Abstract
Background
Cardiac cachexia, loss of muscle mass associated with congestive heart failure (CHF), is associated with increased morbidity and shorter survival times in people, but an association between cardiac cachexia and survival has not been reported in dogs.
Objectives
To determine the prevalence of cachexia and its associations with clinical, laboratory, and survival data in dogs with CHF.
Animals
Two hundred sixty‐nine dogs with CHF.
Methods
Retrospective cohort study. Cachexia was defined by 1 of 2 definitions: (1) mild, moderate, or severe muscle loss or (2) weight loss of ≥5% in 12 months or less. Variables were compared between dogs with and without cachexia.
Results
One hundred thirty of 269 dogs (48.3%) had cardiac cachexia based on muscle loss, whereas 67 of 159 dogs (42.1%) with pre‐evaluation body weights had cachexia based on weight loss. Dogs with cachexia (based on muscle loss) were significantly older (P = .05), more likely to have a cardiac arrhythmia (P = .02), had higher chloride concentrations (P = .04), and had a lower body condition score (P < .001), hematocrit (P = .006), hemoglobin (P = .006), and albumin (P = .004) concentrations. On multivariable analysis, cachexia (P = .05), clinically important tachyarrhythmias (P < .001), azotemia (P < .001), and being under‐ or overweight (both P = .003) were associated with shorter survival times.
Conclusions and Clinical Importance
Cardiac cachexia in common in dogs with CHF and is associated with significantly shorter survival. This emphasizes the importance of preventing, diagnosing, and treating muscle loss in dogs with CHF.
Keywords: degenerative mitral valve disease, dilated cardiomyopathy, muscle condition score, survival, weight loss
Abbreviations
- BCS
body condition score
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- DMVD
degenerative mitral valve disease
- MCS
muscle condition score
1. INTRODUCTION
The effects of congestive heart failure (CHF) are not restricted to the cardiovascular system. A common systemic effect of CHF in both dogs and people is cardiac cachexia, a complex and common condition characterized by muscle loss, with or without weight loss.1, 2 The pathogenesis of cardiac cachexia is multifactorial and complex, and involves decreased energy intake and nutrient absorption, increased energy requirements, and alterations in metabolism.1 Inflammatory cytokines, particularly TNF‐α, IL‐1ß, and IL‐6, play an important role in cardiac cachexia and cause reduced food intake, decreased muscle protein synthesis, and increased protein catabolism.2
In humans, cachexia is linked to poor wound healing, lower exercise capacity, reduced strength, impaired immune function, and perceived poor quality of life.1, 2 Many of these findings are also present in dogs with cardiac cachexia.1 Cachexia in human CHF patients is an independent risk factor for poor prognosis and shorter survival times.3, 4 Cachexia's effect on survival might be compounded in companion animals as some of the signs associated with cachexia are important factors in decisions about euthanasia.5 However, the role of cardiac cachexia in survival of dogs with CHF has not been reported.
Approximately 10% of human CHF patients have cardiac cachexia although, depending on the definition and the patient population, prevalence rates can be up to 42%.2, 4, 6 Despite many years of debate, there is still no single definition for cachexia in humans, which contributes to the variable prevalence. Proposed diagnostic criteria for human cachexia include weight loss of at least 5% in 12 months or less in combination with at least 3 of 5 signs: decreased muscle strength, fatigue, anorexia, low fat‐free mass index, or abnormal serum biochemistry (ie, increased inflammatory markers, anemia, or low serum albumin).7 Despite this published consensus, other definitions have been used for research studies. One of the most common is the loss of at least 5% of body weight over 12 months or less.4, 8, 9 Definitions relying on weight loss as a criterion for the definition of cachexia, with or without additional criteria, however, is problematic in that body weight is an insensitive measure of muscle loss which can delay the diagnosis of cachexia until the disease is more advanced. This is particularly an issue in CHF where there can be fluid accumulation that can mask weight loss. In humans, loss of lean body mass and, more specifically, muscle mass occurs before substantial body weight is lost.10 Therefore, measuring lean body mass or muscle mass might be preferable for the diagnosis of cachexia.11 Loss of muscle can be quantified by advanced imaging modalities such as computed tomography or dual‐energy x‐ray absorptiometry, which require anesthesia or heavy sedation in companion animals, or by ultrasound.11, 12, 13 However, clinically, the easiest approach is to evaluate muscle condition score (MCS).14 Comparing prevalence of cachexia based on weight loss versus muscle loss has not been reported in dogs.
Therefore, the objectives of this study were to determine the prevalence of cachexia in dogs with CHF using definitions of weight loss or muscle loss and to compare clinical and laboratory variables, as well as survival times between dogs with and without cardiac cachexia.
2. METHODS
Electronic medical records were searched to identify dogs diagnosed with CHF caused by degenerative mitral valve disease (DMVD) or dilated cardiomyopathy (DCM) by the Cummings Veterinary Medical Center's Cardiology Service between January 2015 and May 2018. This date range was selected because the hospital's current electronic medical record system was launched in 2015, and MCS has been recorded for all animals evaluated in cardiology appointments since that time. All dogs were assessed by echocardiography (Vivid E9 GE Medical Systems, Milwaukee, Wisconsin). The diagnosis of DMVD was based on signalment, a left apical systolic murmur, typical changes to the mitral valve leaflets on echocardiography, and the presence of mitral regurgitation on color‐flow Doppler. To be included in this study, dogs with DMVD had to have at least a grade 3/6 systolic murmur and evidence of left atrial dilatation, defined as a left atrial‐to‐aortic root ratio of at least 1.6 on 2‐dimensional (2D) echocardiography. A diagnosis of DCM was based on the presence of left atrial enlargement, left ventricular dilatation, and a fractional shortening ≤25% on a 2D and M‐mode echocardiography. Dogs were classified as having CHF based on a combination of clinical signs and echocardiography, along with either radiographic evidence of cardiogenic pulmonary edema or presence of ascites or pleural effusion judged to be cardiogenic in origin. Echocardiography was performed using standard techniques15 by a board‐certified veterinary cardiologist or a supervised cardiology resident.
Exclusion criteria included dogs that were diagnosed with CHF at <1 year of age, dogs with CHF caused by other cardiac diseases, and dogs with other major concurrent diseases that could affect survival (eg, cancer, preexisting chronic kidney disease). Information on other diseases was determined from review of the medical records.
Medical records were retrospectively reviewed using a standardized data form to collect the following data from the visit at which CHF was diagnosed: signalment, body weight, heart rate, arrhythmias, murmur grade, echocardiographic measurements, CBC and serum biochemistry results, underlying disease, ACVIM stage, concurrent diseases, body condition score (BCS, on a 1‐9 scale), and MCS (normal, mild, moderate, or severe muscle loss).14 For the purposes of statistical analysis, MCS was converted to numbers (normal = 3, mild = 2, moderate = 1, severe = 0). Dogs with atrial fibrillation, supraventricular tachycardia, ventricular premature beats, or ventricular tachycardia were defined as having clinically important tachyarrhythmias. Azotemia was defined as a serum creatinine concentration above the upper end of the reference range and anemia was based on a hematocrit below the lower end of the reference range. Body condition scores of 4 or 5 on a 9‐point scale were considered ideal, whereas BCSs < 4/9 were considered underweight and those >5/9 were considered overweight. In addition to data from the date of diagnosis of CHF, body weights 6 and 12 months before diagnosis and 3, 6, 9, and 12 months after diagnosis were also collected when available. If ascites was noted, body weights after abdominocentesis were preferentially collected. If abdominocentesis was not performed, the presence of ascites was noted. The date and cause of death, if not still alive, were also recorded. If survival information was not available in the medical record, the primary care veterinarian was contacted or, if any information was still unavailable, the owner was contacted. If the owner could not be contacted, the dog was considered lost to follow‐up.
2.1. Data analysis
Data distributions were examined graphically before analysis. If visual evaluation was unclear, normality was determined via measures of skewness and kurtosis. Skewness and kurtosis values <2 were considered normally distributed. Dogs were categorized as having cardiac cachexia according to 2 separate definitions:
Muscle loss: Dogs with any muscle loss on the MCS scale (ie, mild, moderate, or severe)14 at the time of diagnosis of CHF were categorized as having cardiac cachexia, whereas dogs with a normal MCS were categorized as having no cardiac cachexia.
Weight loss: Dogs with at least 5% weight loss in 12 months or less before the diagnosis of CHF were categorized as having cardiac cachexia,4 whereas dogs with <5% weight loss were categorized as having no cardiac cachexia.
The percentages of dogs with cachexia based on these 2 definitions were compared by Chi‐square analysis. Chi square analysis was also used to compare categorical variables between dogs with and without cachexia. Independent t tests (for normally distributed variables) or Mann‐Whitney U tests (for skewed variables) were used to compare continuous variables between dogs with and without cachexia. Survival times were calculated from the time of diagnosis of CHF until the time of death/euthanasia. Dogs were right censored if they were alive at the time of analysis or if they were lost to follow‐up. Kaplan‐Meier curves were constructed, and log‐rank analyses were performed to assess the effect of variables on survival. Multivariable analysis was performed to identify potential confounding variables with respect to survival. P values ≤.05 were considered significant. All statistical tests were carried out using commercial statistical software (SYSTAT version 13.0, SYSTAT, San Jose, California; SPSS version 26.0, IBM Corp, Armonk, New York).
3. RESULTS
Of the 269 dogs that met the inclusion criteria, 218 dogs had DMVD and 51 dogs had DCM. Median age at diagnosis of CHF was 11.0 years (range, 1.8‐17.1 years) with 54% male dogs and 46% female dogs (Table 1). The most commonly represented breeds included mixed breed (n = 56), Chihuahua (n = 21), Cavalier King Charles Spaniel (n = 20), Doberman Pinscher (n = 16), Maltese (n = 10), Great Dane (n = 10), Boston Terrier (n = 9), and Dachshund (n = 9), but a variety of other breeds were represented in smaller numbers. All 269 dogs received furosemide, 268 dogs received pimobendan (1 dog was euthanized immediately after the diagnosis of CHF), and 252 dogs received an angiotensin converting enzyme inhibitor. Dogs received a variety of other medications during the course of management of CHF including spironolactone (n = 86), sildenafil (n = 70), diltiazem (n = 46), torsemide (n = 46), digoxin (n = 38), amiodarone (n = 31), amlodipine (n = 23), beta‐blocker (n = 7), and spironolactone/hydrochlorathiazide (n = 3).
Table 1.
Clinical characteristics of 269 dogs with congestive heart failure. Cachexia was defined as dogs with mild, moderate, or severe muscle loss based on the muscle condition score.14 Dogs without cachexia had a normal muscle condition score. Data are presented as number of dogs or median (range)
| Variable | All dogs | Dogs with cachexia | Dogs without cachexia | P value |
|---|---|---|---|---|
| n | 269 | 130 | 139 | ‐ |
| Age (years) | 11.0 (1.8‐17.1) | 11.7 (1.9‐17.1) | 10.4 (1.8‐16.7) | .05 |
| Sex | .11 | |||
| Male | 145 (124 castrated) | 78 (66 castrated) | 67 (58 castrated) | |
| Female | 124 (117 spayed) | 52 (51 spayed) | 72 (66 spayed) | |
| Disease | .05 | |||
| DVMD | 51 | 31 | 20 | |
| DCM | 218 | 99 | 119 | |
| Body weight (kg) | 7.9 (2.1‐82.0) | 9.5 (2.1‐82.0) | 6.9 (2.3‐77.3) | .02 |
| Body condition score | 5 (3‐9) | 5 (3‐8) | 5 (3‐9) | <.001 |
| Muscle condition score | <.001 | |||
| Normal | 139 | 0 | 139 | |
| Mild | 100 | 100 | 0 | |
| Moderate | 23 | 23 | 0 | |
| Severe | 7 | 7 | 0 | |
| Heart rate (/min) | 144 (46‐300) | 148 (80‐250) | 142 (46‐300) | .22 |
| Murmur grade | 5 (0‐6) | 5 (0‐6) | 5 (0‐6) | .23 |
| Blood pressure (mm Hg) | 120 (50‐220) | 112 (80‐190) | 140 (50‐220) | <.001 |
Abbreviations: DCM, dilated cardiomyopathy; DMVD, degenerative mitral valve disease.
One hundred fifty‐nine of the 269 dogs (59.1%) had body weights available at either 12 or 6 months before diagnosis of CHF. Sixty‐seven of 159 (42.1%) had lost at least 5% of their body weight in the 12 months or less before diagnosis of CHF. Thirty‐five of the 269 dogs, or 13.0%, had ascites noted during at least 1 hospital visit. All but 1 dog (n = 268) had a BCS recorded on the date of diagnosis of CHF. Median BCS was 5 (range, 3‐9). Only 12 dogs were underweight (BCS < 4/9; 4.5%), 157 were ideal weight (BCS 4‐5/9; 58.6%), and 99 were overweight (BCS > 5/9; 36.9%). Muscle condition score at the time of diagnosis of CHF was available for all 269 dogs and included 139/269 (51.7%) with normal MCS and 130/269 (48.3%) with some degree of muscle loss: mild (n = 100), moderate (n = 23), or severe (n = 7). Muscle loss was present in 10/12 underweight dogs (83%), 89/157 ideal weight dogs (56.7%), and 30/99 overweight dogs (30%). Body condition score and MCS were significantly correlated (r = .44, P < .001). As only 59.1% of dogs had sufficient body weight information to be able to assess weight loss for a definition of cachexia (and because the prevalence was similar based on both definitions), the definition of cardiac cachexia using MCS was used for all subsequent analyses.
Compared with dogs without cachexia, dogs with cachexia were significantly older (P = .05), more likely to have a clinically important tachyarrhythmia (P = .02), more likely to have DCM compared to DMVD (P = .05) and had lower BCS (P < .001), blood pressure (P < .001), hematocrit (P = .006), hemoglobin (P = .006), and albumin (P = .004) concentrations, but had a higher chloride concentration (P = .04; Tables 1 and 2). No other variables were significantly different between dogs with and without cachexia.
Table 2.
Serum biochemistry and hematologic values of 269 dogs with congestive heart failure. Cachexia was defined as dogs with mild, moderate, or severe muscle loss based on the muscle condition score.14 Dogs without cachexia had a normal muscle condition score. Data are presented as number of dogs or median (range)
| Variable | All dogs | Dogs with cachexia | Dogs without cachexia | P value |
|---|---|---|---|---|
| Hematocrit (%) | 48.6 (28.0‐68.0) | 47.5 (32.0‐65.0) | 50.0 (28.0‐68.0) | .006 |
| Hemoglobin (g/dL) | 16.3 (9.3‐23.6) | 15.9 (11.4‐21.6) | 16.6 (9.3‐23.6) | .006 |
| WBC (1000/μL) | 14.36 (4.50‐45.56) | 14.19 (6.20‐45.56) | 14.39 (4.50‐26.45) | .68 |
| Neutrophils (1000/μL) | 11.34 (3.85‐42.78) | 11.34 (4.26‐42.78) | 11.32 (3.85‐25.48) | .73 |
| Lymphocytes (1000/μL) | 1.48 (0.26‐4.41) | 1.60 (0.26‐3.64) | 1.36 (0.26‐4.41) | .13 |
| Cholesterol (mg/dL) | 221 (107‐447) | 215 (111‐441) | 229 (107‐447) | .09 |
| Potassium (mEq/L) | 4.3 (2.7‐6.7) | 4.4 (2.8‐6.7) | 4.3 (3.0‐5.8) | .08 |
| Chloride (mEq/L) | 110 (83‐130) | 111 (83‐130) | 109 (90‐126) | .04 |
| Sodium (mEq/L) | 147 (128‐159) | 147 (128‐159) | 147 (129‐159) | .53 |
| Globulin (g/dL) | 2.8 (1.3‐9.8) | 2.8 (1.6‐4.4) | 2.9 (1.3‐9.8) | .16 |
| Albumin (g/dL) | 3.6 (1.8‐4.9) | 3.4 (2.0‐4.8) | 3.7 (1.8‐4.9) | .001 |
| Creatinine (mg/dL) | 0.9 (0.2‐2.7) | 0.9 (0.4‐2.5) | 0.9 (0.2‐2.7) | .71 |
| BUN (mg/dL) | 21 (10‐107) | 22 (10‐76) | 21 (10‐107) | .81 |
| Glucose (mg/dL) | 96 (19‐220) | 93 (19‐214) | 100 (31‐220) | .07 |
Abbreviations: BUN, blood urea nitrogen; WBC, white blood cell count.
Sixty‐eight percent of dogs (183/269) died or were euthanized at the time of data analysis; 62 died and 120 were euthanized (cause of death was unknown for 1 dog). Eighty‐six dogs were right‐censored (82 were still alive and 4 were lost to follow‐up). Median survival time for all dogs was 294 days (range, 0‐1264 days). Dogs with cachexia had a survival time of 233 days (range, 0‐1200 days), whereas dogs without cachexia had a survival time of 321 days (range, 1‐1264 days; P = .04; Figure 1). Other variables that were significantly associated with a shorter survival time included BCS (underweight [P = .04; Figure 2] or overweight [P = .03; Figure 2]), DCM (P = .001); clinically important tachyarrhythmias (P = .001); ascites (P = .005); use of digoxin (P = .01), amiodarone (P < .001), or diltiazem (P = .02); azotemia (P = .001), and anemia (P = .03). No other variables were significantly associated with survival time. For the 159 dogs with adequate body weight information, cachexia defined as 5% weight loss within 12 months or less was not significantly associated with survival time (P = .73). On multivariable analysis, arrhythmia (P < .001), azotemia (P < .001), being under‐ or overweight (both P = .003), and cachexia (P = .05) all remained significant independent risk factors for shorter survival times.
Figure 1.
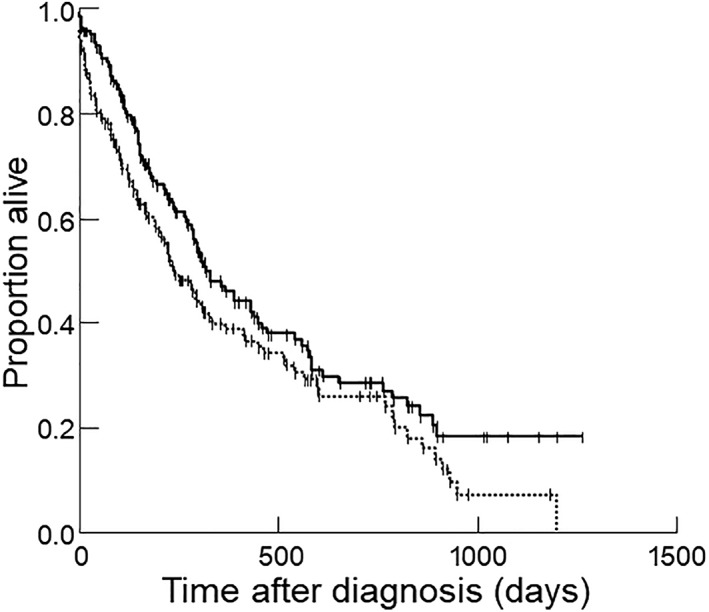
Kaplan‐Meier survival curve for 269 dogs stratified by the presence or absence of cachexia, as defined by muscle condition score. Dogs with cachexia (dotted line) had a significantly shorter survival time compared to dogs without cachexia (solid line; P = .04 on univariate analysis and P = .05 on multivariable analysis)
Figure 2.
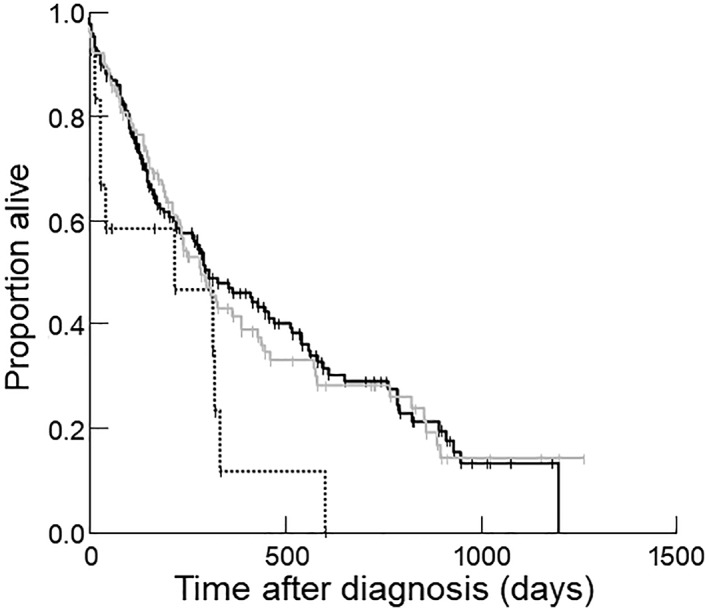
Kaplan‐Meier survival curve for 269 dogs stratified by body condition score (BCS) category, where a BCS < 4/9 was categorized as underweight (dotted line), BCS = 4‐5/9 was categorized as ideal weight (solid line), and BCS > 5/9 was categorized as overweight (gray line). Compared to dogs with ideal BCS, dogs with a BCS < 4/9 (P = .04 on univariate analysis, P = .003 on multivariable analysis) and dogs with a BCS > 5/9 (P = .03 on univariate analysis and P = .003 on multivariable analysis) had shorter survival times
4. DISCUSSION
Results of this study showed that dogs with CHF because of DMVD or DCM that had cachexia, defined as having loss of muscle on a MCS, had a significantly shorter survival time compared with dogs without cachexia. This finding is in agreement with existing survival studies on cachexia in human patients with CHF.3, 4 Causes for shorter survival times in people and dogs with cardiac cachexia are unknown but might be caused by loss of cardiac muscle, impaired metabolism, or reduced immune function.1 Additionally, in dogs, reduced quality of life, reduced food intake, and weakness, often associated with cardiac cachexia, are important factors in the decision to euthanize dogs with CHF which could result in shorter survival times.5
Low BCS at diagnosis was an independent risk factor for death. This is consistent with results of a previous study in which dogs with CHF that lost weight had a significantly shorter survival time compared with dogs that maintained or gained weight.16 Of note, although both BCS and MCS were predictors of death in this study, only 4.5% of dogs were underweight whereas 48.3% of dogs had muscle loss. This distinction is important because BCS and MCS are not the same. Body condition score assesses fat stores, whereas MCS is an assessment of muscle mass. Dogs can be overweight or obese but still have muscle loss (83.3% of dogs with a low BCS had muscle loss but 56.7% of ideal weight dogs and 30.3% of overweight dogs also had muscle loss) whereas conversely, dogs can be thin but have normal MCS (2 of 12 thin dogs [16.7%] in the current study did not have muscle loss). Therefore, it is critical to measure both BCS and MCS, along with body weight, in all dogs.
In addition to low BCS being associated with a shorter survival time, dogs that were overweight or obese (BCS >5/9) also had a shorter survival time compared to those with a BCS = 4‐5/9 at the time of diagnosis of CHF. This is in contrast to many human studies that have found an “obesity paradox,” in which people with heart failure that are overweight or even obese have longer survival times.17, 18 The difference in survival could not be detected based on BCS in dogs with CHF, although dogs that lost weight had shorter survival times than those that maintained or gained weight.16 Similarly, a difference in survival could not be detected based on BCS in cats with CHF, but cats with the lowest or the highest body weights had shorter survival times.19 The inability of these previous studies to detect a difference in BCS could be related to differences in underlying diseases between humans, dogs, and cats or could be because of the small sample size (the dog16 and cat19 studies included 108 and 101 cases, respectively). In addition, the previous study of dogs with CHF had different BCS categories (emaciated [BCS = 1‐2/9], underweight [BCS = 3‐4/9], normal weight [BCS = 5/9], overweight [BCS = 6‐7/9], obese [BCS = 8‐9/9]).16 One possible explanation for the shorter survival time in the current study is that the obesity paradox now appears to be related more to extra lean body mass in most overweight human patients than to obesity per se.20 However, if excess weight is not associated with more lean body mass but is actually associated with cachexia, the beneficial effects are lost. In fact, concurrent obesity and muscle loss in people (“sarcopenic obesity”) might be associated with even shorter survival times than obesity or muscle loss alone.21 In the current study, 30.3% of overweight dogs had muscle loss so sarcopenic obesity might explain the association between overweight dogs and shorter survival times. No matter the cause, the finding of significantly shorter survival time for both low and high BCS in the current study of dogs with CHF warrants further study.
Although a similar percentage of dogs (42.1%) had cachexia based on the definition of weight loss of 5% or more in 12 months or less compared to muscle loss (48.3%), only 59.1% of dogs had sufficient body weights to determine cachexia based on weight loss. This was a consequence of the definition of weight loss requiring information from at least 2 veterinary visits. However, not all dogs had body weights recorded in the 6‐12 month range that was used for one of the current study's definitions of cachexia (some had body weights measured at >12 months or <6 months before diagnosis of CHF). Others had not been taken to a veterinarian during this time period or body weights were not recorded. Additionally, the presence of ascites in 13.0% of the dogs made recorded weights inaccurate in some dogs. Although body weights were collected after abdominocentesis when available, not all dogs underwent abdominocentesis, and not all that did had body weights recorded after the procedure. Thus, it could be that the number of dogs with cachexia based on weight loss was underestimated because of excess fluid masking weight loss. The lack of consistent availability of body weight in this study, in combination with ascites altering weights, makes weight loss an unreliable measure of cardiac cachexia in dogs. Muscle condition score, however, is unaffected by the presence of ascites and can be assessed at a single time point and thus avoids the pitfalls of relying on past records that often have large gaps in weight histories. Finally, even if accurate body weight data were available, it appears to be less sensitive for identifying cachexia than muscle loss in humans and likely in dogs. Of human patients with cancer that did not meet the inclusion criterion for cachexia of ≥5% weight loss, 41% had ≥5% muscle loss over the same period, suggesting that muscle loss is a more sensitive measure of cachexia.10
In addition to MCS and BCS, results of the current study also showed that dogs with important arrhythmias had a shorter survival time compared with dogs without these arrhythmias, as was seen in previous studies.22, 23 Azotemia was also an independent predictor of survival time. Azotemia has also been identified as a risk factor in studies of people with heart failure.24 In addition, serum creatinine concentrations are higher in dogs with increasing severity of heart disease,25 with 23% of dogs with advanced heart failure in 1 study having azotemia.26 However, azotemia results must be interpreted with caution in the current study since laboratory testing was performed at variable time points with relation to the diagnosis of CHF (ie, it was measured on the hospital visit when CHF was first diagnosed, but some were before and others were after diuretic administration). In dogs with CHF, azotemia might be secondary to cardiac medications, as all dogs with known preexisting renal disease were excluded.
In addition to comparing survival times, clinical and laboratory variables were compared between dogs with and without cardiac cachexia. Dogs with cachexia were significantly older, more likely to have DCM, and had lower BCS, blood pressure, hematocrit, hemoglobin, and albumin concentrations and higher chloride concentrations. Lower albumin and hemoglobin concentrations occur in people with cardiac cachexia reflecting malnutrition.27, 28 The findings of lower blood pressure, higher chloride, or the greater likelihood of having DCM have not been previously reported in people or dogs with cardiac cachexia and require further study. It is also important to be aware that muscle loss also occurs in older dogs even in the absence of disease, where it is called sarcopenia. Because CHF occurs more commonly in older dogs, cachexia and sarcopenia can occur concurrently so it is difficult to discern whether muscle loss in these dogs was caused by cardiac cachexia, sarcopenia, or a combination of the two. It also is possible that the weight and muscle loss identified at the time of diagnosis of CHF was because of other undiagnosed diseases.
There are a number of limitations to the current study which are important to consider. This was a retrospective case study, so not all desired information was available in the medical records nor was it collected at the same time points in all dogs, even if it was collected during the hospitalization for the first diagnosis of CHF (eg, body weight and laboratory results). Another limitation is that MCS was not assessed by the same individual and studies in dogs29 and cats30 have shown some inter‐ and intraobserver variability. However, in our hospital's cardiology service, MCS is reviewed and confirmed on every animal by the attending cardiologist or the cardiology resident, so there are a limited number of clinicians assigning these scores.
The high prevalence of cachexia and the association between cardiac cachexia and survival in dogs as well as in people with CHF suggests that muscle loss is important to evaluate in dogs with heart disease. Although few specific treatments are currently available, there is active research on treatments for cachexia in dogs and humans.31, 32 However, it will be important to identify cachexia as early as possible when nutritional or drug treatments are more likely to be successful. The results of this study suggest that MCS is a clinically relevant method to detect cachexia in dogs with CHF at a single time point. This supports the recommendations to measure body weight, BCS, and MCS on every dog at every visit.14, 33
CONFLICT OF INTEREST DECLARATION
Within the past 3 years, Freeman has received research support from Aratana Therapeutics, Nestlé Purina PetCare, and Royal Canin; has consulted with Aratana Therapeutics and Nestlé Purina PetCare; has given sponsored talks for Aratana Therapeutics, Hill's Pet Nutrition, and Nestlé Purina PetCare; and has served on a scientific advisory board for Aratana Therapeutics. Within the past 3 years, Rush has received research support from Aratana Therapeutics, Nestlé Purina PetCare, and Royal Canin and has consulted with Aratana Therapeutics and Nestlé Purina PetCare.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Ineson DL, Freeman LM, Rush JE. Clinical and laboratory findings and survival time associated with cardiac cachexia in dogs with congestive heart failure. J Vet Intern Med. 2019;33:1902–1908. 10.1111/jvim.15566
Funding information National Institutes of Health, Grant/Award Number: OD010963
REFERENCES
- 1. Freeman LM. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J Vet Intern Med. 2011;26(1):3‐17. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Ebner N, dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14:323‐341. [DOI] [PubMed] [Google Scholar]
- 3. Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050‐1053. [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Negassa A, Coats AJS, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet. 2003;361:1077‐1083. [DOI] [PubMed] [Google Scholar]
- 5. Mallery KF, Freeman LM, Harpster NK, Rush JE. Factors contributing to the euthanasia decision in dogs with congestive heart failure. J Am Vet Med Assoc. 1999;214:1201‐1204. [PubMed] [Google Scholar]
- 6. Christensen HM, Kistorp C, Schou M, et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626‐634. [DOI] [PubMed] [Google Scholar]
- 7. Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793‐799. [DOI] [PubMed] [Google Scholar]
- 8. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489‐495. [DOI] [PubMed] [Google Scholar]
- 9. Saitoh M, Santos MRD, Emami A, et al. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure. ESC Heart Fail. 2017;4:448‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roeland EJ, Ma JD, Nelson SH, et al. Weight loss versus muscle loss: re‐evaluating inclusion criteria for future cancer cachexia interventional trials. Support Care Cancer. 2017;25(2):365‐369. [DOI] [PubMed] [Google Scholar]
- 11. Heymsfield AM, Gonzalez MC, Jia G, Thomas DM. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle. 2014;5:9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nijholt W, Scafoglieri A, Jager‐Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8:702‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freeman LM, Sutherland‐Smith J, Prantil LR, et al. Quantitative assessment of muscle in dogs using a vertebral epaxial muscle score. Can J Vet Res. 2017;81:255‐260. [PMC free article] [PubMed] [Google Scholar]
- 14. World Small Animal Veterinary Association . Muscle condition score [Internet]. https://www.wsava.org/WSAVA/media/Documents/Committee%20Resources/Global%20Nutrition%20Committee/English/Muscle‐Condition‐Score‐Chart‐for‐Dogs.pdf. Accessed November 21, 2018.
- 15. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 16. Slupe JL, Freeman LM, Rush JE. Association of body weight and body condition with survival in dogs with heart failure. J Vet Intern Med. 2008;22:561‐565. [DOI] [PubMed] [Google Scholar]
- 17. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, et al. Body mass index and mortality in heart failure: a meta‐analysis. Am Heart J. 2008;156:13‐22. [DOI] [PubMed] [Google Scholar]
- 18. Sharma A, Lavie CJ, Borer JS, et al. Meta‐analysis of the relation of body mass index to all‐cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428‐1434. [DOI] [PubMed] [Google Scholar]
- 19. Finn E, Freeman LM, Rush JE, Lee Y. The relationship between body weight, body condition and survival in cats with heart failure. J Vet Intern Med. 2010;24:1369‐1374. [DOI] [PubMed] [Google Scholar]
- 20. Lavie CJ, De Schutter A, Patel DA, et al. Body composition and survival in stable coronary heart disease. J Am Coll Cardiol. 2012;60(15):1374‐1380. [DOI] [PubMed] [Google Scholar]
- 21. Carbone S, Billingsley HE, Rodriguez‐Miguelez P, et al. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol. 2019;00:1‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120‐128. [DOI] [PubMed] [Google Scholar]
- 23. Martin MWS, Stafford Johnson MJ, Stehlau G, King JN. Canine dilated cardiomyopathy: a retrospective study of prognostic findings in 367 clinical cases. J Small Anim Pract. 2010;51(8):428‐436. [DOI] [PubMed] [Google Scholar]
- 24. Dammam K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta‐analysis. J Card Fail. 2007;13(8):599‐608. [DOI] [PubMed] [Google Scholar]
- 25. Yu IB, Huang HP. Prevalence and prognosis of anemia in dogs with degenerative mitral valve disease. Biomed Res Int. 2016;2016:4727054. Epub 2016 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beaumier A, Rush JE, Yang VK, Freeman LM. Clinical findings and survival time in dogs with advanced heart failure. J Vet Intern Med. 2018;32:944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Araujo JP, Lorenco P, Rocha‐Goncalves F, et al. Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol. 2011;146(3):359‐363. [DOI] [PubMed] [Google Scholar]
- 28. Santos NFD, Pinho CPS, Cardoso AJPE, Mendes RML. Cachexia in hospitalized patients with heart failure. Nutr Hosp. 2018;35:669‐676. [DOI] [PubMed] [Google Scholar]
- 29. Freeman LM, Michel KE, Zanghi BM, Vester Boler BM, Fages J. Evaluation of the use of muscle condition score and ultrasonographic measurements for assessment of muscle mass in dogs. Am J Vet Res. 2019;80:595‐600. [DOI] [PubMed] [Google Scholar]
- 30. Michel KE, Anderson W, Cupp C, Laflamme DP. Correlation of a feline muscle mass score with body composition determined by dual‐energy X‐ray absorptiometry. Br J Nutr. 2011;106:S57‐S59. [DOI] [PubMed] [Google Scholar]
- 31. Bethesda: U.S. National Library of Medicine . Clinicaltrials.gov [Internet]. https://clinicaltrials.gov. Accessed May 9, 2019.
- 32. Rolfe M, Kamel A, Ahmed MM, et al. Pharmacological management of cardiac cachexia: a review of potential therapy options. Heart Fail Rev. 2019. Mar 28. 10.1007/s10741-019-09784-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. World Small Animal Veterinary Association Nutritional Assessment Guidelines Task Force . WSAVA nutritional assessment guidelines. J Small Anim Pract. 2011;52:385‐396. [DOI] [PubMed] [Google Scholar]


