainscough JS, Frank Gerberick G, Dearman RJ et al. : Danger, intracellular signaling, and the orchestration of dendritic cell function in skin sensitization. J Immunotoxicol, 10: 223, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Inouye BM, Hughes FM Jr., Sexton SJ et al. : The Emerging Role of Inflammasomes as Central Mediators in Inflammatory Bladder Pathology. Curr Urol, 11: 57, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.