Abstract
Background
Metastasis of appendicular osteosarcoma is most common to the lungs and is generally considered a terminal event in dogs. Behavior and prognosis associated with cutaneous or subcutaneous metastases (CSM) is poorly defined.
Objective
Describe the population and gather prognostic information regarding appendicular osteosarcoma with CSM in dogs.
Animals
Twenty dogs with appendicular osteosarcoma and CSM.
Methods
Retrospective case series. Medical records were searched to identify dogs diagnosed with appendicular osteosarcoma that developed CSM. Demographic data, order of metastatic events, and CSM clinical features were evaluated. Kaplan‐Meier survival curves were constructed and log‐rank tests were used to compare survival between groups of dogs.
Results
In 19 dogs (95%), CSM was an incidental finding. Seventeen dogs (85%) developed pulmonary metastasis, and 1 dog (5%) developed bone metastasis. No other metastatic sites were detected before euthanasia. The median CSM‐free interval and CSM survival time were 160 days (range: 0‐542 days) and 55 days (range: 5‐336 days), respectively. The median CSM survival time was significantly longer for dogs treated with surgery and chemotherapy (94 days) or chemotherapy only (64 days) than for dogs that did not receive these treatments (11 days) (P = .002 and P = .03, respectively). No other factors were associated with survival after diagnosis of CSM.
Conclusion and Clinical Importance
The skin or subcutaneous tissue can be the first osteosarcoma metastatic site detected. After CSM diagnosis, the prognosis is grave with median survival <2 months. Although this finding could have been biased by case selection, treatment with surgery and chemotherapy may improve outcome.
Keywords: oncology, primary bone tumor, prognostic factor, skin metastasis, stage III osteosarcoma
Abbreviations
- ABPs
aminobisphosphonates
- ALP
alkaline phosphatase
- CMetFI
cutaneous metastasis‐free interval
- CMetST
cutaneous metastasis‐survival time
- CSM
cutaneous or subcutaneous metastases
- CT
computed tomography
- L‐MTP
liposome‐encapsulated muramyl tripeptide phosphatidylethanolamine
- MetFI
metastasis‐free interval
- MetST
metastasis‐survival time
- OSA
osteosarcoma
- OST
overall survival time
- RT
radiation therapy
- SRT
stereotactic radiation therapy
1. INTRODUCTION
Osteosarcoma (OSA) is the most common primary bone tumor in dogs and is frequently metastatic.1 The pulmonary parenchyma and other skeletal sites are the most common metastatic locations. At the time of death, approximately 90% of dogs have gross pulmonary metastases, and 34%‐46% of dogs have metastasis to other bones.1, 2, 3, 4 Less commonly, there is metastasis to the lymph nodes (4% of cases) or abdominal organs (0%‐4% of cases).2, 5, 6, 7 Regardless of the metastatic site(s), the prognosis for dogs with metastatic (stage III) OSA is guarded.8 Median survival varies between 2 and 4 months after diagnosis of stage III OSA.2, 5, 7, 8, 9, 10, 11, 12, 13
The skin and subcutaneous tissues are a metastatic site uncommonly reported in the veterinary literature.4, 14, 15 In 3 different studies, 4% (19/470), 6% (3/50), and 11% (2/19) of dogs with OSA developed metastasis to the skin or subcutaneous tissues.4, 14, 15 However, no specific details were provided regarding the cutaneous or subcutaneous metastases (CSM).
To the authors' knowledge, 6 cases of dogs with CSM from appendicular OSA with details regarding treatment and survival have been published.6, 12, 16, 17, 18 Three dogs were diagnosed with appendicular OSA and CSM either concurrently or within 4 weeks of the primary diagnosis.16, 17, 18 All these dogs received some form of anticancer therapy (including amputation, chemotherapy, bisphosphonates, or a combination of these treatments) and were euthanized within 2 months.16, 17, 18 The 3 other dogs developed CSM later in the course of their appendicular OSA treatment.6, 12 Two dogs are reported that developed widespread metastatic disease, which included the subcutaneous tissues, 14 and 40 months after the initial diagnosis of OSA, respectively.12 One dog was euthanized at the detection of CSM, and the other died 3 months later of an unknown cause.12 In a recent report, a dog developed a dermal sarcoma (suspected OSA on histopathology) a year after the initial diagnosis of appendicular OSA.6 This dog had previously developed metastasis to the lungs, ischium, and vertebra, and was euthanized 5 months after detection of the CSM after developing additional metastasis to the liver.6
There is currently a paucity of information in the veterinary literature regarding CSM of appendicular OSA. The goal of this study was to describe the population, clinical characteristics, and gather prognostic information regarding appendicular OSA with CSM in dogs. We hypothesized that, similar to what has been reported for other metastatic locations, CSM is associated with a grave prognosis.
2. MATERIALS AND METHODS
2.1. Dog selection
This was a multi‐institutional retrospective study performed at Oregon State University, Colorado State University, the University of Wisconsin‐Madison, the University of Georgia, and Washington State University. Electronic medical records from client‐owned dogs were searched between January 1, 2006, and April 1, 2018, to identify dogs diagnosed with appendicular OSA that developed metastasis to the skin or subcutaneous tissues. Dogs were eligible for the study if they fulfilled the following criteria: primary appendicular OSA confirmed via cytology or histopathology; cutaneous or subcutaneous metastasis of OSA confirmed via cytology or histopathology; and adequate follow‐up (ie, date of amputation or first OSA‐related treatment; type and schedule of all subsequent OSA‐related treatment; time to development and location of the first metastatic event; time to development and clinical characteristics of CSM; type, schedule, and response to all CSM‐related treatment; cause of progressive disease after diagnosis of CSM; and date and cause of death). Confirmation of the primary OSA and CSM was performed by a board‐certified pathologist in all cases. For cytological evaluation of OSA, alkaline phosphatase (ALP) staining was recorded when available but was not required. Full staging tests at diagnosis or curative‐intent treatment were not inclusion criteria but were recorded when available. If data regarding outcome were missing in the medical record, the dog owner or primary care veterinarian were contacted to gather additional information. Cutaneous or subcutaneous lesions were considered metastatic if diagnosed concurrently or after the diagnosis of primary appendicular OSA. Dogs diagnosed with isolated cutaneous or subcutaneous OSA were considered to have extraskeletal OSA and were not included in the study.
For selected cases, medical records were reviewed for the following information: breed, sex, neuter status, age and weight at diagnosis, abnormalities on physical examination, hematology and serum biochemistry at OSA diagnosis, primary appendicular OSA location, details of treatment if performed, date and location of first metastatic event and all subsequent metastatic events, date and cause of death, and result of autopsy, if available. Information collected regarding the CSM were number, size, location (head/neck, trunk, or limbs), cutaneous versus subcutaneous, inflammation or pain associated, method of detection (incidental finding on physical examination versus imaging), result of cytology or histopathology, time to development from primary OSA, interval between first metastatic event and CSM, treatment undertaken for CSM, and survival after diagnosis of CSM.
2.2. Statistical analysis
Metastasis‐free interval (MetFI) was defined as the interval between the date of amputation or first OSA‐related treatment (if no amputation was performed, the date of the first radiation therapy (RT) or chemotherapy treatment was used) and the date of first metastatic event, regardless of the first metastatic site detected (CSM or non‐CSM). Cutaneous metastasis‐free interval (CMetFI) was defined as the interval between the date of amputation or first OSA‐related treatment and the date of first CSM detected. Overall survival time (OST) was defined as the interval between the date of amputation or first OSA‐related treatment and the date of death from any cause. Metastasis‐survival time (MetST) was defined as the interval between the date of diagnosis of the first metastatic event and the date of death from any cause. Cutaneous/subcutaneous metastasis‐survival time (CMetST) was defined as the interval between the date of CSM diagnosis and the date of death from any cause. Because all dogs died of causes directly related or presumed to be related to OSA, there were no censored observations in this study.
Kaplan‐Meier analysis with 95% confidence interval was performed for survival by the presence of increased serum ALP activity (n = 9) versus ALP activity within reference range at diagnosis (n = 11), proximal humerus location of primary OSA (n = 5) versus other locations (n = 15), >1 CSM (n = 8) versus only 1 CSM (n = 12), and treatment undertaken for CSM. For the latter, 3 treatment groups were considered: surgery + chemotherapy (n = 4), chemotherapy only (n = 9), and other treatments (palliative RT, aminobisphosphonates [ABPs], oral analgesics, or no treatment; n = 7). Due to the small number of dogs with relative monocytosis (n = 2) and increased serum cholesterol concentration (n = 3), these variables were not evaluated. Log‐rank tests were used to compare survival between strata and were adjusted for multiple comparisons by Tukey's test. A significance threshold of 0.05 was used. Normality tests were performed using GraphPad Prism (GraphPad Prism for windows, version 7.04; La Jolla, California). Kaplan‐Meier analysis were performed using software packages SAS (SAS System for PC, version 9.3; SAS Institute, Cary, North Carolina).
3. RESULTS
3.1. Demographic information and primary OSA diagnosis
Review of medical records initially identified 33 dogs with OSA and a potential diagnosis of CSM. However, 13 dogs were excluded from the analysis because CSM cytology was not interpreted by a board‐certified pathologist (n = 6), CSM was consistent with sarcoma via cytology, but report was not definitive for OSA (n = 4), primary OSA was in an axial location (n = 2), and primary bone tumor was diagnosed as sarcoma via histopathology, but report was not definitive for OSA (n = 1). Hence, a total of 20 dogs fulfilled all inclusion criteria for this study.
For the 20 dogs included in this study, median age at diagnosis was 8 years (range, 2.5‐12.8 years). Dogs included 10 (50%) castrated males, 8 (40%) spayed females, and 2 (10%) intact males. The breeds represented were Labrador Retriever (n = 5, 25%), Golden Retriever, Doberman and mixed‐breed (2 each, 10%). Breeds represented by 1 dog were Flat Coated Retriever, Irish Wolfhound, Boxer, Saint‐Bernard, German Shepherd, Collie, American Pitbull, Greyhound, and Vizsla (1 each, 5%). The median body weight at the time of diagnosis was 38.9 kg (range, 28‐62 kg). None of the dogs had any history of neoplastic or nonneoplastic skin disease. Relevant blood work anomalies at the time of diagnosis included increased serum ALP activity and cholesterol concentration (above reference range of referral laboratory) in 9 and 3 dogs, respectively, as well as relative monocytosis (>400 cells/μL) in 2 dogs. No dog had a relative lymphocytosis (>1000 cells/μL).
The clinical characteristics of the primary appendicular OSA for the 20 dogs are summarized in Table 1. The diagnosis of appendicular OSA was confirmed by histopathology (n = 18) or cytology and ALP staining (n = 2). Staging procedures performed at the time of diagnosis of OSA included thoracic radiography (n = 18), thoracic computed tomography (CT; n = 1), and lymph node biopsy (n = 6). In 1 dog, no staging test was performed at the time of diagnosis. Abdominal imaging, scintigraphy, or positron emission tomography/CT was not performed on any cases. Based on the available information, 2 dogs (10%) had metastasis at the time of primary OSA diagnosis: either CSM alone (n = 1) or CSM and pulmonary metastasis (n = 1). Lymph node histopathology was not consistent with metastatic disease in any of the 6 cases in which it was performed.
Table 1.
Characteristics and treatment of primary appendicular OSA for 20 dogs that developed CSM
| Baseline OSA characteristics | Number (%) of dogs |
|---|---|
| Location | |
| Proximal humerus | 5 (25) |
| Distal radius | 4 (20) |
| Distal femur | 4 (20) |
| Distal tibia | 3 (15) |
| Proximal femur | 2 (10) |
| Proximal tibia | 2 (10) |
| Metastasis at diagnosis | |
| Absent | 18 (90) |
| Present | 2 (10) |
| Lung + CSM | 1 (5) |
| CSM only | 1 (5) |
| Treatment for primary OSA | |
| Curative intent | 15 (75) |
| Amputation + adjuvant treatment | 14 (70) |
| SRT + adjuvant treatment | 1 (5) |
| Palliative | 5 (25) |
| RT + chemotherapy/TKI | 2 (10) |
| RT alone | 1 (5) |
| ABPs/NSAIDs/opioids/gabapentin | 2 (10) |
Abbreviations: ABPs, aminobisphosphonates; CSM, cutaneous or subcutaneous metastases; NSAIDs, nonsteroidal anti‐inflammatory drugs; OSA, osteosarcoma; RT, radiation therapy; SRT, stereotactic radiation therapy; TKI, tyrosine kinase inhibitor.
Fifteen dogs received curative‐intent treatment and 5 dogs received palliative treatment for the primary appendicular OSA (Table 1). Adjuvant chemotherapy after amputation or stereotactic radiation therapy (SRT) included carboplatin alone (n = 10), carboplatin and pamidronate or zoledronate (n = 2), carboplatin and HER2 listeria vaccine (n = 1), carboplatin and metronomic cyclophosphamide (n = 1), and carboplatin and liposome‐encapsulated muramyl tripeptide phosphatidylethanolamine (L‐MTP; n = 1). Palliative chemotherapy included administration of carboplatin (n = 1) and toceranib phosphate and cyclophosphamide (n = 1).
3.2. Metastatic events
The first metastatic event detected was pulmonary metastasis in 7 dogs (35%) and CSM in 7 dogs (35%, 4 of which had thoracic radiographs or CT scan performed within a 2 days and documented the absence of pulmonary metastasis at the time of CSM diagnosis). In 6 dogs (30%), metastasis to the lungs and CSM was detected concurrently. No other sites were noted as the first metastatic event.
The chronological order of all metastatic events is illustrated in Table 2. Among the 7 dogs that developed CSM as the first metastatic event, 4 dogs (57%) subsequently developed pulmonary metastasis, whereas 3 dogs (43%) had no other metastatic site detected. Among the 6 dogs with CSM and pulmonary metastasis detected concurrently as the first metastatic event, none had another metastatic site detected before euthanasia.
Table 2.
Order of metastatic events for 20 dogs that developed CSM from an appendicular osteosarcoma
| Chronological order of metastatic sites | Number (%) of dogs |
|---|---|
| Lung/CSM | 6 (30) |
| Lung → CSM | 6 (30) |
| Lung → CSM/bone | 1 (5) |
| CSM → lung | 4 (20) |
| CSM only | 3 (15) |
An arrow was used to indicate metastatic sites which developed 1 after another, while a slash represents metastatic sites detected simultaneously. Only metastatic events detected while dogs were alive are represented.
Abbreviation: CSM, cutaneous or subcutaneous metastases.
Overall, in addition to CSM, 17 dogs (85%) developed pulmonary metastasis and 1 dog (5%) developed bone metastasis (mid‐ulna) during the course of disease. No other metastatic sites were detected while dogs were alive.
3.3. Clinical features of cutaneous and subcutaneous metastasis
The majority of CSM were incidental findings and initially detected on physical examination (n = 14) or by the dog owner at home (n = 5). In 1 case, the means of CSM detection was not reported. No CSM were detected by imaging alone. The final diagnosis of CSM was obtained by cytology of the masses in 19 dogs (95%, including ALP staining in 11 dogs) and biopsy of 2 CSM in 1 dog (5%).
The clinical characteristics of the CSM are described in Table 3. Fifty‐eight CSM were described for the 20 dogs. Twelve dogs (60%) had only 1 CSM detected, while 8 dogs (40%) had >1 CSM detected. The median number of CSM per dog was 1 (range, 1‐21). The size of the CSM was reported in the medical record for 26 of 58 (45%) masses. The median longest dimension was 20 mm (range, 5‐100 mm). Only 1 dog had CSM‐associated inflammation (ulceration), clearly reported in the medical record. No CSM were reported as being painful at initial detection.
Table 3.
Clinical characteristics of 58 CSM in 20 dogs with primary appendicular osteosarcoma
| CSM characteristics | Number (%) of CSM |
|---|---|
| Depth | |
| Cutaneous | 6 (10) |
| Subcutaneous | 31 (54) |
| Not reported | 21 (36) |
| Location | |
| Head/neck | 14 (24) |
| Trunk/ventrum | 38 (66) |
| Limb | 6 (10) |
| Texture | |
| Firm/very firm | 49 (84) |
| Soft | 1 (2) |
| Not reported | 8 (14) |
| Inflammation | |
| Present | 1 (2) |
| Absent | 14 (24) |
| Not reported | 43 (74) |
| Pain | |
| Present | 0 (0) |
| Absent | 14 (24) |
| Not reported | 44 (76) |
Abbreviation: CSM, cutaneous or subcutaneous metastases.
After diagnosis of CSM, 9 dogs (45%) were treated with palliative chemotherapy (gross disease setting), 4 (20%) were treated with surgery and adjuvant chemotherapy, 2 (10%) were treated with ABPs and oral analgesics, and 1 (5%) was treated with palliative RT. In 4 (20%) dogs, no treatment was pursued to address the CSM.
For the 9 dogs that received chemotherapy in a gross disease setting, the median number of chemotherapeutic agents received per dog was 2 (range, 1‐2). These included metronomic cyclophosphamide/chlorambucil (n = 4), toceranib phosphate (n = 3), doxorubicin (n = 3), carboplatin (n = 2), vinorelbine (n = 1), and an investigational c‐Met inhibitor (n = 1). Among these dogs, 2 also received ABPs, 1 received a last dose of HER2 listeria vaccine, and 1 dog received L‐MTP. No objective response was noted for any of these agents. Stable disease was reported in 2 dogs, both of which had an initial recorded measurement of the CSM. One had stable disease for 109 days after treatment with doxorubicin + zoledronate. The other dog had stable disease for 25 days after treatment with an investigational c‐Met inhibitor. Six dogs were classified as having progressive disease based on a >30% increase in the size of the CSM initially recorded. The last dog did not have CSM size initially recorded, but it was reported in the medical record that the size of CSM increases and that additional cutaneous lesions had appeared 23 days after vinorelbine. Therefore, this dog was also classified as having progressive disease.
All 4 dogs treated with surgery had complete excision of the CSM, and all received adjuvant chemotherapy which included doxorubicin single agent (n = 2), doxorubicin + toceranib phosphate + L‐MTP (n = 1), and oral cyclophosphamide (n = 1). One dog was euthanized 56 days after surgery due to severe pain at night, and without recurrence of CSM. No further staging procedures were performed, and necropsy was declined by the owner. One dog developed progression of previously diagnosed lung metastasis and several new CSM 45 days after surgery. One dog developed previously undiagnosed pulmonary metastasis 78 days after surgery. One dog developed previously undiagnosed pulmonary metastasis and several new CSM 270 days after surgery.
One dog was treated with palliative RT directed at the CSM, was not noted to experience a clinical response, subsequently developed pulmonary metastasis, and was euthanized 48 days after diagnosis of CSM.
3.4. Case outcomes
The median MetFI for all dog included in this study was 94 days (range, 0‐373 days), the median MetST was 97 days (range, 5‐334 days), and the median OST was 187 days (range, 5‐707 days, Figure 1). Necropsy was performed in 5 dogs (25%). Results showed metastasis to the lungs in all 5 dogs, kidneys in 3 of 5 dogs, skin or subcutaneous tissues in 3 of 5 dogs, liver in 2 of 5 dogs, brain in 1 of 5 dogs, and tracheobronchial and mesenteric lymph nodes in 1 of 5 dogs. The necropsy report did not mention metastasis to the skin or subcutaneous tissue in 2 dogs, although these 2 dogs had confirmed gross CSM at the time of euthanasia.
Figure 1.
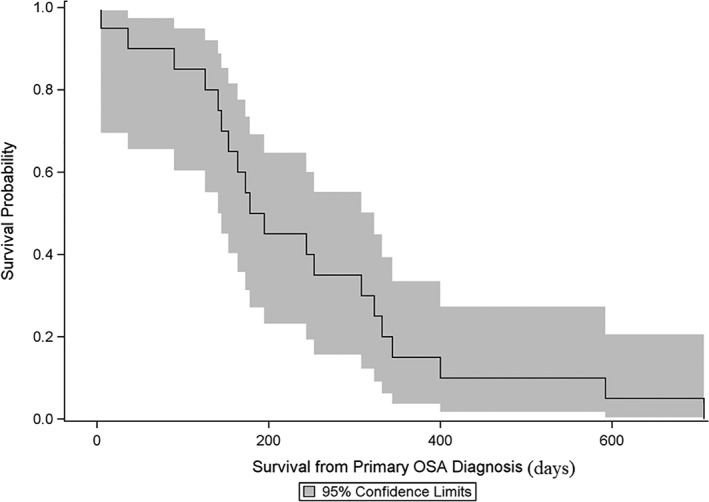
Kaplan‐Meier curve and 95% confidence limits for survival from the initial diagnosis of appendicular osteosarcoma for 20 dogs that developed cutaneous or subcutaneous metastases. The median overall survival time was 187 days (range, 5‐707 days)
The median CMetFI was 160 days (range, 0‐542 days) and the median CMetST was 55 days (range, 5‐336 days, Figure 2). Three dogs (15%) were euthanized for a cause directly related to CSM: 1 dog had 2 CSM which ruptured, were bleeding, and became necrotic; 1 dog due to continued growth of at least 7 CSM; and 1 dog due to a large and ulcerated CSM on the dorsum. The other dogs were euthanized due to decrease in quality of life, energy, and appetite (n = 10) or progressive metastatic disease in the lungs or bone (n = 5). For 2 dogs, the cause of euthanasia was not apparent from the medical record.
Figure 2.
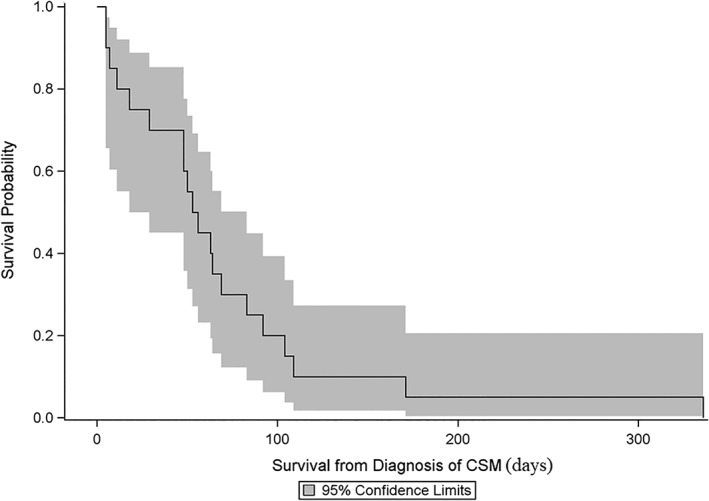
Kaplan‐Meier curve and 95% confidence limits for survival from the diagnosis of cutaneous or subcutaneous metastases for 20 dogs previously diagnosed with appendicular osteosarcoma. The median cutaneous metastasis‐survival time was 55 days (range, 5‐336 days)
3.5. Prognostic factors
No case was censored for this analysis, as all dogs died of OSA‐related causes, and the time of death was known in all cases. The only variable significantly associated with survival from the initial diagnosis of OSA was the location of primary OSA. The median OST was significantly shorter when the primary OSA was located in the proximal humerus (90 days; range, 5‐253 days) versus other locations (244 days; range, 153‐332 days) (P = .003).
The only variable significantly associated with survival from the diagnosis of CSM was the treatment plan. The median CMetST for dogs in which CSM were treated with surgery + chemotherapy or chemotherapy alone (69 days; range, 29‐336 days) was significantly longer than the median CMetST for dogs that did not receive these treatments (11 days; range, 5‐48 days, Figure 3; P < .001). More specifically, the median CMetST for dogs treated with surgery and chemotherapy (94 days; range, 56‐336 days) or chemotherapy only (64 days; range, 29‐171 days) was significantly longer than for dogs that did not receive these treatments (11 days; range, 5‐48 days) (P = .002 and .03, respectively).
Figure 3.
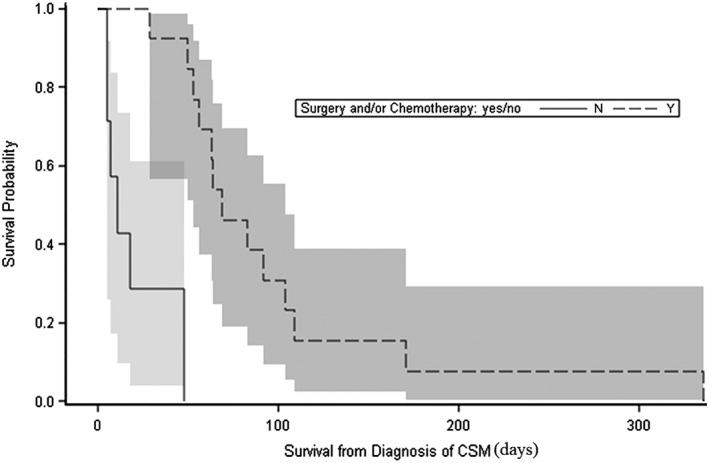
Kaplan‐Meier curves and 95% confidence limits of survival after diagnosis of cutaneous or subcutaneous metastases for dogs treated with surgery + chemotherapy or chemotherapy alone (dashed line) and dogs treated with palliative radiation therapy, aminobisphosphonates, oral analgesics, or no treatments (solid line). The median cutaneous metastasis‐survival time (CMetST) for dogs treated with surgery + chemotherapy or chemotherapy alone (69 days) was significantly longer than the median CMetST for dogs that did not receive these treatments (11 days) (P < .001)
4. DISCUSSION
The aim of our retrospective study was to describe CSM in a group of dogs with appendicular OSA and gather prognostic information. Based on our results, CSM of primary appendicular OSA can occur as a first metastatic event, without concurrent detection of pulmonary metastasis. The time interval between initial OSA diagnosis and the development of CSM is variable, but once CSM is detected, the prognosis is grave. Although this could have been biased by case selection, surgical resection and finding chemotherapy was associated with an improved survival. Overall, the best treatment approach to CSM from OSA in dogs remains to be determined.
The median age (8 years), weight (38.9 kg), male:female ratio (1.5:1), and most common breeds represented in this study are in concordance with dog's characteristics described in previous reports of appendicular OSA in dogs.1, 4, 15, 19 Primary tumor location was slightly more commonly in the hind limb (n = 11) in our population, while previous reports have indicated forelimb locations to be most common.19 The relevance of this finding is unknown; however, it is likely related to bias from the small number of cases included in this report.
The only prognostic factor in this study found to be associated with the OST was the location of the primary tumor. Dogs with a proximal humerus OSA lived significantly shorter than dogs with a primary tumor in other bones (OST = 90 versus 244 days), consistent with other reports.20, 21 Conversely, an increase of serum ALP activity, a negative prognostic indicator,22, 23 was not found to be associated with the OST in this study, likely due to the small number of dogs included.
When examining the first metastatic event, dogs in this study were evenly distributed between those that had pulmonary metastasis first detected (n = 7), those that had CSM first detected (n = 7), and those with concurrent pulmonary and CSM detected (n = 6). All the dogs with pulmonary metastasis first detected subsequently developed CSM after a median of about 5 months (160 days). Of the 7 dogs that had CSM first detected, 4 had thoracic radiographs or CT scan performed within 2 days, which documented the absence of pulmonary metastasis at the time of CSM diagnosis. These 4 dogs eventually developed pulmonary metastasis after a median of 3 months (91 days). On the other hand, 3 dogs with CSM detected as the first metastatic event did not develop other known metastatic sites. This should be interpreted cautiously as no information was available in the medical record of these dogs regarding thoracic or abdominal imaging performed at the time of CSM detection.
In addition to the noted CSM, dogs in this study only had detected metastasis to the lungs or bone before euthanasia. However, based on the information available in the medical record, many dogs did not have a full neurological or ophthalmic evaluation, or abdomen or bone imaging performed during their disease course. Therefore, it is certainly possible that other sites of metastasis were present but not detected. In the 5 dogs that underwent necropsy examination, previously undetected metastases to the kidney, liver, brain, and lymph node were noted.
A majority of humans affected with CSM from OSA are reported to have had other sites of metastasis (lung, bone, lymph nodes, pleura, liver, central nervous system, etc.) detected concurrently or within 6 months.24, 25, 26, 27, 28, 29, 30, 31, 32 Considering the 6 previously reported cases of CSM from OSA in dogs, 2 had CSM diagnosed at the initial diagnosis with no other concomitant metastatic sites,16, 17 and 4 had CSM diagnosed 1,18 6,6 7,12 and 1912 months after the first metastatic event (pulmonary in all cases). All but 1 dog were then diagnosed with widespread metastasis within 4 months of the diagnosis of CSM.6, 12, 16, 18 Unlike previous reports, dogs in our study were not diagnosed with widespread metastatic disease shortly before or after the diagnosis of CSM.
In our study population, the median CMetFI was over 5 months (160 days), but the CMetFI varied considerably between dogs, from 0 days up to 18 months (542 days). In humans, the time between the initial diagnosis of OSA and the detection of skin metastasis is also quite variable and ranges from 0 days to 24 years.24, 25, 26, 27, 29, 30, 33, 34, 35 In the 6 previously reported dogs, the CMetFI ranged from 0 days to 40 months.6, 12, 16, 17, 18 Given the unpredictable timeline of when CSM might develop, any new cutaneous or subcutaneous mass should be investigated in dogs with appendicular OSA, regardless of the time elapsed since the initial diagnosis.
Dogs in our study had varying treatments for CSM. When separating dogs into different groups based on treatment, the sample size was small. Therefore, comparisons of treatment groups via log‐rank testing should be interpreted with caution. It is worth noting that none of the 10 dogs treated with chemotherapy or RT for gross CSM had a clinical response according to the RECIST criteria.36 Furthermore, the 4 dogs treated with surgery and adjuvant chemotherapy had a CMetST of 3 months (94 days), which was significantly longer than dogs that did not receive such specific treatments to address CSM (11 days). The median CMetST for dogs treated with surgery and adjuvant chemotherapy was not significantly different from the CMetST of dogs treated with chemotherapy alone (64 days). The longest surviving dog lived for 11 months (336 days) after diagnosis of CSM, and this dog had been treated with surgical removal of a single CSM followed by doxorubicin, toceranib phosphate, and L‐MTP. Ideally, additional studies including a larger number of dogs should be performed to investigate the most effective treatment for CSM from OSA.
In humans with CSM from OSA, the treatments pursued vary widely. Due to the frequent occurrence of widespread metastatic disease concurrent with CSM, chemotherapy is often recommended. Protocols have included high‐dose methotrexate, ifosfamide, doxorubicin, trimetrexate, bleomycin, cyclophosphamide, actinomycin‐D, and cisplatin25, 27, 28; however, systemic treatment alone has had limited efficacy in most reports. Considering the previously published cases of dogs with CSM, only 1 report described a curative‐intent surgical resection (3 cm margins) of all cutaneous masses.17 In other reported cases, cutaneous metastases were either not removed or biopsied only for diagnostic purposes.6, 12, 16, 18 Systemic treatment in these dogs varied, and it is not clear from these reports if any dog experienced a response to treatment.6, 12, 16, 17, 18
The prognosis for dogs with metastatic OSA is poor with a median survival of 76 days and a 1‐year survival rate of 6.6%.8 In this study, the median MetST was approximately 3 months (97 days), and the median CMetST was less than 2 months (55 days). Therefore, this report indicates that dogs with appendicular OSA that develop CSM have a similarly grave prognosis as dogs that develop other sites of metastasis. Nevertheless, only 3 dogs (15%) were euthanized for a cause directly related to development or progression of CSM. Most dogs were euthanized due to progressive metastasis to the lungs or bone, or noted general decline. The prognosis for humans with CSM from OSA is also guarded due to rapid progression of the disease and poor response to conventional chemotherapies.24, 25, 30, 33, 37
In our study, 66% of CSM were noted over the trunk and ventrum, while the head and neck region were affected in 24% of cases. In humans, skin metastasis from OSA seems to most commonly affect the scalp.24, 25, 28, 29, 30, 32, 35, 37, 38, 39, 40 No site of predilection was apparent from the 6 cases previously reported in dogs, as CSM were noted throughout the entire body16, 17; over the thigh,6, 18 hip, larynx, and tarsus12; or interscapular and dorsal lumbar areas.18 The least common location for CSM in our study was the limbs, representing only 10% of all CSM events.
In this study, CSM were most commonly firm, solitary masses, and were rarely associated with pain, inflammation, or ulceration. The subcutaneous tissue seemed to be affected more frequently than the dermis. On the other hand, most reports in people of CSM from OSA describe dermal, firm, erythematous, and painful lesions.24, 25, 26, 37, 41 Of the 6 reported cases in dogs, 4 cases had subcutaneous masses12, 16, 18 and 2 had dermal masses.6, 17 One report noted that the CSM were firm but not painful or inflamed.17 Based on our study, CSM from OSA in dogs might be less associated with inflammation and pain compared to lesions noted in humans.
Limitations of this study include its retrospective nature and inclusion of cases from multiple hospitals without standardization of treatment protocols. Included dogs received inconsistent staging diagnostics, and the majority did not have a necropsy performed. Thus, it is possible that some metastatic events were missed. Finally, all dogs in this study were euthanized, and, therefore, the date of death was partially owner‐dependent. Consequently, it is possible that rather than a true impact of therapeutic interventions, dogs that did not receive specific treatment for CSM had decreased survival due to pet owners electing euthanasia after discovering metastasis had occurred.
In conclusion, CSM in dogs with appendicular OSA can be the first metastatic site detected and can precede metastases to the lungs. In our study, CSM were more commonly solitary and occurred across the body with a possible predilection for the trunk and ventrum. The subcutaneous tissues appeared affected more commonly than the dermis. In addition, CSM is associated with a grave prognosis. In human medicine, it is recommended to investigate any skin lesion in a patient with OSA to rule out metastasis, regardless of the cancer status.42 Based on our findings, these recommendations should also apply to dogs with OSA.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Parachini‐Winter C, Curran KM, Pellin MK, et al. Cutaneous and subcutaneous metastasis of appendicular osteosarcoma in dogs: 20 cases. J Vet Intern Med. 2019;33:2200–2208. 10.1111/jvim.15557
Funding information Carlson College of Veterinary Medicine, Oregon State University, Corvallis, OR, Grant/Award Number: Internal teaching funds.
REFERENCES
- 1. Ehrhart NP, Ryan SD, Fan TM. Tumors of the skeletal system In: Withrow SJ, Vail DM, Page RL, eds. Withrow & MacEwen's Small Animal Clinical Oncology. Saint Louis: Elsevier Saunders Co; 2013:463‐503. [Google Scholar]
- 2. Hillers KR, Dernell WS, Lafferty MH, Withrow SJ, Lana SE. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986‐2003). J Am Vet Med Assoc. 2005;226:1364‐1367. [DOI] [PubMed] [Google Scholar]
- 3. Forrest LJ, Thrall DE. Bone scintigraphy for metastasis detection in canine osteosarcoma. Vet Radiol Ultrasound. 1994;35:124‐130. [Google Scholar]
- 4. McMahon M, Mathie T, Stingle N, Romansik E, Vail D, London C. Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med. 2011;25:511‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sacornrattana O, Dervisis NG, McNiel EA. Abdominal ultrasonographic findings at diagnosis of osteosarcoma in dogs and association with treatment outcome. Vet Comp Oncol. 2013;11:199‐207. [DOI] [PubMed] [Google Scholar]
- 6. Selmic LE, Griffin LR, Nolan MW, Custis J, Randall E, Withrow SJ. Use of PET/CT and stereotactic radiation therapy for the diagnosis and treatment of osteosarcoma metastases. J Am Anim Hosp Assoc. 2017;53:52‐58. [DOI] [PubMed] [Google Scholar]
- 7. Wallace M, Selmic L, Withrow SJ. Diagnostic utility of abdominal ultrasonography for routine staging at diagnosis of skeletal OSA in dogs. J Am Anim Hosp Assoc. 2013;49:243‐245. [DOI] [PubMed] [Google Scholar]
- 8. Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985‐2004). J Am Vet Med Assoc. 2006;228:1905‐1908. [DOI] [PubMed] [Google Scholar]
- 9. Swift KE, LaRue SM. Outcome of 9 dogs treated with stereotactic radiation therapy for primary or metastatic vertebral osteosarcoma. Vet Comp Oncol. 2018;16:E152‐E158. [DOI] [PubMed] [Google Scholar]
- 10. Turner H, Seguin B, Worley DR, et al. Prognosis for dogs with stage III osteosarcoma following treatment with amputation and chemotherapy with and without metastasectomy. J Am Vet Med Assoc. 2017;251:1293‐1305. [DOI] [PubMed] [Google Scholar]
- 11. Laver T, London CA, Vail DM, Biller BJ, Coy J, Thamm DH. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet Comp Oncol. 2018;16:E23‐e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liptak JM, Monnet E, Dernell WS, Withrow SJ. Pulmonary metastatectomy in the management of four dogs with hypertrophic osteopathy. Vet Comp Oncol. 2004;2:1‐12. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien MG, Straw RC, Withrow SJ, et al. Resection of pulmonary metastases in canine osteosarcoma: 36 cases (1983‐1992). Vet Surg. 1993;22:105‐109. [DOI] [PubMed] [Google Scholar]
- 14. Batschinski K, Dervisis NG, Kitchell BE. Evaluation of ifosfamide salvage therapy for metastatic canine osteosarcoma. Vet Comp Oncol. 2014;12:249‐257. [DOI] [PubMed] [Google Scholar]
- 15. Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin‐based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014;28:554‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniel GB, Avenell JS, Young K, et al. Scintigraphic detection of subcutaneous metastasis in a dog with appendicular osteosarcoma. Vet Radiol Ultrasound. 1996;37:146‐149. [Google Scholar]
- 17. Séllos F, Tostes R, Farias M, et al. Cutaneous metastasis of osteosarcoma in a dog: case report. Braz J Vet Res Anim Sci. 2000;38:240‐242. [Google Scholar]
- 18. Gorman E, Barger AM, Wypij JM, Pinkerton ME. Cutaneous metastasis of primary appendicular osteosarcoma in a dog. Vet Clin Pathol. 2006;35:358‐361. [DOI] [PubMed] [Google Scholar]
- 19. Knecht CD, Priester WA. Musculoskeletal tumors in dogs. J Am Vet Med Assoc. 1978;172:72‐74. [PubMed] [Google Scholar]
- 20. Sottnik JL, Rao S, Lafferty MH, et al. Association of blood monocyte and lymphocyte count and disease‐free interval in dogs with osteosarcoma. J Vet Intern Med. 2010;24:1439‐1444. [DOI] [PubMed] [Google Scholar]
- 21. Kuntz CA, Asselin TL, Dernell WS, et al. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg. 1998;27:417‐422. [DOI] [PubMed] [Google Scholar]
- 22. Ehrhart N, Dernell WS, Hoffmann WE, et al. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990‐1996). J Am Vet Med Assoc. 1998;213:1002‐1006. [PubMed] [Google Scholar]
- 23. Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med. 2000;14:587‐592. [DOI] [PubMed] [Google Scholar]
- 24. Choo JY, Lee JH, Lee JY, Park YM. Cutaneous metastasis of Giant cell‐rich osteosarcoma. Ann Dermatol. 2016;28:247‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collier DA, Busam K, Salob S. Cutaneous metastasis of osteosarcoma. J Am Acad Dermatol. 2003;49:757‐760. [DOI] [PubMed] [Google Scholar]
- 26. Herman TE, McAlister WH. Multifocal synchronous osteosarcoma with cutaneous and muscle metastases. Pediatr Radiol. 2004;34:671‐672. [DOI] [PubMed] [Google Scholar]
- 27. Myhand RC, Hung PH, Caldwell JB, James WD, Sau P, Hargis JB. Osteogenic sarcoma with skin metastases. J Am Acad Dermatol. 1995;32:803‐805. [DOI] [PubMed] [Google Scholar]
- 28. Stavrakakis J, Toumbis‐Ioannou E, Alexopoulos A, Rigatos GA. Subcutaneous nodules as initial metastatic sites in osteosarcoma. Int J Dermatol. 1997;36:606‐609. [DOI] [PubMed] [Google Scholar]
- 29. Delepine F, Leccia N, Schlatterer B, et al. Inaugural cutaneous metastases of an osteosarcoma: a case report. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:719‐723. [DOI] [PubMed] [Google Scholar]
- 30. Ragsdale MI, Lehmer LM, Ragsdale BD, Chow WA, Carson RT. Cutaneous metastasis of osteosarcoma in the scalp. Am J Dermatopathol. 2011;33:e70‐e73. [DOI] [PubMed] [Google Scholar]
- 31. Papachristou DJ, Goodman M, Cieply K, Rao UNM. Extraskeletal osteosarcoma of subcutaneous soft tissue with lymph node and skin metastasis: a case report with fluorescence in situ hybridization analysis. Pathol Oncol Res. 2012;18:107‐110. [DOI] [PubMed] [Google Scholar]
- 32. Ouseph MM, Sharan GK, Sharma P, et al. Osteosarcoma with cutaneous metastases: a case report. Acta Cytologica. 2007;51:102‐106. [DOI] [PubMed] [Google Scholar]
- 33. Setoyama M, Kanda A, Kanzaki T. Cutaneous metastasis of an osteosarcoma: a case report. Am J Dermatopathol. 1996;18:629‐632. [DOI] [PubMed] [Google Scholar]
- 34. Fernandez‐Pineda I, Bahrami A, Green JF, McGregor LM, Davidoff AM, Sandoval JA. Isolated subcutaneous metastasis of osteosarcoma 5 years after initial diagnosis. J Pediatr Surg. 2011;46:2029‐2031. [DOI] [PubMed] [Google Scholar]
- 35. Mariano FV, Correa MB, da Costa MV, et al. Labial mucosa metastasis of fibule giant cell‐rich osteosarcoma: an unusual presentation. Quintessence Int. 2013;44:783‐791. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a veterinary cooperative oncology group (VCOG) consensus document. Vet Comp Oncol. 2015;13:176‐183. [DOI] [PubMed] [Google Scholar]
- 37. Covello SP, Humphreys TR, Lee JB. A case of extraskeletal osteosarcoma with metastasis to the skin. J Am Acad Dermatol. 2003;49:124‐127. [DOI] [PubMed] [Google Scholar]
- 38. Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine‐needle aspiration cytology. Acta Cytol. 2017;61:47‐54. [DOI] [PubMed] [Google Scholar]
- 39. Lee WJ, Lee DW, Chang SE, et al. Cutaneous metastasis of extraskeletal osteosarcoma arising in the mediastinum. Am J Dermatopathol. 2008;30:629‐631. [DOI] [PubMed] [Google Scholar]
- 40. Fernandez‐Anton Martinez MC, Parra‐Blanco V, Aviles Izquierdo JA, et al. Cutaneous metastases of internal tumors. Actas Dermosifiliogr. 2013;104:841‐853. [DOI] [PubMed] [Google Scholar]
- 41. Vaidya S, Pritchard‐Jones K, Fisher C. Osteogenic sarcoma—cutaneous metastases. Med Pediatr Oncol. 2002;38:453‐454. [DOI] [PubMed] [Google Scholar]
- 42. Larsen S, Davis DM, Comfere NI, et al. Osteosarcoma of the skin. Int J Dermatol. 2010;49:532‐540. [DOI] [PubMed] [Google Scholar]


