ABSTRACT
Background
Prospective studies linking dietary pattern and cognitive function in the elderly are limited in Asian populations.
Objective
We examined the associations between various healthful dietary patterns and risk of cognitive impairment in Chinese adults.
Methods
We used data from the Singapore Chinese Health Study of 16,948 men and women who were aged 45–74 y at baseline (1993–1998) and reinterviewed at the third follow-up visit (2014–2016), ∼20 y later. Diet quality at baseline was assessed according to the alternate Mediterranean diet (aMED), the Dietary Approaches to Stop Hypertension (DASH) diet, the alternative Healthy Eating Index (AHEI)-2010, overall plant-based diet index (PDI), and healthful plant-based diet index (hPDI). Cognitive function was evaluated using a Singapore-modified Mini-Mental State Examination during the third follow-up visit when subjects were aged 61–96 y. Multivariable logistic regression models were used to compute ORs and 95% CIs associated with the risk of cognitive impairment defined using education-specific cut-offs.
Results
Cognitive impairment was present in 2443 (14.4%) participants. The OR (95% CI) for cognitive impairment comparing the highest with the lowest quartile of diet quality scores was 0.67 (0.59, 0.77) for aMED, 0.71 (0.62, 0.81) for DASH, 0.75 (0.66, 0.85) for AHEI-2010, 0.82 (0.71, 0.94) for PDI, and 0.78 (0.68, 0.90) for hPDI (all P values for trend <0.001). Each SD increment in different diet quality scores was associated with 7–16% lower risk of cognitive impairment.
Conclusions
These results provide evidence that adherence to healthy dietary patterns in midlife is associated with a lower risk of cognitive impairment in late life in Chinese adults.
Keywords: diet pattern, cognition, Chinese, epidemiological study, cohort study, Mediterranean diet, Dietary Approaches to Stop Hypertension diet, Healthy Eating Index, plant-based diet index
Introduction
The disease burden and economic costs of severe dementia and Alzheimer's disease have increased dramatically and become a global public health priority as life expectancy increases in many countries worldwide (1). Cognitive decline heralds the development of conditions such as mild cognitive impairment and incipient dementia, and these conditions in turn carry an increased risk of Alzheimer's disease or other forms of severe dementia (2). Hence, it is critical to identify modifiable risk factors to retard the progression of cognitive decline with aging, and thus prevent or delay the occurrence of cognitive impairment or dementia.
A number of epidemiologic studies have investigated the impact of individual food or nutrient items on cognitive health (3). However, this approach does not account for the synergistic effects of diverse foods and nutrients consumed together. Hence, increasing attention has been directed to overall dietary patterns (4, 5), as they are able to more comprehensively characterize dietary exposures of related foods and nutrients often consumed together, and account for the possible interactions among them. Findings from studies on dietary patterns also allow better translation of dietary recommendations for the preservation of cognitive function (4, 6).
Various studies have shown that adherence to the Mediterranean diet was associated with a lower risk of cognitive impairment and dementia, as summarized in recent reviews and meta-analyses (7, 8). A few prospective studies have examined Dietary Approaches to Stop Hypertension (DASH) and alternative Healthy Eating Index (AHEI) dietary patterns in relation to cognitive health (9–14), although none has evaluated the associations with the overall plant-based diet index (PDI) and healthful plant-based diet index (hPDI). In addition, most of these studies have been conducted in Western populations, and evidence from Asian populations is limited (8). Since there are marked differences in dietary patterns between Western and Asian populations, it is unclear whether these healthy dietary patterns, derived from Western populations, are related to cognitive health in Asians (15). In addition, to the best of our knowledge, no study performed in an Asian population has systematically evaluated various a priori dietary indexes with cognitive health simultaneously.
In this study, we prospectively evaluated the relations of healthful diets, namely, the alternate Mediterranean diet (aMED), DASH diet index, AHEI-2010, PDI, and hPDI scores, with risk of cognitive impairment in the Singapore Chinese Health Study, a population-based cohort of Chinese living in Singapore.
Methods
Study population
The Singapore Chinese Health Study is an ongoing prospective cohort study designed to evaluate genetic, dietary, and environmental determinants of chronic diseases in Chinese adults living in Singapore. A detailed description of the study has been reported previously (16). In brief, 63,257 participants (27,959 men and 35,298 women) aged 45–74 y were enrolled between April 1993 and December 1998. Study participants belonged to the 2 major dialect groups of Chinese in Singapore (Hokkiens or Cantonese), and were permanent residents or citizens who resided in government-built housing estates, where 86% of the Singaporeans resided during the enrollment period. At baseline, face-to-face interviews were conducted to collect demographic information, usual diet, cigarette smoking, alcohol consumption, physical activity, sleep duration, height, weight, and medical history, using a structured questionnaire. After the baseline interview, participants were recontacted for 3 follow-up visits. The present analysis only included participants who completed cognitive testing during the third follow-up visit (2014 to 2016). We sent 1–2 invitation letters to the surviving participants before the home visits; some participants could not be contacted due to reasons such as moving out of the house, and some were unable to participate in the follow-up visit due to serious diseases or severe cognitive impairment. Meanwhile, the third follow-up visits were only conducted until February 2016 due to limited funding. We did not purposely select certain individuals for the third follow-up visit. The study protocol was approved by the Institutional Review Board of the National University of Singapore. All participants provided written informed consent.
Dietary assessment
At baseline, the usual dietary intake over the past year was assessed using a structured semiquantitative FFQ, which incorporated 165 dietary items commonly consumed by the study population in Singapore. For each food item, participants were asked to choose from 8 frequency categories (ranging from “never or hardly ever” to “2 or more times a day”) and 3 defined portion sizes (small, medium, large) with the aid of photographs. The dietary intake of energy or nutrients for each participant was derived from the Singapore Food Composition Database that was developed especially for our cohort. The FFQ was validated using a series of 24-h dietary recalls and selected biomarkers (16–18). The validation study showed that the correlation coefficients for energy and nutrients ranged from 0.24 to 0.79 between the FFQ and the 24-h dietary recalls (16), similar to a previous validation study reporting diverse populations (19).
Dietary patterns
The scores of 5 dietary patterns (aMED, DASH, AHEI-2010, PDI, hPDI) were calculated based on the relative or absolute intake of the various food items, alcohol, and nutrients, as stipulated in an a priori manner for the composition of each pattern. A higher score represents better adherence. The components and scoring criteria of these dietary patterns have been described in detail elsewhere (20, 21). Briefly, the aMED score consisted of 9 components, and each component was scored 0 or 1 with the total score ranging from 0 to 9. Scoring was based on whether a participant's intake was above or below the cohort-specific median levels of the food items/groups, except for the intake of alcohol, which had 1 point for an intake of 10–25 g/d for men and 5–15 g/d for women and 0 otherwise. The DASH score consisted of 8 components, and each component scored from 1 to 5 (participant's quintiles of intake) with the total score ranging from 8 to 40. For the DASH score, the intake of low-fat dairy was replaced by total dairy because low-fat dairy products were rarely available on the market at the time of recruitment and thus no information relevant to this was collected in the FFQ. The AHEI-2010 generally included 11 food and nutrient components, including alcohol, and each component score ranged from 0 to 10. Due to the very low consumption level and lack of information on trans fat intake in our population, we did not include trans fat in the score and thus the total score included 10 components and ranged from 0 to 100. The PDI and hPDI were developed by Satija et al. (22) based on less healthy and healthy plant foods and animal foods. The PDI assigned positive scores for all plant foods (1 to 5) and inverse scores (5 to 1) for animal foods with increasing consumption levels, but the hPDI assigned positive scores for healthy plant foods (1 to 5) and inverse scores (5 to 1) for less healthy plant foods and animal foods with increasing consumption levels. We included 15 components with sufficiently high and thus practically meaningful intake levels in our analyses. Each food component was scored from 1 to 5 (participant's quintiles of intake) and the total score ranged from 15 to 75.
Assessment of cognitive function
A Singapore-modified version of the Mini-Mental State Examination (SM-MMSE), which has been validated in the Singapore population, was used for the assessment of cognitive function only at the third follow-up visit (23). The MMSE was originally developed by Folstein et al. in 1975 and has been translated into different languages and used in clinical practice and research among diverse populations (24, 25). The MMSE questionnaire is the most widely used cognitive screening tool worldwide and is composed of 30 items assessing orientation (10 points), attention (5 points), language (8 points), immediate recall (3 points), delayed recall (3 points), and construction (1 point) (24). The score ranged from 0 to 30, with higher scores indicating better cognitive function. A cut-off point of 23/24 was typically used to define cognitive impairment in Western countries (25). However, previous studies have shown that the MMSE score is significantly affected by education level (26, 27). In our population, the education level was generally low and thus we used education-specific cut-off points that originated from the Shanghai Dementia Survey, in which the participants had a comparable education level with our study population (26). The cut-off points were 17/18, 20/21, and 24/25 for those with no formal education, primary school education, and secondary school or higher education, respectively.
The SM-MMSE testing was performed via in-person interviews in a quiet environment. An experienced geriatric epidemiologist in our team (LF) systematically trained all the interviewers. We recorded all interviews and randomly selected 20% of the recordings to verify the quality of the interviews during the third follow-up visit. A total of 17,107 participants took part in SM-MMSE testing during the third follow-up visit. After excluding participants with a missing value on the SM-MMSE (n = 55), and participants who were mute (n = 1), blind (n = 55), or deaf (n = 48), we included 16,948 participants in the final analysis and the participant flowchart is shown in Supplementary Figure 1.
Statistical analysis
We compared the characteristics of participants in the lowest and highest quartiles of the 5 dietary scores using descriptive statistics. We also computed Spearman correlation coefficients for various dietary pattern scores. Multivariable logistic regression models were used to estimate ORs and 95% CIs for the associations between dietary patterns and cognitive impairment by using the lowest quartile as the reference group. Model 1 adjusted for age at cognitive status measurement (year). Model 2 further adjusted for other sociodemographic variables including year of recruitment (1993–1995, 1996–1998), sex, dialect group (Hokkiens, Cantonese), marital status (married, separated/divorced, widowed, never married), education level (no formal education, primary school, secondary school or above), and lifestyle factors including cigarette smoking (never, former, and current smokers), physical activity (hours per week spent on moderate activities, strenuous sports, and vigorous work: none, 0.5–3.9, and ≥4.0 h/wk), sleep duration (<6, 6–8, and >8 h/), BMI (<18.5, 18.5–22.9, 23–27.4, and ≥27.5 kg/m2), and total energy intake (kcal/d); Model 2 additionally included alcohol consumption (never, monthly, weekly/daily), tea intake (none, monthly, weekly, daily), and coffee intake (none/less than daily, 1 cup/d, ≥2 cups/d) for the dietary patterns not including these items. Model 3 further adjusted for baseline comorbidities including hypertension, diabetes, cardiovascular disease (CVD; coronary artery disease, stroke), and cancer. Tests for linear trend were performed by treating the median value of dietary quality scores in quartiles as a continuous variable in the final model.
We also used generalized linear models to compare mean SM-MMSE scores by quartile of each dietary quality score with adjustment for the above-mentioned covariates. In this analysis, we also compared the effect size for the associations related to dietary pattern and age (y) to indirectly evaluate the impact of dietary pattern on cognitive status.
We conducted stratified analyses according to age at baseline (<55 and ≥55 y), sex, education level, BMI (<23 and ≥23), and baseline history of hypertension and diabetes in the logistic regression models and generalized linear models. Tests for interaction were performed by adding interaction terms in Model 3.
To test the robustness of our findings, we conducted a series of sensitivity analyses. 1) We excluded those with cancer or CVD at baseline (n = 735) because these 2 types of chronic diseases could substantially change people's diet and lifestyles. 2) We further adjusted for incident hypertension, diabetes, CVD, and cancer events during the follow-up period to examine whether the associations could be influenced or mediated by incident chronic diseases. 3) We adjusted for age at baseline instead of age at SM-MMSE measurement because people could have different follow-up years. 4) We further adjusted for housing type at the third follow-up visit (1–2 rooms, 3 rooms, 4 rooms, 5 rooms, private housing, other housing) as a covariate in the multivariable models to examine whether the associations could be influenced by socioeconomic status. 5) We excluded those with unrealistic daily energy intake (<700 or >3700 kcal for men and <600 or >3000 kcal for women, n = 212) to test whether the results could be influenced by extreme measurement errors in the exposure. 6) We used the SM-MMSE cut-off point of 23/24 to examine whether the results could be influenced by the definition of the outcome. We also compared the baseline characteristics of participants in the baseline survey, follow-up visits 1, 2, and 3, as well as those who did not attend the third follow-up visit to test the influence of selection bias on the results.
All analyses were performed using SAS version 9.4 (SAS Institute). Statistical significance was defined as 2-sided P values <0.05.
Results
Study population characteristics
The characteristics of study participants in the lowest or highest quartile of the 5 dietary scores are shown in Table 1. Of the 16,948 participants, 59.2% were women, and the mean age at baseline was 53.5 y (SD: 6.2) and mean age at SM-MMSE measurement was 73.2 y (SD: 6.4). For all 5 dietary patterns, participants with higher scores were more likely to be women (except for PDI), more educated, never smokers, and physically active. The dietary patterns had a moderate to high correlation with each other (Spearman correlation coefficients ranged from 0.37 to 0.72) (Table 2).
TABLE 1.
Characteristics of study participants according to quartiles of the dietary quality scores1
| aMED | DASH | AHEI-2010 | PDI | hPDI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 |
| n | 5734 | 4076 | 4239 | 4207 | 4237 | 4237 | 4406 | 3950 | 3978 | 4075 |
| Age at interview, mean ± SD, y | 53.4 ± 6.4 | 52.6 ± 6.0 | 52.5 ± 6.1 | 53.5 ± 6.3 | 52.8 ± 6.3 | 53.0 ± 6.1 | 53.3 ± 6.4 | 52.7 ± 6.1 | 52.3 ± 6.1 | 53.6 ± 6.3 |
| Age at follow-up, mean ± SD, y | 73.5 ± 6.5 | 72.8 ± 6.2 | 72.6 ± 6.3 | 73.6 ± 6.4 | 72.8 ± 6.4 | 73.3 ± 6.3 | 73.4 ± 6.6 | 73.0 ± 6.3 | 72.1 ± 6.3 | 74.0 ± 6.5 |
| BMI, mean ± SD, kg/m2 | 23.1 ± 3.3 | 23.1 ± 3.1 | 23.3 ± 3.3 | 22.9 ± 3.1 | 23.2 ± 3.4 | 23.1 ± 3.1 | 23.2 ± 3.2 | 23.1 ± 3.0 | 23.2 ± 3.3 | 23.2 ± 3.2 |
| Men, % | 40.8 | 40.2 | 52.7 | 30.0 | 48.0 | 38.4 | 37.3 | 45.9 | 54.6 | 30.8 |
| Married, % | 87.0 | 90.0 | 89.3 | 87.5 | 86.9 | 89.9 | 87.0 | 89.3 | 89.4 | 88.2 |
| Cantonese dialect, % | 43.7 | 56.3 | 45.0 | 54.0 | 45.6 | 53.4 | 44.4 | 53.6 | 49.9 | 50.7 |
| Higher education2, % | 27.8 | 47.3 | 31.2 | 42.5 | 31.6 | 44.6 | 29.0 | 45.1 | 35.4 | 38.7 |
| Current smokers, % | 17.1 | 8.7 | 22.8 | 5.7 | 19.8 | 8.4 | 14.8 | 11.2 | 19.9 | 8.2 |
| Alcohol drinkers, % | 18.3 | 22.3 | 26.4 | 14.0 | 16.0 | 26.1 | 19.6 | 20.9 | 25.6 | 14.1 |
| Daily tea drinkers, % | 19.2 | 27.3 | 21.9 | 25.2 | 22.1 | 26.7 | 15.4 | 33.0 | 20.8 | 28.4 |
| Daily coffee drinkers, % | 73.1 | 65.0 | 75.4 | 61.1 | 72.5 | 65.5 | 63.8 | 73.0 | 64.4 | 73.2 |
| Physical activity, % | ||||||||||
| None | 69.5 | 54.8 | 68.0 | 56.8 | 67.0 | 56.7 | 70.0 | 55.0 | 62.3 | 60.9 |
| 0.5–3.9 h/wk | 19.4 | 28.9 | 19.7 | 27.9 | 20.2 | 27.4 | 18.7 | 27.8 | 23.6 | 25.0 |
| ≥4 h/wk | 11.1 | 16.3 | 12.3 | 15.3 | 12.8 | 15.9 | 11.3 | 17.2 | 14.1 | 14.1 |
| Sleep duration, % | ||||||||||
| <6 h/d | 9.0 | 7.5 | 8.6 | 8.2 | 9.2 | 7.9 | 8.8 | 8.4 | 7.7 | 8.3 |
| 6–8 h/d | 85.6 | 87.0 | 85.2 | 86.9 | 84.6 | 86.8 | 85.2 | 86.0 | 86.1 | 86.7 |
| >8 h/d | 5.4 | 5.5 | 6.2 | 4.9 | 6.2 | 5.3 | 6.0 | 5.6 | 6.2 | 5.0 |
| Baseline hypertension, % | 18.7 | 19.5 | 17.3 | 20.1 | 18.5 | 20.2 | 17.5 | 21.0 | 17.5 | 21.3 |
| Baseline diabetes, % | 4.5 | 5.8 | 3.9 | 5.8 | 4.1 | 5.5 | 5.6 | 4.3 | 4.3 | 5.8 |
| Baseline cardiovascular disease, % | 2.4 | 2.6 | 1.9 | 3.0 | 2.2 | 2.8 | 2.0 | 2.9 | 2.1 | 2.9 |
| Baseline cancer, % | 1.7 | 1.9 | 1.4 | 2.6 | 1.7 | 2.1 | 2.0 | 1.7 | 1.7 | 2.0 |
Values are means ± SD for continuous and percentages for categorical variables. AHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; PDI, plant-based diet index; Q, quartile.
Secondary school or above.
TABLE 2.
Spearman correlation coefficients between different dietary quality scores1
| Dietary quality scores | aMED | DASH | AHEI-2010 | PDI | hPDI |
|---|---|---|---|---|---|
| aMED | 1 | ||||
| DASH | 0.58 | 1 | |||
| AHEI-2010 | 0.65 | 0.72 | 1 | ||
| PDI | 0.59 | 0.41 | 0.41 | 1 | |
| hPDI | 0.37 | 0.60 | 0.59 | 0.47 | 1 |
AHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; PDI, plant-based diet index.
Dietary patterns and risk of cognitive impairment
The average follow-up duration was 19.7 y from baseline recruitment to the third follow-up interview. The median (IQR) of the SM-MMSE score was 26 (23, 28), and 14.4% of the 16,948 participants were defined as having cognitive impairment using education-specific cut-offs. Compared with those in the lowest quartile, participants in the highest quartile of the dietary pattern scores had a significant reduction, 18% (for PDI) to 33% (for aMED), in the risk of cognitive impairment in the final model (Table 3). After adjustment for potential covariates, the adjusted OR (95% CI) for cognitive impairment was 0.67 (0.59, 0.77) for aMED, 0.71 (0.62, 0.81) for DASH, 0.75 (0.66, 0.85) for AHEI-2010, 0.82 (0.71, 0.94) for PDI, and 0.78 (0.68, 0.90) for hPDI, comparing the highest with the lowest quartiles (all P-trend <0.001, Table 3). Moreover, each 1 SD increment in the dietary pattern scores was associated with a 7% (for PDI) to 16% (for aMED) reduction in the risk of cognitive impairment (Table 3).
TABLE 3.
Relations of quartiles of dietary quality scores with risk of cognitive impairment1
| Dietary quality scores | Median score (range) | Number of cases/Total number of participants | Model 12 OR (95% CI) | Model 23 OR (95% CI) | Model 34 OR (95% CI) |
|---|---|---|---|---|---|
| aMED | |||||
| Q1 | 3 (0, 3) | 934/5734 | 1.00 | 1.00 | 1.00 |
| Q2 | 4 (4, 4) | 520/3563 | 0.90 (0.80, 1.02) | 0.85 (0.75, 0.96) | 0.85 (0.75, 0.96) |
| Q3 | 5 (5, 5) | 481/3575 | 0.84 (0.74, 0.95) | 0.76 (0.66, 0.86) | 0.75 (0.66, 0.86) |
| Q4 | 6 (6, 9) | 508/4076 | 0.79 (0.70, 0.89) | 0.68 (0.59, 0.77) | 0.67 (0.59, 0.77) |
| P-trend5 | <0.001 | <0.001 | <0.001 | ||
| Per SD increment6 | 0.90 (0.86, 0.94) | 0.84 (0.80, 0.89) | 0.84 (0.80, 0.88) | ||
| DASH | |||||
| Q1 | 20 (8, 21) | 653/4239 | 1.00 | 1.00 | 1.00 |
| Q2 | 23 (22, 24) | 635/4368 | 0.88 (0.78, 0.99) | 0.84 (0.75, 0.96) | 0.84 (0.74, 0.95) |
| Q3 | 26 (25, 27) | 563/4134 | 0.78 (0.68, 0.88) | 0.73 (0.64, 0.83) | 0.73 (0.64, 0.83) |
| Q4 | 30 (28, 39) | 592/4207 | 0.79 (0.70, 0.90) | 0.71 (0.62, 0.81) | 0.71 (0.62, 0.81) |
| P-trend5 | <0.001 | <0.001 | <0.001 | ||
| Per SD increment6 | 0.92 (0.88, 0.97) | 0.89 (0.85, 0.93) | 0.89 (0.84, 0.93) | ||
| AHEI-2010 | |||||
| Q1 | 42.6 (22.3, 46.0) | 649/4237 | 1.00 | 1.00 | 1.00 |
| Q2 | 48.4 (46.0, 50.8) | 625/4237 | 0.90 (0.79, 1.02) | 0.88 (0.77, 0.99) | 0.87 (0.77, 0.99) |
| Q3 | 53.0 (50.8, 55.6) | 591/4237 | 0.84 (0.74, 0.95) | 0.80 (0.70, 0.91) | 0.80 (0.70, 0.90) |
| Q4 | 59.1 (55.6, 81.0) | 578/4237 | 0.82 (0.73, 0.94) | 0.75 (0.66, 0.86) | 0.75 (0.66, 0.85) |
| P-trend5 | 0.002 | <0.001 | <0.001 | ||
| Per SD increment6 | 0.93 (0.89, 0.97) | 0.90 (0.85, 0.94) | 0.89 (0.85, 0.94) | ||
| PDI | |||||
| Q1 | 34 (22, 36) | 706/4406 | 1.00 | 1.00 | 1.00 |
| Q2 | 39 (37, 40) | 636/4419 | 0.90 (0.80, 1.01) | 0.87 (0.77, 0.99) | 0.87 (0.77, 0.98) |
| Q3 | 42 (41, 44) | 541/4173 | 0.80 (0.70, 0.90) | 0.75 (0.66, 0.86) | 0.75 (0.66, 0.86) |
| Q4 | 47 (45, 66) | 560/3950 | 0.90 (0.80, 1.02) | 0.82 (0.72, 0.94) | 0.82 (0.71, 0.94) |
| P-trend5 | 0.04 | <0.001 | <0.001 | ||
| Per SD increment6 | 0.96 (0.92, 1.00) | 0.93 (0.88, 0.97) | 0.93 (0.88, 0.97) | ||
| hPDI | |||||
| Q1 | 39 (26, 41) | 562/3978 | 1.00 | 1.00 | 1.00 |
| Q2 | 44 (42, 45) | 626/4440 | 0.90 (0.80, 1.03) | 0.88 (0.77, 1.00) | 0.88 (0.77, 1.00) |
| Q3 | 47 (46, 49) | 656/4455 | 0.90 (0.79, 1.02) | 0.86 (0.75, 0.98) | 0.85 (0.75, 0.97) |
| Q4 | 52 (50, 68) | 599/4075 | 0.85 (0.75, 0.97) | 0.78 (0.68, 0.90) | 0.78 (0.68, 0.90) |
| P-trend5 | 0.02 | <0.001 | <0.001 | ||
| Per SD increment6 | 0.95 (0.91, 0.99) | 0.92 (0.88, 0.97) | 0.92 (0.88, 0.97) | ||
AHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; PDI, plant-based diet index; Q, quartile.
Model 1: adjusted for age at cognitive status measurement.
Model 2: adjusted for variables in Model 1 plus year of baseline interview, sex, dialect group, marital status, education level, smoking status, physical activity, sleep duration, BMI, total energy intake. Analyses for the DASH, PDI, and hPDI indexes were additionally adjusted for alcohol consumption, and analyses for the aMED, DASH, and AHEI-2010 were additionally adjusted for tea and coffee intake.
Model 3: adjusted for variables in Model 2 plus baseline history of hypertension, diabetes, cardiovascular disease, and cancer.
Tests for a linear trend were calculated by fitting median scores for quartiles as continuous variables in logistic regression models.
SD values are as follows: 1.67 for aMED, 4.33 for DASH, 7.32 for AHEI-2010, 5.76 for PDI, and 5.60 for hPDI.
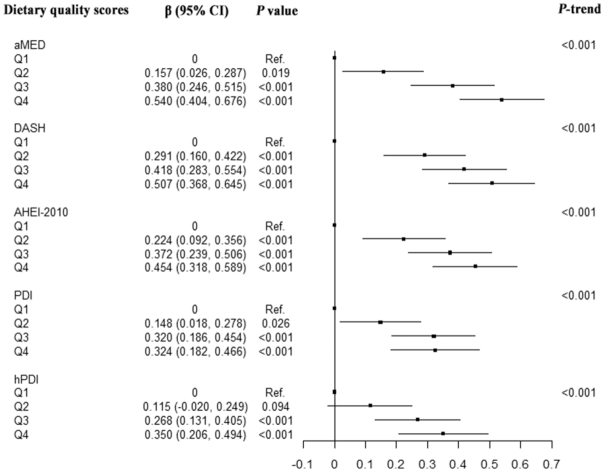
In the generalized linear models, the adjusted mean SM-MMSE scores were higher with increasing quartiles of 5 dietary scores (all P-trend <0.001; Figure 1). Compared with those in the lowest quartile, participants in the highest quartile of aMED (mean difference β: 0.540; 95% CI: 0.404, 0.676; P <0.001), DASH (mean difference β: 0.507; 95% CI: 0.368, 0.645; P <0.001), AHEI-2010 (mean difference β: 0.454; 95% CI: 0.318, 0.589; P <0.001), and PDI (mean difference β: 0.324; 95% CI: 0.182, 0.466; P <0.001) and hPDI scores (mean difference β: 0.350; 95% CI: 0.206, 0.494; P <0.001) had higher adjusted MMSE scores (Figure 1 and Supplemental Table 1). In the final model, each 1 y increment in age was associated with a decrease of 0.185 in the SM-MMSE score. Therefore, the differences of the adjusted mean scores between the highest and the lowest quartiles were equivalent to differences observed among participants 2.93 y younger in age for aMED, 2.72 y younger for DASH, 2.45 y younger for AHEI-2010, 1.76 y younger for PDI, and 1.89 y younger for hPDI.
FIGURE 1.
Relations of quartiles of dietary quality scores with the SM-MMSE score. The multivariable-adjusted model was adjusted for age at cognitive status measurement, year of baseline interview, sex, dialect group, marital status, education level, smoking status, physical activity, sleep duration, BMI, total energy intake, baseline history of hypertension, diabetes, cardiovascular disease, and cancer. Analyses for the DASH, PDI, and hPDI indexes were additionally adjusted for alcohol consumption, and analyses for the aMED, DASH, and AHEI-2010 were additionally adjusted for tea and coffee intake. Tests for a linear trend were calculated by fitting median scores for quartiles as continuous variables in generalized linear models. AHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; PDI, plant-based diet index; Q, quartile; SM-MMSE, Singapore-modified version of Mini-Mental State Examination.
Stratified analyses
The associations between dietary patterns and cognitive impairment were not significantly modified by sex, education level, BMI group, baseline hypertension, or diabetes (all P-interaction >0.05; Supplemental Table 2). The only difference was found in the stratified analysis by age at baseline (<55 and ≥55 y; P-interaction = 0.02) for the association between hPDI and cognitive impairment, with differences seen in the third quartile but similar point estimates in the fourth quartile (Supplemental Table 2).
The associations between dietary patterns and the SM-MMSE score were not significantly modified by BMI, baseline hypertension, or diabetes (all P-interaction >0.05; Supplemental Table 3). A difference was found in the stratified analysis of age (P-interaction = 0.002), sex (P-interaction = 0.02), and education level (P-interaction = 0.01) for the association between aMED and the SM-MMSE score, with a stronger association observed in older adults, women, and those with no formal education. Meanwhile, the association between AHEI-2010 and the SM-MMSE score was stronger in women (P-interaction = 0.004); the association between hPDI and the SM-MMSE score was stronger in those with no formal education (P-interaction = 0.02; Supplemental Table 3).
Sensitivity analyses
The results were generally robust in various sensitivity analyses excluding those with cancer or CVD at baseline, adjusting for incident chronic diseases during the follow-up period, adjusting for age at baseline, adjusting for housing type at the third follow-up, excluding those who had unrealistic daily energy intake at baseline, or using 23/24 as the SM-MMSE cut-off point (Supplemental Table 4). Compared with participants who attended the baseline survey, and follow-up visits 1 and 2 only, those who participated in the third follow-up visit were generally younger, more likely to be female, married, never smokers, have a higher education level, lower prevalence of baseline comorbidities, but similar dietary pattern scores (Supplemental Table 5).
Discussion
In this large prospective cohort study with a mean follow-up period of almost 2 decades, we found that 5 predetermined healthful dietary patterns were inversely associated with the risk of cognitive impairment in the Singapore Chinese population. The strongest association was found between aMED and cognitive function.
Our observed inverse association between aMED and cognitive impairment is consistent with existing evidence (7, 8). Although no significant associations were found in several smaller cohort studies (12, 28–30), a recent meta-analysis of 15 cohort studies and 2 randomized clinical trials observed a beneficial effect of the Mediterranean diet on global cognitive function among healthy older adults in both Mediterranean and non-Mediterranean regions (8). To the best of our knowledge, only 1 cohort study has been conducted in China (1650 Chinese adults with an average of 5.3 y follow-up), and it was noted that a higher Mediterranean diet score was associated with a slower rate of cognitive decline among adults ≥65 y but not in adults aged 55–65 y (31). The authors hypothesized that the rate of cognitive decline was relatively small in younger adults thus the impact of diet might be difficult to detect. However, in our study with a much larger sample size and longer follow-up period, we did not see significant differences between older and younger participants. A 6.5-y randomized clinical trial found that the Mediterranean diet, supplemented with olive oil or nuts, improved global cognition compared with a low-fat diet (32).
Prospective studies from Western countries found that higher adherence to a DASH diet pattern was associated with better cognitive function in the Nurses’ Health Study (9), the Cache County Memory Study (10), and the Memory and Aging Project (11), although null association was also reported in a prospective study involving 6425 American postmenopausal women (12). In a 4-mo randomized trial conducted in 124 overweight and sedentary participants with hypertension, the DASH diet group showed better psychomotor speed than the usual diet control group (33). Our findings confirmed an inverse association between the DASH diet and cognitive impairment in a Chinese population.
The association between AHEI and cognitive function is conflicting in previous studies (12–14). In a multinational cohort study of 27,860 middle-aged and elderly people at high risk of CVD, greater adherence to modified AHEI was associated with a lower risk of cognitive decline over 5 y of follow-up (13). However, the Women's Health Initiative Memory Study found no significant associations between AHEI-2010 and cognitive decline in participants aged ≥65 y over a median follow-up of 9.1 y (12). A Canadian cohort study in participants aged 67–84 y initially did not find a significant association between the Canadian version HEI and cognitive function over 3 y of follow-up (14). However, in a later analysis, the authors found that the association depended on socioeconomic position: a high adherence to the prudent pattern was associated with less cognitive decline only in those with low composite socioeconomic position, whereas adherence to the Western pattern was associated with more cognitive decline only in those with low education attainment (34). Our study participants had lower education attainment relative to most Western populations, and the inverse association between AHEI-2010 score and cognitive impairment was consistent with this Canadian study (34).
In our study, the 5 predetermined healthy dietary patterns were all associated with a lower risk of cognitive impairment, although the effect sizes varied across scores with the strongest association found for the Mediterranean diet score. Our findings indicate that these dietary patterns all capture key aspects of healthful diets in a Chinese population. Several explanations have been provided for the protective role of these healthy dietary patterns on cognitive health. First, human studies have consistently found that adherence to healthy dietary patterns is related to a lower risk of chronic diseases (35), which are major contributors to age-related cognitive decline (36). However, adjusting for incident chronic diseases did not significantly alter the observed associations in our study. Second, healthy dietary patterns could decrease chronic inflammation and oxidative stress, which are also related to the pathophysiology of neurodegenerative diseases (37, 38). Third, emerging evidence from animal models and human studies suggests that some components of these dietary patterns (vegetables, fruits, and whole grains) are beneficial for the gastrointestinal microbiome (39), which can influence the central nervous system (40). Taken together, the healthy dietary patterns and related components could improve cognition via favorable changes in metabolic, inflammatory, and microvascular function.
The strengths of the study include the prospective study design, long follow-up duration, and thorough assessment of potential confounders. Other strengths include a detailed FFQ specifically developed and validated for our population, and the investigation of multiple diet index scores in a single study. Some limitations should be considered. First, cognitive function was only evaluated during the third follow-up visit, thus we were not able to capture cognitive decline over time and reverse causation is also possible. However, the possibility is small given that the mean age at baseline was still young and all participants could answer the comprehensive questionnaires indicating good cognitive status. Second, SM-MMSE testing was used for screening purposes rather than clinical diagnosis and may have poor sensitivity for the detection of very severe or very mild cognitive impairment. Third, the self-reporting of dietary intake and other covariates through baseline interviews is susceptible to measurement errors, which may have resulted in some nondifferential misclassification and underestimation of the associations observed in the cohort study. Fourth, dietary intake was only assessed at baseline and subsequent changes during follow-up were not captured. However, changes in diet would be expected to lead to nondifferential misclassification and most likely underestimate the observed associations because of the prospective design. Fifth, self-selection bias is possible since participants in the third follow-up visit did not include those who died or could not attend the third follow-up visit. As expected, those who attended the third follow-up visit were younger and generally had a healthier lifestyle and less comorbidities at baseline compared with those who did not attend the third follow-up visit. However, exposure status (dietary pattern scores) was not substantially different between participants who attended the third follow-up visit and participants who did not attend the third follow-up visit, thus the associations observed in our study were more likely to be underestimated because of the nondifferential loss-to-follow-up if the selection bias had an impact on the results. Sixth, we did not collect information on APOE Ɛ4 status, and some previous small studies have suggested that APOE genotype may modify the associations between dietary patterns and cognitive function (28) or Alzheimer's disease (41), but the direction of the modification effect in those studies was not consistent and certainly more studies are still needed. Finally, we did not collect information on household income or other socioeconomic status variables in our study except for education level. However, further adjustment for housing type at the third follow-up did not materially change the results. Nevertheless, we acknowledge that our findings should be interpreted cautiously given that residual confounding is inevitable in the observational study.
In conclusion, we found that higher adherence to healthy dietary patterns, represented by the aMED, DASH, AHEI-2010, PDI, and hPDI scores, in midlife were associated with a lower risk of cognitive impairment in late life in Chinese adults. These findings suggest that maintaining a healthy dietary pattern is important for the prevention of onset and delay of cognitive impairment. Future longitudinal studies with repeated measures of diet and cognition and clinical diagnosis of dementia among diverse populations are needed. This would help identify the critical window for diet intervention to have a major impact on cognition and identify the potential population that would benefit the most.
Supplementary Material
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and Renwei Wang for the maintenance of the cohort study database.
The authors’ contributions were as follows—WJ: conceived the study, analyzed data, drafted the initial manuscript, and critically revised and edited the final manuscript; XYS: checked the statistical analysis and data in the manuscript, reviewed and contributed to discussions about the results; G-CC, NN, and RMvD: data conversion and calculation of the dietary pattern scores; reviewed and edited the final manuscript; LF: contributed to the data collection of MMSE interviews, reviewed and edited the final manuscript; J-MY: supervised the data collection, reviewed and edited the final manuscript; W-PK and AP: supervised the data collection, conceived the study, contributed to discussions about the results, critically revised and edited the final manuscript, were the guarantors of the work, had full access to all of the data in the study, and took primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by grants from the National Medical Research Council, Singapore (NMRC/CSA/0055/2013) and the NIH (R01 CA144034 and UM1 CA182876). The Saw Swee Hock School of Public Health, National University of Singapore also provided funding for the implementation of follow-up visits of the cohort. AP is supported by the National Key Research and Development Program of China (2017YFC0907504) and Hubei Province Science Fund for Distinguished Young Scholars (2018CFA033).
Supplemental Tables 1–5 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean diet; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; hPDI, healthful plant-based diet index; PDI, plant-based diet index; SM-MMSE, Singapore-modified Mini-Mental State Examination.
REFERENCES
- 1. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohenmansfield J. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 2. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. [DOI] [PubMed] [Google Scholar]
- 3. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, Schilardi A, D'Introno A, La Montagna M, Calvani M et al.. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59(3):815–49. [DOI] [PubMed] [Google Scholar]
- 4. Alles B, Samieri C, Feart C, Jutand MA, Laurin D, Barberger-Gateau P. Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev. 2012;25(2):207–22. [DOI] [PubMed] [Google Scholar]
- 5. van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 7. Cao L, Tan L, Wang HF, Jiang T, Zhu XC, Lu H, Tan MS, Yu JT. Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53(9):6144–54. [DOI] [PubMed] [Google Scholar]
- 8. Loughrey DG, Lavecchia S, Brennan S, Lawlor BA, Kelly ME. The impact of the Mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv Nutr. 2017;8(4):571–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berendsen AAM, Kang JH, van de Rest O, Feskens EJM, de Groot L, Grodstein F. The Dietary Approaches to Stop Hypertension diet, cognitive function, and cognitive decline in American older women. J Am Med Dir Assoc. 2017;18(5):427–32. [DOI] [PubMed] [Google Scholar]
- 10. Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr. 2013;98(5):1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, Morris MC. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, Neuhouser ML, Wassertheil-Smoller S. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women's Health Initiative Memory Study. J Acad Nutr Diet. 2016;116(6):921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smyth A, Dehghan M, O'Donnell M, Anderson C, Teo K, Gao P, Sleight P, Dagenais G, Probstfield JL, Mente A et al.. Healthy eating and reduced risk of cognitive decline – a cohort from 40 countries. Neurology. 2015;84(22):2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shatenstein B, Ferland G, Belleville S, Gray-Donald K, Kergoat MJ, Morais J, Gaudreau P, Payette H, Greenwood C. Diet quality and cognition among older adults from the NuAge study. Exp Gerontol. 2012;47(5):353–60. [DOI] [PubMed] [Google Scholar]
- 15. Chen YC, Jung CC, Chen JH, Chiou JM, Chen TF, Chen YF, Tang SC, Yeh SJ, Lee MS. Association of dietary patterns with global and domain-specific cognitive decline in Chinese elderly. J Am Geriatr Soc. 2017;65(6):1159–67. [DOI] [PubMed] [Google Scholar]
- 16. Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39(2):187–95. [DOI] [PubMed] [Google Scholar]
- 17. Seow A, Shi CY, Franke AA, Hankin JH, Lee HP, Yu MC. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7(2):135–40. [PubMed] [Google Scholar]
- 18. Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7(9):775–81. [PubMed] [Google Scholar]
- 19. Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AM, Earle ME, Nagamine FS et al.. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151(4):358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neelakantan N, Koh WP, Yuan JM, van Dam RM. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese adults. J Nutr. 2018;148:1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen GC, Koh WP, Neelakantan N, Yuan JM, Qin LQ, van Dam RM. Diet quality indices and risk of type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol. 2018;187(12):2651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng L, Chong MS, Lim WS, Ng TP. The Modified Mini-Mental State Examination test: normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singapore Med J. 2012;53(7):458–62. [PubMed] [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell AJ. A meta-analysis of the accuracy of the Mini-Mental State Examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411–31. [DOI] [PubMed] [Google Scholar]
- 26. Katzman R, Zhang MY, Ouang Ya Q, Wang ZY, Liu WT, Yu E, Wong SC, Salmon DP, Grant I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41(10):971–8. [DOI] [PubMed] [Google Scholar]
- 27. Lipnicki DM, Crawford JD, Dutta R, Thalamuthu A, Kochan NA, Andrews G, Lima-Costa MF, Castro-Costa E, Brayne C, Matthews FE et al.. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 2017;14(3):e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olsson E, Karlstrom B, Kilander L, Byberg L, Cederholm T, Sjogren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2014;43(1):109–19. [DOI] [PubMed] [Google Scholar]
- 29. Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation in Cancer and Nutrition). Public Health Nutr. 2008;11(10):1054–62. [DOI] [PubMed] [Google Scholar]
- 30. Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97(2):369–76. [DOI] [PubMed] [Google Scholar]
- 31. Qin B, Adair LS, Plassman BL, Batis C, Edwards LJ, Popkin BM, Mendez MA. Dietary patterns and cognitive decline among Chinese older adults. Epidemiology. 2015;26(5):758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84(12):1318–25. [DOI] [PubMed] [Google Scholar]
- 33. Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the Dietary Approaches to Stop Hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parrott MD, Shatenstein B, Ferland G, Payette H, Morais JA, Belleville S, Kergoat MJ, Gaudreau P, Greenwood CE. Relationship between diet quality and cognition depends on socioeconomic position in healthy older adults. J Nutr. 2013;143(11):1767–73. [DOI] [PubMed] [Google Scholar]
- 35. Galbete C, Schwingshackl L, Schwedhelm C, Boeing H, Schulze MB. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol. 2018;33(10):909–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129(15):1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steele M, Stuchbury G, Munch G. The molecular basis of the prevention of Alzheimer's disease through healthy nutrition. Exp Gerontol. 2007;42(1–2):28–36. [DOI] [PubMed] [Google Scholar]
- 38. Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A, Solfrizzi V. Nutraceutical properties of Mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimers Dis. 2010;22(3):715–40. [DOI] [PubMed] [Google Scholar]
- 39. Jacka FN. Nutritional psychiatry: where to next?. Ebiomedicine. 2017;17(C):24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–42. [DOI] [PubMed] [Google Scholar]
- 41. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.