Abstract
Background
The pathogenic role of mycoplasmas in the lower respiratory tract (LRT) of dogs is debated, because mycoplasmas can be isolated from both healthy and sick dogs.
Objectives
To critically assess available data from controlled observational studies on the role of 4 mycoplasma species in LRT disease of dogs.
Design
Systematic review and meta‐analyses.
Methods
Seven electronic databases were searched for relevant publications. Risk of bias was assessed by the Newcastle‐Ottawa Scale. Meta‐analyses, stratified by mycoplasmal species, were performed using a random effects Bayesian model with noninformative priors to estimate pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between Mycoplasma cynos, Mycoplasma canis, Mycoplasma spumans, and Mycoplasma edwardii and LRT disease in dogs.
Results
Five studies were included from 1201 references identified. All studies dealt with M. cynos, whereas 3 dealt with the other mycoplasma species. A significant association was found between M. cynos and LRT disease (Bayesian OR, 3.60; CI, 1.31‐10.29). Conversely, M. canis, M. spumans, and M. edwardii were not significantly associated with LRT signs (Bayesian OR, 1.06; CI, 0.10‐14.63; Bayesian OR, 3.40; CI, 0.16‐54.27; and Bayesian OR, 1.04; CI, 0.05‐23.54, respectively).
Conclusions and Clinical Importance
Results support a pathogenic role of M. cynos and a commensal role of M. canis and M. edwardii in LRT in dogs. Although the association was not significant based on the CI, the point estimate of the Bayesian OR was relatively high for M. spumans, making its role less clear. Mycoplasma cynos‐specific polymerase chain reaction should be considered on samples from dogs with LRT.
Keywords: bronchitis, canine, commensal, Mycoplasma cynos, pathogen, pneumonia
Abbreviations
- CI
confidence interval
- LRT
lower respiratory tract
- NOS
Newcastle‐Ottawa Scale
- OR
odds ratio
- PCR
polymerase chain reaction
1. INTRODUCTION
Mycoplasmas are bacteria that occur as commensal organisms or opportunistic pathogens in plants, mammals, reptiles, birds, and insects.1, 2 Because of limited metabolic capability, which is a consequence of their small genome and lack of cell wall, they are fragile and challenging to culture. Therefore, culture and sensitivity may be a poor method to diagnose mycoplasmal infection.3, 4, 5 Furthermore, each species cannot be differentiated on morphology alone. Species may be identified by biochemical, nutritional, or serological studies. However, cross reactivity among species remains possible.5, 6 Polymerase chain reaction (PCR) has been developed to detect mycoplasmal organisms from culture samples and clinical specimens and to accurately identify species.1, 7 By amplifying mycoplasmal DNA, PCR may be more sensitive than culture.8, 9 However, it may not indicate active infection, decreasing its specificity. Serology may be used, but a 2‐ to 4‐fold increase in the titer is needed to establish a diagnosis of active infection, as with other immunoglobulin G‐based serology testing.8
In dogs, many Mycoplasma spp. are thought to form part of the normal bacterial flora of the upper airways.4 A recent study evaluating the microbiome of healthy dogs showed that mycoplasmas occurred in decreasing frequency from the nasal cavity to the lower airways.10 This could suggest that colonization of the lower respiratory tract (LRT) by mycoplasmas may arise from the upper airways.10 Mycoplasmas have been isolated in pure culture from dogs with LRT disease, suggesting that they may be primary respiratory pathogens in dogs.11 However, another study showed that dogs with LRT disease that had Mycoplasma spp. identified by PCR in bronchoalveolar lavage fluid were 5.1 times more likely to have evidence of oral bacterial contamination.12 This finding could suggest that, although Mycoplasma spp. have been found in dogs with LRT disease, they could simply represent oropharyngeal contamination.
Mycoplasmas may be isolated from the LRT more often in dogs that are <1 year old.13 As such, Mycoplasma spp. may play a role in the infectious respiratory disease complex in dogs.13 It is possible, however, that clinical signs may be more attributable to viruses or Bordetella sp. rather than to primary mycoplasmal infection in puppies.
In cats, some mycoplasmas, such as Mycoplasma felis, may be more pathogenic than others.14 This also may be the case in dogs1; Mycoplasma canis, Mycoplasma cynos, Mycoplasma edwardii, and Mycoplasma spumans all have been reported in dogs with LRT disease, among others.3, 15
These potential biases could explain why some studies have shown an association between some mycoplasma species and LRT infection in dogs, yet others have not. It is difficult to make any definitive conclusion on the basis of these individual studies. It may be possible to determine the role of Mycoplasma spp. in LRT disease in dogs more definitively by means of a systematic review, which selects only well‐designed studies and identifies bias in a standardized way in each individualized study, and by a meta‐analysis, which reassesses the association between Mycoplasma spp. and LRT diseases after attributing a weight to each study based on its precision.16, 17 The purpose of our study was to critically assess and analyze, by means of a critical review and meta‐analyses, the role of 4 mycoplasma species in the development of LRT disease in dogs.
2. MATERIALS AND METHODS
The systematic review and meta‐analysis were performed and reported in accordance with the Preferred Reporting Items for Systemic Reviews and Meta‐Analyses guidelines for systematic reviews and meta‐analyses.18
2.1. Review question and literature selection
The review question was developed according to the population, exposure, comparator, and outcome format for assessing the effect of an exposure on a disease.19 In dogs with clinical signs of LRT disease, the presence of mycoplasmal organisms was determined by PCR or bacterial culture of lower airway samples, or by seroconversion using paired serum samples. The primary outcome measure investigated was presence of Mycoplasma spp. in LRT samples or a 2‐fold or higher increase in specific antibody titers. Studies using oral or upper airway sampling techniques were not included.12, 20
Seven indexing and abstracting services (PubMed, Scopus, ISI Web of Science, EBSCO, ProQuest, Google Scholar, and the Cochrane Library) were searched from July to August 2017 to identify relevant primary research related to the research question. For all 7 databases, the following search terms were used: “mycoplasma AND (dogs OR canine) AND (bronchitis OR (lower AND airway) OR respiratory).” A filter for citations from the veterinary field only was used when searching Scopus and ProQuest databases. The “bronchitis OR (lower AND airway)” terms were used to focus the search on mycoplasma infection of the LRT. The search in Google Scholar returned 9010 articles but only the first 925 were available for review. Two of the authors (A.J. and E.R.) independently assessed articles for inclusion. Discrepancies in articles chosen were resolved with further review by the third author (K.L.B.). All articles, without restrictions on language or time period, were considered for inclusion. However, articles published in any language other than English were considered for inclusion only if sufficient information could be retrieved after translation by Google Translate to assess eligibility and extract relevant data.
All retrieved citations were screened to determine whether they met the following 4 eligibility criteria:
Was the study relevant to the review question? An answer of no was assigned if the study was obviously irrelevant (eg, the study was not about Mycoplasma spp.).
Was the research conducted in dogs with clinical signs of respiratory tract disease? An answer of no was assigned if the study was conducted in any species other than dogs or if the study was conducted in dogs that did not have clinical signs of respiratory tract disease.
Did the study assess the presence of Mycoplasma spp. in the study population? An answer of no was assigned if the presence of mycoplasmal organisms was not determined by sampling of the LRT or evidence of seroconversion on paired blood samples.
Did the study compare the presence of mycoplasmal organisms in case dogs with the presence in control dogs free from clinical signs of respiratory tract disease? An answer of no was assigned if the study did not include healthy control dogs.
Studies were included in the review only if the response to all 4 eligibility criteria was yes. When it was not possible to determine from the abstract and title whether a study met the eligibility criteria, the full text was evaluated. Manual searches for additional eligible studies were performed by reviewing the reference lists of studies selected for inclusion, along with published reviews on respiratory mycoplasmosis in dogs.1, 21, 22
2.2. Data extraction
The following data were extracted from studies that met the eligibility criteria: name of the first author, publication year, numbers of case and control dogs, number of samples positive for Mycoplasma spp., clinical signs shown by case dogs, how the diagnosis was made, the technique used to obtain samples from the lower airways, the diagnostic method used to identify mycoplasmal organisms (PCR assay, bacterial culture of organisms from the LRT, or serology), the mycoplasma species identified, and the environment of case and control dogs.
2.3. Risk of bias assessment
For the studies considered for inclusion in the review, the Newcastle‐Ottawa Scale (NOS) with minor modifications, as used in previous critical reviews and meta‐analyses, was used to assess the risk of bias.14, 23 The scale consisted of 3 domains, with each domain composed of several criteria. Each criterion was scored either 1 or 0, for a low or high risk of bias, respectively. The 3 domains included selection of the cases and the controls (4 criteria), comparability of the 2 groups (1 criterion composed of 2 parts), and exposure assessment (2 criteria). The exposure assessment domain originally was composed of 3 criteria, but 1 criterion (nonresponse rate) was withdrawn, because it was not relevant for the included studies.
2.4. Exclusion of studies
Studies that met the eligibility criteria were excluded if the specific species of mycoplasma was not identified.
2.5. Statistical analysis
In dogs, mycoplasmal organisms may be commensal or pathogenic; this is variable for each mycoplasma species.2 Stratified analyses therefore were performed to evaluate the effect of each species of mycoplasma identified in causing LRT disease in dogs. The mycoplasma species evaluated included M. cynos, M. canis, M. spumans, and M. edwardii, because they were the most common species implicated in LRT disease in dogs.3, 15
For each study included in the review, odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated on the basis of numbers of mycoplasma positive and negative dogs and numbers of dogs with and without clinical signs of LRT disease in each group. For calculation of ORs, the formula was (number of mycoplasma‐positive dogs with LRT signs)/(number of mycoplasma‐negative dogs with LRT signs) divided by (number of mycoplasma‐positive dogs without LRT signs)/(number of mycoplasma‐negative dogs without LRT signs).
Accurate estimation of the effect size may be problematic when a small number of studies is included in a meta‐analysis. According to the most recent literature, the Bayesian method is recommended to estimate the effect size in meta‐analyses with few studies.24, 25, 26 Therefore, a random‐effects Bayesian model was used to assess the association between the presence of mycoplasmal organisms in dogs and the presence of clinical signs of LRT disease. The Bayesian analysis used Markov chain Monte Carlo with Gibbs sampling to make inferences. The Markov chain Monte Carlo chain was run for 32 498 iterations with a burn‐in of 2500 and thinning of 3 iterations, which allowed satisfactory convergence (as assessed graphically). Noninformative normal (0, 10) and inverse gamma (0.01, 0.01) prior distributions were considered for the parameters θ (overall mean log OR) and τ2 (between‐trial variance). For all meta‐analyses, a pooled OR of approximately 1.5 was considered a mild association, a pooled OR of approximately 2.5 was considered a moderate association, a pooled OR of 4 was considered a strong association, and a pooled OR of approximately 10 was considered a very strong association.27
Heterogeneity was assessed to determine whether the true effect in all studies was the same.28 The chi‐squared test was used to evaluate if there was heterogeneity among the studies in the meta‐analyses; a P‐value >.1 indicated there was no significant heterogeneity in the studies detected. The I 2 (measure of inconsistency) also was used to evaluate heterogeneity; this measure describes the percentage of variation in ORs across studies attributable to heterogeneity rather than chance.28, 29 Values of I 2 between 0 and 40% were considered indicative of unimportant heterogeneity, values between 30 and 60% were considered indicative of moderate heterogeneity, values between 50 and 90% were considered indicative of substantial heterogeneity, and values between 75 and 100% were considered indicative of considerable heterogeneity.30 Sensitivity analysis was conducted to determine whether the exclusion of any single study would result in a significant change in the final results. All analyses were performed using STATA version 14.2 software (StataCorp LLC, College Station, Texas).
3. RESULTS
3.1. Study selection and characteristics
Of the 1201 reports that were screened for inclusion in the review, only 6 fulfilled the eligibility criteria.13, 31, 32, 33, 34, 35 One study was excluded because it did not specify which mycoplasma species was evaluated,13 leaving 5 studies included in the systematic review and meta‐analyses (Figure 1).
Figure 1.
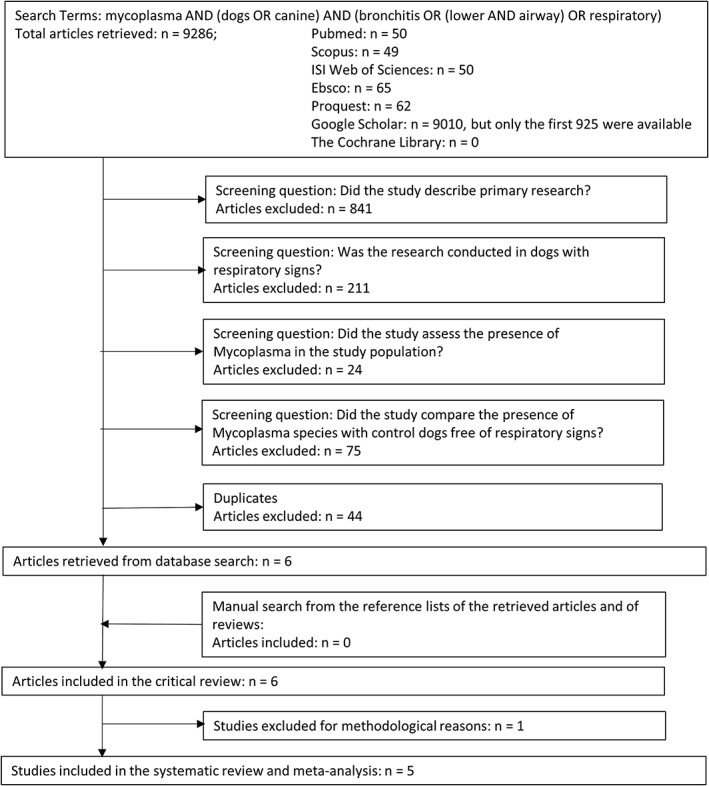
Flow of the study selection process
3.1.1. Mycoplasma cynos
All 5 studies evaluated the presence of M. cynos in case dogs versus control dogs (Table 1). A total of 188 clinically ill dogs (median, 26 dogs; range, 8‐108 dogs) and 122 control dogs (median, 16 dogs; range, 10‐65 dogs) were assessed. Age distribution was known for 2 studies31, 35; median age of case dogs was 0.5 years (range, 0.2‐3.8)31 and 6 years (range, 2‐11),35 and median age of control dogs was 6 years (range, 0.4‐14.2)31 and 9 years (range, 3.1‐17.4).35 Animals came from a variety of environments: dogs were from kennel or shelter environments in 3 studies32, 33, 34; whereas dogs were privately owned and presented to a veterinary hospital in 2 other studies.31, 35 However, 2 of the 10 control dogs came from a shelter in 1 study.31 Clinical signs were specifically assessed in 3 studies.31, 33, 35 One study reported that all case dogs had a chronic, hacking, productive cough.31 Another study graded severity of respiratory signs, from no clinical signs to coughing, nasal discharge, inappetence, and lethargy.33 One study reported clinical signs including coughing, respiratory noise, and respiratory distress.35
Table 1.
Characteristics of studies included in the systematic review of the association between Mycoplasma cynos, Mycoplasma canis, Mycoplasma spumans, and Mycoplasma edwardii and lower respiratory tract disease in dogs
| Study | No of dogs positive for M. cynos | No of dogs positive for M. canis | No of dogs positive for M. spumans | No of dogs positive for M. edwardii | Definition of LRT disease | Diagnostic method | Sampling site | Ante‐/postmortem | Median age in years (min‐max) | Environment | Chronicity of clinical signs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canonne31 | Cases | 9/17 | NA | NA | NA | Chronic cough | PCR | BALF | Ante | 0.5 (0.2‐3.8) | Hospital | 2 months |
| Controls | 2/10 | NA | NA | NA | PCR | BALF | Ante | 6 (2‐11) | Private (8); shelter (2) | NA | ||
| Chalker33 | Cases | 30/108 | 18/120 | 15/123 | 7/131 | Cough, nasal discharge, depression | Culture | BALF | Post | Unknown | Shelter | Unknown |
| Controls | 7/65 | 9/63 | 4/68 | 3/69 | Culture | BALF | Post | Unknown | Shelter | Unknown | ||
| Rosendal32 | Cases | 1/8 | 0/8 | 2/8 | 1/8 | Pneumonia at necropsy | Culture | Lung | Post | Unknown | Kennel | Unknown |
| Controls | 0/15 | 0/15 | 0/15 | 0/15 | Culture | Lung | Post | Unknown | Kennel | Unknown | ||
| Rycroft34 | Cases | 12/26 | NA | NA | NA | Imprecise respiratory signs | Serology | serum | Ante | Unknown | Shelter | Unknown |
| Controls | 3/16 | NA | NA | NA | Serology | serum | Ante | Unknown | Shelter | Unknown | ||
| Schulz35 | Cases | 4/29 | 3/29 | 1/29 | 0/29 | Nasal discharge, cough, dyspnea | Culture, PCR | BALF | Ante | 6 (0.4‐14.2) | Hospital | Unknown |
| Controls | 0/16 | 2/16 | 0/16 | 1/16 | Culture, PCR | BALF | Post | 9 (3.1‐17.4) | Hospital | Unknown | ||
Abbreviations: BALF, bronchoalveolar lavage fluid, LRT, lower respiratory tract; PCR, polymerase chain reaction.
Only 1 study used M. cynos‐specific PCR to detect organisms from the respiratory tract.31 Another study used both culture and PCR to identify evidence of mycoplasmal infection; the rate of positive results was not significantly different between culture and PCR.35 Two studies used culture to detect mycoplasma from the respiratory tract of dogs.32, 33 Species identification was performed by serology in another study,32 and by PCR in yet another study.33 One study evaluated the serological response to M. cynos in dogs by measuring paired serum titers in dogs that were admitted to a rehoming kennel, and by comparing serology results between dogs that remained well over a 3‐week period to those that developed LRT signs.34
Bronchoalveolar lavage samples were used for diagnostic testing in most studies.31, 33, 35 Bronchoalveolar lavage was performed in dogs under anesthesia,31, 34, 35 or immediately after death.33, 35 One study used lung tissue samples obtained during necropsy for culture,32 and another used serum for serology.34
One study found no significant difference in rate of detection of M. cynos between dogs with LRT signs and healthy controls.31 Conversely, another study found a significant association between detection of M. cynos and LRT disease in dogs.33 One study also found a significant relationship between clinical LRT disease and increasing antibody titers to M. cynos.34 The other studies did not specifically determine if a statistical association existed between M. cynos and respiratory disease, but did note that no mycoplasmal organisms were found in the lower airways of healthy dogs, whereas M. cynos was found in the lower airways of 1/8 and 4/29 sick dogs, respectively.32, 35
3.1.2. Mycoplasma canis, Mycoplasma spumans, and Mycoplasma edwardii
Three of the 5 studies evaluated isolation of M. canis, M. spumans, and M. edwardii in case and control dogs (Table 1).32, 33, 35 Although the numbers of case and control dogs were the same as those presented for M. cynos,32, 35 the number varied depending on the mycoplasma species: 120, 123, and 131 case dogs and 63, 68, and 69 control dogs for M. canis, M. spumans, and M. edwardii, respectively.33
Two studies did not specifically determine if a statistical association existed between each mycoplasma species and respiratory disease.32, 35 However, 1 study found no evidence of M. canis in the LRT of sick or healthy dogs, whereas M. spumans and M. edwardii only were isolated from the lungs of 2/8 and 1/8 ill dogs, respectively.32 The other study found M. canis in bronchoalveolar lavage fluid of 3/29 dogs with respiratory disease and 2/16 control dogs.35 Mycoplasma spumans only was isolated from a single sick dog, whereas M. edwardii only was found in a single control dog.35 Finally, another study did not find any significant differences in rate of isolation of M. canis, M. spumans, and M. edwardii between dogs with LRT signs and healthy controls.33
3.2. Risk of bias assessment
The risk of bias assessment showed that only 1 study35 of the 5 included studies used an appropriate case definition (Table 2). In this study, the clinical signs that case dogs showed were clearly described (eg, cough, nasal discharge, difficulty breathing, and lethargy), and respiratory disease was determined by objective measurements (physical examination by a veterinarian, radiography, and tracheobronchoscopy).35 The other studies all stated that case dogs had signs of respiratory disease but did not precisely indicate whether these signs were evaluated by a veterinarian, veterinary technician, or trained veterinary student. Furthermore, respiratory disease was not specifically confirmed using objective criteria.31, 32, 33, 34 Finally, cases primarily were included based on Bordetella bronchiseptica infection diagnosis rather than based on the presence of LRT signs in 1 study.31
Table 2.
Risk of bias assessment for studies included in the systematic review of the association between Mycoplasma cynos, Mycoplasma canis, Mycoplasma spumans, and Mycoplasma edwardii and lower respiratory tract disease in dogs. Data represent scores for the Newcastle‐Ottawa Scale (NOS)
| Study | Definition of casesa | Representativeness of casesb | Selection of controlsc | Definition of controlsd | Control for age or other confounderse | Ascertainment of exposuref | Same ascertainment methodg |
|---|---|---|---|---|---|---|---|
| Canonne31 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Chalker33 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Rosendal32 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Rycroft34 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Schulz35 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
A score of 1 was assigned if the study clearly stated how dogs were identified as cases (ie, which clinical signs) and if clinical signs were independently validated by an objective measure (eg, radiography) or were identified during a physical examination performed by a veterinarian, veterinary technician, or trained veterinary student.
A score of 1 was assigned if the geographic area, timeframe, and selection method were defined and adequate.
A score of 1 was assigned if control dogs used in the study were selected from the same population as the case dogs.
A score of 1 was assigned if it was explicitly stated that control dogs had no history of clinical signs of respiratory tract disease; a score of 0 was assigned if the possibility of respiratory tract disease in the recent past was not considered.
A maximum score of 2 was assigned if case and control dogs were matched in the study design and the analysis was adjusted for confounders. A statement that there was no differences between groups or that differences were not statistically significant was not considered sufficient for establishing comparability. If the study adjusted for only 1 confounding factor, a score of 1 was assigned. If the study adjusted for >1 confounding factor, a score of 2 was assigned.
A score of 1 was assigned if a validated polymerase chain reaction (PCR) assay was used to detect M. cynos, M. canis, M. spumans, and M. edwardii in samples obtained from case and control dogs; a score of 0 was assigned if bacterial culture or serology was used.
Score of 1 was assigned if the same diagnostic method was used for the case and control groups.
For 4 of the 5 studies, selected cases were representative of a well‐characterized population in terms of a defined period of time and location.31, 33, 34, 35 Two studies evaluated dogs with respiratory signs presented to a veterinary hospital,31, 35 and 2 studies assessed case dogs with respiratory signs that were from a kennel or shelter environment.33, 34 Lastly, 1 study simply stated that all case dogs came from a kennel, but did not further define the setting.32
Selection of control patients was adequate in 4 of the 5 studies.32, 33, 34, 35 In these studies, the control dog population was from the same population as the cases. One study used a control population that was different from the case population, because case dogs were client‐owned dogs with B. bronchiseptica infection referred to a veterinary teaching hospital, whereas controls were either healthy dogs belonging to veterinary staff or shelter dogs.31
Only 2 studies adequately defined control cases; these explicitly stated that control dogs had no history of respiratory disease.31, 35 The remaining 3 studies only mentioned that control dogs were free of respiratory signs during the study period.32, 33, 34
None of the studies matched case and control dogs for age, and no studies adjusted the statistical analysis for potential confounders. Although not sufficient for establishing comparability, control and case dogs were compared in terms of demographic data (age and sex) in 1 study and no statistically significant difference was found between the 2 groups.35 A concurrent nonmycoplasmal respiratory pathogen (B. bronchiseptica) only was investigated in 1 study, but no adjustment was made for its potential confounding effect.31
In all studies, the diagnostic method used to identify Mycoplasma spp. in case and control dogs was the same.
3.3. Pathogenic role of each mycoplasma species
A significant association was found between M. cynos and LRT disease in dogs (Bayesian pooled OR, 3.60; 95% CI, 1.31‐10.29; Figure 2). No heterogeneity was found among studies (I 2 = 0%; P = .99). On the other hand, no significant association was found between M. canis and LRT signs in dogs (Bayesian pooled OR, 1.06; 95% CI, 0.10‐14.63; Figure 3). No heterogeneity was found among studies (I 2 = 0%; P = .93).
Figure 2.
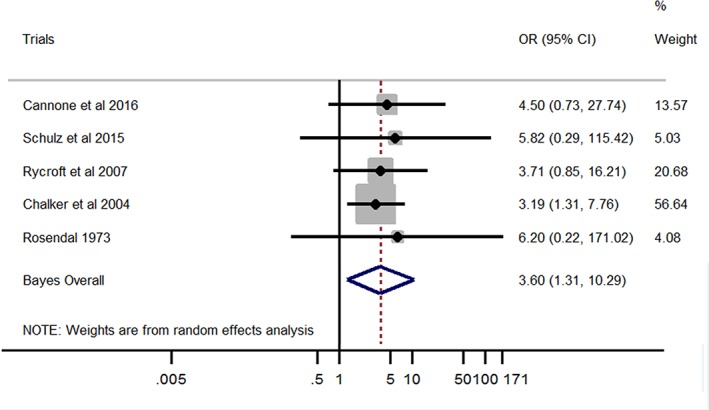
Forest plot of the association between Mycoplasma cynos and lower respiratory tract disease in dogs. Gray boxes represent estimated odds ratios (ORs) for each study; the area of the boxes is proportional to the weight attributed to each study, as determined from random‐effects analysis. Horizontal solid lines represent 95% confidence intervals (CIs). The vertical line represents an OR of 1 (ie, no association). The diamond represents the Bayesian random‐effects pooled OR calculated from all studies included in the meta‐analysis
Figure 3.
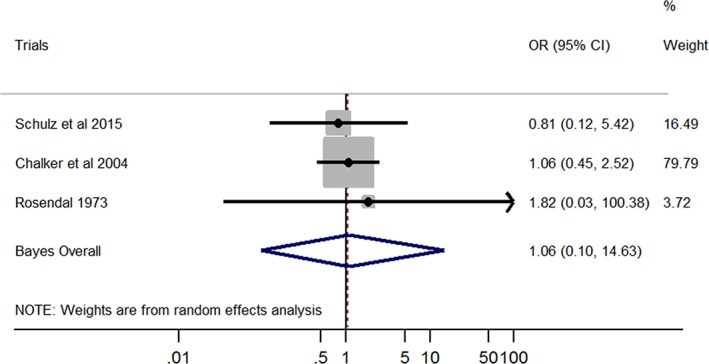
Forest plot of the association between Mycoplasma canis and lower respiratory tract disease in dogs. Gray boxes represent estimated odds ratios (ORs) for each study; the area of the boxes is proportional to the weight attributed to each study, as determined from random‐effects analysis. Horizontal solid lines represent 95% confidence intervals (CIs). The vertical line represents an OR of 1 (ie, no association). The diamond represents the Bayesian random‐effects pooled OR calculated from all studies included in the meta‐analysis
Although the effect size between M. spumans and LRT signs in dogs was similar to that between M. cynos and LRT disease in dogs (Bayesian OR, 3.40 and 3.60, respectively), the association between M. spumans and LRT signs was not statistically significant based on the Bayesian CI (pooled OR, 3.40; 95% CI, 0.16‐54.27; Figure 4). No heterogeneity was found among studies (I 2 = 0%; P = .6).
Figure 4.
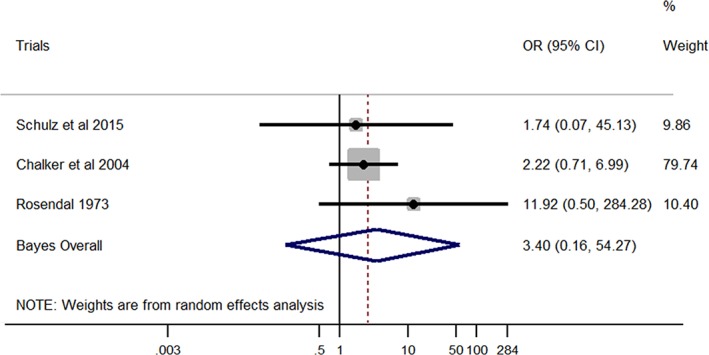
Forest plot of the association between Mycoplasma spumans and lower respiratory tract disease in dogs. Gray boxes represent estimated odds ratios (ORs) for each study; the area of the boxes is proportional to the weight attributed to each study, as determined from random‐effects analysis. Horizontal solid lines represent 95% confidence intervals (CIs). The vertical line represents an OR of 1 (ie, no association). The diamond represents the Bayesian random‐effects pooled OR calculated from all studies included in the meta‐analysis
Finally, no significant association was found between M. edwardii and LRT disease (Bayesian pooled OR, 1.04; 95% CI, 0.05‐23.54; Figure 5). Unimportant inconsistency was detected among studies (I 2 = 12.4%), but this was not found to be statistically significant (P = .32).
Figure 5.
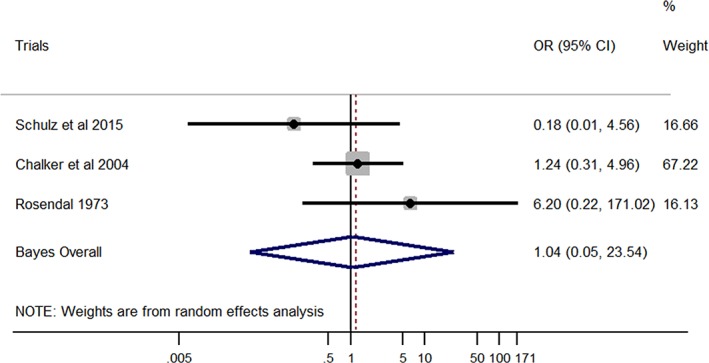
Forest plot of the association between Mycoplasma edwardii and lower respiratory tract disease in dogs. Gray boxes represent estimated odds ratios (ORs) for each study; the area of the boxes is proportional to the weight attributed to each study, as determined from random‐effects analysis. Horizontal solid lines represent 95% confidence intervals (CIs). The vertical line represents an OR of 1 (ie, no association). The diamond represents the Bayesian random‐effects pooled OR calculated from all studies included in the meta‐analysis
3.4. Sensitivity analysis and publication bias
Sensitivity analysis showed that for all 4 meta‐analyses, omission of 1 study in each meta‐analysis did not significantly influence the overall results of the study (Figure 6). Publication bias was not assessed, because all 4 meta‐analyses included fewer than 10 studies.
Figure 6.
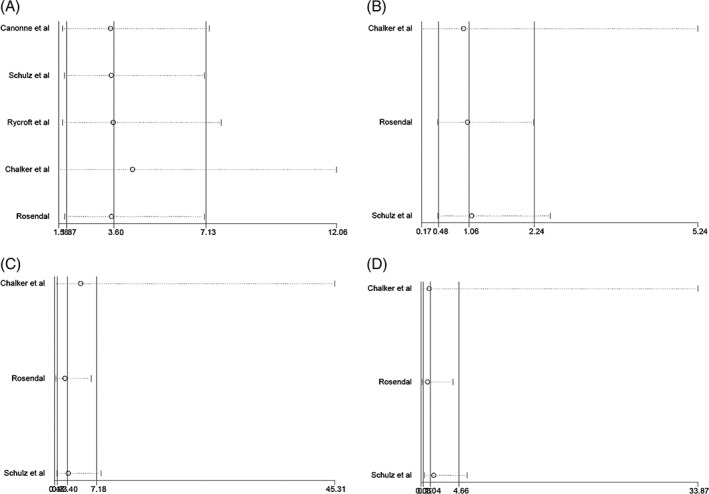
Sensitivity analysis for the association between A, Mycoplasma cynos; B, Mycoplasma canis; C, Mycoplasma spumans; and D, Mycoplasma edwardii and lower respiratory tract disease in dogs. The vertical straight line represents the pooled odds ratio (OR). Circles represent the change in the pooled OR when each study was omitted from the analysis. Horizontal lines represent the 95% confidence interval (CI) for the change in pooled OR. Influential studies would be detected if the pooled OR and the 95% CI varied significantly (ie, the circles move away from the vertical straight line and the horizontal lines do not cross the vertical straight line) when the studies are removed from the analysis. No influential studies were detected in the present meta‐analyses
4. DISCUSSION
Results of our meta‐analyses provided evidence of a significant association between M. cynos and LRT disease in dogs. However, no significant association was found between LRT disease and the other mycoplasma species studied (M. canis, M. spumans, and M. edwardii).
The association between M. cynos and LRT disease could be either causal or consequential. In the latter hypothesis, a primary respiratory pathogen, such as distemper virus, canine adenovirus type 2, Streptococcus sp., or B. bronchiseptica, induces primary lesions and decreases the host's defense, allowing mycoplasma to colonize the LRT.3, 13, 36 However, M. cynos has been isolated in pure culture from the lungs of puppies, in which viruses (distemper virus, canine adenovirus type 1 and 2, herpesvirus), aerobic and anaerobic bacteria, fungi, and parasites were searched for but not found.37 Our study strongly suggests a primary pathogenic role of M. cynos.
It is challenging to definitively demonstrate that an association is indeed causal; some criteria, referred to as Bradford Hill criteria, have been proposed to help assess causality.38 Although they are not all applicable to the association between M. cynos and LRT disease, most criteria can be examined, such as strength of association, analogy, temporality, coherence and consistency, biological plausibility, and experimental evidence. The association between M. cynos and LRT disease was determined to be moderate to strong according to the pooled OR (3.6). The OR represents the number of mycoplasma‐positive dogs divided by the number of mycoplasma‐negative dogs in cases (ie, dogs with signs of LRT disease) versus controls. Given that there are many other known causes of LRT disease in dogs, at least some of which are likely more common than respiratory mycoplasmosis, the number of mycoplasma‐negative dogs is expected to be higher than the number of mycoplasma‐positive dogs among the cases, and this tends to skew the OR toward lower values.39, 40 Interestingly, the pooled OR of 3.6 is analogous to the pooled OR of 2.4 found in another meta‐analysis on the association between M. felis and upper respiratory tract diseases in nonshelter cats.14 A causal relationship requires that the exposure (ie, M. cynos infection) starts before the outcome (ie, LRT signs) occurs.38 The design of 1 study allowed investigation of this temporality criterion, because all of the dogs were free of LRT signs when entering the rehoming kennel.34 The fact that 46% (12/26) dogs mounted an antibody response to M. cynos antigen among those that developed clinical signs supports an exposure to M. cynos, followed by a disease state. However, 3/16 dogs that did not develop respiratory signs also underwent seroconversion in this study. All 3 dogs had demonstrated antibody to M. cynos since the first day, indicating a likely past exposure to this agent and possible protection against M. cynos. As for M. felis in shelter cats, nonclinical carriage of M. cynos strains for months has been suspected in kenneled dogs.14, 41
No heterogeneity was detected in our meta‐analysis, confirming coherence and consistency of the association between M. cynos and LRT signs. Failure to demonstrate this association in individual studies seems to be a consequence of a lack of statistical power according to our forest plot (Figure 2).
Biological plausibility is supported by the identification of several virulence factors, including sialidase activity and hemagglutinin HapA, in M. cynos.42, 43, 44 Hemagglutinin lipoprotein HapA is thought to mediate binding to host cell receptors, allowing infection and colonization of LRT. Sialidase is an enzyme that assists in microbial colonization and dissemination within the host.45 It also can exert a direct toxic effect on the host cells as well as interfere with host defense mechanisms. The pathological changes associated with isolation of M. cynos include lung consolidation, loss of cilia on bronchial and bronchiolar epithelial cells, low‐grade serofibrinous pleuritis, severe acute generalized catarrhal‐suppurative and hemorrhagic or fibrinonecrotizing bronchopneumonia with infiltration of mononuclear cells, pulmonary edema, exudation of neutrophils and macrophages into alveolar spaces, and hyperplasia and exfoliation of type II pneumocytes.36, 37, 46 The fact that the immunohistochemistry signal of antibodies to M. cynos was centered on areas of severe neutrophilic inflammation in affected puppies suggests that the inflammation was caused by M. cynos in these dogs.37 Finally, pneumonia, destruction and loss of cilia, and alveolar infiltration with neutrophils and macrophages were produced experimentally by endobronchial infection with M. cynos and by exposure to dogs with M. cynos.3, 47 This evidence, along with the results of the present meta‐analysis, strongly supports a primary pathogenic role of M. cynos in LRT disease of dogs.
On the other hand, no significant association was found between LRT disease and M. canis, M. spumans, and M. edwardii. For M. canis and M. edwardii, the Bayesian pooled OR was very close to 1, indicating that these mycoplasma species have been isolated from both case and control dogs with relatively the same frequency. The pooled OR for the association between M. spumans and LRT disease is more difficult to interpret, because it was relatively high (approximately 3). On the other hand, the association clearly was not significant according to the Bayesian model. Only 3 studies were included in the meta‐analysis, and the number of dogs in 2 of the studies was relatively small.32, 35 Various statistical methods (eg, fixed‐effects and random‐effects DerSimonian‐Laird models or Bayesian models) can be used to perform meta‐analyses. All methods have limitations, especially when only a few studies are included in the meta‐analysis.24, 25, 26 Fixed‐effects models are appropriate for small meta‐analyses, because a summary based on ≥2 studies yields a more precise estimate of the true effect than either study alone.48 However, fixed‐effects models assume that all studies arise from a unique population, which does not fit reality in our situation (ie, the environments of the dogs are different in all 3 studies, thus indicating 3 different populations). Random‐effects DerSimonian‐Laird models take into account effect size heterogeneity among studies and therefore are more realistic. However, their accuracy is impacted by the number of studies included in the meta‐analysis. Confidence intervals for the effect size are narrower on average than they should be and P‐values associated with hypothesis testing are smaller than expected when the number of studies is modest.49 Conversely, Bayesian meta‐analysis is considered more appropriate when a small number of studies is included or when studies have less event data.50 The Bayesian OR CI might therefore be more accurate, thus indicating no significant association between LRT disease and M. spumans. This does not necessarily mean that no such association exists, but this association cannot be demonstrated based on the currently available evidence. Unlike M. cynos, experimental infection with M. canis and M. spumans failed to reproduce respiratory disease in dogs, which argues against a primary pathogenic role of M. spumans.3 Nevertheless, an investigation of an outbreak in a closed colony of Labrador Retrievers resulted in the isolation of M. spumans and canine parainfluenza virus from tracheal swabs and lung tissues of affected animals, whereas no other bacteria or viruses were recovered.21 Hence, if M. spumans cannot be considered a primary respiratory pathogen based on our results, the high OR found in our meta‐analysis and the conflicting evidence found in the literature should encourage more studies to properly characterize its role in LRT disease of dogs.
Our systematic review and associated meta‐analyses had several limitations, mainly pertaining to the low number of studies included. This low number of studies more likely reflects the paucity of data available on the topic, rather than being because of study omission. Indeed, a comprehensive electronic search was conducted that included 7 databases. The large number of databases was used to screen for as many articles as possible, regardless of their language and source, as recommended by the Cochrane Collaboration.51 This also is important to avoid overestimation of the effect size, because studies with positive findings are reportedly more likely to be published in English and in journals indexed in Medline.51, 52 The low number of included studies may have impacted both effect size precision and heterogeneity estimation.53 In 3 of the 4 meta‐analyses performed, I 2 was 0%, whereas I 2 was 12% for the meta‐analysis assessing the association between M. edwardii and LRT disease. By examining the forest plots, it can be seen that heterogeneity truly seems small, because all individual ORs tend to line up. The variance within studies is relatively large (ie, individual studies are underpowered) and fully explains the variance among studies. Put another way, given the imprecision of the studies, we would expect the effect size to vary somewhat from 1 study to the next. Therefore, we believe that heterogeneity is truly small in all 4 meta‐analyses. When a meta‐analysis has few studies and true heterogeneity is small, I 2 will tend to be overestimated.53 Hence, consistency among studies is very likely in our meta‐analyses. Reliability of the effect size estimation is very dependent on the quality of the studies included. The risk of bias was assessed with the NOS. The Cochrane Collaboration's tool for assessing risk of bias generally is considered superior to the use of scales, but this tool is designed for randomized trials only, not for observational studies.54 For nonrandomized noninterventional studies, the Cochrane Collaboration recognizes the NOS as 1 of the most useful tools for assessing the risk of bias. The NOS is simple to apply, and ratings usually are easily interpreted. Risk of bias was identified in most studies regarding definition of cases and controls, adjustment for confounders, and the laboratory technique used to ascertain exposure to mycoplasmas. Definition of cases and controls can affect the magnitude and statistical significance of the size effect, as well as being a source of heterogeneity among studies.55 Indeed, studies are not necessarily comparable if they did not include the same populations of dogs (cases may be restricted to dogs with respiratory distress or to dogs with cough only, which do not represent the same population of patients). Confounders suspected to potentially bias the relationship between mycoplasmas and LRT disease include young age, coinfections, and oropharyngeal contamination.12, 13 Age rarely was described in the included studies, and no studies adjusted the statistical analysis or matched cases and controls on age. Potential coinfections rarely were assessed. Of the 3 studies that used bronchoalveolar lavage as a sampling method, 2 excluded samples with evidence of oropharyngeal contamination (ie, exclusion of samples showing Simonsiella spp. or squamous cells).31, 35 Finally, the laboratory techniques used to identify exposure to mycoplasmas and to characterize the species were different among studies. Although PCR may be more sensitive than culture, there is no clear evidence to ascertain a difference in diagnostic accuracy between the 2 techniques.8, 9 When mycoplasmal culture was performed, 1 study used PCR for species identification33 whereas another used serology.32 Because cross‐reaction among species is possible with serology, proper species identification cannot be completely ascertained in the latter study.5, 53 One study used Western immunoblot serology for both mycoplasmal exposure and species identification.34 Western immunoblotting currently is considered the most sensitive and specific serological technique for detection of anti‐Mycoplasma pneumoniae antibodies in humans.8 A previous study showed that dogs experimentally inoculated with M. cynos developed antibody titers solely to this bacteria.3 Despite these flaws in study quality, the absence of heterogeneity among studies in the 4 meta‐analyses suggests a limited risk of bias. Publication bias could not be assessed because there were <10 included studies.54
In conclusion, despite a paucity of data on the association between M. cynos, M. canis, M. spumans, and M. edwardii and LRT disease in dogs, the current literature provides convincing evidence on a pathogenic role of M. cynos, and a commensal role of M. canis and M. edwardii. There is a lack of conclusive evidence to support a pathogenic role of M. spumans. Additional well‐designed controlled studies on the association between M. spumans and LRT disease therefore are warranted. Finally, our results support the use of species‐specific PCR directed toward M. cynos in practice for detection from the LRT in dogs with respiratory clinical signs, whereas the use of nonspecific primers for Mycoplasma spp. should be discouraged.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Preliminary results were presented as a poster at the European College of Veterinary Internal Medicine Congress, Rotterdam, September 2018.
Jambhekar A, Robin E, Le Boedec K. A systematic review and meta‐analyses of the association between 4 mycoplasma species and lower respiratory tract disease in dogs. J Vet Intern Med. 2019;33:1880–1891. 10.1111/jvim.15568
Correction added on 18 July 2019, after first online publication: in the third paragraph of Section 2.5, Statistical analysis, “Noninformative normal (0, 10) and inverse gamma (0.01, 0.01) before distributions were considered” was corrected to “Noninformative normal (0, 10) and inverse gamma (0.01, 0.01) prior distributions were considered”.
REFERENCES
- 1. Chalker VJ. Canine mycoplasmas. Res Vet Sci. 2005;79:1‐8. [DOI] [PubMed] [Google Scholar]
- 2. Greene CE, Chalker VJ. Nonhemotropic mycoplasmal, ureaplasmal, and L‐form infections In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier Health Sciences; 2002:319‐324. [Google Scholar]
- 3. Rosendal S. Canine mycoplasmas: pathogenicity of mycoplasmas associated with distemper pneumonia. J Infect Dis. 1978;138:203‐210. [DOI] [PubMed] [Google Scholar]
- 4. Koshimizu K, Ogata M. Characterization and differentiation of mycoplasmas of canine origin. Nippon Juigaku Zasshi. 1974;36:391‐406. [DOI] [PubMed] [Google Scholar]
- 5. Barile MF, DelGiudice RA, Carski TR, Yamashiroya HM, Verna JA. Isolation and rapid identification of Mycoplasma species from canine tissues by plate immunofluorescence. Proc Soc Exp Biol Med. 1970;134:146‐148. [DOI] [PubMed] [Google Scholar]
- 6. Edward DG, Fitzgerald WA. The isolation of organisms of the pleuropneumonia group from dogs. J Gen Microbiol. 1951;5:566‐575. [DOI] [PubMed] [Google Scholar]
- 7. Spergser J, Rosengarten R. Identification and differentiation of canine Mycoplasma isolates by 16S‐23S rDNA PCR‐RFLP. Vet Microbiol. 2007;125:170‐174. [DOI] [PubMed] [Google Scholar]
- 8. Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003;9:263‐273. [DOI] [PubMed] [Google Scholar]
- 9. Reed N, Simpson K, Ayling R, Nicholas R, Gunn‐Moore D. Mycoplasma species in cats with lower airway disease: improved detection and species identification using a polymerase chain reaction assay. J Feline Med Surg. 2012;14:833‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ericsson AC, Personett AR, Grobman ME, Rindt H, Reinero CR. Composition and predicted metabolic capacity of upper and lower airway microbiota of healthy dogs in relation to the fecal microbiota. PLoS One. 2016;11:e0154646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988‐1999). J Am Anim Hosp Assoc. 2002;38:111‐119. [DOI] [PubMed] [Google Scholar]
- 12. Chan CM, Ridgway MD, Mitchell MA, Maddox CW. Association between Mycoplasma‐specific polymerase chain reaction assay results and oral bacterial contamination of bronchoalveolar lavage fluid samples from dogs with respiratory tract disease: 121 cases (2005‐2012). J Am Vet Med Assoc. 2013;243:1573‐1579. [DOI] [PubMed] [Google Scholar]
- 13. Randolph JF, Moise NS, Scarlett JM, Shin SJ, Blue JT, Bookbinder PR. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease. Am J Vet Res. 1993;54:387‐391. [PubMed] [Google Scholar]
- 14. Le Boedec K. Association between mycoplasma and feline upper and lower respiratory tract diseases: a critical review and meta‐analysis. J Am Vet Med Assoc. 2017;250:397‐407. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong D, Morton V, Friedman MH, Steger L, Tully JG. Canine pneumonia associated with mycoplasma infection. Am J Vet Res. 1972;33:1471‐1478. [PubMed] [Google Scholar]
- 16. Field AP, Gillett R. How to do a meta‐analysis. Br J Math Stat Psychol. 2010;63:665‐694. [DOI] [PubMed] [Google Scholar]
- 17. Haidich AB. Meta‐analysis in medical research. Hippokratia. 2010;14:29‐37. [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Connor AM, Sargeant JM. Meta‐analyses including data from observational studies. Prev Vet Med. 2014;113:313‐322. [DOI] [PubMed] [Google Scholar]
- 20. Sumner CM, Rozanski EA, Sharp CR, Shaw SP. The use of deep oral swabs as a surrogate for transoral tracheal wash to obtain bacterial cultures in dogs with pneumonia. J Vet Emerg Crit Care. 2011;21:515‐520. [DOI] [PubMed] [Google Scholar]
- 21. Bemis DA. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet Clin North Am Small Anim Pract. 1992;22:1173‐1186. [DOI] [PubMed] [Google Scholar]
- 22. Priestnall SL, Mitchell JA, Walker CA, Erles K, Brownlie J. New and emerging pathogens in canine infectious respiratory disease. Vet Pathol. 2014;51:492‐504. [DOI] [PubMed] [Google Scholar]
- 23. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 10, 2018.
- 24. Valentine JC, Wilson SJ, Rindskopf D, et al. Synthesizing evidence in public policy contexts: the challenge of synthesis when there are only a few studies. Eval Rev. 2017;41:3‐26. [DOI] [PubMed] [Google Scholar]
- 25. Friede T, Röver C, Wandel S, Neuenschwander B. Meta‐analysis of few small studies in orphan diseases. Res Synth Methods. 2017;8:79‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seide SE, Röver C, Friede T. Likelihood‐based random‐effects meta‐analysis with few studies: empirical and simulation studies. BMC Med Res Methodol. 2019;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenthal JA. Qualitative descriptors of strength of association and effect size. J Soc Serv Res. 1996;21:37‐59. [Google Scholar]
- 28. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 30. Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta‐analyses. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; 2011. http://handbook-5-1.cochrane.org/. Accessed December 10, 2018.
- 31. Canonne AM, Billen F, Tual C, et al. Quantitative PCR and cytology of bronchoalveolar lavage fluid in dogs with Bordetella bronchiseptica infection. J Vet Intern Med. 2016;30:1204‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosendal S. Canine mycoplasmas. I. Cultivation from conjunctivae, respiratory‐ and genital tracts. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973;81:441‐445. [DOI] [PubMed] [Google Scholar]
- 33. Chalker VJ, Owen WMA, Paterson C, et al. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491‐3497. [DOI] [PubMed] [Google Scholar]
- 34. Rycroft AN, Tsounakou E, Chalker V. Serological evidence of Mycoplasma cynos infection in canine infectious respiratory disease. Vet Microbiol. 2007;120:358‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schulz BS, Raufeisen K, Weber K, Laberke S, Hartmann K. Comparison of the prevalence of Mycoplasma species in dogs with and without respiratory disease. Berl Munch Tierarztl Wochenschr. 2015;128:304‐309. [PubMed] [Google Scholar]
- 36. Chvala S, Benetka V, Möstl K, Zeugswetter F, Spergser J, Weissenböck H. Simultaneous canine distemper virus, canine adenovirus type 2, and Mycoplasma cynos infection in a dog with pneumonia. Vet Pathol. 2007;44:508‐512. [DOI] [PubMed] [Google Scholar]
- 37. Zeugswetter F, Weissenböck H, Shibly S, Hassan J, Spergser J. Lethal bronchopneumonia caused by Mycoplasma cynos in a litter of golden retriever puppies. Vet Rec. 2007;161:626‐627. [DOI] [PubMed] [Google Scholar]
- 38. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sykes JE. Canine viral respiratory infections In: Sykes JE, ed. Canine and Feline Infectious Diseases. 1st ed. St. Louis, MO: W.B. Saunders; 2014:170‐181. [Google Scholar]
- 40. Sykes JE. Bacterial bronchopneumonia and pyothorax In: Sykes JE, ed. Canine and Feline Infectious Diseases. 1st ed. St. Louis, MO: W.B. Saunders; 2014:847‐858. [Google Scholar]
- 41. Mannering SA, McAuliffe L, Lawes JR, Erles K, Brownlie J. Strain typing of Mycoplasma cynos isolates from dogs with respiratory disease. Vet Microbiol. 2009;135:292‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. May M, Brown DR. Secreted sialidase activity of canine mycoplasmas. Vet Microbiol. 2009;137:380‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berčič RL, Cizelj I, Benčina M, et al. Demonstration of neuraminidase activity in Mycoplasma neurolyticum and of neuraminidase proteins in three canine Mycoplasma species. Vet Microbiol. 2012;155:425‐429. [DOI] [PubMed] [Google Scholar]
- 44. Kastelic S, Cizelj I, Narat M, et al. Molecular characterisation of the Mycoplasma cynos haemagglutinin HapA. Vet Microbiol. 2015;175:35‐43. [DOI] [PubMed] [Google Scholar]
- 45. Corfield T. Bacterial sialidases–roles in pathogenicity and nutrition. Glycobiology. 1992;2:509‐521. [DOI] [PubMed] [Google Scholar]
- 46. Hong S, Kim O. Molecular identification of Mycoplasma cynos from laboratory beagle dogs with respiratory disease. Lab Anim Res. 2012;28:61‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosendal S, Vinther O. Experimental mycoplasmal pneumonia in dogs: electron microscopy of infected tissue. Acta Pathol Microbiol Scand B. 1977;85:462‐465. [DOI] [PubMed] [Google Scholar]
- 48. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. When does it make sense to perform a meta‐analysis? In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, eds. Introduction to Meta‐Analysis. 1st ed. Chichester, UK: John Wiley & Sons; 2009:357‐364. [Google Scholar]
- 49. Guolo A, Varin C. Random‐effects meta‐analysis: the number of studies matters. Stat Methods Med Res. 2017;26:1500‐1518. [DOI] [PubMed] [Google Scholar]
- 50. Lewis MG, Nair SN. Review of applications of Bayesian meta‐analysis in systematic reviews. GJMPH. 2015;4:1‐9. [Google Scholar]
- 51. Egger M, Zellweger‐Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350:326‐329. [DOI] [PubMed] [Google Scholar]
- 52. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867‐872. [DOI] [PubMed] [Google Scholar]
- 53. von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta‐analyses. BMC Med Res Methodol. 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0; 2011. http://handbook-5-1.cochrane.org/. Accessed December 10, 2018.
- 55. Sebastiani P, Bae H, Gurinovich A, Soerensen M, Puca A, Perls TT. Limitations and risks of meta‐analyses of longevity studies. Mech Ageing Dev. 2017;165:139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]


