Abstract
Background
Famotidine is sometimes administered as a continuous rate infusion (CRI) to treat gastrointestinal ulceration in critically ill dogs. However, clinical studies have not evaluated the efficacy of a famotidine CRI in dogs.
Hypothesis/Objectives
To evaluate the efficacy of famotidine at raising intragastric pH when it is administered as a CRI in dogs. We hypothesized that CRI treatment with famotidine would meet clinical goals for raising intragastric pH ≥3 and 4.
Animals
Nine healthy Beagle dogs.
Methods
Randomized 2‐way crossover. All dogs received 1.0 mg/kg IV q12h famotidine or CRI famotidine at 1.0 mg/kg IV loading dose and 8.0 mg/kg/d for 3 consecutive days. Beginning on day 0 of treatment, intragastric pH monitoring was used to continuously record intragastric pH. Mean percentage times (MPTs) for which intragastric pH was ≥3 and ≥4 were compared between groups using analysis of variance.
Results
There was a statistically significant difference (P < .05) in MPT ≥3 and ≥4 between the CRI and IV q12h groups on all treatment days. On days 1, 2, and 3, the MPTs ± SD for which pH was ≥3 were 92.1 ± 8.5, 96.3 ± 6.2, and 90.0 ± 15.7 for the CRI treatment group and 49.3 ± 27.3, 42.2 ± 19.6, and 45.8 ± 10.1, respectively, for the twice‐daily group.
Conclusions and Clinical Importance
These results suggest that a famotidine CRI, but not standard doses of famotidine, achieves the clinical goals established in people to promote healing of gastric tissue injury and offers an alternative to intravenous treatment with proton pump inhibitors in dogs.
Keywords: acid suppressant, bravo monitoring, canine, histamine‐2 receptor antagonist
Abbreviations
- CRI
continuous rate infusion
- GI
gastrointestinal
- H2RA
histamine‐2 receptor antagonists
- MPT
mean percentage time
- PPI
proton pump inhibitor
1. INTRODUCTION
Gastrointestinal (GI) tissue injury and bleeding are sequelae to many conditions in dogs, including GI neoplasia and ulcerogenic drug administration.1, 2, 3 The healing of upper GI tissue injury in humans is in part dependent on the percentage of the day (mean percentage time [MPT]) that intragastric pH is maintained ≥3.0 and 4.0.4, 5 pH goals for the treatment of GI tissue injury and bleeding in dogs are unknown but could be similar. Thus, the treatment for acid‐related disorders in dogs includes promoting a sustained increase in the intragastric pH, which is accomplished through the use of acid‐suppressant medications including proton pump inhibitors (PPIs, eg, pantoprazole) and histamine‐2 receptor antagonists (H2RA, eg, famotidine and ranitidine). The PPIs are more efficacious in raising the intragastric pH compared with H2RA.6 Despite this, H2RA are still commonly utilized by veterinary practitioners for several reasons. The PPIs take several days to reach maximal efficacy, a commonly utilized IV PPI, pantoprazole, is occasionally unavailable, and both short‐ and long‐term use of PPIs has been associated with adverse effects in humans.7, 8 Famotidine has some advantages, including that it is maximally effective on the first day of treatment in dogs, can be administered with a meal, and is widely available.9, 10 Famotidine is commonly administered to dogs as twice‐daily bolus injections. However, twice‐daily administration of IV famotidine and ranitidine had a moderate effect on intragastric pH and did not meet the aforementioned clinical pH goals.11 In studies performed in critically ill human patients, famotidine continuous rate infusions (CRIs) administered IV provided superior acid suppression and met the clinical goals for MPT pH ≥3.0 and 4.0.12, 13 Moreover, in a subsequent study, the authors concluded that intermittent IV bolus dosing of H2RAs should be discontinued in favor of a CRI.14
To the authors' knowledge, there are no published studies in which the efficacy of famotidine‐administered IV as a CRI has been evaluated in dogs. As such, the objective of this study was to determine if famotidine administered as a CRI in healthy dogs meets the aforementioned clinical goals for the treatment of acid‐related disorders. We hypothesized that the famotidine CRI would reach clinical goals for raising intragastric pH whereas twice‐daily standard IV dosing would not.
2. MATERIALS AND METHODS
2.1. Study animals
The Institutional Animal Care and Use Committee (IACUC) at the University of Tennessee, Knoxville, Tennessee (UTK) approved the protocol for this study (#2580‐0318). The subjects of this study were 9 healthy adult purpose‐bred Beagles from a research colony at UTK (5 neutered males, 4 spayed females), aged 1.66 to 1.8 years (median, 1.66 years), and weighing 8.6‐12.4 kg (median, 9.5 kg). Dogs included in the study were deemed healthy on the basis of normal physical examinations and normal CBC, serum biochemistry profile, and centrifugation fecal flotation performed within approximately 1 year of study entry. In addition, the dogs received a monthly heartworm preventative, had no history of GI disease (eg, vomiting, diarrhea, and anorexia), and had a normal packed cell volume, total serum protein, lactate, and blood glucose concentration at study entry. To comply with IACUC guidelines and to ensure the inclusion of healthy dogs, dogs were excluded from the study if they developed inappetence (defined as eating less than 50% of a meal for 3 consecutive meals), diarrhea (defined as a Purina fecal score ≥ 5) observed for greater than 48 hours, or persistent vomiting that was unresponsive to supportive care (defined as more than 3 episodes of vomiting in a 24‐hour period).
2.2. Study design
A randomized, open label, 2‐way crossover study was performed. Using a random number generator, all dogs were randomized into a treatment group to receive famotidine (Famotidine 200 mg/20 mL injection, Westward Pharmaceuticals, LLC, Eatontown, New Jersey) as every 12 hour IV boluses or CRI first. To obtain baseline pH data, all dogs received 0.9% saline every 6 hours for the first 24 hours of intragastric pH monitoring before administration of either treatment. Twice‐daily IV bolus treatments were dosed at 1.0 mg/kg IV q12h for 3 consecutive days. The CRI treatments were dosed at 1.0 mg/kg IV as a 1‐time loading bolus dose, followed by continuous infusion of 8.0 mg/kg/d for 3 consecutive days. The CRI dose was chosen based on its frequent use at the authors' institutions and the goal to achieve a dose higher than 40 mg/d for each dog. The bolus dose was administered as a steady IV injection administered over 15 seconds and the CRI was maintained using a syringe pump. The dogs were not sedated and engaged in normal daily activities throughout the 3‐day treatment period. The 3‐day treatment period was followed by a minimum 7‐day washout period before the dogs crossed over to receive the other treatment. Famotidine was resuspended to a concentration of 1.0 mg/mL in 0.9% saline immediately before administration of the CRI. The pump infusion speed varied according to the weight of the dog. Unused resuspended famotidine was discarded after 72 hours, which was more stringent than manufacturer recommendations to discard after 7 days after resuspension in saline. Famotidine was stored at a controlled cold temperature and protected from light. The dogs' peripheral IV catheters were flushed with 0.9% saline every 6 hours throughout the treatment period to assess and maintain catheter patency. Dogs in the twice‐daily famotidine group were medicated at 8:00 am and 8:00 pm daily. All dogs were fed a maintenance diet (Purina ONE Smartblend Lamb & Rice Formula, Nestlé Purina PetCare Company, St. Louis, Missouri) at 8:30 am and 8:30 pm. The dogs had unlimited access to water throughout the pH monitoring period. Clinical signs including changes in mentation, food consumption, vomiting, number of defecations, and fecal consistency were recorded a minimum of 4 times daily. The feces were graded from 1 to 7 using a standardized fecal scoring system (Fecal Scoring System, Nestlé Purina PetCare Company). For the purposes of this study, diarrhea was defined as a fecal score of ≥5. Food consumption was defined as the percent consumed of the previous meal at the time of the next meal. The dog's food cup was used to estimate the percentage of food consumed.
2.3. Placement of intragastric pH monitor
On the morning of day 0 of each treatment period, the morning meal was withheld and the dogs were sedated for digital radiology‐assisted placement of a Bravo pH capsule. Based on temperament, the dogs were sedated with dexmedetomidine at 0.005 mg/kg (Dexdomitor 0.5 mg/mL injection, Orion Pharma, Espoo, Finland) and butorphanol at 0.4 mg/kg (Torbugesic 10 mg/mL injection, Zoetis Inc., Kalamazoo, Michigan) IM followed by placement of an IV catheter or the IV catheter was placed and sedation with dexmedetomidine (0.005 mg/kg) and butorphanol (0.4 mg/kg) was administered IV. An additional dose of dexmedetomidine (0.005 mg/kg) was administered IV through the IV catheter once if needed for adequate sedation. The sedation protocol was kept consistent between treatments for each individual dog. Before use, all pH capsules and receivers were calibrated as previously described according to the manufacturer's instructions.6 After sedation, dogs were placed in right lateral recumbency and the pH capsule was blindly introduced transorally. The measurements on the capsule delivery device were used to measure the distance from the upper incisor teeth to the area of capsule placement—this measurement was recorded and used to keep the location of each pH capsule consistent in each dog between the treatment groups. Location of the capsule with the delivery device in respect to the stomach was assessed using a lateral radiograph. The capsule was placed as previously described.10 Confirmation of capsule adherence to the gastric fundus was ascertained using orthogonal (lateral and ventrodorsal) abdominal radiographs. After capsule placement, the sedation was reversed with atipamezole at 0.05‐0.10 mg/kg IM (Antisedan 5 mg/mL injection, Orion Pharma).
2.4. pH recordings
Intragastric pH recordings were obtained telemetrically at 6‐second sampling intervals for 96 hours after capsule placement starting at baseline (day 0) and continuing through treatment day 3 or until the capsule detached. The corresponding data receivers were kept on the front of each dog's run or cage during the data acquisition phase. When the dogs were walked, the receivers remained with the caretaker within 6 feet of the dogs. pH data were uploaded to the computer by manufacturer software (Polygram Net Software, Given Imaging, Yokneam, Israel) every 24 hours for each monitoring period. After the data were uploaded, the data from the receiver were cleared and the receiver was used to obtain data for the subsequent 24 hours. The mean pH and MPT for which intragastric pH was ≥3.0 and ≥4.0 were calculated by the manufacturer software.
2.5. Statistical analysis
A 2‐factor repeated‐measures mixed‐effects crossover design was performed to evaluate mean intragastric pH, MPT that intragastric pH was ≥3, and MPT that intragastric pH was ≥4. Each response was analyzed with repeated‐measures mixed model analysis of variance (ANOVA) to evaluate for treatment, time, the treatment by time interaction, and period differences.15 During each treatment phase, the dogs received either CRI or famotidine IV bolus, and each response was measured at 4 time points (baseline and days 1‐3). Two additional comparisons were also performed on day 1 hours 0‐6 versus 6‐12 and day 1 hours 0‐12 versus 12‐24 to determine how rapidly mean gastric pH changed under each treatment. Unstructured Kronecker product variance/covariance structures were incorporated into each model.16 In each analysis, post hoc and specific contrasts tests were developed to test for within day and between treatment differences. A Shapiro‐Wilk test for normality and QQ plots were used to evaluate normality of ANOVA residuals. Levene's equality of variances test was used to evaluate equality of treatment variances. All statistical assumptions regarding normality and equality of variances were met and no transformations were required. Statistical significance was defined as P < .05. Statistical analysis was performed using commercial software (SAS software, version 9.4, Cary, North Carolina, Release TS1M5). Descriptive statistics were used to investigate the occurrence of adverse events.
3. RESULTS
3.1. Bravo pH monitoring system results
Nineteen of 21 pH capsules were successfully attached to the fundic mucosa. On 1 occasion, the pH capsule failed to deploy from the delivery device; a new pH capsule was placed in the gastric fundus without complication. On the second occasion, the dog's first capsule detached after 20 hours in the stomach and capsule replacement was unsuccessful because the dog had been recently fed and the presence of food particles in the stomach obstructed the capsule's suction to the mucosa.
Regarding capsules that were successfully adhered, the Bravo pH capsule detached and exited the stomach before the end of the 96‐hour monitoring period on 8 occasions. In the dogs receiving twice‐daily boluses of famotidine, premature detachment of the capsule occurred in 1 dog on treatment day 1, 1 dog on treatment day 2, and 3 dogs on treatment day 3. In the dogs receiving the famotidine CRI, capsule detachment occurred in 2 dogs on treatment day 2 and 1 dog on treatment day 3. Data from these dogs were not included in the treatment comparisons on days in which the data were not available.
In 4 dogs, capsules remained in the stomach for longer than the 96‐hour monitoring period. This occurred in 1 dog in the twice‐daily bolus treatment group, 2 dogs in the CRI treatment group, and 1 dog in both the twice‐daily bolus and CRI treatment groups. In these cases, additional data were gathered but not included in the statistical analyses. The longest period that a capsule was in the stomach was 8 days, which occurred in 1 dog in the twice‐daily bolus treatment group. Body weight did not appear to influence retention of capsule as both the smallest dog and the largest dog by weight had a capsule remain in the stomach beyond the 96‐hour monitoring period.
3.2. Famotidine treatment and intragastric pH recordings
The median (and range) total daily famotidine dose was 19 (17.2‐24.8) mg for the twice‐daily group on days 1‐3 and 85.5 mg (77.4‐111.6 mg) and 76 mg (68.8‐99.2 mg) on days 1 and days 2‐3, respectively, for the CRI group. The MPT in a 24‐hour period for which the intragastric pH was ≥3 and 4 and the mean pH were used to perform comparative analyses of treatments over time. Significant treatment by time interactions was observed for mean intragastric pH, MPT intragastric pH ≥3, and MPT intragastric pH ≥ 4, thereby indicating that each intragastric pH measure changed differently over time depending on the treatment each animal received (P = .001, P = .002, and P = .001, respectively). No significant differences were found between periods or between treatments on day 0 for any pH response measure. Thus, no significant carryover effects were found indicating the washout period between treatments was adequate.
Contrasts and post hoc tests revealed significant differences in MPT ≥3 between treatments on days 1, 2, and 3 (P < .001, P < .001, and P < .001, respectively). At baseline (day 0), the MPT ± SD intragastric pH was ≥3 was 28.5 ± 19.3 for the CRI treatment group and 27.5 ± 16.0 in the twice‐daily treatment group (Figure 1). The MPT ± SD pH was ≥3 was 92.1 ± 8.5 on day 1, 96.3 ± 6.2 on day 2, and 90.0 ± 15.7 on day 3 for the CRI treatment group (Figure 1). Under the effects of the CRI, significant changes over time were identified when comparing baseline (day 0) and all other days (P < .001). No additional significant differences were detected between days 1 through 3. In the twice‐daily treatment group, the MPT ± SD pH ≥3 were 49.3 ± 27.3, 42.2 ± 19.6, and 45.8 ± 10.1 on days 1, 2, and 3, respectively. Under the effects of twice‐daily administration, a significant difference was only found between day 0 and day 1 (P = .03); however, treatment days 2 and 3 did not differ from day 0, day 1, or each other.
Figure 1.
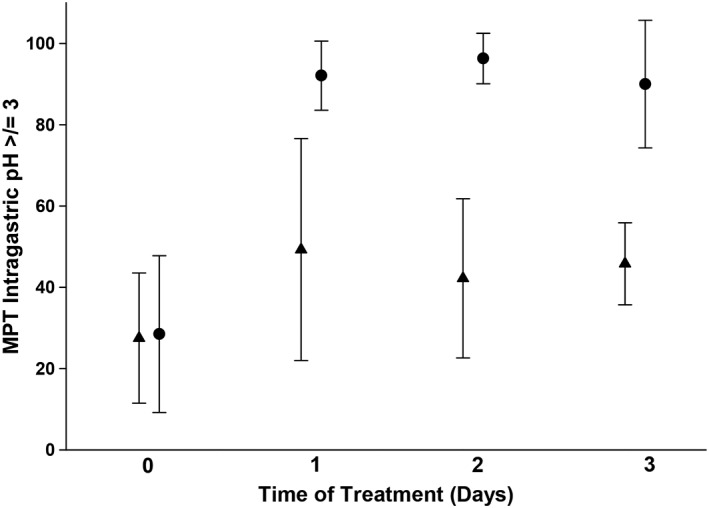
Efficacy of injectable famotidine over time as assessed by mean percent time (MPT) intragastric pH ≥3. Circles represent the MPT ± SD for dogs receiving continuous rate infusion (CRI). Triangles represent the MPT ± SD for dogs receiving twice‐daily IV dosing. There was a statistically significant difference (P < .05) in MPT ≥3 between the CRI and IV q12h groups on all treatment days. Please refer to Table 1 for the number of animals on each day for both treatment groups
Significant treatment differences in MPT ≥4 on days 1, 2, and 3 were found using contrasts and post hoc tests (P < .001, P < .001, and P = .001, respectively). On day 0, the MPT ± SD intragastric pH was ≥4 was 19.5 ± 17.5 in the CRI treatment group and 18.4 ± 14.2 in the twice‐daily treatment group (Figure 2). In the CRI treatment group, the MPT ± SD intragastric pH ≥4 were 83.1 ± 14.2, 90.5 ± 13.9, and 79.0 ± 26.9 for days 1‐3, respectively (Figure 2). Under the effects of the CRI, significant changes over time were found between baseline (day 0) and all other days (P < .001). No additional significant differences were detected between days 1 through 3. The MPT ± SD pH ≥4 in the twice‐daily treatment group were 39.5 ± 27.1 on day 1, 27.1 ± 16.1 on day 2, and 30.8 ± 5.2 on day 3. Under the effects of twice‐daily administration, a significant difference was only found between day 0 and day 1 (P = .02); however, treatment days 2 and 3 did not differ from day 0, day 1, or each other. Thus, a partial reversion to baseline MPT4 values on days 2 and 3 was observed under the effects of twice‐daily administration.
Figure 2.
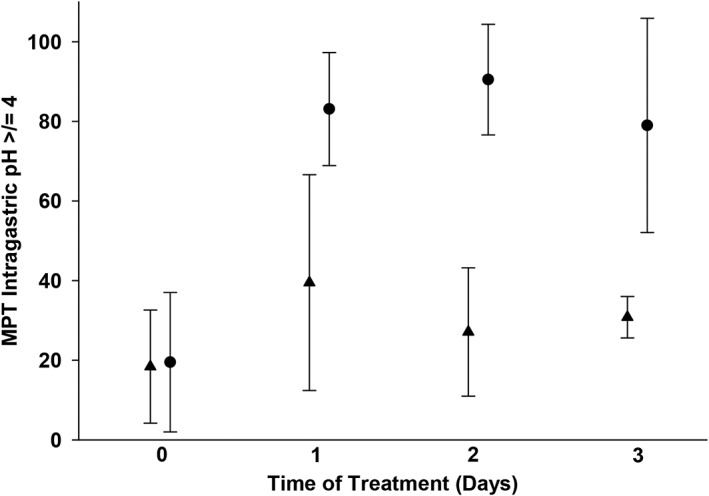
Efficacy of injectable famotidine over time as assessed by mean percent time (MPT) intragastric pH ≥4. Circles represent the MPT ± SD for dogs receiving continuous rate infusion (CRI). Triangles represent the MPT ± SD for dogs receiving twice‐daily IV dosing. There was a statistically significant difference (P < .05) in MPT ≥4 between the CRI and IV q12h groups on all treatment days. Please refer to Table 1 for the number of animals on each day for both treatment groups
The mean ± SD intragastric pH on baseline day 0 and in dogs receiving CRI and twice‐daily IV treatments on days 1‐3 are listed in Table 1. Contrast and post hoc tests revealed significant differences in mean intragastric pH between the treatment groups on days 1, 2, and 3 (P < .001, P < .001, and P = .004, respectively). In the CRI treatment group, significant changes over time were found between baseline (day 0) and all other days (P < .001). No statistically significant difference was detected between days 1 and 2 and between days 1 and 3, but a significant difference was found between treatment days 2 and 3 (P = .03), which corresponded to a mean decrease of 0.8 between days 2 and 3. Under the effects of twice‐daily administration, a significant difference was only found between days 0 and 1 (P = .009); however, treatment days 2 and 3 did not differ from day 0, day 1, or each other. To determine how rapidly mean gastric pH changed under the effects of famotidine treatment, comparisons were made between and within treatments on day 1 for hours 0‐6 and hours 6‐12 as well as hours 0‐12 and hours 12‐24 (Figure 3). Significant treatment by time interactions were observed while considering the first 12 hours and first 24 hours thereby indicating mean pH measures changed differently over time dependent on the treatment received (P = .008 and P = .006, respectively). Regarding the first 12 hours of treatment (ie, 0‐6 versus 6‐12 hours after administration), there was a statistically significant change over time with the twice‐daily famotidine treatment (P = .02) but not with CRI. There was no difference between treatments for the first 6 hours. However, by hours 6‐12, there was a difference between treatments (P = .009). When comparing the 1st 12 hours of treatment to the 2nd 12 hours of treatment on day 1 (ie, 0‐12 versus 12‐24 hours), there was a statistically significant change over time with the CRI treatment (P = .0006) but not with the twice‐daily treatment. There were differences between treatments at hours 0‐12 (P = .04) and hours 12‐24 (P = .001).
Table 1.
Mean ± SD intragastric pH in dogs receiving continuous rate infusion (CRI) or twice‐daily famotidine
| CRI treatment | Twice‐daily treatment | |
|---|---|---|
| Day 0 (baseline) | 2.6 ± 0.72 (n = 9) | 2.6 ± 0.70 (n = 9) |
| Day 1 | 5.2 ± 0.84 (n = 9) | 3.6 ± 1.2 (n = 8) |
| Day 2 | 5.7 ± 0.76 (n = 8) | 3.0 ± 0.63 (n = 7) |
| Day 3 | 4.9 ± 0.92 (n = 7) | 3.3 ± 0.17 (n = 4) |
Figure 3.
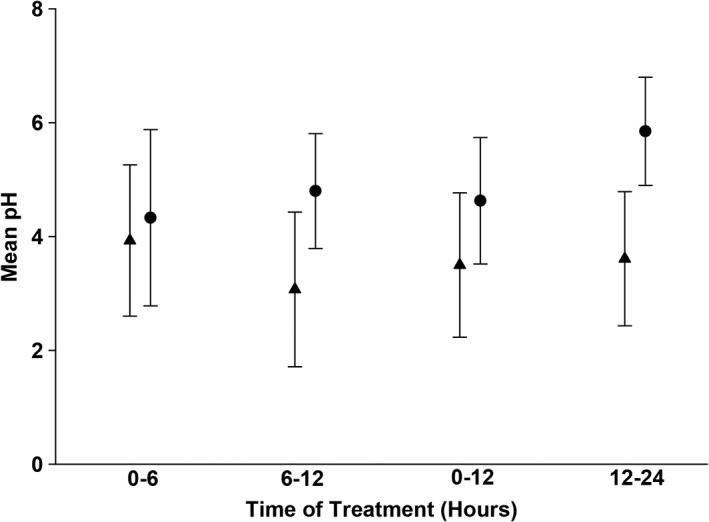
Onset of famotidine action as assessed by mean pH on day 1. Circles represent the mean pH ± SD for dogs receiving continuous rate infusion (CRI). Triangles represent the mean pH ± SD for dogs receiving twice‐daily IV dosing. Please refer to Table 1 for the number of animals on each day for both treatment groups
3.3. Adverse events
No dogs were excluded from the study. Emesis was noted in 5 dogs with a total occurrence of 15 episodes of vomiting. There were 4 episodes of vomiting in the twice‐daily treatment group with 2 episodes on day 1 and 1 episode each on days 2 and 3 of treatment. Eleven episodes of vomiting occurred in the CRI treatment group with 3 episodes on day 1, 6 episodes on day 2, and 2 episodes on day 3. No vomiting episodes occurred during the placebo before treatment for either group. To comply with animal welfare guidelines, maropitant citrate (Cerenia 10 mg/mL injection, Zoetis Inc.) was administered IV once at 1 mg/kg to 3 dogs in the CRI treatment group—2 of which vomited 2 or more times in a 24‐hour period and to 1 dog that exhibited persistent signs of nausea (ptyalism, eructation). Diarrhea was defined as a fecal score ≥ 5. There were 99 defecations over the 8‐day study period. Of these defecations, diarrhea was noted in 7 dogs with a total occurrence of 10 episodes. There were a total 3 episodes of diarrhea in the intermittent bolus group, 2 episodes in the CRI group, 3 episodes in the placebo before intermittent bolus group, and 2 episodes in the placebo before CRI group. All of these diarrheic events were graded as a fecal score of 5 or 6 and occurred before the end of treatment day 2. Dogs in the placebo before treatment group did not have more than 1 episode of diarrhea. The mean ± SD fecal score was 2.9 ± 1.0 for the CRI group and 2.5 ± 0.9 for the twice‐daily IV bolus group. There were no changes in mentation or food consumption associated with either treatment. The mean ± SD percent food consumed was 98 ± 4.3, 91 ± 15.7, 92 ± 18.4, and 99 ± 2.2 for days 0, 1, 2, and 3, respectively, for the famotidine CRI group and 97 ± 8.3, 99 ± 1.7, 100 ± 0, and 100 ± 0.83 for days 0, 1, 2, and 3, respectively, for the twice‐daily group.
4. DISCUSSION
In the present study, we evaluated the effect of famotidine when administered as an intravenous CRI for a consecutive 72 hours in healthy dogs. In humans, the treatment goals for duodenal ulceration and gastroesophageal reflux disease include maintaining an intragastric pH ≥3 for at least 75% of a 24‐hour period and a pH ≥4 for at least 67% of the day, respectively.4, 5 On all treatment days, the CRI treatment group met or exceeded these clinical goals whereas the standard twice‐daily famotidine treatment group did not meet the clinical goals on any day of treatment.
On treatment days 1 and 2, the CRI treatment provided excellent gastric acid suppression, achieving an MPT intragastric pH ≥3 of 92 and 96%, respectively, and a, MPT intragastric pH ≥4 of 83 and 90%, respectively. Famotidine CRI was superior to twice‐daily injections on all days with significant differences on intragastric pH noted after the first 12 hours of treatment. On day 3 of treatment, the CRI had a slightly diminished effect, achieving an MPT ≥3 of 90% and an MPT ≥4 of 79% but still met the clinical goals for gastric acid suppression on this day. Thus, we cannot comment as to whether tolerance might start to develop after 3 days of famotidine CRI. Prolonged oral administration of famotidine in dogs results in decreased efficacy over time.10 The same phenomenon occurs in people with prolonged parenteral administration of H2RA.17 The development of tolerance to a famotidine CRI in dogs should be investigated further and could be achieved by repeating this study over a treatment period longer than 3 days.
In this study, we chose to investigate an IV CRI dose of a 1.0 mg/kg loading dose followed by 8.0 mg/kg/d for 3 consecutive days. To the authors' knowledge, there is no published study in which the effect of a famotidine CRI in dogs has been evaluated. The dose chosen in this study was based on doses used at our and other academic veterinary institutions as well as a study in humans that showed that a CRI of 40 mg famotidine per day was insufficient to raise gastric pH to a degree that would aid in healing of bleeding duodenal ulceration.18 It is unknown if a lower dose or intermittent injections with the same total daily dose would be equally effective and should be investigated.
It is likely that the excellent gastric acid suppression achieved by the IV CRI compared to the twice‐daily IV treatment was largely because of the higher total daily administered dose in the CRI treatment group. However, in a study performed in human patients in which an equivalent total daily dose of famotidine was administered as either a CRI or intermittent IV boluses, the CRI was the more efficacious of the 2 treatments in maintaining a sustained increase in intragastric pH.13 It is the authors' belief, therefore, that the maximal acid suppression achieved in the CRI treatment group was also because of the drug being administered continuously rather than as bolus injections.
Dogs had no detectable change in food consumption with either treatment. Only 10 episodes of diarrhea in 7 dogs occurred over the course of the study. All episodes of diarrhea were graded as a fecal score of 5 and occurred before day 2, including on days in which dogs received no treatment; thus, we believe that these diarrheic events may have been associated with the stress of the study rather than an effect of treatment. The administration of famotidine CRI did appear to be associated with vomiting, as more vomiting events were noted in this treatment group; however, the study was underpowered to detect a significant difference between groups. A follow‐up study in dogs with underlying GI disease is warranted to determine the clinical significance of these vomiting events and their potential causation with CRI of famotidine.
The crossover design of this study allowed each of the dogs to serve as their own control. This study was performed in a small group of dogs with no history, physical exam, or laboratory indicators of GI disease. A study performed in dogs clinically affected with GI disorders is warranted, as the response achieved by the famotidine CRI in healthy dogs may not be representative of that in diseased animals. The baseline day when dogs received only saline treatment was used to identify a significant effect of treatment on gastric pH; however, this analysis would have been strengthened by the inclusion of a placebo control group especially to determine the significance of diarrheic events.
In conclusion, administration of famotidine IV by CRI is effective in increasing the intragastric pH in healthy dogs to a degree that may help to promote healing of upper GI acid‐related injury.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The IACUC at The University of Tennessee approved the protocol for this study (Approval #2580‐0318).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Bernie Hansen, Adam Birkenheuer, Randy Buddington, Skylar Bowers, Tammy Moyers, Gina Galyon, Brittany Nelson, Nicole Meagher, Gordon Conklin, Jackie Bellan, and Rachel Feuerstein for their technical support. This subject was presented at the 2019 ACVIM Forum, Seattle, Washington.
Hedges K, Odunayo A, Price JM, Hecht S, Tolbert MK. Evaluation of the effect of a famotidine continuous rate infusion on intragastric pH in healthy dogs. J Vet Intern Med. 2019;33:1988–1994. 10.1111/jvim.15558
Adesola Odunayo and M. Katherine Tolbert contributed equally to this work and should be considered as co‐corresponding authors.
REFERENCES
- 1. Gonzalez‐Gonzalez JA, Garcia‐Compean D, Vazquez‐Elizondo G, et al. Nonvariceal upper gastrointestinal bleeding in patients with liver cirrhosis. Clinical features, outcomes and predictors of in‐hospital mortality. A prospective study. Ann Hepatol. 2011;10:287‐295. [PubMed] [Google Scholar]
- 2. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346‐352. [DOI] [PubMed] [Google Scholar]
- 3. Luna SPL, Basilio AC, Steagall PVM, et al. Evaluation of adverse effects of long‐term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res. 2007;68:258‐264. [DOI] [PubMed] [Google Scholar]
- 4. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345‐351. [DOI] [PubMed] [Google Scholar]
- 5. Bell NJV, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastroesophageal reflux disease. Digestion. 1992;51:59‐67. [DOI] [PubMed] [Google Scholar]
- 6. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med. 2011;25:47‐54. [DOI] [PubMed] [Google Scholar]
- 7. Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Physician. 2002;66:273‐280. [PubMed] [Google Scholar]
- 8. Johnson DA, Oldfield EC. Reported side effects and complications of long‐term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol. 2013;11:458‐464. [DOI] [PubMed] [Google Scholar]
- 9. Huang JQ, Hunt RH. Pharmacological and pharmacodynamic essentials of H‐2‐receptor antagonists and proton pump inhibitors for the practising physician. Best Pract Res Clin Gastroenterol. 2001;15:355‐370. [DOI] [PubMed] [Google Scholar]
- 10. Tolbert MK, Graham A, Odunayo A, et al. Repeated famotidine administration results in a diminished effect on intragastric pH in dogs. J Vet Intern Med. 2017;31:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bersenas AME, Mathews KA, Allen DG, Conlon PD. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res. 2005;66:425‐431. [DOI] [PubMed] [Google Scholar]
- 12. Heiselman DE, Hulisz DT, Fricker R, et al. Randomized comparison of gatric pH control with intermittent and continuous intravenous‐infusion of famotidine in ICU patients. Am J Gastroenterol. 1995;90:277‐279. [PubMed] [Google Scholar]
- 13. Baghaie AA, Mojtahedzadeh M, Levine RL, Fromm RE Jr, Guntupalli KK, Opekun AJ. Comparison of the effect of intermittent administration and continuous infusion of famotidine on gastric pH in critically ill patients: results of a prospective, randomized, crossover study. Crit Care Med. 1995;23:687‐691. [DOI] [PubMed] [Google Scholar]
- 14. Ballesteros MA, Hogan DL, Koss MA, et al. Bolus or intravenous infusion of ranitidine: effects on gastric pH and acid secretion. A comparison of relative efficacy and cost. Ann Intern Med. 1990;112:334‐339. [DOI] [PubMed] [Google Scholar]
- 15. Brown H. Applied Mixed Models in Medicine. 3rd ed. Chichester, England: Wiley; 2015. [Google Scholar]
- 16. Littell RC. SAS for Mixed Models. 2nd ed. Cary, NC: SAS Institute, Inc.; 2006. [Google Scholar]
- 17. Merki HS, Wilder‐Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology. 1994;106:60‐64. [DOI] [PubMed] [Google Scholar]
- 18. Delchier JC, Amine I, Roudot‐Thoraval F, et al. Maintenance of gastric pH above 6 with intravenous famotidine in patients with a bleeding duodenal ulcer. Aliment Pharmacol Ther. 1995;9(2):191‐196. [DOI] [PubMed] [Google Scholar]


