Abstract
Background
Two‐dimensional shear wave elastography (2D‐SWE) can noninvasively evaluate hepatic elastic modulus as shear wave velocity (SWV). Additionally, it may predict the presence of clinical relevant hepatic fibrosis (≥F2) in dogs with hepatic disease.
Objectives
To investigate whether SWV measured by 2D‐SWE can differentiate between dogs with (≥F2) and without (F0‐1) clinically relevant hepatic fibrosis.
Animals
Twenty‐eight client‐owned dogs with hepatic disease and 8 normal healthy Beagle dogs were enrolled.
Methods
In this cross‐sectional prospective study, SWVs were measured using 2D‐SWE in all dogs. Hepatic fibrosis stages and necroinflammatory activity grades were histopathologically evaluated using a histological scoring scheme that was adapted from the Ishak schema used in human medicine.
Results
Median SWVs were significantly higher in dogs with clinically relevant hepatic fibrosis (2.04 m/s; range, 1.81‐2.26 m/s) than in healthy dogs (1.51 m/s; range, 1.44‐1.66 m/s; P = .007), and dogs without clinically relevant hepatic fibrosis (1.56 m/s; range, 1.37‐1.67 m/s; P < .001). However, no significant difference was found in the SWVs between dogs without clinically relevant hepatic fibrosis and healthy dogs (P = .99). Furthermore, median SWVs were not significantly different among dogs with necroinflammatory activity, those without necroinflammatory activity, and healthy dogs (Kruskal‐Wallis test, P = .12).
Conclusions and Clinical Importance
The 2D‐SWE may be useful for predicting the presence of hepatic fibrosis in dogs with hepatic disease.
Keywords: 2D‐SWE, canine, liver biopsy, liver disease, SWV
Abbreviations
- 2D‐SWE
two‐dimensional shear wave elastography
- HA
hyaluronic acid
- SWE
shear wave elastography
- SWV
shear wave velocity
- Tchol
total cholesterol
- US
ultrasound
1. INTRODUCTION
Hepatic fibrosis is histopathologically characterized by the progressive accumulation of extracellular matrix in the liver parenchymal tissue, which subsequently leads to distortion of hepatic architecture. Hepatic fibrosis in dogs usually is caused by chronic hepatobiliary diseases, such as chronic hepatitis and cholangiohepatitis.1, 2 Progression of hepatic fibrosis involves an increase in the liver elastic modulus and resistance of hepatic blood flow, which consequently result in liver cirrhosis and portal hypertension. In humans with hepatic fibrosis, the stage of hepatic fibrosis and its progression are essential for determination of optimal treatment and prediction of prognosis.3
Liver biopsy is the gold standard for diagnosing the severity of hepatic fibrosis in dogs and humans. However, the limitations of liver biopsy include the risk of bleeding and anesthesia, invasiveness, and possibility of sampling errors.4, 5 Hence, noninvasive and easily accessible methods of predicting stages of hepatic fibrosis have been developed in human medicine. Consequently, in human patients with hepatic fibrosis, hyaluronic acid (HA), which is an essential component of the extracellular matrix, is widely used as a direct marker for the assessment of hepatic fibrosis.6, 7, 8 Similarly, in veterinary medicine, several studies have reported that the blood HA concentration is increased in dogs with hepatic disease.9, 10 However, another report did not find a positive correlation between serum HA concentration and the stage of hepatic fibrosis in dogs with hepatic disease.11
Shear wave elastography (SWE) with the use of acoustic radiation force impulses is a newly developed imaging technology that can quantitatively assess the elastic modulus of the tissue by measuring the shear wave propagation speed within the tissue.12 Shear wave velocity (SWV) in meter per second represents the liver elastic modulus. Many studies in human patients with hepatic fibrosis have indicated that SWV is correlated with the stage of hepatic fibrosis, and SWE is widely applied in the clinical setting to predict the stage of hepatic fibrosis in humans.13, 14, 15, 16, 17 Recently, we reported that two‐dimensional SWE (2D‐SWE) is a feasible technique for assessing SWVs in healthy dogs.18 However, to the best of our knowledge, no reports have evaluated the use of 2D‐SWE for the diagnosis of hepatic fibrosis in dogs with spontaneous hepatic disease.
Our aim was to evaluate the usefulness of 2D‐SWE for the detection of clinically relevant hepatic fibrosis (≥F2) in dogs with hepatic disease. We hypothesized that SWVs measured by 2D‐SWE would be different between dogs without clinically relevant hepatic fibrosis (F0‐1) and dogs with clinically relevant hepatic fibrosis (≥F2).
2. MATERIALS AND METHODS
2.1. Animals
Our study was a prospective, cross‐sectional observational study. From June 2017 to January 2019, 32 client‐owned dogs with histopathologically diagnosed acute or chronic hepatobiliary disease examined at the Hokkaido University Veterinary Teaching Hospital were included. Signalment that consisted of age, breed, sex, and body weight were recorded at the time of recruitment. Laboratory findings, including CBC and serum biochemistry, were extracted from the medical records of all the included dogs. Dogs with congenital portosystemic shunt, rupture of the gallbladder, obstructive jaundice, or hepatic tumors were excluded based on abdominal ultrasound (US) and computed tomography findings. Informed owner's consent was obtained in all cases.
Eight healthy beagles were included in the study: 3 were intact males and 5 were intact females. Their age range was 1‐4 years and body weight was 9.7‐15 kg. All dogs were confirmed healthy based on physical examination, CBC, serum biochemistry, echocardiography, and abdominal US. All animal experimental procedures were conducted in accordance with the standard operation protocols of the institutional animal experimental committee reviewed by the Association for Assessment and Accreditation of Laboratory Animal Care international (AAALAC International). All animal experiments were approved by the Hokkaido University Veterinary Teaching Hospital.
2.2. Measurement of SWV
In all the dogs, 2D‐SWE was performed without sedation using an Aplio 500 ultrasound system (Canon Medical Systems, Otawara, Tochigi) and using a 3.5‐MHz convex probe (PVT‐375 BT; Canon Medical Systems, Otawara, Tochigi). According to our previous study18 and the recommended guidelines for the clinical use of elastography in humans,19 all dogs were positioned in left lateral recumbency for imaging of the right liver lobe. The probe was placed parallel to and within the intercostal space, and sufficient gel was applied to minimize rib shadowing. Liver image acquisition was positioned within the right lobe parenchyma using the intercostal approach under visual control in the 2‐dimensional B mode. If gas in the duodenum, colon, or lung hindered imaging of right liver lobe, the probe was positioned more dorsally. Dogs with ascites also were included in the study. Subsequently, 2D‐SWE of the liver was performed during the normal end‐expiratory phase to minimize the effects of respiratory motion. The reliability of data obtained by 2D‐SWE was confirmed by use of the proper propagation mode as described in our previous study (Figure 1A, C).18 Data reliability is high when the contour lines are nearly straight and regularly parallel to each other, but when the contour lines are irregularly distorted and chaotic, data reliability is low. Additionally, the speed map (range, 0.5‐6.5 m/s) displayed as the SWVs was displayed by gradual colors, with increasing elastic modulus indicated in an ascending order of blue, green, yellow, and red (Figure 1B, D). Regions that were not color‐coded on the elasticity images reflected the absence of a shear wave. The diameter of the region of interest was set as 10 mm and placed on the areas with parallel contour lines in the hepatic parenchyma. At least 10 validated measurements were made for each dog. The median values of the SWVs were representative of the liver modulus values.
Figure 1.
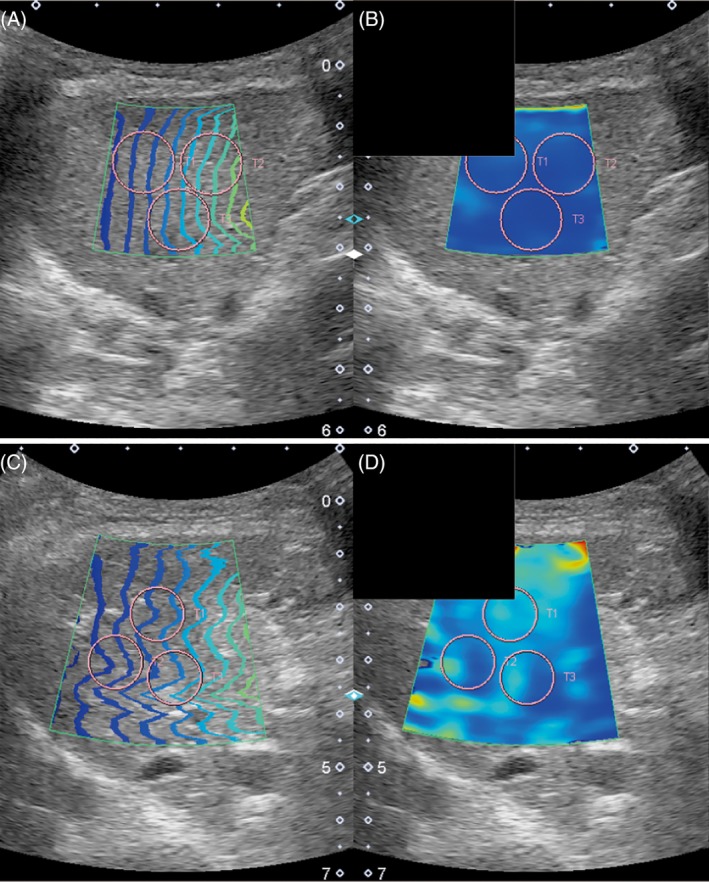
Representative images of the right lobe of the liver using the intercostal approach for the speed mode in a dog without clinically relevant hepatic fibrosis (F0, A0; A and B) and a dog with clinically relevant hepatic fibrosis (F3, A0; C and D). Images are the proper propagation map (A, C) and the speed map (B, D). Notice the consistent parallel contour lines for the speed modes, and the region of interest (T1, T2, and T3; 10 mm) used for the measurements (circles)
The SWVs of all dogs with hepatic disease were measured using 2D‐SWE before liver biopsy or euthanasia and necropsy. The measurements of SWVs were performed by a single sonographer (M.T.) to ensure consistent imaging conditions throughout the study. The SWVs of dogs with hepatic disease were compared to those of 8 clinically healthy Beagle dogs; the latter were obtained from our previous study evaluating the feasibility of 2D‐SWE.18
2.3. Histopathological examination and scoring of fibrosis or necroinflammation of liver
Liver biopsy specimens in all dogs were collected by surgical laparotomy, laparoscopy, or necropsy. Liver biopsy specimens were fixed in formalin and embedded in paraffin, and the paraffin section was stained with hematoxylin and eosin. All histological examinations were performed by an American College of Veterinary Pathologists board‐certified veterinary pathologist (Y.K.) according to the criteria developed by the World Small Animal Veterinary Association Liver Standardization Group.20 After routine histopathological examination, hepatic fibrosis and necroinflammatory activity were evaluated semiquantitatively according to a histological scoring scheme that was adapted from the Ishak scheme used in human medicine; this procedure was performed by a single pathologist (Y.K.), who was blinded to the SWV variable.20, 21 In this scoring scheme, hepatic fibrosis is staged as follows: 0: absent, 1: mild, 2: moderate, 3: marked, or 4: very marked. Furthermore, all dogs were divided into the following groups: dogs without clinically relevant hepatic fibrosis (F0‐1) and dogs with clinically relevant hepatic fibrosis (≥F2). Necroinflammatory activity was graded as follows: 0: absent, 1: slight, 2: mild, 3: moderate, 4: marked, or 5: very marked. Additionally, all dogs were divided into those without necroinflammatory activity (A0) and those with necroinflammatory activity (≥A1).
2.4. Measurement of serum HA concentration
The serum HA concentrations were compared between dogs with and without clinically relevant hepatic fibrosis. Serum samples were obtained from dogs with hepatic disease before surgical laparotomy, laparoscopy, or necropsy and stored at −80°C until HA measurement. Serum HA concentrations were measured using a commercially available enzyme‐linked immunosorbent assay kit (Hyaluronan ELISA Kit, Echelon Biosciences, Salt Lake City, Utah), which was used for the measurement of serum HA concentration in dogs in a previous study.11 Absorbance was read at 405 nm using a microplate reader (Benchmark Microplate Reader; BIO‐RAD, Hercules, California). All samples were examined in duplicate, and the mean optical density was calculated.
2.5. Statistical analysis
Statistical analyses were performed using a commercially available statistical program (JMP Pro version 14.1.0; SAS Institute, Cary, North Carolina). All continuous variables are presented as median and ranges. The Mann‐Whitney U‐test was used to compare laboratory findings and HA concentrations between dogs with and without clinically relevant hepatic fibrosis. The Kruskal‐Wallis test was used to analyze the overall difference among the 3 groups, and post hoc multiple comparisons were determined by the Steel‐Dwass test. The difference was considered statistically significant when P values were <.05.
3. RESULTS
3.1. Study population
Thirty‐two dogs with hepatic disease initially were enrolled in the study, but 4 dogs were excluded because of poor US image quality (ie, unable to obtain proper contour lines in the propagation mode) for the measurement of SWV; imaging of the right liver lobe was interfered with because of shadowing of gas in the duodenum, colon, or lung in 2 dogs and because of uncontrollable panting in 2 dogs. Finally, 28 dogs with hepatobiliary disease were included in the study (Table 1). The following breeds were presented: 4 Miniature Schnauzers, 3 Miniature Dachshunds, 3 Beagles, 3 Toy Poodles, 3 Shih Tzus, 2 American Cocker Spaniels, 2 Chihuahuas, 2 mixed dogs, and 1 each of Cairn Terrier, Scottish Terrier, Pug dog, Border Collie, Pomeranian, and Yorkshire Terrier. Histopathological diagnosis of 20 of the 28 dogs with hepatic disease was as follows: 11 cases of cholangiohepatitis; 3 vacuolar hepatopathies; 3 chronic hepatitis; and 3 primary hypoplasia of the portal vein. The last 8 of the 28 dogs were diagnosed with minimal nonspecific changes, including mildly scattered lipogranulomas or mononuclear infiltrates with hydropic or histologically normal liver. All hepatic samples were considered adequate and the stages of hepatic fibrosis and necroinflammatory activity grades were evaluated (Table 1). Three dogs that had clinically relevant hepatic fibrosis also had mild ascites.
Table 1.
Evaluation of stages of hepatic fibrosis and necroinflammatory activity grades
| Characteristics | Dogs with hepatic disease (n = 28) |
|---|---|
| Age, y | 11 (1‐15) |
| Sex, number of dogs) | 3 M, 4MC, 4F, 17FS |
| Body weight, kg | 6.1 (2.2‐22.2) |
| Fibrosis staging | |
| F0 (absent) | 17 |
| F1 (mild) | 5 |
| F2 (moderate) | 2 |
| F3 (marked) | 1 |
| F4 (very marked) | 3 |
| Necroinflammatory activity grading | |
| A0 (absent) | 17 |
| A1 (slight) | 6 |
| A2 (mild) | 4 |
| A3 (moderate) | 1 |
| A4 (marked) | 0 |
| A5 (very marked) | 0 |
Age and body weight are reported as medians with the ranges in parentheses. Numbers in the fibrosis staging and necroinflammatory activity grading indicate number of dogs.
Abbreviations: FS, female spayed; MC, male castrated.
3.2. Laboratory findings and serum HA concentration
The results of laboratory findings and serum HA concentrations in all dogs with hepatic disease are presented in Table 2. The laboratory findings were measured in all dogs, whereas serum HA concentration was measured in 20 of the 22 dogs without clinically relevant hepatic fibrosis and 5 of the 6 dogs with clinically relevant hepatic fibrosis. Only serum albumin and total cholesterol (Tchol) concentrations were significantly lower in dogs with clinically relevant hepatic fibrosis than in those without clinically relevant hepatic fibrosis (P = .001 and P = .02, respectively). Serum HA concentration was not significantly different between dogs with (median, 192 ng/mL; range, 151‐305 ng/mL) and without clinically relevant hepatic fibrosis (median, 162 ng/mL; range, 117‐211 ng/mL, P = .11).
Table 2.
Routine laboratory findings and hyaluronic acid concentration are reported as medians with the ranges in parentheses
| F0‐1 dogs (n = 22) | ≥F2 dogs (n = 6) | P value | |
|---|---|---|---|
| Platelet (14.8‐48.4 × 104/μL) | 41.9 (23.6‐132) | 38.9 (27.5‐57) | .54 |
| ALT (17‐44 IU/L) | 261 (46‐ > 1000) | 708 (164‐952) | .09 |
| Albumin (2.6‐4.0 g/dL) | 3.4 (2.7‐4.3) | 2.6 (2.2‐3.1) | .001 |
| Glucose (75‐128 mg/dL) | 103 (84‐119) | 108 (95‐117) | .38 |
| T‐Bil (0.1‐0.5 mg/dL) | 0.1 (0.1‐1.0) | 0.2 (0.1‐2.6) | .58 |
| Tchol (111‐312 mg/dL) | 265 (129‐451) | 179 (63‐258) | .02 |
| HA (ng/mL) | 162 (117‐211) | 192(151‐305) | .11 |
Reference intervals are listed after each laboratory finding in the first column.
Abbreviations: ALT, alanine aminotransferase; HA, hyaluronic acid; T‐Bil, total bilirubin; Tchol, total cholesterol.
3.3. Comparison of SWV among hepatic fibrosis stages
We compared SWV results among healthy dogs (n = 8), dogs without clinically relevant hepatic fibrosis (n = 22), and dogs with clinically relevant hepatic fibrosis (n = 6). The speed map of SWVs of dogs without clinically relevant hepatic fibrosis is displayed in blue (Figure 1B), and that of dogs with clinically relevant hepatic fibrosis is displayed in green in the major parts and blue in minimal parts (Figure 1D). Figure 2 shows the box plots of SWVs measured by 2D‐SWE in healthy dogs, dogs without clinically relevant hepatic fibrosis, and dogs with clinically relevant hepatic fibrosis. The median SWVs in healthy dogs, dogs without clinically relevant hepatic fibrosis, and dogs with clinically relevant hepatic fibrosis were 1.51 (range, 1.44‐1.66) m/s, 1.56 (range, 1.37‐1.67) m/s, and 2.04 (range, 1.81‐2.26) m/s, respectively (Kruskal‐Wallis test, P < .001). The SWVs measured by 2D‐SWE in dogs with clinically relevant hepatic fibrosis were significantly higher than those in healthy dogs (P = .007) and dogs without clinically relevant hepatic fibrosis (P < .001). Conversely, SWVs in dogs without clinically relevant hepatic fibrosis were similar to those of healthy dogs (P = .95).
Figure 2.
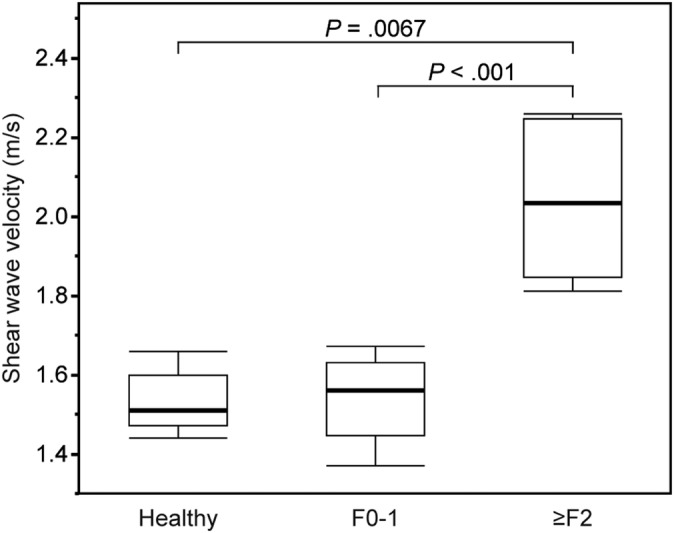
Shear wave velocity in healthy dogs (n = 8), dogs without clinically relevant hepatic fibrosis (F0‐1, n = 22), and dogs with clinically relevant hepatic fibrosis (≥F2, n = 6). The box extends from 25% to 75% percentile with the median and the whiskers extend to limits of the data
3.4. Comparison of SWV among different amounts of necroinflammatory activity
Figure 3 shows the SWVs measured by 2D‐SWE for the 3 groups: healthy dogs (n = 8), dogs without inflammatory activity (n = 17), and dogs with inflammatory activity (n = 11). The data obtained for healthy dogs are the same as shown in Figure 3. The median SWV was 1.57 (range, 1.37‐2.26) m/s for dogs without inflammatory activity and 1.61 (range, 1.45‐2.24) m/s for dogs with inflammatory activity. No significant difference was found in the values among healthy dogs, dogs without inflammatory activity, and dogs with inflammatory activity (Kruskal‐Wallis test, P = .11).
Figure 3.
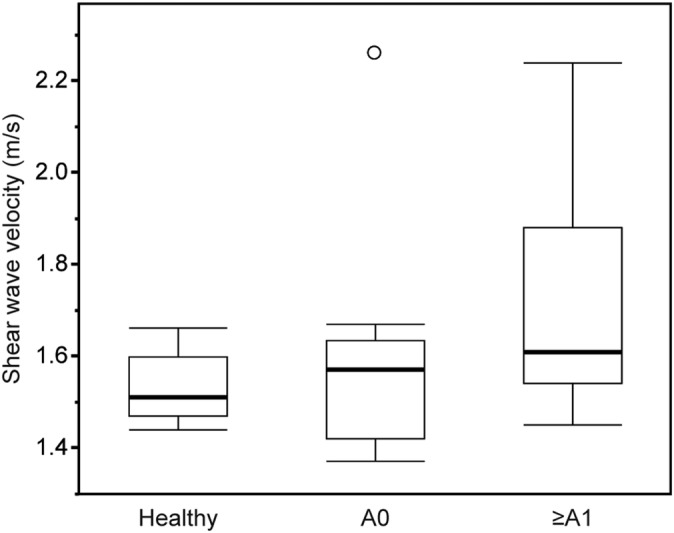
Shear wave velocity in healthy dogs (n = 8), dogs without necroinflammatory activity (A0, n = 17), and dogs with necroinflammatory activity (≥A1, n = 11). The box extends from 25% to 75% percentile with the median and the whiskers extend to limits of the data
4. DISCUSSION
Liver biopsy is a criterion‐referenced method for the definitive diagnosis and monitoring of hepatic disease and hepatic fibrosis. Repeated liver biopsies are required to evaluate the progression of disease and monitor treatment efficacy, but it is sometimes difficult to perform liver biopsy because of the patient's clinical condition and the risk of life‐threatening complications. Conventional B‐mode US would be useful for diagnosing hepatic parenchymal disorders such as chronic hepatitis, especially in the advanced stage, because of its ability to detect surface irregularity and regenerative nodules in the liver. However, it is considered a nonspecific and insensitive method for the diagnosis of hepatic fibrosis. Thus, more specific and sensitive methods are needed.
Elastography is a relatively new US technology that can qualitatively or quantitatively assess the elastic modulus of tissues. There are 2 major categories of elastography techniques introduced in clinical practice: the older strain imaging technique and the newer shear wave imaging technique.12, 19, 22, 23 Strain imaging is an elastography technique that involves the application of stress to the organ by external compression using an US transducer or internal physiological pulsation, such as heartbeats or respiration. Several studies have reported the feasibility of strain imaging in the liver, spleen, kidneys, and prostate gland of healthy dogs and cats.24, 25 Furthermore, recent studies have reported the usefulness of strain imaging for differentiating malignant lymph nodes and SC mass lesions from benign lesions.26, 27 However, a major disadvantage of strain imaging is its inability to quantitatively assess the elastic modulus of the tissues.12, 22, 23 Conversely, shear wave imaging can quantitatively evaluate the elastic modulus of the tissues. Two types of shear wave imaging techniques currently are used in clinical practice: transient elastography and SWE (eg, 2D‐SWE). Transient elastography is a widely used and validated technique for the evaluation of hepatic fibrosis in human patients. A disadvantage of transient elastography, however, is its inability to provide an anatomical B‐mode image during the assessment of the elastic modulus of the tissue. Moreover, a previous study reported that 2D‐SWE showed higher accuracy than transient elastography for the assessment of the severity of hepatic fibrosis in humans with chronic hepatitis C.28 Therefore, we used the 2D‐SWE for the assessment of the liver elastic modulus in our study.
In our study, we investigated whether 2D‐SWE could differentiate between dogs with clinically relevant hepatic fibrosis (≥F2) and dogs without clinically relevant hepatic fibrosis (F0‐1). Consequently, SWVs in dogs with clinically relevant hepatic fibrosis were significantly higher than in dogs without clinically relevant hepatic fibrosis. Similar to findings in human patients, this result indicated that the liver elastic modulus assessed by 2D‐SWE in dogs successfully reflected the severity of hepatic fibrosis secondary to hepatic parenchymal disorders such as chronic hepatitis or cholangiohepatitis. However, no significant difference was found between healthy dogs and dogs without clinically relevant hepatic fibrosis, even though all dogs without clinically relevant hepatic fibrosis were clinically diagnosed with hepatic disease and had increased hepatic enzyme activity. In humans, a report indicated that SWVs measured using 2D‐SWE overlapped among F0, F1, and the healthy control group.29 Thus, our results indicated that it is difficult to predict the absence of or mild hepatic fibrosis (F0 or F1 stage) using 2D‐SWE in dogs.
On the other hand, no significant difference was found in the SWVs among healthy dogs, dogs with necroinflammatory activity, and dogs without necroinflammatory activity. Accordingly, we presume that the necroinflammatory activity has a much lesser effect on SWVs than does hepatic fibrosis. However, several previous studies in humans have reported that the SWVs would be affected by conditions of severe liver inflammation such as acute hepatitis, and this finding is attributed to the presence of inflammatory cells infiltrates, tissue edema, and hepatocyte swelling in the liver with severe necroinflammatory activity.30, 31 In our study, the majority of dogs with hepatic disease were graded as having only mild necroinflammatory activity (A0 to A2), and only 1 dog was graded as A3. Thus, it was difficult to determine the effect of necroinflammatory activity on SWV because of the small number of cases. Further studies with a larger sample of dogs with severe necroinflammatory activity are warranted.
We also found that serum albumin and Tchol concentrations were significantly lower in dogs with clinically relevant hepatic fibrosis than in those without clinically relevant hepatic fibrosis, although the concentrations in most dogs with hepatic disease were within reference ranges. In dogs with hepatic disorders, hypoalbuminemia tends to occur when approximately 70% of liver function has been lost. Hypocholesterolemia also could be detected in end‐stage hepatic disease in dogs.32 In humans, several indirect markers of hepatic failure have been reported for predicting the presence of hepatic fibrosis in clinical settings. In patients with chronic hepatitis B, serum albumin concentrations have been reported to be significantly lower in patients with clinically relevant hepatic fibrosis (≥F2) than in patients without clinically relevant hepatic fibrosis (F0‐1).33 Although the decreases in albumin and Tchol concentrations could help predict the presence of severe hepatic fibrosis in dogs as an indirect indicator, these changes are not specific for hepatic disorders.
Conversely, HA was not significantly different between dogs with and without clinically relevant hepatic fibrosis in our study. Hyaluronic acid is a high‐molecular‐weight glycosaminoglycan primary synthesized by hepatic stellate cells and degraded by sinusoidal endothelial cells. It had been reported to possess high diagnostic accuracy for the noninvasive evaluation of hepatic fibrosis and cirrhosis in human patients.6, 7, 8 However, in veterinary medicine, only 3 reports have investigated the usefulness of HA in predicting hepatic fibrosis. One report indicated that plasma HA concentration was significantly increased in dogs with cirrhosis,9 whereas another report indicated that the serum HA concentration correlated with hepatic fibrosis stage.10 One study,11 however, found no positive correlation between serum HA concentration and hepatic fibrosis stage, which is similar to the results of our study. Discrepancy in results may be caused by differences in assay types, sample size, or histological sample population.
Our study had several limitations. First, sample size was small, especially for dogs with clinically relevant hepatic fibrosis. However, despite small sample size, our results indicated the usefulness of 2D‐SWE as a noninvasive diagnostic tool for the assessment of hepatic fibrosis. Further studies with larger sample size are needed to determine the sensitivity, specificity, and the cutoff value of 2D‐SWE for distinguishing among fibrosis groups. Second, sampling errors might have occurred in some dogs with hepatic diseases, possibly because the region measured by 2D‐SWE may not have completely coincided with that of liver biopsy. Additionally, it was impossible to collect biopsy specimens from multiple liver lobes because of the high risk of the bleeding. In the previous study of 70 dogs undergoing necropsy, some histological changes such as hepatic fibrosis were nonuniformly distributed among lobes.34 Conversely, several previous reports in humans indicated that minimal or no significant differences in hepatic lesion were detected among hepatic lobes.35, 36, 37 Further studies are needed to elucidate the effect of histopathological variations among liver lobes and the result of SWE measurement. Third, although our study included 3 dogs with mild ascites secondary to clinically relevant hepatic fibrosis, mild ascites did not separate the right liver lobe from the abdominal wall in these 3 dogs. Transient elastography is limited to patients that do not have perihepatic ascites or fluid in the abdomen surrounding the liver and cannot be performed in patients with ascites. On the other hand, the recommended guidelines for the clinical use of elastography in humans indicated that SWE can measure the SWVs of patients with ascites because the US push beam, which generates the shear waves, propagates through fluids and appears not to be influenced by the presence of ascites.19 Because the influence of ascites on the measurement of SWVs using 2D‐SWE is not well understood in veterinary medicine, further studies also are needed to evaluate the influence of ascites on SWVs in dogs.
In conclusion, our prospective preliminary study showed that SWVs measured by 2D‐SWE are increased in dogs with clinically relevant hepatic fibrosis compared to dogs without clinically relevant hepatic fibrosis and healthy dogs. These results indicated that 2D‐SWE may be a useful method for noninvasively predicting clinically relevant hepatic fibrosis in dogs. Further studies potentially would be able to distinguish different stages of hepatic fibrosis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This work was approved by the Animal Ethical Committee of Graduate School of Veterinary Medicine, Hokkaido University.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Tamura M, Ohta H, Shimbo G, et al. Usefulness of noninvasive shear wave elastography for the assessment of hepatic fibrosis in dogs with hepatic disease. J Vet Intern Med. 2019;33:2067–2074. 10.1111/jvim.15598
Funding information Japan Society for the Promotion of Science KAKENHI, Grant/Award Number: 18J21189
REFERENCES
- 1. Eulenberg VM, Lidbury JA. Hepatic fibrosis in dogs. J Vet Intern Med. 2018;32:26‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison JL, Turek BJ, Brown DC, Bradley C, Callahan Clark J. Cholangitis and cholangiohepatitis in dogs: a descriptive study of 54 cases based on histopathologic diagnosis (2004–2014). J Vet Intern Med. 2018;32:172‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horowitz JM, Venkatesh SK, Ehman RL, et al. Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY). 2017;42:2037‐2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. Hepatology. 2000;32:477‐481. [DOI] [PubMed] [Google Scholar]
- 5. Bravo AA, Sheth SG, Chopra S. Liver Biopsy. N Engl J Med. 2001;344:495‐500. [DOI] [PubMed] [Google Scholar]
- 6. Parise ER, Oliveira AC, Figueiredo‐Mendes C, et al. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int. 2006;26:1095‐1099. [DOI] [PubMed] [Google Scholar]
- 7. Saitou Y, Shiraki K, Yamanaka Y, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL‐40, in patients with HCV‐associated liver disease. World J Gastroenterol. 2005;11:476‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orasan OH, Ciulei G, Cozma A, Sava M, Dumitrascu DL. Hyaluronic acid as a biomarker of fibrosis in chronic liver disease of different etiologies. Clujul Med. 2016;89:24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanemoto H, Ohno K, Sakai M, et al. Blood hyaluronic acid as a marker for canine cirrhosis. J Vet Med Sci. 2009;71:1251‐1254. [DOI] [PubMed] [Google Scholar]
- 10. Glińanemoto K, Orłowska A, Spuzak J, Jankowski M, Kubiak K. Suitability of using serum hialuronic acid concentrations in the diagnosis of canine liver fibrosis. Pol J Vet Sci. 2015;18:873‐878. [DOI] [PubMed] [Google Scholar]
- 11. Lidbury JA, Hoffmann AR, Fry JK, Suchodolski JS, Steiner JM. Evaluation of hyaluronic acid, procollagen type III N‐terminal peptide, and tissue inhibitor of matrix metalloproteinase‐1 as serum markers of canine hepatic fibrosis. Can J Vet Res. 2016;80:302‐308. [PMC free article] [PubMed] [Google Scholar]
- 12. Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osaki A, Kubota T, Suda T, et al. Shear wave velocity is a useful marker for managing nonalcoholic steatohepatitis. World J Gastroenterol. 2010;16:2918‐2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takeda T, Yasuda T, Nakayama Y, et al. Usefulness of noninvasive transient elastography for assessment of liver fibrosis stage in chronic hepatitis C. World J Gastroenterol. 2006;12(48):7768‐7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferraioli G, Tinelli C, Lissandrin R, et al. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787‐4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two‐dimensional shear‐wave elastography performance in the noninvasive evaluation of liver fibrosis in patients with chronic hepatitis B: comparison with serum fibrosis indexes. Radiology. 2017;283:873‐882. [DOI] [PubMed] [Google Scholar]
- 17. Frulio N, Trillaud H. Ultrasound elastography in liver. Diagn Interv Imaging. 2013;94:515‐534. [DOI] [PubMed] [Google Scholar]
- 18. Tamura M, Ohta H, Nisa K, et al. Evaluation of liver and spleen stiffness of healthy dogs by use of two‐dimensional shear wave elastography. Am J Vet Res. 2019;80:378‐384. [DOI] [PubMed] [Google Scholar]
- 19. Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med Biol. 2015;41:1161‐1179. [DOI] [PubMed] [Google Scholar]
- 20. Van den Ingh TSGAM, Van Winkle T, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver In: WSAVA , ed. Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. 1st ed. Philadelphia, PA: Saunders Elsevier; 2006:85‐101. [Google Scholar]
- 21. Lidbury JA, Rodrigues Hoffmann A, Ivanek R, et al. Interobserver agreement using histological scoring of the canine liver. J Vet Intern Med. 2017;31:778‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barr RG, Ferraioli G, Palmeri ML, et al. Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology. 2015;276:845‐861. [DOI] [PubMed] [Google Scholar]
- 23. Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126‐1147. [DOI] [PubMed] [Google Scholar]
- 24. Jeon S, Lee G, Lee SK, Kim H, Yu D, Choi J. Ultrasonograpahic elastography of the liver, spleen, kidneys, and prostate in clinically normal beagle dogs. Vet Radiol Ultrasound. 2015;56:425‐431. [DOI] [PubMed] [Google Scholar]
- 25. White J, Gay J, Farnsworth R, Mickas M, Kim KG, Mattoon J. Ultrasound elastography of the liver, spleen, and kidneys in clinically normal cats. Vet Radiol Ultrasound. 2014;55:428‐434. [DOI] [PubMed] [Google Scholar]
- 26. Seiler GS, Griffith E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet Radiol Ultrasound. 2018;59:79‐88. [DOI] [PubMed] [Google Scholar]
- 27. Longo M, Bavcar S, Handel I, Smith S, Liuti T. Real‐time elastosonography of lipomatous vs. malignant subcutaneous neoplasms in dogs: preliminary results. Vet Radiol Ultrasound. 2018;59:198‐202. [DOI] [PubMed] [Google Scholar]
- 28. Ferraioli G, Tinelli C, Dal Bello B, et al. Accuracy of real‐time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125‐2133. [DOI] [PubMed] [Google Scholar]
- 29. Leung VY, Shen J, Wong VW, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear‐wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910‐918. [DOI] [PubMed] [Google Scholar]
- 30. Yoshioka K, Kawabe N, Hashimoto S. Transient elastography: applications and limitations. Hepatol Res. 2008;38:1063‐1068. [DOI] [PubMed] [Google Scholar]
- 31. Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380‐384. [DOI] [PubMed] [Google Scholar]
- 32. Cocker S, Richter K. Hepatobiliary disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 8th ed. Philadelphia, PA: WB Saunders; 2017:1611‐1621. [Google Scholar]
- 33. Hongbo L, Xiaohui L, Hong K, Wei W, Yong Z. Assessing routine and serum markers of liver fibrosis in CHB patients using parallel and serial interpretation. Clin Biochem. 2007;40:562‐566. [DOI] [PubMed] [Google Scholar]
- 34. Kemp SD, Zimmerman KL, Panciera DL, Monroe WE, Leib MS. Histopathologic variation between liver lobes in dogs. J Vet Intern Med. 2015;29:58‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giesen CP, Koepsell JE, Hastings EV, Lindert MC. Correlation of punch liver biopsy with autopsy material. Am J Dig Dis. 1951;18:304‐307. [DOI] [PubMed] [Google Scholar]
- 36. Mallory TB. The pathology of epidemic hepatitis. J Am Med Assoc. 1947;134:655‐662. [DOI] [PubMed] [Google Scholar]
- 37. Waldstein SS, Szanto PB. Accuracy of sampling by needle biopsy in diffuse liver disease. Arch Pathol. 1950;50:326‐328. [PubMed] [Google Scholar]


