Abstract
Background
Hepatocellular carcinoma (HCC) is the most common primary liver tumor in dogs. Abnormalities in hepatic copper, iron, zinc, and selenium concentrations increase risk for HCC development in other species, but trace mineral concentrations have not been evaluated in dogs with HCC.
Objectives
To investigate hepatic trace mineral concentrations in dogs with HCC.
Animals
Archived liver specimens from 85 dogs with HCC and 85 control dogs.
Methods
Retrospective case‐control study. A histopathology database was searched to identify dogs with HCC (test population) and an age‐matched control population. Demographic information was retrieved, and H&E and rhodanine stained slides were reviewed for all cases. Copper, iron, zinc, and selenium concentrations were determined in noncancerous liver tissues (test and control population) and in HCC tissues (test population) using inductively coupled plasma mass spectrometry.
Results
Hepatic copper concentrations (non‐neoplastic hepatic tissue) were greater in test population dogs (median, IQR; 294.9 μg/g, 233.5‐475.9 μg/g) than in control dogs (202.8 μg/g, 135.0‐295.3 μg/g; P < .001). Hepatic zinc concentrations in test (132.1 μg/g,108.6‐163.2 μg/g) and control dogs (151.5 μg/g, 117.1‐184.5 μg/g) also were different (P = .03). Within test population dogs, all trace mineral concentrations were decreased in the HCC tissue as compared to the non‐neoplastic hepatic tissue (all P < .001).
Conclusions and Clinical Importance
Hepatic copper accumulation and other abnormalities in hepatic trace mineral concentrations could be involved in the pathogenesis of HCC in some dogs.
Keywords: copper toxicosis, hepatitis, liver cancer, trace elements
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor in both dogs and humans.1, 2 It is the 5th most common cancer in men and the 7th most common in women, and nearly 10% of all new cases of cancer in humans are HCC.3, 4 The exact prevalence in dogs is unknown, but 0.5% of dogs undergoing necropsy at 1 institution had HCC.5 In humans, HCC is rapidly progressive and has 1 of the shortest survival times of any cancer in both adult males and females.2 Median survival times in dogs with HCC are less than 1 year without surgical intervention.1 The metastatic rate in dogs ranges from 37% to 100% with low‐grade and massive subtypes having a low metastatic rate as compared to high‐grade and diffuse forms.1
The epidemiology of HCC in humans has been well characterized, and HCC often occurs in the setting of advanced liver disease.2, 6, 7, 8, 9, 10 Viral hepatitis, alcohol abuse, metabolic syndrome, and cirrhosis independent of cause are common risk factors for HCC development.2 Imbalances in hepatic trace mineral concentrations also are associated with increased risk for HCC.7, 8 Humans with hemochromatosis, a hereditary disorder leading to excess hepatic iron concentrations, have a 20‐ to 200‐fold increased risk for HCC as compared to the general population.8, 11 Various rodent models of Wilson's disease, a hereditary disorder resulting in pathologic hepatic copper accumulation in humans, frequently develop HCC or cholangiocellular carcinoma, but a link between copper induced liver disease and HCC in actual Wilson's disease patients is controverisal.12, 13, 14, 15, 16 There are inverse relationships between hepatic selenium and zinc concentrations and HCC.10, 17, 18 Excess copper and iron induce oxidative stress in tissues, whereas both zinc and selenium have important antioxidant properties.19, 20, 21, 22 Oxidative stress and injury associated with these trace mineral abnormalities likely contribute to carcinogenesis.8, 17, 23
The pathogenesis of HCC in dogs is poorly characterized. However, trace mineral abnormalities have been increasingly recognized in dogs with noncancerous liver disease in recent years.24, 25 Copper‐associated hepatitis is one of the most common causes of progressive liver disease in dogs, and it shares many clinicopathologic features with Wilson's disease.24, 26 Hemochromatosis has not been described in dogs, but hepatic iron concentrations are often increased as a result of hepatitis.27 Although hepatic trace mineral concentrations are commonly investigated in dogs with inflammatory liver disease, similar investigations have not been reported in dogs with HCC. Trace mineral abnormalities could be involved in the pathogenesis of HCC in dogs considering our understanding of HCC in other species. The primary objectives of this study were to investigate hepatic copper, iron, zinc, and selenium concentrations in archived specimens from dogs with HCC and in an age‐matched control population. Hepatic specimens also were assessed for concurrent parenchymal disease because of the frequent associations of hepatitis and cirrhosis with HCC in other species.2 Our primary hypotheses were that hepatic copper and iron concentrations would be greater in dogs with HCC as compared to the control population, whereas hepatic selenium and zinc concentrations would be lower in dogs with HCC.
2. METHODS
2.1. Study design
A study of trace mineral concentrations in dogs with HCC was conducted using archived specimens from the Michigan State University Veterinary Diagnostic Laboratory (MSU‐VDL). The electronic histopathology database at the MSU‐VDL was searched to identify all cases in which HCC had been diagnosed between January 1, 2012, and March 1, 2018. Cases in which formalin‐fixed and paraffin‐embedded liver specimens were available and contained both neoplastic and non‐neoplastic hepatic tissues were included in the study (test population). An age‐ and time‐period‐matched control population was selected from the MSU‐VDL necropsy database using a random number generator. The general histopathology database was not utilized, as surgical biopsies are often performed for further evaluation of chronic hepatitis, which is frequently caused by copper‐associated hepatitis.24, 25 This would have selected a control population with a high rate of copper‐associated hepatitis and precluded accurate hypothesis testing. For the same reason, cases were excluded from the control population search if hepatic disease was listed as the primary reason for necropsy. The identification of hepatic disease (ie, incidentally discovered hepatic disease) on histopathologic assessment did not result in exclusion as long as hepatic disease was not the clinical reason for tissue evaluation. Information retrieved from the submission records for both populations included breed, age, sex, neuter status, and date of biopsy or necropsy. Within test population dogs, HCC cases were further classified into massive, nodular, or diffuse subtypes when diagnostic imaging and surgery reports were available for review.1
2.2. Hepatic histopathology and HCC characterization
Hematoxylin and eosin stained slides were reviewed by a board‐certified veterinary pathologist (R. C. Smedley) who was blinded to quantitative mineral concentrations. Slide review was performed to confirm and characterize HCC (test population) and to document concurrent hepatic pathology. Also, cases with marked autolysis, insufficient tissue for quantitative mineral determinations, severe freeze‐thaw artifact, and marked loss of hepatocytes within normal tissue caused by compression atrophy and replacement by fibrous connective tissue or massive necrosis were excluded as they were likely to preclude accurate histologic assessment or accurate determination of mineral concentrations.28, 29 Sections of both neoplastic and non‐neoplastic hepatic tissue were evaluated for HCC cases, and representative sections of liver tissue were evaluated for control cases. HCCs were classified as well differentiated, moderately differentiated, or poorly differentiated.30, 31 Briefly, well‐differentiated HCCs are defined as being composed of trabeculae of neoplastic hepatocytes that closely resemble normal hepatocytes, whereas poorly differentiated HCCs are composed of pleomorphic neoplastic cells that are not easily recognized as hepatocellular in origin. Poorly differentiated neoplasms are more likely to contain giant cells and have a higher mitotic count than well‐differentiated and moderately differentiated forms. Moderately differentiated neoplasms have intermediate features between well‐differentiated and poorly differentiated neoplasms. This category primarily was used for more solid neoplasms that lacked the degree of cellular pleomorphism seen in poorly differentiated neoplasms. Hepatic tissues in both test and control populations were assessed for vacuolation, fibrosis, necrosis, bile duct proliferation, and inflammation according to World Small Animal Veterinary Association guidelines.32 Rhodanine stained slides were reviewed for all cases and scored for copper accumulation using slight adaptations of a previously described scoring system.33 Scores ranged from 0 (no detected copper granules) to 5 (panlobular presence of large numbers of copper granules in hepatocytes). An additional score of 1.5 was included to describe liver tissue in which there were greater than 2 hepatocytes within a 10× field with copper accumulation, which would be greater than grade 1, but that did not meet the criteria needed for a grade 2, which is defined as obvious small to moderate numbers of copper granules in <50% of zone 3 hepatocytes (or < 4 clusters/10× field).
2.3. Quantitative mineral analysis
Representative sections of hepatic tissue from both test and control population cases were obtained for mineral determinations, and a representative section of neoplastic tissue from test population cases also was obtained for mineral determinations. Hepatic copper, iron, zinc, and selenium concentrations were determined in the tissue specimens using slight modifications of previously described methodology for deparaffinization and analysis.25 Briefly, tissues were removed from the specimen blocks by melting the wax if the whole block was used or by using a disposable biopsy punch (Sklar Tru‐Punch, Sklar Instruments, West Chester, PA) to remove the desired sections. The tissues were deparaffinized by adding 900 μL of CitriSolv and vortexing until the paraffin was completely dissolved. Samples then were centrifuged for 5 minutes at 21 000g, and the supernatant was removed. The pellet was washed with 900 μL of ethanol, vortexed, and again centrifuged at 21 000g for 5 minutes. Excess ethanol was removed, a second ethanol wash was completed, and samples were air‐dried. The deparaffinized tissues were dried overnight in a 95°C oven. The next day, samples were digested overnight in a 95°C oven, using at least 10× the dry tissue mass of nitric acid. The digested samples were then diluted with water to at least 100× the dried tissue mass. Elemental analysis followed the method of Wahlen et al using an Agilent 7500 inductively coupled plasma‐mass spectrometer (ICP‐MS; Agilent 7500ce ICP‐MS, Agilent Technologies, Santa Clara, California).34 Additional descriptions of the ICP‐MS methodology can be found elsewhere,25 and in‐house, single laboratory validation statements can be found on file at the MSU‐VDL, which is an American Association of Veterinary Laboratory Diagnosticians (AAVLD) accredited laboratory.
2.4. Data analysis
Data sets were evaluated for normality using Shapiro‐Wilk testing and boxplot analyses. Normally distributed data were characterized by means and SD, whereas ordinal and non‐normally distributed data were characterized by medians and interquartile ranges (IQR). Statistical analyses were performed to evaluate potential differences in hepatic trace mineral concentrations and rhodanine scores between test and control populations using Mann‐Whitney U testing. Within the test population, trace mineral concentrations and rhodanine scores also were compared between neoplastic tissue and non‐neoplastic hepatic tissue using Wilcoxon signed‐rank testing. Proportionate differences related to mineral concentrations and histologic features were compared between test and control populations using Fisher exact testing. Statistical analyses were performed with commercially available software (GraphPad Prism Version 6.0, Graphpad Software Inc, La Jolla, CA), and for all analyses, P ≤.05 was considered significant.
3. RESULTS
3.1. Animal demographics
One hundred seventy dogs—85 test population dogs and 85 control population dogs—were included in this study. The test population consisted of 25 mixed breed dogs, 9 Golden Retrievers, 6 Labrador Retrievers, 5 Fox Terriers, and 4 Beagles. The control population consisted of 18 mixed breed dogs, 6 Bernese Mountain Dogs, 5 German Shepherds, 5 Pugs, and 4 Labrador Retrievers. All other breeds were represented by 3 or fewer cases. There was a greater proportion of Golden Retrievers in the test population (9 of 85 dogs) as compared to the control population (1 of 85 dogs; P = .02). The mean ± SD age of dogs was 10.6 ± 1.9 years in both populations. There were 44 male dogs (1 intact, 43 neutered) and 41 female dogs (1 intact, 40 spayed) in the test population whereas there were 46 male dogs (9 intact, 33 neutered, 4 unknown) and 39 female dogs (10 intact, 27 spayed, 2 unknown) in the control population. There were more spayed or neutered dogs in the test population (83 of 85 dogs) than in the control population (60 of 79 dogs; P < .001). Within the test population, clinical HCC characterization was possible for 41 cases, 39 of which had massive HCC, whereas diffuse and nodular subtypes were represented by 1 dog each.
3.2. Histologic features
In the representative sections examined, 82 of the 85 HCC cases were classified histologically as well‐differentiated HCC. Fifteen of the well‐differentiated HCC had marked vacuolation. Two of the 85 HCC cases were moderately differentiated, and 1 was poorly differentiated with marked vacuolation. Evaluation of the non‐neoplastic hepatic tissue in the test population dogs revealed few histologic abnormalities. Three test population dogs had evidence of chronic hepatitis. Fourteen dogs had vacuolar hepatopathy. Other lesions included pigmented granulomas (6 dogs), varying degrees of periportal to bridging fibrosis (6 dogs), varying degrees of necrosis (4 dogs), regenerative nodule formation (3 dogs), bile duct hyperplasia (2 dogs), extrahepatic cholestasis (2 dogs), dilated lymphatics (1 dog), severe bridging fibrosis with regenerative nodule formation and stromal collapse (1 dog), and extramedullary hematopoiesis (1 dog). In the control population, hepatic histologic abnormalities included varying degrees of periportal to bridging fibrosis (3 dogs), hyperplastic nodules (3 dogs), vacuolar hepatopathy (2 dogs), and chronic hepatitis (1 dog). Vacuolar hepatopathy was more common in test population dogs (16.5%) than in control dogs (2.4%; P = .003).
3.3. Mineral concentrations and rhodanine scoring
Mineral concentration determinations and rhodanine scoring were performed on non‐neoplastic hepatic tissue in test population dogs, HCC (neoplastic) tissue in test population dogs, and hepatic tissue in control population dogs. The median hepatic copper concentration (non‐neoplastic tissue) of 294.9 μg/g (IQR, 233.5‐475.9 μg/g) in test population dogs was greater than the median hepatic copper concentration of 202.8 μg/g (IQR, 135.0.‐295.3 μg/g) in control population dogs (P < .001; Figure 1). Twenty‐nine of 85 (34%) test population dogs had hepatic copper concentrations (non‐neoplastic tissue) > 400 μg/g whereas only 12 of 85 (14%) control population dogs had hepatic copper concentrations >400 μg/g (P = .004). Eight of 85 (9%) test population dogs and 2 of 85 (2%) control dogs had hepatic copper concentrations >700 μg/g (P = .10), and only 1 dog in each population had copper concentrations exceeding 1000 μg/g. The median hepatic copper concentrations in the non‐neoplastic hepatic tissue of dogs with well‐differentiated (n = 82) and moderately or poorly differentiated (n = 3) HCC were 295.7 μg/g (IQR, 233.5‐475.9 μg/g) and 276.4 μg/g (range, 203.7‐382.5 μg/g), respectively. Copper concentrations in the non‐neoplastic hepatic tissue of test population dogs with vacuolar hepatopathy (median, 255.6 μg/g; IQR, 205.2‐333.9 μg/g) and without vacuolar hepatopathy (median, 313.1 μg/g; IQR, 232.6‐479.9 μg/g) were not different (P = .12). Copper concentrations in the neoplastic HCC tissue were less than the non‐neoplastic hepatic tissue in test population dogs (P < .001; Figure 1).
Figure 1.
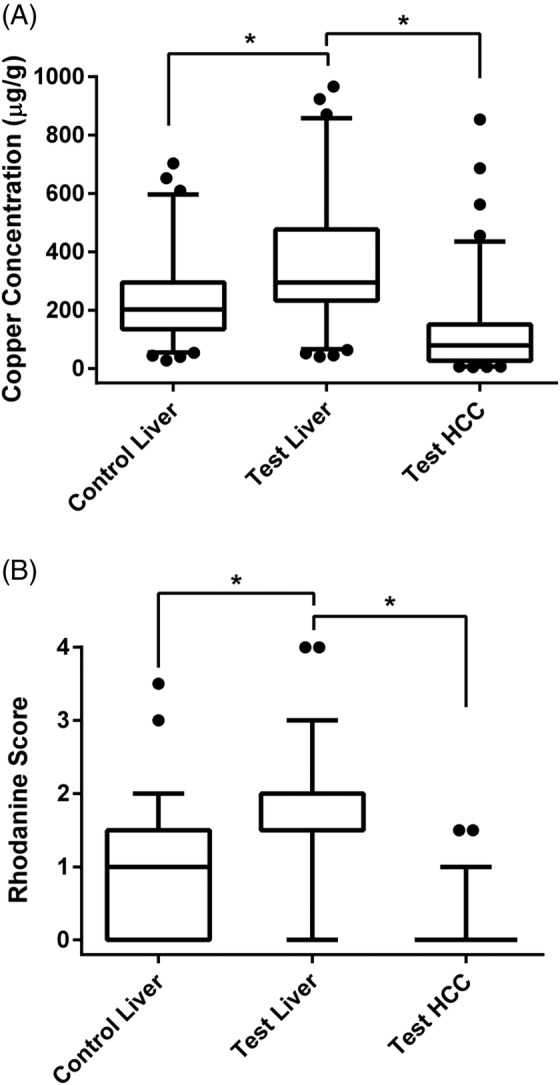
Box and whisker plot depicting copper concentrations (A) and rhodamine scores (B) in test and control population dogs. The horizontal line within each box represents the median; the lower and upper boundaries of each box represent the first and third quartiles; and the whiskers represent the 5th to 95th percentile range. Dots represent individual values outside of this range. Two dogs (1 test liver and 1 control liver) with copper concentrations >1000 μg/g are not depicted in Figure 1A, but they were included in analyses. Control liver, hepatic tissue from control population dogs; Test liver, non‐neoplastic hepatic tissue from test population dogs; Test HCC, neoplastic tissue from test population dogs. *P < .001
Qualitative copper assessments also were different between populations as the median rhodanine score of 1.5 (IQR, 1.5‐2.0) in non‐neoplastic hepatic tissue of test population dogs was greater than the median rhodanine score of 1 (IQR, 0.0‐1.5) in control population dogs (P < .001; Figure 1). The median rhodanine score of 0 (IQR, 0‐0) in the neoplastic hepatic tissues was also less than the median rhodanine score of 1.5 (IQR, 1.5‐2) in the non‐neoplastic hepatic tissue in test population dogs (P < .001). Lobular localization of copper in the non‐neoplastic liver tissue also was assessed in the 78 test population dogs with rhodanine scores ≥1. Fifty‐four of 78 cases had random patchy localization of copper, 22 cases had a predominantly centrilobular localization, 1 case had periportal localization, and 1 case had centrilobular and periportal localization. In the control population, 56 dogs had rhodanine scores ≥1. This included 41 dogs with random patchy localization and 14 dogs with primarily centrilobular localization. No control livers had periportal localization.
The median hepatic zinc concentration of 132.1 μg/g (IQR, 108.6‐163.2 μg/g) in non‐neoplastic hepatic tissue of test population dogs was less than the median hepatic zinc concentration of 151.5 μg/g (IQR, 117.1‐184.5 μg/g) in control population dogs (P = .03). Although median zinc concentrations were different, the proportion of test (5 of 85) and control (1 of 85) dogs with hepatic zinc concentrations less than the lower‐limit of the reference interval were not different (P = .21). Hepatic iron and selenium concentrations were not different between test and control population dogs (Table 1). Within the test population, concentrations of iron, zinc, and selenium were greater in the non‐neoplastic hepatic tissue as compared to the neoplastic tissue (P < .001 for all comparisons; Table 1).
Table 1.
Trace mineral concentrations in test and control population dogs
| Mineral concentrations (μg/g) | |||
|---|---|---|---|
| Mineral (RI) | Control liver | Test liver | Test HCC |
| Zinc (75‐225 μg/g) | 151.5† | 132.1*, † | 93.8* |
| (117.1‐184.5) | (108.6‐163.2) | (72.2‐134.3) | |
| Selenium (1.5‐4.3 μg/g) | 2.1 | 2.0* | 1.3* |
| (1.8‐2.5) | (1.6‐2.4) | (1.1‐1.7) | |
| Iron (500‐4150 μg/g) | 1691 | 1716* | 947* |
| (1019‐2441) | (1003‐2473) | (563‐1463) | |
Note: Values represent the median concentrations (μg/g) for each mineral, and the IQR is provided in parentheses. Control liver, hepatic tissue from the control population dogs; Test liver, non‐neoplastic hepatic tissue from the test population dogs; Test HCC, neoplastic tissue from test population dogs.
Abbreviation: HCC, hepatocellular carcinoma; RI, reference interval.
P < .001.
P = .03.
4. DISCUSSION
This study provides unique insight into hepatic trace mineral concentrations in dogs with HCC. Hepatic copper concentrations were 45% greater in dogs with HCC as compared to controls, and the proportion of test population dogs with copper concentrations exceeding 400 μg/g was 2.4 times that of the control population. Reasons for differences between populations are unclear because of the cross‐sectional study design, and it is important to note that approximately 65% of test population dogs had hepatic copper concentrations considered to be normal. Copper is an important transition metal that acts as a cofactor for hundreds of enzymatic reactions throughout the body, but excessive tissue copper promotes free radical formation.35, 36 This sustained environment of oxidative stress and subsequent hepatocyte injury contributes to carcinogenesis in rodent models of copper overload.13, 37, 38 One possibility is that increased hepatic copper contributed to carcinogenesis in some of the dogs in this study. It is also possible that increased hepatic copper in test population dogs occurred as a result of the HCC rather than being present before tumor development. An expanding HCC could result in cholestasis and impaired biliary copper excretion, but dogs are generally resistant to cholestasis‐induced copper accumulation.39 Other environmental factors could have independently increased HCC risk while simultaneously increasing hepatic copper stores. Our results only establish that copper concentrations were different between test and control populations, and an evaluation of HCC prevalence in dogs with and without increased hepatic copper concentrations as well as a longitudinal investigation of dogs with varying hepatic copper loads would be necessary to further characterize potential relationships between HCC and copper in dogs.
Hepatocellular carcinomas typically occur in the setting of chronic hepatitis and cirrhosis in both rodents and humans.2, 8, 15, 16 The majority of dogs in our study did not have chronic hepatitis which is consistent with earlier reports of HCC in dogs.5, 40 It is unknown if oxidative stress associated with hepatic trace mineral imbalances can induce carcinogenesis without concurrent inflammation. The magnitude of copper accumulation in test population dogs also is mild considering that copper concentrations frequently exceed 1000 μg/g in dogs with copper‐associated hepatitis.24, 41 Conversely, there is no established threshold at which copper predictably results in liver damage, and hepatic copper concentrations in the range of 400 to 1000 μg/g have been associated with inflammation in some dogs.42, 43 It is also important to note that the absence of inflammation does not preclude the possibility of tissue damage. Various noninflammatory histologic abnormalities frequently are observed in the liver of Wilson's disease patients.44 Routine liver histology is even normal in some Wilson's disease patients despite presence of ultrastructural abnormalities.44 Similarly, genes associated with oxidative stress are differentially expressed in histologically normal hepatic tissue from Labrador Retrievers with increased hepatic copper stores as compared to Labrador Retrievers with normal copper concentrations.45 However, studies of the cellular consequences and long‐term clinical effects of mild to moderate copper accumulation in dogs without hepatitis are limited.
Hepatic zinc concentrations were lower in test population dogs than in control population dogs. Although a statistical difference between populations was detected, the exact clinical implication is unclear as only 5 of 85 test population dogs had hepatic zinc concentrations less than the reference interval. Trace mineral concentrations were substantially less in neoplastic tissue as compared to non‐neoplastic hepatic tissue within test population dogs. Perhaps mutated or defective mineral transport proteins or even an accelerated metabolic rate within the neoplastic tissue contributed to decreased trace mineral concentrations. A similar finding is observed in some rodent models of copper overload.13 These results could have therapeutic implications. Selenium deficiency contributes to oxidative stress and enhances tumor progression whereas selenium supplementation can slow HCC progression.46, 47 Copper is essential for angiogenesis, which typically is accelerated in solid tumors.48 A small reduction in copper within HCC tissue could delay tumor growth whereas a reduction of similar magnitude in the non‐neoplastic parenchyma would be unlikely to have adverse clinical effects given the already increased copper concentrations. Indeed, copper chelation is being investigated as an adjunctive antineoplastic agent in multiple species including humans and dogs.49, 50
The increased proportions of sexually altered dogs and Golden Retrievers in the test population were unexpected findings. Golden Retrievers are predisposed to other malignancies, but they are not considered to be predisposed to pathologic copper accumulation.51 Neuter status might be a risk factor for some malignancies in dogs, and gonadal and related hormones are involved in the pathogenesis of HCC in humans.52, 53 Androgens can activate transcription of oncoproteins in viral hepatitis whereas estradiol and estrogen receptors are thought to protect hepatocytes from oxidative stress.53, 54 A sex disparity was not apparent in our report, but the possibility for hormonal factors in HCC epidemiology in dogs is intriguing. The breed and neuter findings should be interpreted cautiously though as utilization of a necropsy control population could have introduced an unknown bias. For instance, owners of an expensive pure breed dog might be more likely to obtain an ante‐mortem diagnosis for any serious health condition, thereby precluding postmortem evaluation. A similar phenomenon could exist for neuter status. Consequently, the association of Golden Retrievers and neuter status with HCC should be evaluated in larger population‐based studies.
A limitation of our study is that well‐differentiated HCCs were overrepresented (82 of 85 cases). Dogs with massive forms of HCC also appeared to be overrepresented (39 of 41 cases) although this clinical classification was not possible for many dogs. Dogs with diffuse or poorly differentiated forms of HCC, which frequently metastasize,1 might be less likely to undergo surgical intervention or biopsy. Obvious differences in copper concentrations between the tumor subtypes were not apparent, but applications of our results to dogs with poorly differentiated or nonmassive forms of HCC should be done with caution because of the limited sample size. Another limitation is that many risk factors for HCC exist in other species, and diet, environment, comorbid conditions, and medication history were not evaluated in our study. Although these variables should be considered in future studies, this information was not available for many cases and was beyond the scope of our investigation.
In summary, hepatic copper concentrations in dogs with HCC were increased compared to the control population as demonstrated by both quantitative mineral determinations and scoring of rhodanine stained slides, but the nature of this relationship cannot be determined. Additional studies are needed to elucidate potential roles of hepatic trace mineral abnormalities in the development, progression, and outcome of HCC in dogs.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Portions of this work have been presented in poster form at the Michigan State University College of Veterinary Medicine annual Phi Zeta Research Day in October, 2018.
Harro CC, Smedley RC, Buchweitz JP, Langlois DK. Hepatic copper and other trace mineral concentrations in dogs with hepatocellular carcinoma. J Vet Intern Med. 2019;33:2193–2199. 10.1111/jvim.15619
Funding information Michigan State University College of Veterinary Medicine Trinket Fund; National Institutes of Health, Grant/Award Number: T35OD016477‐14
REFERENCES
- 1. Balkman C. Hepatobiliary neoplasia in dogs and cats. Vet Clin North Am Small Anim Pract. 2009;39:617‐625. [DOI] [PubMed] [Google Scholar]
- 2. Kew MC. Hepatocellular carcinoma: epidemiology and risk factors. J Hepatocell Carcinoma. 2014;1:115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferlay J, Shin H, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893‐2917. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 5. Patnaik AK, Hurvitz AI, Lieberman PH, Johnson GF. Canine hepatocellular carcinoma. Vet Pathol. 1981;18:427‐438. [DOI] [PubMed] [Google Scholar]
- 6. Flores A, Marrero JA. Emerging trends in hepatocellular carcinoma: focus on diagnosis and therapeutics. Clin Med Insights Oncol. 2014;8:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Himoto T, Fujita K, Nomura T, et al. Roles of copper in hepatocarcinogenesis via the activation of hypoxia‐inducible factor‐1α. Biol Trace Elem Res. 2016;174:58‐64. [DOI] [PubMed] [Google Scholar]
- 8. Radford‐Smith DE, Powell EE, Powell LW. Haemochromatosis: a clinical update for the practising physician. Intern Med J. 2018;48:509‐516. [DOI] [PubMed] [Google Scholar]
- 9. Wachsmann J, Peng F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J Gastroenterol. 2016;22:221‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Z, Bi M, Liu Q, Yang J, Xu S. Meta‐analysis of the correlation between selenium and incidence of hepatocellular carcinoma. Oncotarget. 2016;7:77110‐77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fracanzani AL, Conte D, Fraquelli M, et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non‐iron related chronic liver disease. Hepatology. 2001;33:647‐651. [DOI] [PubMed] [Google Scholar]
- 12. Huster D, Finegold MJ, Morgan CT, et al. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol. 2006;168:423‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sawaki M, Enomoto K, Hattori A, Tsuzuki N, Sugawara N, Mori M. Role of copper accumulation and metallothionein induction in spontaneous liver cancer development in LEC rats. Carcinogenesis. 1994;15:1833‐1837. [DOI] [PubMed] [Google Scholar]
- 14. van Meer S, de Man RA, van den Berg AP, et al. No increased risk of hepatocellular carcinoma in cirrhosis due to Wilson disease during long‐term follow‐up. J Gastroenterol Hepatol. 2015;30:535‐539. [DOI] [PubMed] [Google Scholar]
- 15. Walshe JM, Waldenström E, Sams V, et al. Abdominal malignancies in patients with Wilson's disease. QJM. 2003;96:657‐662. [DOI] [PubMed] [Google Scholar]
- 16. Xu R, Hajdu CH. Wilson disease and hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2008;4:438‐439. [PMC free article] [PubMed] [Google Scholar]
- 17. Ebara M, Hiroyuki F, Ryoji H, et al. Metal contents in the liver of patients with chronic liver disease caused by hepatitis C virus: reference to hepatocellular carcinoma. Oncology. 2003;65:323‐330. [DOI] [PubMed] [Google Scholar]
- 18. Maeda T, Shimada M, Harimoto N, et al. Role of tissue trace elements in liver cancers and non‐cancerous liver parenchyma. Hepatogastroenterology. 2005;52:187‐190. [PubMed] [Google Scholar]
- 19. Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147‐163. [DOI] [PubMed] [Google Scholar]
- 20. Pietrangelo A. Iron, oxidative stress, and liver fibrogenesis. J Hepatol. 1998;28:8‐13. [DOI] [PubMed] [Google Scholar]
- 21. Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130:1447S‐1454S. [DOI] [PubMed] [Google Scholar]
- 22. Yao HD, Wu Q, Zhang ZW, et al. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochem Biophys Acta. 2013;1830:3112‐3120. [DOI] [PubMed] [Google Scholar]
- 23. Siziba K, Asare GA, Mossanda KS, et al. Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology. 2005;219:41‐52. [DOI] [PubMed] [Google Scholar]
- 24. Dirksen K, Fieten H. Canine copper‐associated hepatitis. Vet Clin North Am Small Anim Pract. 2017;47:631‐644. [DOI] [PubMed] [Google Scholar]
- 25. Strickland JM, Buchweitz JP, Smedley RC, et al. Hepatic copper concentrations in 546 dogs (1982‐2015). J Vet Intern Med. 2018;32:1943‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu X, Leegwater PA, Fieten H. Canine models for copper homeostasis disorders. Int J Mol Sci. 2016;17:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cedeño Y, López‐Alonso M, Miranda M. Hepatic concentrations of copper and other metals in dogs with and without chronic hepatitis. J Small Anim Pract. 2016;57:703‐709. [DOI] [PubMed] [Google Scholar]
- 28. Thornburg LP, Shaw D, Dolan M, et al. Hereditary copper toxicosis in West Highland White Terriers. Vet Pathol. 1986;23:148‐154. [DOI] [PubMed] [Google Scholar]
- 29. Miyamura H, Nakanuma Y, Kono N. Survey of copper granules in liver biopsy specimens from various liver abnormalities other than Wilson's disease and biliary diseases. Gastroenterol Jpn. 1988;23:633‐638. [DOI] [PubMed] [Google Scholar]
- 30. Kondo F, Wada K, Nagato Y, et al. Biopsy diagnosis of well‐differentiated hepatocellular carcinoma based on new morphologic criteria. Hepatology. 1989;9:751‐755. [DOI] [PubMed] [Google Scholar]
- 31. Head KW, Cullen JM, Dubielzig RR, et al. Histological classification of tumors of the alimentary system of domestic animals In: Head KW, ed. Histologic Classification of Tumors of the Alimentary System of Domestic Animals. 2nd ed. Washington DC: Armed Forces Institute of Pathology;2003:122‐124. [Google Scholar]
- 32. Van den Ingh TSGAM, Van Winkle TJ, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver In: Rothuizen J, Cullen JM, eds. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. 1st ed. Philadelphia: Saunders Elsevier;2006:85‐101. [Google Scholar]
- 33. Center SA, McDonough SP, Bogdanovic L. Digital image analysis of rhodanine‐stained liver biopsy specimens for calculation of hepatic copper concentrations in dogs. Am J Vet Res. 2013;74:1474‐1480. [DOI] [PubMed] [Google Scholar]
- 34. Wahlen R, Evans L, Turner J, Hearn R. The use of collision/reaction cell ICP‐MS for the determination of elements in blood and serum samples. Spectroscopy. 2005;20:84‐89. [Google Scholar]
- 35. Sakurai H, Satoh H, Hatanaka A, et al. Unusual generation of hydroxyl radicals in hepatic copper‐metallothionein of LEC (Long‐Evans Cinnamon) rats in the presence of hydrogen peroxide. Biochem Biophys Res Commun. 1994;199:313‐318. [DOI] [PubMed] [Google Scholar]
- 36. Suzuki K, Rui M, Ueda J, Ozawa T. Production of hydroxyl radicals by copper‐containing metallothionein: roles as prooxidant. Toxicol Appl Pharmacol. 1996;141:231‐237. [PubMed] [Google Scholar]
- 37. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals, and antioxidants in oxidative stress‐induced cancer. Chem Biol Interact. 2006;160:1‐40. [DOI] [PubMed] [Google Scholar]
- 38. Mori M, Hattori A, Sawaki M, et al. The LEC rat: a model for human hepatitis, liver cancer, and much more. Am J Pathol. 1994;141:200‐204. [PMC free article] [PubMed] [Google Scholar]
- 39. Azumi N. Copper and liver injury‐‐experimental studies on the dogs with biliary obstruction and copper loading. Hokkaido Igaku Zasshi. 1982;57:331‐349. [PubMed] [Google Scholar]
- 40. Trigo FJ, Thompson H, Breeze RG, Nash AS. The pathology of liver tumours in the dog. J Comp Pathol. 1982;92:21‐39. [DOI] [PubMed] [Google Scholar]
- 41. Langlois DK, Smedley RC, Schall WD, Kruger JM. Acquired proximal renal tubular dysfunction in 9 Labrador Retrievers with copper‐associated hepatitis (2006‐2012). J Vet Intern Med. 2013;27:491‐499. [DOI] [PubMed] [Google Scholar]
- 42. Mandigers PJ, van den Ingh TS, Bode P, Rothuizen J. Improvement in liver pathology after 4 months of D‐penicillamine in 5 doberman pinschers with subclinical hepatitis. J Vet Intern Med. 2005;19:40‐43. [DOI] [PubMed] [Google Scholar]
- 43. Fieten H, Biourge VC, Watson AL, Leegwater PAJ, van den Ingh TSGAM, Rothuizen J. Nutritional management of inherited copper‐associated hepatitis in the Labrador Retriever. Vet J. 2014;199:429‐433. [DOI] [PubMed] [Google Scholar]
- 44. Pronicki M. Wilson disease – liver pathology. Handb Clin Neurol. 2017;142:71‐75. [DOI] [PubMed] [Google Scholar]
- 45. Dirksen K, Spee B, Penning LC, et al. Gene expression patterns in the progression of canine copper‐associated chronic hepatitis. PLoS One. 2017;12(5):e0176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Novotny L, Rauko P, Kombian SB, Edafiogho IO. Selenium as a chemoprotective anti‐cancer agent: reality or wishful thinking? Neoplasma. 2010;57:383‐391. [DOI] [PubMed] [Google Scholar]
- 47. Rohr‐Udilova N, Sieghart W, Eferl R, et al. Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology. 2012;55:1112‐1121. [DOI] [PubMed] [Google Scholar]
- 48. Raju KS, Alessandri G, Ziche M, Gullino PM. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982;69:1183‐1188. [PubMed] [Google Scholar]
- 49. Kent MS, Madewell BR, Dank G, Dick R, Merajver SD, Brewer GJ. An anticopper antiangiogenic approach for advanced cancer in spontaneously occurring tumors using tetrathiomolybdate: a pilot study in a canine model. J Trace Elem Exp Med. 2004;17:9‐20. [Google Scholar]
- 50. Xu M, Casio M, Range DE, Sosa JA, Counter CM. Copper chelation as targeted therapy in a mouse model of oncogenic BRAF‐driven papillary thyroid cancer. Clin Cancer Res. 2018;24:4271‐4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dobson JM. Breed‐predispositions to cancer in pedigree dogs. ISRN Vet Sci. 2012;2013:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith AN. The role of neutering in cancer development. Vet Clin North Am Small Anim Pract. 2014;44:965‐975. [DOI] [PubMed] [Google Scholar]
- 53. Ruggieri A, Gagliardi MC, Anticoli S. Sex‐dependent outcome of hepatitis B and C viruses infections: synergy of sex hormones and immune responses? Front Immunol. 2018;9:2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu Z, Liu J, Jianxin C, Yongliang Z, Pan X. 17β‐Estradiol inhibits testosterone‐induced cell proliferation in HepG2 by modulating the relative ratios of 3 estrogen receptor isoforms to the androgen receptor. J Cell Biochem. 2018;119:8659‐8671. [DOI] [PubMed] [Google Scholar]


