Abstract
Animals make use of changes in photoperiod to adapt their physiology to the forthcoming breeding season. Comparative studies have contributed to our understanding of the mechanisms of seasonal reproduction in vertebrates. Birds are excellent models for studying these phenomena because of their rapid and dramatic responses to changes in photoperiod. Deep brain photoreceptors in birds perceive and transmit light information to the pars tuberalis (PT) in the pituitary gland, where the thyroid-stimulating hormone (TSH) is produced. This PT-TSH locally increases the level of the bioactive thyroid hormone T3 via the induction of type 2 deiodinase production in the mediobasal hypothalamus, and an increased T3 level, in turn, controls seasonal gonadotropin-releasing hormone secretion. In mammals, the eyes are the only photoreceptive structure, and nocturnal melatonin secretion encodes day-length information and regulates the PT-TSH signaling cascade. In Salmonidae, the saccus vasculosus plays a pivotal role as a photoperiodic sensor. Together, these studies have uncovered the universality and diversity of fundamental traits in vertebrates.
Keywords: photoperiodism, circadian clock, thyrotropin, thyroid hormone, photoreceptor, opsin
1. Introduction
Organisms living in a seasonal world alter their physiology and behavior to adapt to annual fluctuations in the environment. Animals display a wide variety of responses to the changing seasons, such as alterations in the growth rate, metabolism, and immune function as well as the initiation of molting, migration, nesting, hibernation, and reproductive activity. Anticipating and aligning with periodic, geophysical cycle-generated changes to ensure the precise timing of these annual processes is essential for their survival and reproductive success. A number of environmental factors, such as temperature, rainfall, and day length, vary with seasons. However, seasonal fluctuations in temperature and precipitation are relatively unpredictable because there are occasional warm winter or dry rainy seasons. In contrast, changes in the day length (photoperiod) are the most reliable seasonal cues because solstices and equinoxes occur at almost identical times each year, suggesting that organisms use photoperiod to estimate the time of year. The principal role of photoperiod in the seasonal responses was conclusively demonstrated in various organisms in the 1920s1–3) and these day length-dependent responses are collectively referred to as photoperiodism.
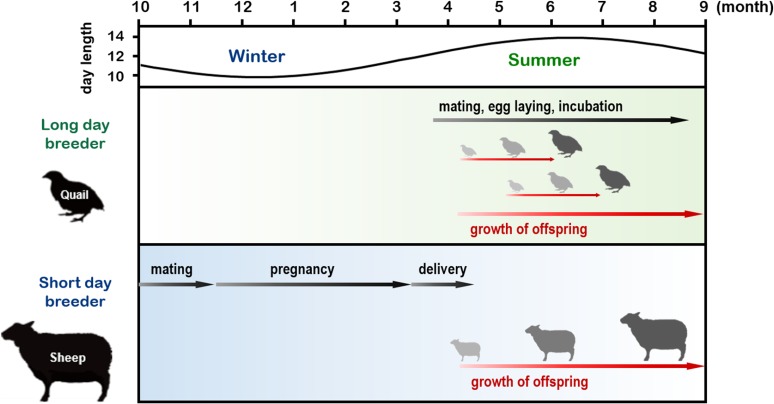
Strategies for reproduction in animals can be classified into the following two categories: seasonal and nonseasonal. Nonseasonal reproduction is mainly observed in animals inhabiting tropical areas, where only relatively minor changes in the environment occur throughout the year. Animals in these areas reproduce almost throughout the year. In contrast, most animals living outside the tropics limit their reproductive activity to a specific time of the year. The advantages of seasonal reproduction are obvious; animals can avoid expending energy for reproduction at an inappropriate time of the year (e.g., winter), and the survival of offspring will be maximized if they are delivered and raised in seasons with a moderate climate. For example, most birds breed during spring and summer and are called long-day (LD) breeders. Because the incubation period of most birds lasts only a few weeks, their offspring are born during spring and summer. Conversely, relatively large mammals, such as goats and sheep, breed during autumn and are called short-day (SD) breeders. Because these animals have a gestation period of approximately six months, their offspring are also born and raised during spring and summer (Fig. 1). As a result, the offspring of both LD and SD breeders can grow in favorable conditions with a moderate climate and abundant food.
Figure 1.
(Color online) Calendar of seasonal breeders. Small species with short gestation or incubation periods mate in spring and summer [long-day (LD) breeders]. In contrast, relatively large species with approximately six-month gestation periods mate in fall [short-day (SD) breeders]. Consequently, the offspring of both LD and SD breeders are born and raised in spring/summer. Figure adapted from Shinomiya et al. (2014)104) with permission under the terms of the Creative Commons Attribution License (CC BY) © 2014 Shinomiya, Shimmura, Nishiwaki-Ohkawa, and Yoshimura.
Reproduction in vertebrates is primarily regulated by the hypothalamic–pituitary–gonadal (HPG) axis. Gonadotropin-releasing hormone (GnRH), released from the hypothalamus, induces the secretion of gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) from the pars distalis (PD) of the pituitary (also known as the anterior pituitary). Gonadotropins, in turn, activate gonadal function and trigger reproductive activity. In this context, seasonal reproduction is achieved via activation of the HPG axis at an appropriate time of the year. For seasonal breeders, activation of the HPG axis results in a dramatic change in the gonadal size, particularly in birds, in which it increases more than a hundred fold during the breeding season.4) In addition to this gonadal response, birds have more sophisticated photoperiodic mechanisms than other vertebrate species. They have very short breeding seasons, and the HPG axis is automatically turned off after breeding; the gonads start to regress although the photoperiod still increases, a phenomenon known as photorefractoriness, and gonadal weight is significantly reduced during the nonbreeding season.5) Such robust responses and a restricted breeding season appear to be adaptations of birds to flight. Accordingly, birds have often been investigated in studies on photoperiodic responses. Among mammals, hamsters and sheep are frequently studied to understand the mechanisms of photoperiodism because they also show robust photoperiodic responses. However, the magnitude of gonadal regression is less dramatic in mammals than in birds and only involves a several-fold change.6)
2. Models for photoperiodic time measurement
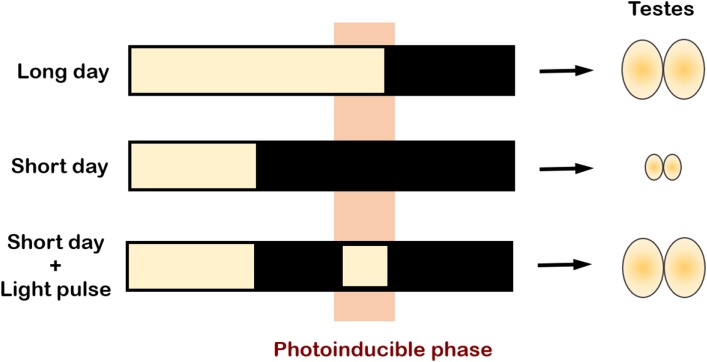
One of the most important questions in the field of photoperiodism is how organisms discriminate between LDs and SDs. Organisms show photoperiodic responses when the day length reaches or exceeds the so-called “critical day length”. Most seasonal breeders possess a highly accurate mechanism to respond to small changes in photoperiod; for instance, testicular recrudescence (gonadal growth) can only be seen in hamsters transferred from SD to photoperiods of ≥12.5 h.7,8) In Japanese quail, days that are >11.5 h induce testicular growth.9) Somewhere between the receptors receiving environmental day-length information and the HPG axis must be the capacity to measure the photoperiod. The easiest and most intuitive way to conceptualize this is to assume that a substance (chemical or physiological agent) required for a seasonal event (e.g., gonad development) gradually accumulates in response to light (or dark). The seasonal event is then initiated when the level of this substance reaches a critical value or threshold at the appropriate day length.10) However, this model, referred to as the hourglass model, has long been refuted because a light pulse given at a specific time of day (called the photoinducible phase) is sufficient to induce testicular development (Fig. 2).11) This finding reveals that organisms possess a daily rhythm of light sensitivity and that it is the time point or phase at which the light pulse occurs that matters, rather than the total duration of light exposure. This implicates the involvement of the endogenous circadian clock in photoperiodic time measurement.
Figure 2.
(Color online) Involvement of the circadian clock in photoperiodic time measurement. A light pulse given at a specific time of the day, called the “photoinducible phase” (highlighted in orange), is sufficient to induce gonadal growth. The circadian clock is thought to be involved in generating this photoinducible phase, but the exact mechanism remains unclear. Figure adapted with permission from RightsLink Permissions Springer Nature Customer Service Centre GmbH: Springer Nature. Avian Reproduction. Advances in Experimental Medicine and Biology, vol 1001. Molecular and Neuroendocrine Mechanisms of Avian Seasonal Reproduction, Tamai T.K., Yoshimura T.,105) © Springer Nature Singapore Pte. Ltd. 2017.
Two models, referred to as “external coincidence”12) and “internal coincidence”,13) have been proposed to address the role of the circadian clock in seasonal time measurement. The former model is based on Bünning’s hypothesis,14) which states that when an external light stimulus is applied during the photosensitive phase (generated by the circadian oscillator), a photoperiodic response is induced. External coincidence refers to the requirement of the concurrence of an external stimulus with an internal rhythm of photosensitivity. Conversely, the internal coincidence model hypothesizes the existence of multiple oscillators, of which one oscillator couples with dawn and the other with dusk, and it also hypothesizes that the phase relationship between the two oscillators changes between seasons. In this model, the coincidence of two (or more) internal oscillators leads to seasonal responses. Although the exact nature of these endogenous rhythms of photosensitivity remains elusive, these theoretical models have helped us to better understand photoperiodism and have promoted the discovery of molecular mechanisms for photoperiodic responses in vertebrates as well as in plants and insects.
Photoperiodic time measurement is a process that describes how environmental light information is perceived, assessed, transduced, and finally translated into a neuroendocrine event in organisms. It consists of three components: (1) a light input pathway that perceives external light/dark information, (2) a biological clock that measures the day length within a 24 h cycle, and (3) a neuroendocrine output pathway that regulates various aspects of physiology and behavior. In the following section, we present an updated overview of the mechanisms that regulate photoperiodism in vertebrates.
3. Mechanism of seasonal reproduction in birds
3.1. Involvement of deep brain photoreceptors in seasonal reproduction in birds.
Organisms detect and transduce light signals through a functional unit known as the photoreceptor. In vertebrates, photoreceptors are light-sensing cells (usually neurons) that contain a photopigment (comprising an opsin protein and a vitamin A derivative, retinal), which is capable of phototransduction.15) Classic photoreceptors are the cone and rod cells within the eyes (retina), which are responsible for image formation.16) In addition, there are non-image-forming photoreceptors found in the eyes, which are involved in light entrainment of circadian rhythms.17) In marked contrast to mammals, in which the eyes are the only photoreceptive structure, nonmammalian vertebrates have extraocular photoreceptors. Apart from the eyes, the pineal gland is the best-known photoreceptive organ; the photopigment pinopsin has been isolated from chicken pineal gland.18) It was observed that the photoperiodic response is not disrupted by the removal of the eyes and pineal gland in quail,19) suggesting an extraretinal, extrapineal pathway in mediating photoperiodic light responses. In a number of studies investigating the light input pathway in birds, the involvement of deep brain photoreceptors was suggested. These are photosensitive cells located deep within the brain and are capable of perceiving light penetrating through the scalp. Benoit first demonstrated that blind ducks, capable of photo-induced gonadal development, were incapable of providing photoperiodic responses when black caps were placed on their heads.20) Moreover, injection of India ink under the scalp of blinded, pinealectomized sparrows blocked the photoperiodic response.21) Finally, local illumination experiments highlighted the critical importance of deep brain photoreception. The implantation of radium luminous-painted beads into the septal region of the telencephalon or mediobasal hypothalamus (MBH) led to testicular growth in quail under SD conditions, indicating two candidate sites where avian deep brain photoreceptors might exist.22) Several photopigments identified in a variety of brain regions have been proposed as candidates for deep brain photoreception, including the photopigments rhodopsin,23,24) melanopsin,25–27) and vertebrate ancient (VA) opsin.28,29) Subsequent identification of OPN5 (also known as Opsin 5 and neuropsin) in quail30) added another strong candidate for the putative deep brain photopigment-mediating seasonal responses. OPN5 is localized to the cerebrospinal fluid (CSF)-contacting neurons in the paraventricular organ (PVO) within the MBH.30,31) Notably, lesions around PVO prevent photoperiodic responses in quail,32) and CSF-contacting neurons are morphologically similar to photoreceptor cells in the developing retina,33) implying a potential role for OPN5 in photoperiodism. Functional analysis demonstrated that OPN5 is a short-wavelength-sensitive (SWS) photopigment,30) and this is consistent with an observation that short-wavelength light, ranging from blue light to UV-B,34) is sufficient to induce gonadal growth. Despite the argument of ineffective penetration of short-wavelength light into the brain, it is confirmed that short-wavelength light can penetrate the hypothalamus of quail.35) These findings suggest that OPN5-positive, CSF-contacting neurons of PVO are the long-sought deep brain photoreceptors that regulate seasonal reproduction in birds. It is noteworthy that Foster et al.34) reported an action spectrum for photoperiodic responses and suggested the involvement of photoreceptors that have a high sensitivity around 480 nm. In this context, it is also possible that the presence of other photopigments, such as melanopsin and/or VA opsin, accounts for the reported sensitivity at 480 nm. Owing to the fact that sunlight comprises a wide range of wavelengths, birds might utilize multiple photopigments and wavelengths to measure the photoperiod.
3.2. Birds have multiple circadian pacemakers.
Light information perceived by photoreceptors has to be interpreted or measured, and this process, as described previously, involves the endogenous circadian clock. The existence of a circadian clock has been demonstrated virtually in all tissues and cell types. These cell-autonomous peripheral oscillators are synchronized by the so-called master circadian pacemaker, which is thought to be the site responsible for photoperiodic time measurement. In mammals, the master pacemaker is localized to the suprachiasmatic nucleus (SCN) of the hypothalamus.36) However, unlike mammals, birds have multiple circadian pacemakers in the pineal organ, eyes, and hypothalamic SCN, but the relative importance of these three pacemakers varies among avian species.37–41) Intriguingly, the photoperiodic response of birds is not affected by the removal of either the eyes20) or pineal organ19) or the presence of lesions around the SCN.42) Therefore, it is still unclear which tissue clocks are responsible for measuring the photoperiodic response. Alternatively, several studies have implicated the MBH in photoperiodic time measurement.22,32,43) Moreover, OPN5-positive neurons send projections to the external layer of the median eminence within the MBH,30) suggesting that light information received by deep brain photoreceptors might be interpreted or measured in this region.
3.3. Local activation of thyroid hormone (TH) in the MBH is critical for the photoperiodic response.
A key to understanding the mechanism of photoperiodism in birds came with the discovery of local regulation of TH metabolism in the MBH of quail. In quail, the MBH is considered to be crucial for seasonal reproduction. This is primarily based on the observations that the MBH lesions disrupt seasonal LH secretion32,44) and that LD activates neurons in the MBH, as evidenced by the induction of the neuronal activation marker c-Fos.45) As described above, light pulses delivered during the photoinducible phase stimulate testicular development, indicating that key light-regulated events may be taking place within the MBH during this period. By analyzing differentially expressed genes in the MBH during the photoinducible phase, the type 2 deiodinase (DIO2) gene was found to be induced by light stimulation.46) DIO2 encodes a TH-activating enzyme that converts the precursor thyroxine (T4) to bioactive triiodothyronine (T3). The expression of DIO2 is upregulated under LD and suppressed under SD conditions in the ependymal cells (ECs, also known as tanycytes) that line the ventrolateral walls of the third ventricle within the MBH.46) This suggests that a local increase in the T3 level may contribute to the LD response. Later studies further revealed that DIO3, a TH-inactivating enzyme that converts T4 and T3 into inactive reverse T3 (rT3) and 3,3′-diiodothyronine (T2), respectively, is coexpressed with DIO2 in ECs. Conversely, the expression of DIO3 is upregulated under SD and downregulated under LD conditions.46,47) These findings implicate a role of reciprocal switching of DIO2 and DIO3, which, in turn, fine-tunes the local TH concentration within the MBH to regulate seasonal responses. Consistent with this hypothesis is evidence showing that the concentration of T3 within the MBH is approximately ten-fold higher under LD than under SD conditions, whereas plasma concentrations are similar during both photoperiods.46) The functional significance of this locally enhanced TH activity was demonstrated by intracerebroventricular (ICV) infusion of T3 and a DIO2 inhibitor; T3 administration under SD conditions induced testicular growth in a dose-dependent manner, whereas the administration of a DIO2 inhibitor under LD conditions attenuated testicular growth.46) These studies identifying DIO2 as a key regulator are critical because later studies demonstrated that a similar mechanism also underlies the seasonal response in mammals and fish (see Sections 4.2 and 5.1).
The connection between the photoinduction of T3 in the MBH and activation of the HPG axis is hinted at by its potential target sites, such as the location of its cognate receptors. TH receptors (THRα, THRβ, and RXRα) are expressed in the median eminence46) at the base of the hypothalamus, functioning as a gateway for the release of hypothalamic hormones into the portal capillary that leads to the anterior pituitary. This suggests that T3 from the MBH is involved in the regulation of GnRH release. It is thought that direct contact of the nerve terminals of GnRH neurons to the pericapillary space (i.e., the basal lamina) is required for secretion of the hypothalamic neurohormone from the hypothalamus into the portal capillary.48) Ultrastructural analysis of the quail median eminence revealed that many GnRH nerve terminals are covered by the end-feet of glial processes and do not come into contact with the basal lamina under SD conditions. In contrast, under LD conditions, many GnRH nerve terminals are in close proximity to the basal lamina.49) Particularly, local T3 administration to the MBH under SD conditions mimics these LD-induced morphological changes as well as testicular growth,49,50) indicating that the MBH T3 regulates or modulates seasonal GnRH release by altering neuroglial plasticity in the median eminence.
3.4. Thyrotropin in the pars tuberalis (PT) is a springtime hormone.
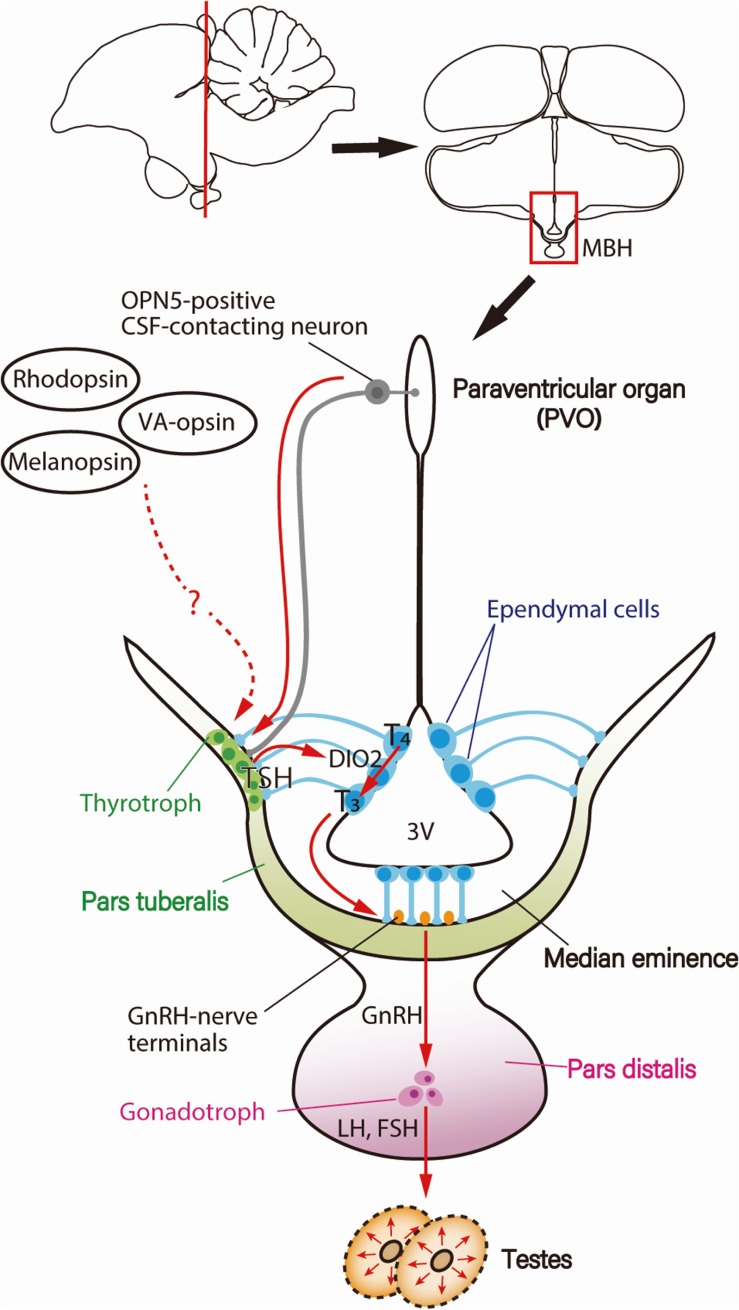
An increase in the plasma LH level, which reflects the activation of the HPG axis, is observed before the end of the first LD when quails are transferred from SD to LD.51,52) This photoperiodic response is the core feature of the so-called “first-day release model” and has been used to unravel the regulatory mechanisms of photoperiodism. Consistent with our working model, the aforementioned reciprocal switching of DIO2 and DIO3 precedes the photoperiodic induction of LH release from the anterior pituitary by a couple of hours.47) The next emerging question was “What is the link between the light stimulus and the T3-mediated cascade in the MBH?” In other words, it is unclear whether there are events occurring earlier to regulate the switching of DIO2/DIO3 and subsequent photoperiodic signaling. A genome-wide gene expression analysis conducted with Japanese quail during the transition from SD to LD conditions revealed a critical role of thyroid-stimulating hormone (TSH, thyrotropin). The TSHB gene, which encodes the TSH β-subunit, was found to be induced 4 h prior to DIO2/DIO3 switching (i.e., 14 h after dawn) in PT of the pituitary gland.53) PT comprises thin layers of cells and is present at the interface between the median eminence and PD of the pituitary gland. Although TSHB is not expressed in ECs where DIO2/DIO3 switching occurs, the fact that TSH is a hormone indicates that it acts on sites remote from PT. Critically, the expression of the TSH receptor (TSHR) and binding of 125I-labeled TSH were observed in ECs.53) Furthermore, ICV TSH administration stimulated DIO2 expression and suppressed DIO3 expression in ECs even under SD conditions, whereas passive immunization against TSH mitigated LD induction of DIO2 expression,53) highlighting the significant role of TSH as a springtime hormone. Notably, the magnitude of testicular growth induced by ICV TSH infusion was nearly identical to that observed in LD-exposed birds, suggesting that LD-induced PT-TSH is a master regulator of seasonal reproduction in birds. Because OPN5-positive neurons project to the external layer of the median eminence adjacent to PT,30) it appears that PT is an important hub for photoperiodic regulation (Fig. 3).
Figure 3.
(Color online) Photoperiodic signal transduction cascade for seasonal reproduction in birds. Light information received by deep brain photoreceptors, such as OPN5-positive cerebrospinal fluid (CSF)-contacting neurons, is transmitted to the pars tuberalis (PT) of the pituitary gland, inducing thyroid-stimulating hormone (TSH). PT-derived TSH then acts on the ependymal cells to induce a thyroid hormone-activating enzyme, type 2 deiodinase (DIO2), which converts the thyroid hormone precursor T4 into the active form T3. T3 regulates morphological changes in GnRH nerve terminals and glial processes, thereby facilitating GnRH secretion, resulting in the secretion of gonadotropin and gonadal development. Figure reproduced from Nakane and Yoshimura (2014)106) with permission under the terms of the Creative Commons Attribution License (CC BY) © 2014 Nakane and Yoshimura. Abbreviations: 3V, third ventricle; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; MBH, mediobasal hypothalamus.
4. Mechanism of seasonal reproduction in mammals
4.1. Melatonin encodes day-length information.
In mammals, removal of the eyes completely abolishes the photoperiodic response,54,55) clearly demonstrating that the eyes are the only photoreceptive structure/organ to perceive light for seasonal responses. However, the identity of the photoreceptors that mediate this response remains unknown. Seasonal changes in day length are detected by the eyes, which send projections to the master circadian pacemaker in the SCN, where the light information is decoded.55,56) The SCN readout of the photoperiodic time is then transmitted to the pineal gland via a multisynaptic pathway, resulting in different secretion profiles of melatonin,55,56) thus converting the photic signal into a rhythmic endocrine message. During periods of darkness (e.g., night), melatonin is synthesized and secreted, whereas exposure to light immediately suppresses the synthesis of melatonin. The resulting rhythmic secretion of melatonin, which closely reflects the duration of the night, provides the biochemical signal for photoperiodic information in mammals. The SCN–pineal melatonin pathway appears to be crucial for mammalian seasonal reproduction because animals that have SCN lesions or are pinealectomized are incapable of generating photoperiodic responses.55,56) However, the underlying mechanism of how the SCN decodes the photoperiodic information is still unclear.
4.2. Involvement of TH in seasonal reproduction in mammals.
It has been known for decades that TH can affect reproduction in mammals and in birds. Thyroidectomy blocks the normal seasonal response,57–60) whereas thyroxine replacement restores it.61,62) After the discovery of photoperiodic DIO2/DIO3 switching in birds, photoperiodic regulation of DIO2 and/or DIO3 within the MBH was reported in a number of mammalian species, such as hamsters63,64) and SD breeding sheep65) and goats.66) In addition, seasonal changes in the morphology of GnRH neurons and glial cells have also been observed.67) Thus, the T3-mediated neuroglial interaction likely regulates or modulates seasonal changes in GnRH secretion in both birds and mammals.
4.3. PT-TSH mediates melatonin signaling for seasonal reproduction in mammals.
As mentioned above, melatonin, which encodes day-length information, and the local switching of DIO2/DIO3 both play crucial roles in the photoperiodic regulation of reproduction in mammals. Following secretion by the pineal gland, melatonin is immediately released and travels to target tissues via the bloodstream, where it exerts its action through the G-protein-coupled melatonin receptors. Two types of melatonin receptors, called MT1 and MT2, have been identified in mammals.68,69) However, the absence of melatonin receptor expression in ECs70,71) raises the question of how melatonin regulates the seasonal switching of DIO2/DIO3. Evidence indicates that the vital link is PT-derived TSH. First, the MT1 receptor is highly expressed in the thyrotrophs of PT,72) and the binding of melatonin is observed in PT in a wide range of seasonally breeding mammalian species.73) Second, consistent with birds, the expression of PT-TSH in mammals is induced by LD, and TSHR is highly expressed in ECs.65,74) Moreover, local administration of TSH in the MBH upregulates DIO2 expression.65,74,75) Finally, the administration of melatonin has no effect on DIO2/DIO3 expression in TSHR-null mice,74) demonstrating the crucial role of TSH–TSHR signaling in mediating melatonin action on seasonal responses. Notably, some of these discoveries were made with laboratory mice, a well-known, nonseasonal breeding species. Although murine gonads do not exhibit a photoperiodic response and most inbred strains of mice are melatonin deficient,76) melatonin treatment can mimic the photoperiodic response in gene expression in the MBH.74) In addition, in the melatonin-proficient CBA mouse, TSHB, DIO2, and DIO3 are clearly regulated photoperiodically.74) These results, along with the availability of genetic modification techniques, suggest that mice are a valuable model for the study of the molecular mechanisms of photoperiodism at the hypothalamic–pituitary level. Taken together, the studies on mammals suggest that the photoperiodic signaling pathway, which is formed by TSH, DIO2/DIO3 switching, and the resultant local activation of T3 in the MBH, appears to be conserved between birds and mammals, despite the differences in their light input mechanisms. In addition, although remarkable strides have been made, an interesting question remains regarding the discrepancy in T3 action between LD and SD breeders. As the LD-induced, PT-TSH-mediated mechanism has also been reported in SD breeders, such as sheep,65) the mechanism by which the resultant local T3 activation shows opposite effects in LD and SD breeders (i.e., stimulates the HPG axis in LD breeders and suppresses the HPG axis in SD breeders) needs to be assessed. Further studies are required to determine the underlying mechanism because they may provide new critical insights into the regulation of seasonal reproduction.
4.4. Tissue-specific glycosylation prevents functional crosstalk between TSHs.
It is well known that TSH is a hormone secreted by PD (PD-TSH) and that it stimulates the thyroid gland to synthesize and secrete THs, which, in turn, regulate growth and metabolism in the body. However, the aforementioned PT-derived TSH is secreted in response to melatonin signaling and acts on the adjacent ECs to regulate seasonal physiology. Thus, it has been a great mystery how the two TSHs trigger distinct biological processes without interfering with one another. Results of mass spectrometric analysis show that both PT-TSH and PD-TSH have the same protein structure, but each is glycosylated differently.77) PD-TSH has sulfated, biantennary carbohydrate chains, which are easily metabolized. In contrast, PT-TSH has sialylated, multibranched carbohydrate chains, which are easily trapped and inactivated by the immunoglobulin and albumin present in the blood.77) Thus, tissue-specific glycosylation in PT and PD differentiates the two TSHs, preventing functional crosstalk within the body.
4.5. Involvement of kisspeptin and RFamide-related peptides in mammalian seasonal reproduction.
Kisspeptin is encoded by Kiss1, and three RFamide-related peptides (RFRPs) are encoded by the Npvf gene and cleaved from propeptides, which belong to the RFamide superfamily.78,79) These peptides are suggested to be involved in the regulation of the reproductive activity. Kisspeptin induces GnRH release via the G-protein-coupled receptor GPR54, which is expressed in GnRH neurons, resulting in the activation of the HPG axis. In LD-breeding rodents, melatonin treatment (as an SD signal) suppresses the secretion of kisspeptin from the hypothalamic arcuate nucleus,80,81) whereas the administration of kisspeptin restores reproductive function in SD conditions,80,82) suggesting a role of kisspeptin in activating the HPG axis downstream of melatonin signaling. The RFRP-1 and RFRP-3 peptides were originally identified as mammalian orthologs of the gonadotropin inhibitory hormone, which inhibits the HPG axis in birds.79) In mammals, the effects of RFRPs on seasonal reproduction vary among species. The injection of RFRP-3 suppresses the activity of GnRH neurons in sheep83) but induces secretion of GnRH under the control of melatonin in Syrian hamsters.84) In addition, the effect of RFRP appears to be photoperiod-dependent in Siberian hamsters, in which it inhibits LH release in LD conditions but stimulates LH release in SD conditions.85) There is no consistent evidence suggesting a role for RFRP in the photoperiodic signaling mechanism. Additional work is required to clarify whether these peptides are the direct targets of inhibitory melatonin signaling or they lie downstream of the TSH–TH signaling cascade.
5. Mechanism of seasonal reproduction in fish
5.1. Saccus vasculosus (SV) is the seasonal sensor in Salmonidae.
Fish show robust seasonal changes in physiology and behavior, akin to terrestrial species;86) for example, Japanese medaka fish (Oryzias latipes) are seasonal breeders that undergo gonadal development in response to LDs. In contrast, Salmonidae fish are SD seasonal breeders that swim upstream to their home river in autumn for spawning. Based on the observation that removal of the eyes and pineal gland has a limited effect on photoperiodic responses, it has been suggested that deep brain photoreceptors might also exist in fish.86) However, the photoreceptors mediating photoperiodicity have long been unknown in fish. In addition, there is no conclusive evidence that melatonin plays a major physiological role in seasonal reproduction in fish.86) Moreover, fish do not possess an anatomically distinct PT,87) which is a regulatory hub for seasonal reproduction in birds and mammals. Thus, the signal transduction pathway for seasonal reproduction in fish remains unclear.
In a study on the masu salmon (Oncorhynchus masou masou), it was revealed that the key elements involved in vertebrate seasonal reproduction, such as photopigments, TSH, TSHR, and DIO2, may be integrated into a fish-specific organ known as SV.88) The role of SV in fish physiology has long been a mystery since its discovery in 1685.89) SV is located at the floor of the hypothalamus, posterior to the pituitary gland. Anatomical study showed that SV comprises coronet, CSF-contacting, and supporting cells.88) Coronet cells have a crown-like morphology with globule-tipped cilia protruding from the cell bodies. Immunohistochemical analysis revealed that TSH and the photopigments SWS1 (SWS opsin 1) and melanopsin are expressed in the globules of cilia, whereas the DIO2 protein is expressed in the cell bodies,88) implying that SV plays a pivotal role as a photoperiodic sensor in fish. Subsequent work supports this hypothesis; isolated SV shows photoperiodic changes in gene expression, and removal of SV prevents photoperiodically induced gonadal development.88) Notably, not all fish have SV; therefore, how fish that lack an SV, such as medaka and zebrafish, measure and process photoperiodic time information is currently under investigation.
5.2. Seasonal regulation of color perception in medaka.
In addition to gonadal development, other physiological processes related to reproductive success also exhibit seasonal responses; for example, during the breeding season, medaka develop black stripes and spots on their fins, and their orange-red color becomes much more intense.90) This phenomenon, known as nuptial coloration, implies that medaka are attracted to orange-red mates. Recent work using virtual fish generated by 3D computer graphics revealed that color perception in medaka was influenced by the seasons; only breeding (spring/summer) medaka exhibited a preference for virtual fish with nuptial coloration, whereas nonbreeding (winter) medaka showed no such preference.91) Consistent with these findings, the long-wavelength-sensitive (LWS) opsin, which is potentially responsible for the perception of the orange-red color,92) was found to be upregulated in summer medaka.91) Knockout of LWS attenuated the preference for virtual fish with nuptial coloration under summer conditions,91) suggesting that summer-induced LWS opsin is crucial for the emergence of mate preference.
6. Domestication and the photoperiodic signaling pathway
Seasonal reproduction is one of the critical rate-limiting factors in the production of animals. In this context, the photoperiodic signaling pathway represents a potential target that can facilitate human-driven domestication. An obvious example of this is laboratory mice, of which most inbred strains lack the enzyme activity for melatonin biosynthesis.76,93,94) Evidence of selective sweeps has also been found at the TSHR locus in all domestic chickens,95) implicating a role of TSHR as a domestication locus in this species. Therefore, genes involved in the photoperiodic signaling pathway emerge as useful selection targets for the domestication of wild animals and improvement of livestock production in agriculture.
7. Conclusions and perspectives
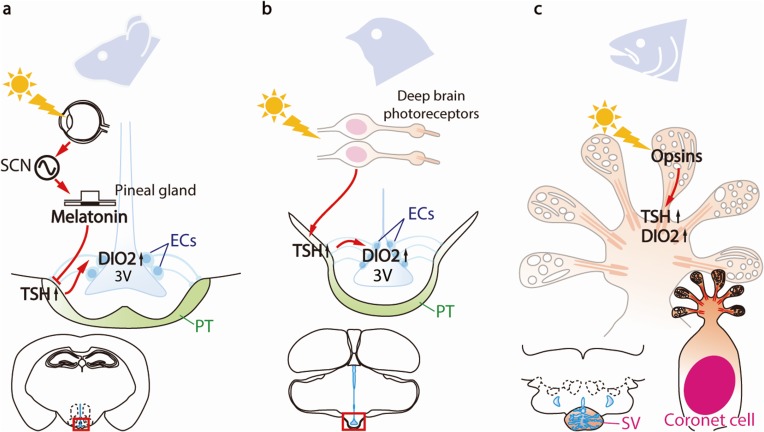
The mechanisms of vertebrate seasonal reproduction have long been a mystery; however, recent comparative studies have uncovered signal transduction pathways that regulate seasonal reproduction across various vertebrate species. The results of these studies have revealed the universality and diversity of these photoperiodic mechanisms. The molecules (TSH, DIO2, and THs) involved are conserved, but the tissues/organs responsible for these seasonal effects appear to be different (Fig. 4).
Figure 4.
(Color online) Universality and diversity in the photoperiodic signal transduction pathways in vertebrates. (a) In mammals, light information is detected via the eyes and transmitted through the suprachiasmatic nucleus (SCN) to the pineal gland. The profile of melatonin secretion from the pineal gland encodes photoperiodic information and regulates the thyroid-stimulating hormone (TSH) in the pars tuberalis (PT) of the pituitary gland. (b) In birds, light information is directly received by deep brain photoreceptors and is then transmitted to PT to trigger TSH secretion. (c) In fish, all of the key elements required for seasonal reproduction (from photoreceptors to neuroendocrine output) are integrated within the coronet cells located in saccus vasculosus (SV). Figure reproduced from Nakane and Yoshimura (2014)106) with permission under the terms of the Creative Commons Attribution License (CC BY) © 2014 Nakane and Yoshimura. Abbreviations: 3V, third ventricle; DIO2, type 2 deiodinase; ECs, ependymal cells.
Although humans are not considered seasonal, changes in various physiological processes and susceptibility to external agents of disease have been observed in different seasons. The incidence of multiple types of diseases, such as infectious diseases, heart diseases, cerebrovascular diseases, and lung cancer, has been suggested to be seasonal.96) Furthermore, the seasons also affect our mood and behavior and are strongly associated with the occurrence of depression, mania, and suicide.97–99) Seasonal affective disorder (SAD) is a syndrome characterized by recurrent depression that begins and ends at approximately the same time every year,100) usually appearing from late fall to winter and disappearing during spring and summer. The susceptibility of humans to SAD or other seasonally related syndromes continues to be a much discussed, but poorly understood, topic. Understanding the biological process of seasonality in animals may help us better understand seasonality in humans.
An emerging theme in chronobiology is the development of compounds that interfere with the circadian clock. Several small molecules that act directly on clock proteins to lengthen the circadian period have been discovered,101) and extended efforts have been made to develop novel period-shortening molecules by conducting structure–activity relationship analyses.102) Furthermore, via a drug-repurposing approach, several circadian clock modulators have been identified from existing drugs.103) Clock-modulating compounds will provide useful tools for understanding how the circadian clock regulates seasonal reproduction, particularly for studies on nonmodel species, in which genetic modification approaches are not yet applicable. Moreover, because disruption of the circadian clock has been reported in patients with SAD, these compounds might help develop better treatments for these patients.
Acknowledgments
This work was supported in part by the JSPS KAKENHI “Grant-in-Aid for Specially Promoted Research” (26000013), “Grant-in-Aid for JSPS Fellows” (17F17107), and the Human Frontier Science Program (RGP0030/2015). WPI-ITbM is supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Abbreviations
- CSF
cerebrospinal fluid
- DIO2
type2 deiodinase
- DIO3
type3 deiodinase
- EC
ependymal cell
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic–pituitary–gonadal
- ICV
intracerebroventricular
- LD
long day
- LH
luteinizing hormone
- LWS
long-wavelength-sensitive
- MBH
mediobasal hypothalamus
- PD
pars distalis
- PT
pars tuberalis
- PVO
paraventricular organ
- RFRPs
RFamide-related peptides
- SAD
seasonal affective disorder
- SCN
suprachiasmatic nucleus
- SD
short day
- SV
saccus vasculosus
- SWS
short-wavelength-sensitive
- TSH
thyroid-stimulating hormone
- TSHR
thyroid-stimulating hormone receptor
- TH
thyroid hormone
- VA
vertebrate ancient
Biographies
Profile
Ying-Jey Guh was born and brought up in Taiwan. He earned his B.S. degree in Animal Science, M.S. degree in Animal Science, and Ph.D. degree in Life Science from the National Chung Hsing University, National Taiwan University, and National Defense Medical Center, respectively, in Taiwan. Thereafter, he began his postdoctoral training at Academia Sinica, Taiwan. He received the JSPS Postdoctoral Fellowship and moved to Japan in 2017, where he was employed in the Nagoya University and National Institute for Basic Biology under the supervision of Prof. Takashi Yoshimura. His research interest is environmental physiology, with primary focus on understanding the modulation of physiological mechanisms in aquatic animals to cope with the changing environment, such as changes between seasons as well as perturbations in ambient salinity, pH, or temperature. He is currently working as a postdoctoral research fellow in the WPI Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University.
Takako Katherine Tamai was born in California in 1960 and graduated from the University of California, Davis, in 1986. She received her Ph.D. degree in Biological Chemistry from the University of Michigan, Ann Arbor, in 1993 and worked as a postdoctoral research fellow in Germany and France. She moved to London in 2001 and worked at University College London as a senior scientist in the laboratory of Prof. David Whitmore, where she investigated circadian rhythms and tissue light sensitivity in zebrafish. She then moved to Japan in 2015 to work with Prof. Takashi Yoshimura at the WPI Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, where she screened for circadian clock modulators and studied seasonal rhythms in the medaka fish.
Takashi Yoshimura was born in Shiga Prefecture in 1970 and graduated from Nagoya University. He received his Ph.D. degree from Nagoya University in 1999 and is currently a Professor in the WPI Institute of Transformative Bio-Molecules (WPI-ITbM) and the Graduate School of Bioagricultural Sciences, Nagoya University. He was appointed as a Visiting Professor in the National Institute for Basic Biology from 2013 to 2019. Work conducted in Yoshimura’s laboratory is focused on understanding the molecular mechanism of seasonal adaptation in vertebrates. The uniqueness of his research lies in the use of various vertebrate species, such as the Japanese quail, chicken, hamster, mouse, salmon, and medaka, together with an interdisciplinary approach. He currently serves as the Vice President of the Japanese Society for Chronobiology and is a fellow of the Royal Society of Biology.
References
- 1).Garner W.W., Allard H.A. (1920) Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J. Agric. Res. 18, 553–606. [Google Scholar]
- 2).Marcovitch S. (1923) Plant lice and light exposure. Science 58, 537–538. [DOI] [PubMed] [Google Scholar]
- 3).Rowan W. (1925) Relation of light to bird migration and developmental changes. Nature 115, 494. [Google Scholar]
- 4).Dawson A., King V.M., Bentley G.E., Ball G.F. (2001) Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365–380. [DOI] [PubMed] [Google Scholar]
- 5).Nicholls T.J., Goldsmith A.R., Dawson A. (1988) Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 68, 133–176. [DOI] [PubMed] [Google Scholar]
- 6).Watanabe M., Yasuo S., Watanabe T., Yamamura T., Nakao N., Ebihara S., et al. (2004) Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: Possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology 145, 1546–1549. [DOI] [PubMed] [Google Scholar]
- 7).Elliott J.A. (1976) Circadian rhythms and photoperiodic time measurement in mammals. Fed. Proc. 35, 2339–2346. [PubMed] [Google Scholar]
- 8).Goldman B.D. (2001) Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms 16, 283–301. [DOI] [PubMed] [Google Scholar]
- 9).Follett B.K., Maung S.L. (1978) Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural daylengths. J. Endocrinol. 78, 267–280. [DOI] [PubMed] [Google Scholar]
- 10).Nelson, R. (2010) Photoperiodism in vertebrates. In Photoperiodism: The Biological Calendar (eds. Nelson, R.J., Denlinger, D.L. and Somers, D.E.). Oxford University Press, Oxford, pp. 365–370. [Google Scholar]
- 11).Follett B.K., Sharp P.J. (1969) Circadian rhythmicity in photoperiodically induced gonadotrophin release and gonadal growth in the quail. Nature 223, 968–971. [DOI] [PubMed] [Google Scholar]
- 12).Pittendrigh C.S., Minis D.H. (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am. Nat. 98, 261–294. [Google Scholar]
- 13).Pittendrigh C.S. (1972) Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc. Natl. Acad. Sci. U.S.A. 69, 2734–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Bünning E. (1936) Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber. Dtsch. Bot. Ges. 54, 590–607. [Google Scholar]
- 15).Wald G. (1968) Molecular basis of visual excitation. Science 162, 230–239. [DOI] [PubMed] [Google Scholar]
- 16).Dowling, J.E. (1987) The Retina: An Approachable Part of the Brain. Harvard University Press, Cambridge, Massachusetts. [Google Scholar]
- 17).Berson D.M., Dunn F.A., Takao M. (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- 18).Okano T., Yoshizawa T., Fukada Y. (1994) Pinopsin is a chicken pineal photoreceptive molecule. Nature 372, 94–97. [DOI] [PubMed] [Google Scholar]
- 19).Siopes T.D., Wilson W.O. (1974) Extraocular modification of photoreception in intact and pinealectomized coturnix. Poult. Sci. 53, 2035–2041. [DOI] [PubMed] [Google Scholar]
- 20).Benoit J. (1935) Le role des yeux dans l’action stimulante de la lumiere sure le developpement testiulaire chez le canard. C. R. Soc. Biol. 118, 669–671. [Google Scholar]
- 21).Menaker M., Roberts R., Elliott J., Underwood H. (1970) Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. U.S.A. 67, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Homma, K., Ohta, M. and Sakakibara, Y. (1979) Photoinducible phase of the Japanese quail detected by direct stimulation of the brain. In Biological Rhythms and Their Central Mechanism (eds. Suda, M., Hayaishi, O. and Nakagawa, H.). Elsevier, Amsterdam, pp. 85–94. [Google Scholar]
- 23).Silver R., Witkovsky P., Horvath P., Alones V., Barnstable C.J., Lehman M.N. (1988) Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 253, 189–198. [DOI] [PubMed] [Google Scholar]
- 24).Wada Y., Okano T., Adachi A., Ebihara S., Fukada Y. (1998) Identification of rhodopsin in the pigeon deep brain. FEBS Lett. 424, 53–56. [DOI] [PubMed] [Google Scholar]
- 25).Bailey M.J., Cassone V.M. (2005) Melanopsin expression in the chick retina and pineal gland. Brain Res. Mol. Brain Res. 134, 345–348. [DOI] [PubMed] [Google Scholar]
- 26).Chaurasia S.S., Rollag M.D., Jiang G., Hayes W.P., Haque R., Natesan A., et al. (2005) Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): Differential regulation of expression in pineal and retinal cell types. J. Neurochem. 92, 158–170. [DOI] [PubMed] [Google Scholar]
- 27).Kang S.W., Leclerc B., Kosonsiriluk S., Mauro L.J., Iwasawa A., El Halawani M.E. (2010) Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience 170, 200–213. [DOI] [PubMed] [Google Scholar]
- 28).Davies W.I., Turton M., Peirson S.N., Follett B.K., Halford S., Garcia-Fernandez J.M., et al. (2012) Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol. Lett. 8, 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Halford S., Pires S.S., Turton M., Zheng L., Gonzalez-Menendez I., Davies W.L., et al. (2009) VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 19, 1396–1402. [DOI] [PubMed] [Google Scholar]
- 30).Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., et al. (2010) A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. U.S.A. 107, 15264–15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Yamashita T., Ohuchi H., Tomonari S., Ikeda K., Sakai K., Shichida Y. (2010) Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. U.S.A. 107, 22084–22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Sharp P.J., Follett B.K. (1969) The effect of hypothalamic lesions on gonadotrophin release in Japanese quail (Coturnix coturnix japonica). Neuroendocrinology 5, 205–218. [DOI] [PubMed] [Google Scholar]
- 33).Vigh B., Vigh-Teichmann I. (1998) Actual problems of the cerebrospinal fluid-contacting neurons. Microsc. Res. Tech. 41, 57–83. [DOI] [PubMed] [Google Scholar]
- 34).Foster R.G., Follett B.K., Lythgoe J.N. (1985) Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature 313, 50–52. [DOI] [PubMed] [Google Scholar]
- 35).Foster R.G., Follett B.K. (1985) The involvement of a rhodopsin-like photopigment in the photoperiodic response of the Japanese quail. J. Comp. Physiol. A 157, 519–528. [Google Scholar]
- 36).Herzog E.D., Tosini G. (2001) The mammalian circadian clock shop. Semin. Cell Dev. Biol. 12, 295–303. [DOI] [PubMed] [Google Scholar]
- 37).Ebihara S., Kawamura H. (1981) The role of the pineal organ and the suprachiasmatic nucleus in the control of circadian locomotor rhythms in the Java sparrow, Padda oryzivora. J. Comp. Physiol. 141, 207–214. [Google Scholar]
- 38).Menaker M. (1968) Extraretinal light perception in the sparrow. I. Entrainment of the biological clock. Proc. Natl. Acad. Sci. U.S.A. 59, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Steele C.T., Zivkovic B.D., Siopes T., Underwood H. (2003) Ocular clocks are tightly coupled and act as pacemakers in the circadian system of Japanese quail. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R208–R218. [DOI] [PubMed] [Google Scholar]
- 40).Takahashi J.S., Menaker M. (1982) Role of the suprachiasmatic nuclei in the circadian system of the house sparrow, Passer domesticus. J. Neurosci. 2, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Yoshimura T., Yasuo S., Suzuki Y., Makino E., Yokota Y., Ebihara S. (2001) Identification of the suprachiasmatic nucleus in birds. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1185–R1189. [DOI] [PubMed] [Google Scholar]
- 42).Davies D.T., Follett B.K. (1975) The neuroendocrine control of gonadotrophin release in the Japanese quail. II. The role of the anterior hypothalamus. Proc. R. Soc. Lond. B Biol. Sci. 191, 303–315. [DOI] [PubMed] [Google Scholar]
- 43).Yasuo S., Watanabe M., Okabayashi N., Ebihara S., Yoshimura T. (2003) Circadian clock genes and photoperiodism: Comprehensive analysis of clock gene expression in the mediobasal hypothalamus, the suprachiasmatic nucleus, and the pineal gland of Japanese quail under various light schedules. Endocrinology 144, 3742–3748. [DOI] [PubMed] [Google Scholar]
- 44).Juss T.S., Meddle S.L., Servant R.S., King V.M. (1993) Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnix japonica). Proc. Biol. Sci. 254, 21–28. [DOI] [PubMed] [Google Scholar]
- 45).Meddle S.L., Follett B.K. (1997) Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J. Neurosci. 17, 8909–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Yoshimura T., Yasuo S., Watanabe M., Iigo M., Yamamura T., Hirunagi K., et al. (2003) Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426, 178–181. [DOI] [PubMed] [Google Scholar]
- 47).Yasuo S., Watanabe M., Nakao N., Takagi T., Follett B.K., Ebihara S., et al. (2005) The reciprocal switching of two thyroid hormone-activating and -inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology 146, 2551–2554. [DOI] [PubMed] [Google Scholar]
- 48).Prevot V., Croix D., Bouret S., Dutoit S., Tramu G., Stefano G.B., et al. (1999) Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: Implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience 94, 809–819. [DOI] [PubMed] [Google Scholar]
- 49).Yamamura T., Hirunagi K., Ebihara S., Yoshimura T. (2004) Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 145, 4264–4267. [DOI] [PubMed] [Google Scholar]
- 50).Yamamura T., Yasuo S., Hirunagi K., Ebihara S., Yoshimura T. (2006) T3 implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res. 324, 175–179. [DOI] [PubMed] [Google Scholar]
- 51).Follett, B.K., King, V.M. and Meddle, S.L. (1998) Rhythms and photoperiodism in birds. In Biological Rhythms Photoperiodism in Plants (eds. Lumsden, P.J. and Miller, A.J.). Biostatistics Scientific, Oxford, pp. 231–242. [Google Scholar]
- 52).Nicholls T.J., Follett B.K., Robinson J.E. (1983) A photoperiodic response in gonadectomized Japanese quail exposed to a single long day. J. Endocrinol. 97, 121–126. [DOI] [PubMed] [Google Scholar]
- 53).Nakao N., Ono H., Yamamura T., Anraku T., Takagi T., Higashi K., et al. (2008) Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452, 317–322. [DOI] [PubMed] [Google Scholar]
- 54).Legan S.J., Karsch F.J. (1983) Importance of retinal photoreceptors to the photoperiodic control of seasonal breeding in the ewe. Biol. Reprod. 29, 316–325. [DOI] [PubMed] [Google Scholar]
- 55).Reiter R.J. (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1, 109–131. [DOI] [PubMed] [Google Scholar]
- 56).Arendt, J. (1994) Melatonin and the Mammalian Pineal Gland. Chapman & Hall, London. [Google Scholar]
- 57).Dawson A. (1993) Thyroidectomy progressively renders the reproductive system of starlings (Sturnus vulgaris) unresponsive to changes in daylength. J. Endocrinol. 139, 51–55. [DOI] [PubMed] [Google Scholar]
- 58).Dawson A. (1998) Thyroidectomy of house sparrows (Passer domesticus) prevents photo-induced testicular growth but not the increased hypothalamic gonadotrophin-releasing hormone. Gen. Comp. Endocrinol. 110, 196–200. [DOI] [PubMed] [Google Scholar]
- 59).Moenter S.M., Woodfill C.J., Karsch F.J. (1991) Role of the thyroid gland in seasonal reproduction: Thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology 128, 1337–1344. [DOI] [PubMed] [Google Scholar]
- 60).Parkinson T.J., Follett B.K. (1995) Thyroidectomy abolishes seasonal testicular cycles of Soay rams. Proc. Biol. Sci. 259, 1–6. [DOI] [PubMed] [Google Scholar]
- 61).Follett B.K., Nicholls T.J. (1985) Influences of thyroidectomy and thyroxine replacement on photoperiodically controlled reproduction in quail. J. Endocrinol. 107, 211–221. [DOI] [PubMed] [Google Scholar]
- 62).Wilson F.E., Reinert B.D. (2000) Thyroid hormone acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) for vernal and autumnal components of seasonality. J. Neuroendocrinol. 12, 87–95. [DOI] [PubMed] [Google Scholar]
- 63).Revel F.G., Saboureau M., Pevet P., Mikkelsen J.D., Simonneaux V. (2006) Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology 147, 4680–4687. [DOI] [PubMed] [Google Scholar]
- 64).Yasuo S., Yoshimura T., Ebihara S., Korf H.W. (2007) Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology 148, 4385–4392. [DOI] [PubMed] [Google Scholar]
- 65).Hanon E.A., Lincoln G.A., Fustin J.M., Dardente H., Masson-Pevet M., Morgan P.J., et al. (2008) Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 18, 1147–1152. [DOI] [PubMed] [Google Scholar]
- 66).Yasuo S., Nakao N., Ohkura S., Iigo M., Hagiwara S., Goto A., et al. (2006) Long-day suppressed expression of type 2 deiodinase gene in the mediobasal hypothalamus of the Saanen goat, a short-day breeder: Implication for seasonal window of thyroid hormone action on reproductive neuroendocrine axis. Endocrinology 147, 432–440. [DOI] [PubMed] [Google Scholar]
- 67).Jansen H.T., Cutter C., Hardy S., Lehman M.N., Goodman R.L. (2003) Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: Changes in identified GnRH inputs and glial association. Endocrinology 144, 3663–3676. [DOI] [PubMed] [Google Scholar]
- 68).Reppert S.M., Godson C., Mahle C.D., Weaver D.R., Slaugenhaupt S.A., Gusella J.F. (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. U.S.A. 92, 8734–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Reppert S.M., Weaver D.R., Ebisawa T. (1994) Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13, 1177–1185. [DOI] [PubMed] [Google Scholar]
- 70).Schuster C., Gauer F., Guerrero H., Lakhdar-Ghazal N., Pevet P., Masson-Pevet M. (2000) Photic regulation of mt1 melatonin receptors in the Siberian hamster pars tuberalis and suprachiasmatic nuclei: Involvement of the circadian clock and intergeniculate leaflet. J. Neuroendocrinol. 12, 207–216. [DOI] [PubMed] [Google Scholar]
- 71).Song C.K., Bartness T.J. (2001) CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R666–R672. [DOI] [PubMed] [Google Scholar]
- 72).Klosen P., Bienvenu C., Demarteau O., Dardente H., Guerrero H., Pevet P., et al. (2002) The mt1 melatonin receptor and RORβ receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J. Histochem. Cytochem. 50, 1647–1657. [DOI] [PubMed] [Google Scholar]
- 73).Morgan P.J., Barrett P., Howell H.E., Helliwell R. (1994) Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem. Int. 24, 101–146. [DOI] [PubMed] [Google Scholar]
- 74).Ono H., Hoshino Y., Yasuo S., Watanabe M., Nakane Y., Murai A., et al. (2008) Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 18238–18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Klosen P., Sebert M.E., Rasri K., Laran-Chich M.P., Simonneaux V. (2013) TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB J. 27, 2677–2686. [DOI] [PubMed] [Google Scholar]
- 76).Ebihara S., Marks T., Hudson D.J., Menaker M. (1986) Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231, 491–493. [DOI] [PubMed] [Google Scholar]
- 77).Ikegami K., Liao X.H., Hoshino Y., Ono H., Ota W., Ito Y., et al. (2014) Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep. 9, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Tsutsui K., Bentley G.E., Kriegsfeld L.J., Osugi T., Seong J.Y., Vaudry H. (2010) Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: New key neuropeptides controlling reproduction. J. Neuroendocrinol. 22, 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Tsutsui K., Ubuka T., Bentley G.E., Kriegsfeld L.J. (2013) Review: Regulatory mechanisms of gonadotropin-inhibitory hormone (GnIH) synthesis and release in photoperiodic animals. Front. Neurosci. 7, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Ansel L., Bolborea M., Bentsen A.H., Klosen P., Mikkelsen J.D., Simonneaux V. (2010) Differential regulation of kiss1 expression by melatonin and gonadal hormones in male and female Syrian hamsters. J. Biol. Rhythms 25, 81–91. [DOI] [PubMed] [Google Scholar]
- 81).Revel F.G., Saboureau M., Masson-Pevet M., Pevet P., Mikkelsen J.D., Simonneaux V. (2006) Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr. Biol. 16, 1730–1735. [DOI] [PubMed] [Google Scholar]
- 82).Ansel L., Bentsen A.H., Ancel C., Bolborea M., Klosen P., Mikkelsen J.D., et al. (2011) Peripheral kisspeptin reverses short photoperiod-induced gonadal regression in Syrian hamsters by promoting GnRH release. Reproduction 142, 417–425. [DOI] [PubMed] [Google Scholar]
- 83).Clarke I.J., Sari I.P., Qi Y., Smith J.T., Parkington H.C., Ubuka T., et al. (2008) Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 149, 5811–5821. [DOI] [PubMed] [Google Scholar]
- 84).Ancel C., Bentsen A.H., Sebert M.E., Tena-Sempere M., Mikkelsen J.D., Simonneaux V. (2012) Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: The exception proves the rule. Endocrinology 153, 1352–1363. [DOI] [PubMed] [Google Scholar]
- 85).Ubuka T., Inoue K., Fukuda Y., Mizuno T., Ukena K., Kriegsfeld L.J., et al. (2012) Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 153, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Borg, B. (2010) Photoperiodism in fishes. In Photoperiodism: The Biological Calendar (eds. Nelson, R.J., Denlinger, D.L. and Somers, D.E.). Oxford University Press, Oxford, pp. 371–398. [Google Scholar]
- 87).Cyr D.G., Bromage N.R., Duston J., Eales J.G. (1988) Seasonal patterns in serum levels of thyroid hormones and sex steroids in relation to photoperiod-induced changes in spawning time in rainbow trout, Salmo gairdneri. Gen. Comp. Endocrinol. 69, 217–225. [DOI] [PubMed] [Google Scholar]
- 88).Nakane Y., Ikegami K., Iigo M., Ono H., Takeda K., Takahashi D., et al. (2013) The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat. Commun. 4, 2108. [DOI] [PubMed] [Google Scholar]
- 89).Gottsche C.M. (1835) Vergleichende Anatomie des Gehirns der Grätenfische. Arch. Anat. Physiol. Wiss. Med., 433–486. [Google Scholar]
- 90).Niwa H.S. (1965) Effects of castration and administration of methyl testosterone on the nuptial coloration of the Medaka (Oryzias latipes). Embryologia (Nagoya) 9, 289–298. [DOI] [PubMed] [Google Scholar]
- 91).Shimmura T., Nakayama T., Shinomiya A., Fukamachi S., Yasugi M., Watanabe E., et al. (2017) Dynamic plasticity in phototransduction regulates seasonal changes in color perception. Nat. Commun. 8, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Matsumoto Y., Fukamachi S., Mitani H., Kawamura S. (2006) Functional characterization of visual opsin repertoire in Medaka (Oryzias latipes). Gene 371, 268–278. [DOI] [PubMed] [Google Scholar]
- 93).Kasahara T., Abe K., Mekada K., Yoshiki A., Kato T. (2010) Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. U.S.A. 107, 6412–6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Shimomura K., Lowrey P.L., Vitaterna M.H., Buhr E.D., Kumar V., Hanna P., et al. (2010) Genetic suppression of the circadian clock mutation by the melatonin biosynthesis pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 8399–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Rubin C.J., Zody M.C., Eriksson J., Meadows J.R., Sherwood E., Webster M.T., et al. (2010) Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464, 587–591. [DOI] [PubMed] [Google Scholar]
- 96).Stevenson T.J., Visser M.E., Arnold W., Barrett P., Biello S., Dawson A., et al. (2015) Disrupted seasonal biology impacts health, food security and ecosystems. Proc. Biol. Sci. 282, 20151453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Carney P.A., Fitzgerald C.T., Monaghan C.E. (1988) Influence of climate on the prevalence of mania. Br. J. Psychiatry 152, 820–823. [DOI] [PubMed] [Google Scholar]
- 98).Maes M., Meltzer H.Y., Suy E., De Meyer F. (1993) Seasonality in severity of depression: Relationships to suicide and homicide occurrence. Acta Psychiatr. Scand. 88, 156–161. [DOI] [PubMed] [Google Scholar]
- 99).Parker G., Walter S. (1982) Seasonal variation in depressive disorders and suicidal deaths in New South Wales. Br. J. Psychiatry 140, 626–632. [DOI] [PubMed] [Google Scholar]
- 100).Rosenthal N.E., Sack D.A., Gillin J.C., Lewy A.J., Goodwin F.K., Davenport Y., et al. (1984) Seasonal affective disorder: A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 41, 72–80. [DOI] [PubMed] [Google Scholar]
- 101).Hirota T., Lee J.W., St. John P.C., Sawa M., Iwaisako K., Noguchi T., et al. (2012) Identification of small molecule activators of cryptochrome. Science 337, 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Oshima T., Yamanaka I., Kumar A., Yamaguchi J., Nishiwaki-Ohkawa T., Muto K., et al. (2015) C-H activation generates period-shortening molecules that target cryptochrome in the mammalian circadian clock. Angew. Chem. Int. Ed. Engl. 54, 7193–7197. [DOI] [PubMed] [Google Scholar]
- 103).Tamai T.K., Nakane Y., Ota W., Kobayashi A., Ishiguro M., Kadofusa N., et al. (2018) Identification of circadian clock modulators from existing drugs. EMBO Mol. Med. 10, e8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).Shinomiya A., Shimmura T., Nishiwaki-Ohkawa T., Yoshimura T. (2014) Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front. Endocrinol. 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Tamai, T.K. and Yoshimura, T. (2017) Molecular and neuroendocrine mechanisms of avian seasonal reproduction. In Avian Reproduction. Advances in Experimental Medicine and Biology (ed. Sasanami, T.). Springer, Singapore, pp. 125–136. [DOI] [PubMed] [Google Scholar]
- 106).Nakane Y., Yoshimura T. (2014) Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Front. Neurosci. 8, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]