Abstract
Background
Development of management strategies for lumbosacral stenosis in dogs is hampered by the lack of objective diagnostic criteria and outcome measures.
Objective
To explore the suitability of electrodiagnostic tests as ancillary diagnostic aids, inclusion criteria, or outcome measures.
Sample population
Sixty‐one client‐owned dogs with clinical signs of lumbosacral foraminal stenosis.
Methods
A blinded, cross‐sectional cohort study. Fifty‐one dogs exhibiting apparent lumbosacral pain or pelvic limb lameness with no detected orthopedic cause had blinded review of magnetic resonance imaging (MRI), allowing classification as affected with foraminal stenosis (25 dogs), unaffected (20 dogs), or another diagnosis (6 dogs). The presence of electromyographic changes and tibial neurography variables were compared between groups.
Results
Cord dorsum potential onset latency, F‐wave onset latency (both corrected for limb length), and F‐ratio were increased in dogs with lumbosacral foraminal stenosis versus those without, although there was overlap of the values between groups. The proportion of dogs with electromyographic changes was not significantly greater in MRI‐affected dogs.
Conclusion
Electrophysiological testing is a useful ancillary test, either to provide stricter inclusion criteria and outcome measures or to aid clinical decision‐making in equivocal cases.
Keywords: dog, electrodiagnostics, electromyography, lumbosacral, radiculopathy
Abbreviations
- CDP
cord dorsum potential
- CMAP
compound motor action potential
- DLSS
degenerative lumbosacral stenosis
- EMG
electromyography
- MRI
magnetic resonance imaging
1. INTRODUCTION
The term degenerative lumbosacral stenosis (DLSS) describes an acquired condition in which the vertebral canal, the intervertebral foramina at L7‐S1, or a combination of both structures become narrowed by a combination of intervertebral disc degeneration and protrusion plus bone and soft tissue proliferation, which might be exacerbated by intervertebral movement and congenital stenosis.1 Degenerative stenosis can lead to compression of the nerve roots traversing this joint space; most frequently, the L7 spinal nerve but also the sacral nerves. This can cause clinical signs consistent with lower motor neuron paresis (muscle atrophy, hyporeflexia of the pelvic limbs, tail, or anus with or without fecal or urinary incontinence), pain (lameness, reluctance to jump, climb or stand, spontaneous vocalization, or excessive reaction to manipulation), or a combination of both. A substantial proportion (10%‐30%)1 of dogs fail to improve with surgical or medical treatment, and recurrence reported between 3% and 27% in those dogs that do.2, 3, 4
Several obstacles impede development of effective management strategies for DLSS,5 including that (i) objective outcome measures are not widely used and most studies rely upon subjective, nonblinded owner or veterinary assessment; (ii) the disease can be ill‐defined, so studies include a heterogeneous mix of conditions affecting the L7‐S1 junction meaning meta‐analysis of the data is impossible; and (iii) magnetic resonance imaging (MRI) is generally used as the definitive diagnostic test, despite having only moderate ability to detect a cause of low back pain in people.
Electrodiagnostic tests could partially overcome these obstacles because they can objectively assess neurologic function. Reduced function of afferent (sensory) axons and the dorsal nerve root can delay the onset or reduce the amplitude of the cord dorsum potential (CDP).6, 7 Reduced function of efferent (motor) axons and the ventral nerve root can cause spontaneous activity on electromyography (EMG) of innervated muscles, reduce the amplitude of the compound motor action potential (CMAP) and delay the onset of the F‐wave, reduce its latency or increase its duration.8, 9, 10
Although it is established that EMG and CDPs are abnormal in some dogs with DLSS,11, 12, 13, 14 previous studies have included dogs with overt motor neurological deficits such as paresis and muscle atrophy, which are likely to exhibit electrophysiologic abnormalities. Furthermore, multiple modalities have not been assessed concurrently, meaning that it is difficult to compare their relative diagnostic efficiencies.
The aim of the study was to explore the suitability of electrodiagnostic tests as ancillary diagnostic aids to improve the management of DLSS in dogs and define outcome measures that could be used as inclusion criteria in future studies. To that end, we designed a prospective cross‐sectional cohort study by examining the effect of 1 subtype of DLSS (lumbosacral foraminal stenosis) on motor and sensory electrophysiology measurements in dogs exhibiting only signs of pain.
2. MATERIALS AND METHODS
This study was performed under institutional ethical approval from the University of Bristol Animal Welfare and Ethical Review Body (VIN/16/053). Dogs presenting for investigation of apparent pain in the caudal lumbar/sacral vertebral column or pelvic limb lameness but without an obvious orthopedic cause were considered for inclusion in this prospective study. Clinical signs of apparent pain that were consistent with inclusion in the study were 1 or more of altered posture (kyphosis of lumbar spine), reluctance to jump, stand, or climb stairs; subjectively increased reaction to forced lordosis of the lumbar vertebral column; or ventrally directed pressure on the L7‐S1 intervertebral junction. Animals were excluded at this stage if their signs had been present for less than 7 days or they exhibited motor neurological deficits (including fecal/urinary incontinence, pelvic limb paresis, postural reaction deficits, or hyporeflexia). Dogs with sensory deficits such as nerve root signature or auto‐mutilation were not excluded.
After physical examination, each dog underwent electrodiagnostic testing as part of their diagnostic workup. This was performed by a blinded operator before interpretation of MR images. Standard electrodiagnostic equipment, including 15 mm, monopolar 26‐gauge stainless steel needle electrodes (ME‐FC‐9065, MedEvolve, Surrey, UK) and a portable electrodiagnostic unit (Cadwell Sierra Wave, MedEvolve) were used for all tests unless otherwise stated. After electrodiagnostic testing, 1.5 T MR images of the lumbosacral region were acquired with the dogs in dorsal recumbency with their legs extended but no forced lordosis of the lumbar spine, similar to the “neutral” position described by Zindl et al (8). Images obtained included sagittal and transverse T2 weighted images and 3D volume T1 weighted sequences for each case.
These MR sequences were interpreted blinded to the results of the electrodiagnostic tests. Dogs were excluded from analysis based on MRI appearance or classified as MRI‐affected or MRI‐unaffected. Dogs with attenuation of the fat signal through the intervertebral foramen AND a swollen L7 nerve root were considered as MRI‐affected cases. Dogs with no compression of the cauda equina and unimpeded fat signal around the L7 nerve root throughout the intervertebral foramina were considered as MRI‐unaffected animals. Dogs with degenerative change of the lumbosacral junction, including some loss of fat signal in the foramen, but no swelling of the L7 nerve root, 6 or 8 lumbar vertebrae, or compression of the midline cauda equina only were considered equivocal cases and not included in further analysis. Dogs diagnosed with neoplastic/inflammatory diseases were also excluded.
2.1. EMG recording
Twenty‐three‐gauge concentric needles were used for EMG analysis (ME‐FC‐9006, MedEvolve). The pedal interosseous, cranial tibial, gastrocnemius, biceps femoris, semimembranosus, and sacral epaxial musculature were all examined at 5 sites and at 2 depths. The muscle was considered abnormal if it contained 2 or more sites of abnormal spontaneous activity.8
2.2. CMAP recording
The CMAP was recorded from the pedal interosseous muscles after stimulation of the distal tibial nerve. The tibial nerve was stimulated using needle electrodes inserted subdermally between the calcaneal tendon and tibia just proximal to the tarsus with the cathode placed 10‐20 mm distal to the anode. A ground electrode was placed subdermally over the lateral aspect of the stifle. The active recording electrode was inserted in the body of the pedal interosseous muscles and repositioned until the initial deflection of the CMAP was in an upward (negative) direction to indicate recording was close to the motor point of the muscle. The reference electrode was placed over the lateral aspect of digit 5. The recording electrodes were connected to a digital amplifier with the active electrode in the inverting channel. Electrical stimulation was applied as a rectangular wave of 0.2‐millisecond duration at a frequency of 1 Hz with an intensity that was 20% greater than that required to give a CMAP of maximum amplitude. The CMAP onset latency (used in F‐wave analysis below) was recorded from the beginning of the initial negative deflection, and the CMAP amplitude (used in analysis) was recorded from the largest negative peak to the largest positive trough.
2.3. F‐wave recording
To record the F‐wave, needles were positioned as for the CMAP recording except the polarity of the cathode and anode were reversed. Traces were obtained until 10 F‐waves were recorded and then 3 variables of the F‐wave were calculated: the F‐latency delay, the F‐ratio, and the F‐wave duration. The F‐latency delay was the difference between the observed minimum F‐latency and the expected minimum F‐latency based on a previously derived equation:
where the limb length was measured from the greater trochanter to the tip of digit 3.10
The F‐ratio was calculated using another previously derived equation9 where,
The F‐wave duration was the difference in milliseconds between minimum F‐wave onset latency and the maximum F‐wave termination latency when all 10 recorded F‐waves were examined.
2.4. CDP recording
Cord dorsum potentials were generated using a previously described protocol6; 40‐mm long, 30‐gauge, Teflon‐coated, stainless steel needles with 10‐mm bare terminals were used as recording electrodes (202253‐000, MedEvolve). Stimulus electrode positioning was identical to that for F‐wave recording. Repetitive, rectangular impulses of 0.2‐millisecond duration were delivered to the tibial nerve at a frequency of 3 Hz. The intensity was adjusted to 50% greater than that which gave a visible foot twitch (to ensure stimulation was supramaximal). The active recording electrode was inserted dorsoventrally between L4 and L5 vertebrae until it contacted the dorsal lamina. The impedance of the circuit was determined before each recording and needles replaced if it was more than 8 kΩ.
Recording electrodes were connected to a digital signal amplifier, with the active electrode connected to the inverting channel. Band‐pass on the amplifier was set at 30 Hz to 3 kHz. Sensitivity of the amplifier was set at 10 μV. Between 256 and 512 stimuli were applied to the tibial nerve and the recorded potentials averaged into 1 trace. To ensure a recorded trace was not the product of background noise, CDPs were accepted only if 2 consecutive averaged recordings overlay one another. Both pelvic limbs were tested.
Two measurements from the CDP were analyzed: the maximum amplitude (peak to trough) and the CDP onset delay. The CDP onset delay was calculated in a similar manner to the F‐latency delay. The expected CDP onset latency was calculated with a previously derived equation6:
This expected onset latency was then subtracted from the observed value to give the CDP onset delay.
2.5. Statistical analysis
Once all data were collected, measurements from 1 side only were used for each dog. If the dog presented with lameness, the most severely affected side was used. If signs were symmetrical (eg, slow rising, apparent pain only), then the side with a swollen L7 nerve root on MRI was used for MRI‐affected dogs and the left side was arbitrarily chosen in unaffected dogs.
Analysis was conducted on 2 groups: a pilot group and a study population using commercially available software (Prism version 7.0d for Mac OS X, GraphPad Software, La Jolla, California). Ten apparently painful dogs (6 MRI‐affected and 4 unaffected) acted as a pilot study group and had their F‐latency delay, F‐ratio, CDP amplitude, and CPD latency delay compared using 1‐tailed Mann‐Whitney for differences in these continuous variables. These were not included in further analysis but provided hypotheses to inform further data collection. Subjective analysis of data from these dogs also suggested that CMAP amplitude might be reduced and F‐wave duration might be increased in MRI‐affected dogs, so these variables were also analyzed in the study population.
Pilot data suggested that there might be differences in CDP onset delay, F‐wave latency delay, and F‐ratio. Power calculations were performed on these data for 1‐tailed Wilcoxon‐Mann‐Whitney analysis with an alpha error probability of 0.05 and power of 0.95.15 These analyses suggested that the minimum numbers of dogs required to detect these pilot differences were 7, 8, and 8 dogs per group for CDP onset delay, F‐latency delay and F‐ratio, respectively.
The second group of dogs acted as the study population. First, the EMG test results were analyzed using 2 Fisher's exact tests. One compared the proportions with 1 or more muscle groups affected (termed “Any EMG changes”). The second compared the proportions with any muscle group excluding the pedal interosseous affected (termed “Sacral and limb changes only”). This second analysis was performed because clinical experience gained before the study suggested that the pedal interosseous was most commonly affected in otherwise normal dogs but other muscle groups rarely were.
Next, the same variables that were investigated in the pilot group (CDP amplitude and onset delay, F‐ratio, F‐latency delay) were analyzed in the study population, using Mann‐Whitney or t‐test analysis. In addition, CMAP amplitude and F‐wave duration were also analyzed, using Mann‐Whitney tests. These tests were 1‐tailed because we predicted that DLSS would only slow nerve conduction or reduce waveform amplitude. Cord dorsum potential duration was compared using a 2 tailed Mann‐Whitney test.
Limb length was considered to be a potential confounding variable because longer nerves might conduct more slowly than shorter nerves (because of a longer tapering segment), so limb length between the MRI‐affected and MRI‐unaffected dogs was also compared with a 2‐tailed unpaired t‐test.
3. RESULTS
3.1. Pilot group
Data recorded from this group showed that F‐latency delay (P < .005), CDP onset delay (P = .02), and F‐ratio (P < .005) were all increased in MRI‐affected dogs compared to MRI‐unaffected dogs. Cord dorsum potential amplitude was no different between the groups (Figure 1).
Figure 1.
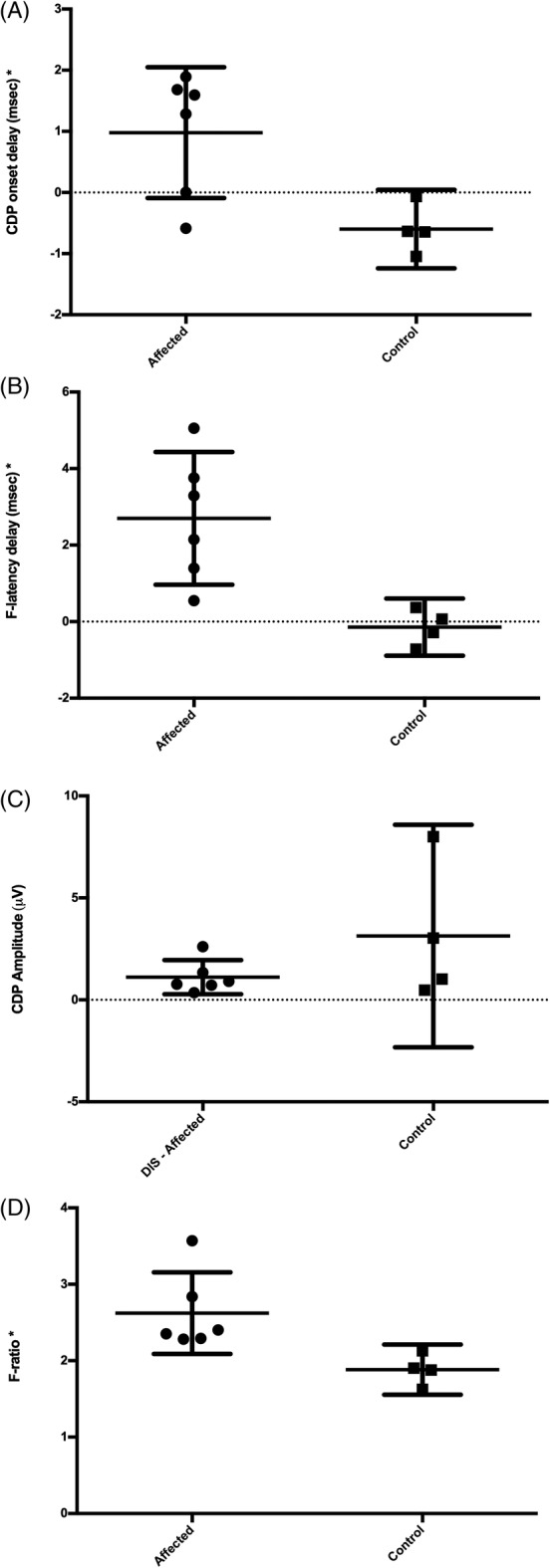
Data from the 10 dogs in the pilot group. Individual values are represented with error bars to show the mean and 95% confidence interval. As indicated by asterisk, F‐latency delay, F‐ratio, and CDP onset delay were increased in MRI‐affected dogs (1‐tailed Mann–Whitney, P < .05), while CDP amplitude was not different between groups
3.2. Study group
Data for the study group were recorded from 51 dogs, 25 of which were considered affected after reviewing the MR images. From the remaining 26 dogs, 4 were excluded because they had degenerative changes of the lumbosacral junction and vertebral canal stenosis, but no L7 nerve root enlargement, 1 was excluded with an L7/L8 lateralized disc protrusion, and 1 with an ilial wing tumor that was causing nerve root entrapment and enlargement. This left 20 MRI‐unaffected dogs. Two MRI‐affected dogs did not have a CDP recorded (because of time constraints under anesthesia).
Limb length was not different between MRI‐unaffected (mean 498 mm) and affected (mean 515 mm) groups (t‐test, P = .60, Figure 2A).
Figure 2.
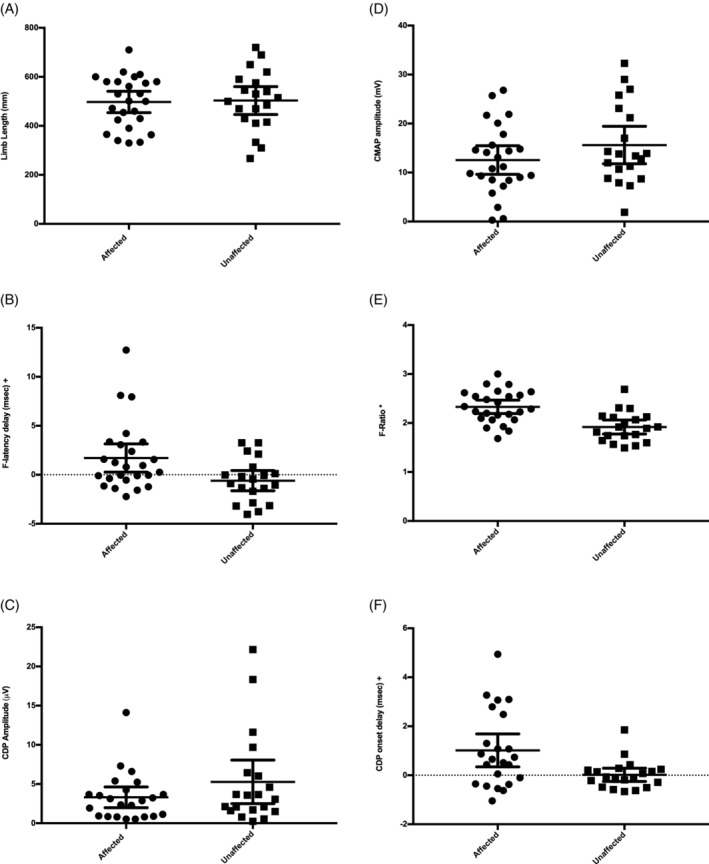
Analysis of continuous data in the study group. The symbol * denotes significant difference with an independent t‐test (P < .05) and the symbol + denotes significant difference with a Mann‐Whitney U test (P < .05). Solid lines represent the means and 95% confidence intervals. The 3 dogs with an F‐latency delay >5 milliseconds in graph C also had CDP onset delays >2 milliseconds
3.2.1. EMG analysis
Eleven of the 25 (44%) MRI‐affected dogs had at least 1 muscle group affected on EMG (“Any EMG changes”), compared to 3 of the 20 (15%) MRI‐unaffected dogs (Table 1). These proportions were not significantly different (Fisher's exact test, P = .06). Seven of the 25 (28%) affected dogs had EMG activity in muscles other than the pedal interosseous compared to 1 of the 20 (5%) MRI‐unaffected dogs. These proportions were not significantly different (Fisher's exact test, P = .06).
Table 1.
EMG changes in MRI‐affected and MRI‐unaffected dogs showing the distribution of changes in both groups
| Any EMG activity | Sacral and/or limb activity | Pedal activity only | Limb activity only | Sacral activity only | Pedal and limb activity only | Sacral and pedal activity only | Sacral and limb activity only | Pedal, limb and sacral activity | |
|---|---|---|---|---|---|---|---|---|---|
| MRI‐affected dogs | 11/25 | 7/25 | 4/25 | 2/25 | 1/25 | 1/25 | 0/25 | 2/25 | 1/25 |
| MRI‐unaffected dogs | 4/20 | 1/20 | 3/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 1/20 |
The data from the first 2 columns were compared and the proportions of MRI‐affected versus MRI‐unaffected dogs with EMG changes were not found to be significantly different.
Abbreviations: EMG, electromyography; MRI, magnetic resonance imaging.
3.2.2. CMAP analysis
The mean CMAP amplitude of MRI‐affected dogs (12.6 mV, SD 7.1 mV) was not significantly smaller than the mean CMAP amplitude of the MRI‐unaffected dogs (15.6 mV, SD 8.2 mV, unpaired, 1‐tailed t‐test, P = .09; Figure 2B).
3.2.3. F‐wave analysis
The mean F‐latency delay of MRI‐affected dogs (1.7 milliseconds, SD 3.5 millisecond) was longer than MRI‐unaffected dogs (−0.6 milliseconds, SD = 2.2 milliseconds, 1‐tailed Mann‐Whitney, P = .008, Figure 2C). The mean F‐ratio of MRI‐affected dogs (2.33, SD 0.33) was larger than the mean F‐ratio of MRI‐unaffected dogs (1.92, SD 0.31, unpaired, 1‐tailed t‐test, P < .001; Figure 2D). The mean F‐wave duration was not significantly different between MRI‐affected dogs (17.1 milliseconds; SD 11.3 milliseconds) and MRI‐unaffected dogs (16.3 milliseconds; SD 9.2 milliseconds) when compared (2‐tailed Mann‐Whitney, P = 0.94).
3.2.4. CDP analysis
Two MRI‐affected dogs did not have their CDP recorded. Mean CDP amplitude was 3.31 μV (SD 3.07 μV) in the MRI‐affected dogs and 5.27 μV (SD 5.93 μV) in the MRI‐unaffected dogs. These groups were not significantly different when compared (1‐tailed Mann‐Whitney, P = .23; Figure 2E). Mean CDP onset delay was higher in MRI‐affected dogs (1.01 milliseconds; SD 1.55 milliseconds) versus MRI‐unaffected dogs (0.02 milliseconds; SD = 0.58 milliseconds; 1‐tailed Mann‐Whitney, P = .009; Figure 2F).
4. DISCUSSION
Data from our pilot group and our study group show that CDP and F‐wave onset were significantly delayed and F‐ratio was significantly increased in our MRI‐affected groups of dogs. This is consistent with decreased conduction velocity in the proximal segment of the nerve and affecting both motor and sensory axons and supports the hypothesis that lumbosacral foraminal stenosis can delay both motor and sensory nerve conduction in dogs with apparent pain but without neurological deficits.
Data from Figure 2 suggests that there was considerable overlap between groups, but some dogs in the MRI‐affected group had substantially delayed F‐waves and CDPs compared to the MRI‐unaffected group, suggesting that these electroneurographic abnormalities existed in some, but not all, MRI‐affected dogs. We did not attempt to classify dogs as “normal” or “abnormal” based on their values because we did not have an established reference interval to compare the groups to.
The 3 dogs with long F‐latency delays (>5 milliseconds) also had long CDP onset delays (>2 milliseconds), while there were 4 dogs with long CDP onset delays that did not appear to have delayed F‐latencies. This suggests that CDP onset delay is a more sensitive indicator of dysfunction than F‐latency delay and therefore that sensory nerves are more vulnerable to the effects of compression than motor nerves. Further analysis would be required to establish this, particularly as our case selection included dogs with sensory disturbance (pain) but not motor deficits (eg, paresis).
Our EMG findings are consistent with 2 previous studies on dogs with DLSS with pain as the only clinical sign. These studies showed EMG abnormalities in a total of 8 of 16 dogs (4/1216 and 4/614), compared with our findings of abnormalities in 11 of 25 MRI‐affected dogs. These proportions appear to be similar, but we did not find them to be higher than the MRI‐unaffected dogs. One reason for this might be there was relatively high proportion of spontaneous activity in the pedal interosseous muscles of MRI‐unaffected dogs, suggesting this can be a “normal” finding. Another reason is that our sample size could have been too small to detect a significant difference. A post hoc sample size calculation suggested that we would need 57 dogs in each group to establish if the difference in proportions we had observed (44% vs 15%) was significantly different using a Fisher's exact test.
Tibial nerve CDP latency has been suggested to increase, and amplitude decrease, in dogs with DLSS compared to normal controls.12 We did not find a difference in CDP amplitude in our dogs. This might have been because we only measured the baseline to first negative peak amplitude of the evoked potential (equivalent to N1 published by Meij et al12) which was also not different in their study group.
Of the 51 dogs included as having signs of possible back pain, 20 did not have a demonstrable lesion on MRI (our “MRI‐unaffected” group). This 40% non‐diagnosis rate for apparent back pain is similar to rates reported in humans, in which only around 50% of patients with an abnormal extension test are found to have disc herniation on MRI.17 We included a defined range of historical and physical examination abnormalities as inclusion criteria for possible back pain. If these were altered (eg, to include subjective assessment of muscle mass18), then this diagnostic accuracy might have been improved. There are, however, no studies evaluating which combination of abnormalities best predict MRI abnormalities in dogs.
One reason that we failed to detect MRI abnormalities in some dogs could have been that our diagnostic criteria were too strict. We used MRI as the gold standard of diagnosis and required that dogs had foraminal stenosis (defined by loss of fat in the intervertebral foramen) and a swollen L7 nerve root to be defined as MRI‐affected. This was based on the experimental studies that suggest root‐swelling is a typical radicular response to compression and associated with clinical signs,19 and a human imaging study using nerve‐root anesthesia that suggested nerve swelling had an 80% positive predictive value for radicular pain.20 We also excluded dogs we felt had an equivocal MRI as they had changes to the foramen and lumbosacral junction but no nerve root swelling. Because of this, we believe that our MRI‐affected and MRI‐unaffected groups had clearly different MRI appearances.
Another reason we could have inappropriately categorized MRI‐affected dogs as MRI‐unaffected is that we only performed MRI in a neutral position, with the dogs in dorsal recumbency. The cross‐sectional area and the volume of the canine lumbosacral intervertebral foramen has been consistently shown to be larger in flexion (kyphosis) and smaller in extension (forced lordosis) versus a (variably defined) neutral position.21, 22, 23 The relevance of this for the diagnosis of symptomatic foraminal stenosis is less well established because there is no significant differences in foraminal measurements of clinically affected or unaffected dogs21 or small statistical differences with large overlap of measurements between affected and unaffected groups. Because of this, we felt it was unlikely that a foraminal change would be dramatic enough to turn a normal, MRI‐unaffected dog into an abnormal, MRI‐affected dog if measured in a different position. For this reason, dynamic views were not performed.
The fact that we could not demonstrate electrophysiological evidence of dysfunction in all MRI‐affected dogs (and that some apparently MRI‐unaffected dogs did have evidence of dysfunction) could arise for 3 main reasons. First, in dogs with a contribution to the tibial nerve from the L6 nerve root, there might have been sufficient normal axons to give normal electrophysiological function. From anatomical dissections, vivisection, and electrophysiological studies on cats, dogs are thought to predominantly rely upon axons from the L7 spinal nerve to make up the tibial nerve, but there are likely to be contributions from L6 and S1.24, 25 We partially addressed this by using EMG, because several nerve roots are required to innervate all the limb muscles, in particular, the sacral paraspinal muscles that are thought to be segmentally innervated.26 There is evidence that the common peroneal nerve is also affected by L7 root compression because stimulation of the nerves at the caudolateral stifle to cause tarsal flexion and digit extension (a peroneal nerve function) has been shown to delay the onset of the CDP and reduce its amplitude.12
Second, MRI might not have been an adequate gold standard. Some “unaffected” dogs might have had abnormal nerve function as the source of their apparent pain but a normal‐looking nerve on MRI. Conversely, the enlarged nerve root might not have been the source of clinical signs in some dogs classified as “affected” because although enlarged, it might have been functioning normally and not the source of back pain. This could be addressed by selecting only dogs with neurological signs to evaluate, because their nerve dysfunction might be more severe. Of the previously published cases, EMG changes were apparent in 17 of 22 dogs with neurological deficits, compared with 8 of 16 dogs with apparent pain only.11, 13, 14 This suggests neurologically deficient dogs might have more electrodiagnostic abnormalities, but not that EMG will universally be abnormal in them either.
Third, we could have examined some dogs at the wrong time point in their disease to detect electrophysiological abnormalities. Electromyographic abnormalities take several days to become apparent after nerve injury,8 and some human electrodiagnostic texts suggest that EMG changes can diminish in time, so that the optimum window is around 3 weeks after the onset of signs.27 We do not think that the variable timing of our examinations will have had a large influence as all dogs had their signs for more than 7 days, and human research shows that the change of finding EMG changes is not hugely influenced by the duration of clinical signs28 and experimental research on cauda‐equina compression in dogs shows that motor and sensory nerve root function is unlikely to return to normal if clinical signs are persistent.29
We performed 9 separate, preplanned statistical analyses; 4 Mann‐Whitney tests, 3 t‐tests, and 2 Fisher's exact tests. Three of these tests returned statistically significant differences (at the .05 level). We did not apply a correction for multiple testing—for several reasons: (1) this is an exploratory study in which we were aiming to define the electrophysiologic tests that might be most helpful in diagnosing this condition; (2) the tested variables were derived from pilot data that suggested hypotheses for further testing and so were prespecified (reducing the opportunity for “p‐hacking”); (3) application of corrections for multiple testing is difficult for these tests as they are very likely to be correlated (if 1 test of nerve conduction disorder is significantly affected, it is highly likely another will be too), meaning that conventional formulae for multiple testing correction are not appropriate.
We have not calculated sensitivity or specificity values for the various measured variables because these would only have provided information on the value of electrodiagnostics to predict MRI changes, rather than assessing how useful electrodiagnostic tests could be versus MRI for diagnosis of the cause of apparent pain, which would require a different “gold standard” such as response to treatment.
Our findings support the hypothesis that L7 nerve root compression can affect the motor and sensory nerve conduction in some dogs. The tests we performed are no more demanding than routine nerve conduction studies and quick to perform. Because of this, we would recommend that further research is conducted to establish whether electrodiagnostic tests (particularly the F‐ratio and CDP onset delay) can provide a useful line of evidence for the diagnosis of DLSS in dogs that is independent of imaging findings. For example, if MRI can be overdiagnostic for the clinical significance of nerve root compression, it might be that dogs with electrodiagnostic changes respond better to decompressive surgery (eg, foraminotomy) than those who do not, and that those that do not respond might have another source of pain.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This work was carried out under ethical approval from the University of Bristol, UK.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Data from the pilot group of dogs was presented as part of a lecture at the ECVN congress in Cambridge, UK, 2010.
Harcourt‐Brown TR, Granger NP, Fitzpatrick N, Jeffery ND. Electrodiagnostic findings in dogs with apparently painful lumbosacral foraminal stenosis. J Vet Intern Med. 2019;33:2167–2174. 10.1111/jvim.15589
REFERENCES
- 1. Meij BP, Bergknut N. Degenerative lumbosacral stenosis in dogs. Vet Clin North Am Small Anim Pract. 2010;40:983‐1009. [DOI] [PubMed] [Google Scholar]
- 2. Danielsson F, Sjostrom L. Surgical treatment of degenerative lumbosacral stenosis in dogs. Vet Surg. 1999;28:91‐98. [DOI] [PubMed] [Google Scholar]
- 3. De Risio L, Sharp NJ, Olby NJ, et al. Predictors of outcome after dorsal decompressive laminectomy for degenerative lumbosacral stenosis in dogs: 69 cases (1987‐1997). J Am Vet Med Assoc. 2001;219:624‐628. [DOI] [PubMed] [Google Scholar]
- 4. Suwankong N, Meij BP, Van Klaveren NJ, et al. Assessment of decompressive surgery in dogs with degenerative lumbosacral stenosis using force plate analysis and questionnaires. Vet Surg. 2007;36:423‐431. [DOI] [PubMed] [Google Scholar]
- 5. Jeffery ND, Barker A, Harcourt‐Brown T. What progress has been made in the understanding and treatment of degenerative lumbosacral stenosis in dogs during the past 30 years? Vet J. 2014;201:9‐14. [DOI] [PubMed] [Google Scholar]
- 6. Cuddon PA, Delauche AJ, Hutchison JM. Assessment of dorsal nerve root and spinal cord dorsal horn function in clinically normal dogs by determination of cord dorsum potentials. Am J Vet Res. 1999;60:222‐226. [PubMed] [Google Scholar]
- 7. Holliday TA. Electrodiagnostic examination. Somatosensory evoked potentials and electromyography. Vet Clin North Am Small Anim Pract. 1992;22:833‐857. [DOI] [PubMed] [Google Scholar]
- 8. Cuddon PA. Electrophysiology in neuromuscular disease. Vet Clin North Am Small Anim Pract. 2002;32:31‐62. [DOI] [PubMed] [Google Scholar]
- 9. Poncelet L, Balligand M. Nature of the late potentials and F‐ratio values in dogs. Res Vet Sci. 1991;51:1‐5. [DOI] [PubMed] [Google Scholar]
- 10. Steiss JE. Linear regression to determine the relationship between F‐wave latency and limb length in control dogs. Am J Vet Res. 1984;45:2649‐2650. [PubMed] [Google Scholar]
- 11. Chambers JN, Selcer BA, Oliver JE Jr. Results of treatment of degenerative lumbosacral stenosis in dogs by exploration and excision. Vet Comp Orthop Traumatol. 1988;3:130‐133. [Google Scholar]
- 12. Meij BP, Suwankong N, van den Brom WE, et al. Tibial nerve somatosensory evoked potentials in dogs with degenerative lumbosacral stenosis. Vet Surg. 2006;35:168‐175. [DOI] [PubMed] [Google Scholar]
- 13. Oliver JE Jr, Selcer RR, Simpson S. Cauda equina compression from lumbosacral malarticulation and malformation in the dog. J Am Vet Med Assoc. 1978;173:207‐214. [PubMed] [Google Scholar]
- 14. Sisson AF, LeCouteur RA, Ingram JT, et al. Diagnosis of cauda equina abnormalities by using electromyography, discography, and epidurography in dogs. J Vet Intern Med. 1992;6:253‐263. [DOI] [PubMed] [Google Scholar]
- 15. Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175‐191. [DOI] [PubMed] [Google Scholar]
- 16. Chambers JN, Selcer BA, Sullivan SA, Coates JR. Diagnosis of lateralized lumbosacral disk herniation with magnetic resonance imaging. J Am Anim Hosp Assoc. 1997;33:296‐299. [DOI] [PubMed] [Google Scholar]
- 17. van der Windt DAWM, Simons E, Riphagen II, et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low‐back pain. Cochrane Database Syst Rev. 2010;(2):CD007431. [DOI] [PubMed] [Google Scholar]
- 18. Godde T, Steffen F. Surgical treatment of lumbosacral foraminal stenosis using a lateral approach in twenty dogs with degenerative lumbosacral stenosis. Vet Surg. 2007;36:705‐713. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression ‐ part 1: Intraradicular inflammatory changes induced by mechanical compression. J Orthop Res. 2004;22:170‐179. [DOI] [PubMed] [Google Scholar]
- 20. Park SH, Jeon I, Kim SW. Diagnostic values of ProSet magnetic resonance coronal source imaging for detecting symptomatic lesion in multiple lumbar foraminal stenosis. Clin Neurol Neurosurg. 2016;150:185‐189. [DOI] [PubMed] [Google Scholar]
- 21. Jones JC, Davies SE, Werre SR, Shackelford KL. Effects of body position and clinical signs on L7‐S1 intervertebral foraminal area and lumbosacral angle in dogs with lumbosacral disease as measured via computed tomography. Am J Vet Res. 2008;69:1446‐1454. [DOI] [PubMed] [Google Scholar]
- 22. Worth AJ, Hartman A, Bridges JP, Jones BR, Mayhew JIG. Computed tomographic evaluation of dynamic alteration of the canine lumbosacral intervertebral neurovascular foramina. Vet Surg. 2017;46:255‐264. [DOI] [PubMed] [Google Scholar]
- 23. Zindl C, Tucker RL, Jovanovik J, Gomez Alvarez C, Price D, Fitzpatrick N. Effects of image plane, patient positioning, and Foraminal zone on magnetic resonance imaging measurements of canine lumbosacral intervertebral foramina. Vet Radiol Ultrasound. 2017;58:206‐215. [DOI] [PubMed] [Google Scholar]
- 24. Bennett D, Vaughan LC. Peroneal nerve paralysis in the cat and dog: an experimental study. J Small Anim Pract. 1976;17:499‐506. [DOI] [PubMed] [Google Scholar]
- 25. Sherrington CS. Notes on the arrangement of some motor fibres in the Lumbo‐sacral plexus. J Physiol. 1892;13:621‐772.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaggy A, Oliver JE Jr, Purinton PE. Distribution of Myotomes to the Longissimus Lumborum and Multifidus Lumborum muscles in the dog. Progress Vet Neurol. 1990;1:333‐339. [Google Scholar]
- 27. Campbell W, Radiculopathies W. Essentials of Electrodiagnostic Medicine. 2nd ed. New York: Demos Medical; 2014. [Google Scholar]
- 28. Dillingham TR, Pezzin LE, Lauder TD, et al. Symptom duration and spontaneous activity in lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000;79:124‐132. [DOI] [PubMed] [Google Scholar]
- 29. Kim NH, Yang IH. A study of motor and sensory evoked potentials in chronic cauda equina compression of the dog. Eur Spine J. 1996;5:338‐344. [DOI] [PubMed] [Google Scholar]


