Abstract
Background
Protein‐losing enteropathy (PLE) because of chronic inflammatory enteropathy (CIE) in dogs is often treated with a combination of glucocorticoids and second‐line immunosuppressant (SLI). This combined approach might not be necessary in all dogs.
Hypothesis/objectives
To describe diagnostic features and outcomes of dogs with PLE treated with glucocorticoids alone (group P) or with glucocorticoids and SLI (group S).
Animals
Thirty‐one dogs with PLE.
Material and methods
Retrospective analysis of signalment data from diagnostic procedures, treatment, and outcome of dogs with CIE/PLE (from 2015 to 2017), using the hospital's digital case database. Dogs with hypoalbuminemia and CIE were included. Because of a stepwise treatment algorithm, dogs were allocated to group P or S. Time to serum albumin concentrations ≥20 g/L and survival data were collected. Dogs were additionally categorized by their albumin and cobalamin serum concentrations. Multivariate and univariate analysis as well as Pearson's correlation and Kaplan‐Maier survival analysis were performed.
Results
Seventeen dogs were included in group P and 14 in group S. World Small Animal Veterinary Association score of the duodenum was different between groups (P = .05), but none of the other examined data. Median time until serum albumin reached >20 g/L was 13 days. Median survival time after start of treatment was 85 days (range, 13‐463 days) in group P and 166 days (range, 8‐390 days) in group S.
Conclusion and Clinical Importance
No routine diagnostic test was predictive of clinical response, treatment group, or outcome. Glucocorticoid treatment alone can be appropriate in dogs with PLE.
Keywords: chronic enteropathy, cobalamin, hypoalbuminemia, inflammatory bowel disease, prognosis
Abbreviations
- CCECAI
Canine Chronic Enteropathy Clinical Activity Index
- CIBDAI
Canine Inflammatory Bowel Disease Activity Index
- CIE
chronic inflammatory enteropathy
- MANOVA
multivariate analysis of variance
- PLE
protein‐losing enteropathy
- SLI
second‐line immunosuppressive drug
- WSAVA
World Small Animal Veterinary Association
1. INTRODUCTION
Protein‐losing enteropathy (PLE) is a syndrome characterized by excessive loss of serum albumin across the intestinal wall.1, 2, 3 Many different dog breeds can be affected, with some at increased risk.1, 4, 5, 6 Underlying causes vary and include primary (congenital) and secondary (acquired) lymphangiectasia, chronic inflammatory enteropathies (CIE), most commonly lymphoplasmacytic enteritis, intestinal neoplasia (principally alimentary lymphoma), and infectious disease.3 Even though hypoalbuminemia is the hallmark of PLE, additional laboratory abnormalities occur, including lymphopenia, hypocholesterolemia, hypoglobulinemia, hypocalcemia, and hypomagnesemia.2, 3 The severity of clinical signs (often assessed by the canine IBD activity index [CIBDAI]7 or the canine chronic enteropathy clinical activity index [CCECAI]8), serum albumin concentration (<20 g/L [2.0 g/dL]), and hypocobalaminemia are negative prognostic indicators.2, 3, 8, 9 Even though hypocobalaminemia is common in dogs with PLE, and serum concentrations of cobalamin and albumin correlate,8 the prevalence of hypocobalaminemia in dogs with PLE because of CIE has not been described. Other negative prognostic indicators include clinical abnormalities such as chronic vomiting,10 low body weight,1, 11, 12 failure to normalize CIBDAI or CCECAI,13 laboratory abnormalities (eg, decreased10 or increased12, 13 blood urea nitrogen concentration), high endoscopic scores, and duodenal inflammation.8 Elevations in serum C‐reactive protein concentration and decreases in 25(OH) vitamin D serum concentration also have prognostic value.11, 14, 15
In cases of PLE, abdominal ultrasonography identifies intestinal lesions, concurrent lymphadenopathy and allows better distinction between inflammatory and neoplastic causes.2, 3 Hyperechoic mucosal stippling or striations are associated with lacteal dilation, having a sensitivity of 75% and a specificity of 96% for PLE.16, 17 However, diagnostic imaging findings have not been evaluated for their prognostic value. Endoscopy and histopathology scores are inconsistent predictors of diagnosis and prognosis of PLE.8, 15, 17
Given survival rates of dogs with PLE of 30%‐40%,3, 15 most treatment protocols involve an aggressive and multimodal approach, combining dietary modification, administration of antimicrobials, and immunosuppressive drugs. Recently, immunosuppressive treatment in PLE has come under some scrutiny because of adverse effects of glucocorticoid treatment and an encouraging clinical response to various dietary interventions alone, for example, in Yorkshire Terriers with PLE.10, 15 Also, immunosuppressive treatment is significantly associated with a negative outcome compared with dietary management of PLE.15 However, most of the studies demonstrating a beneficial effect of diet alone are restricted to PLE occurring in a single breed (mostly Yorkshire Terriers), so it is unknown if these dogs have a dietary‐responsive phenotype of the disease or if this treatment could also be applied to a wider population of dogs with PLE. The idea that steroids should be combined with a second‐line immunosuppressant (SLI) is based on a study investigating risk factors for a negative outcome in CIE, in which none of the 10 dogs with PLE responded to glucocorticoid treatment alone.8 Subsequent addition of cyclosporine rescued 7 of 10 dogs from euthanasia.8 Treatment with prednisolone alone, the combinations of prednisolone and azathioprine, cyclosporine, or methotrexate are all described.1, 8, 18 Increasingly, mycophenolate mofetil is also empirically used, and in some refractory cases more than 1 SLI is prescribed. A chlorambucil‐prednisolone combination for treatment of CIE with concurrent PLE resulted in significantly improved serum albumin concentration and weight gain compared to treatment with an azathioprine‐prednisolone combination.1 Median survival time was not reached for the chlorambucil‐prednisolone treated dogs.1 However, no comparison of chlorambucil with cyclosporine as a treatment for PLE has been published. The percentage of dogs with PLE/CIE needing a SLI or if this can be associated with any routinely obtained clinicopathological, diagnostic imaging, endoscopic, or histological test is unknown.
The aim of the present study was to retrospectively determine the proportion of dogs treated with glucocorticoids for inflammatory PLE that respond to this treatment alone (without the subsequent requirement for an SLI) and to describe data from history, laboratory evaluations, ultrasound, endoscopy, histology, and clinical outcome. Routine diagnostic tests were assessed for their potential association with treatment requirements, prognosis, or outcome.
2. MATERIALS AND METHODS
Retrospective case acquisition was performed by using the electronic client data management system's (Tristan Veterinary Software Ltd, version 1.8.3.914, Orion Engineering Services, Orion House, Inverness, United Kingdom) search tool. Search terms used within both the free text clinical record as well as VeNom (veterinary nomenclature) diagnostic code were “protein losing enteropathy,” “cobalamin,” “inflammatory bowel disease,” “(chronic) enteropathy,” “food intolerance,” and “hypoalbuminemia.” The search was conducted for a time frame spanning from January 1, 2015, to December 31, 2017. Search results were exported into commercial software (Microsoft Excel spreadsheet software, Microsoft Corporation, Redmond, Washington). Duplicate files were removed manually. Only first visits of dogs were included. Of the remaining files, the first serum albumin concentration on record as well as the final diagnosis was revisited by 1 of the authors (S.S.S.). Dogs with a serum albumin concentration within the reference range (26‐35 g/L) were excluded. Files of dogs with acute disease, intestinal neoplasia, GI bleeding, concurrent diseases not allowing the accurate diagnosis of PLE, and dogs without a final diagnosis were excluded. Of the remaining dogs, available data from history, physical examination, clinicopathological values, endoscopy (including scores as previously reported19), histopathology, treatment, and outcome were collated. Because a stepwise treatment algorithm for dogs with PLE was in place (Supporting Information Figure S1), dogs could be allocated to 1 of 2 treatment groups: group P consisted of dogs that had received supportive treatment, dietary management and glucocorticoids (prednisolone), but no SLI; and group S, which consisted of dogs that had received similar treatments but the addition of a SLI. For both groups, time to reach a serum albumin ≥20 g/L (identified as an acceptable short‐term treatment target) as well as survival data was collected. Dogs were additionally categorized by their albumin and cobalamin serum concentrations, respectively, to allow assessment of potential impact of these data on outcome. Determination of serum cobalamin concentrations was done in the same commercial laboratory (IDEXX Laboratories Ltd, Wetherby, West Yorkshire, United Kingdom; for all dogs apart from 1) with a reference range of >250 ng/mL and a lower detection limit of the assay of <150 ng/mL, hence categories were chosen to represent severe hypocobalaminemia (<150 ng/mL), moderate hypocobalaminemia (150‐250 ng/mL) and normal serum cobalamin concentrations (>250 ng/mL).
Ultrasound findings were assessed based on the initial diagnostic report (by board‐certified or board‐eligible diagnostic imagers); images were not reviewed specifically for the purpose of this study. Gastrointestinal abnormalities recorded were categorized by section of the GI tract (stomach, small intestine, large intestine) and into the presence or absence of 1 or more of the following: mucosal lesions (specifically hyperechogenicity, hyperechoic striations, and speckles), changes of the entire wall or loss of layering, subjective dysmotility or ileus, as well as abnormalities of intra‐abdominal lymph nodes and presence of free peritoneal fluid.
Endoscopy scores were available from initial reports issued by the board‐certified or board‐eligible specialist in small animal internal medicine performing the endoscopy. For this, the grading system proposed by the World Small Animal Veterinary Association (WSAVA) International Gastrointestinal Standardization Group was used.20 Briefly, each segment of the GI tract is evaluated for the absence/ presence and severity of a given list of lesions, which can each be graded as normal (=0), mild (=1), moderate (=2), or severe (=3). Maximum achievable scores based on this system are 30 for stomach and duodenum, 27 for the ileum, and 21 for the colon. Sums of these scores by GI segment were used for analysis in this study.
Histopathological diagnosis was based on the initial case file information, however, for the purpose of this study, all slides were reexamined (or recut if not considered adequate) and scored according to WSAVA guidelines21 by a single board‐certified pathologist (LM).
2.1. Statistical analysis
Normality of data was assessed using Shapiro‐Wilk test. For multivariate analysis of variance (MANOVA), missingness of data was imputed by median replacement before postprocessing, but not used for univariate downstream analysis. Multivariate analysis of variance (general linear model) was performed separately for treatment group (P or S), survival status (deceased or alive) and cobalamin status (>250 ng/mL, between 150 and 250 ng/mL, <150 ng/mL) as fixed factors. Interactions between variables were also examined using this model. Least significant difference was used as a post hoc test where appropriate. Student's t tests or Mann‐Whitney U tests were used for univariate comparison of selected data between treatment groups and for survival status. Tukey's correction was used to adjust for multiple comparisons. Pearson's correlation analysis of clinicopathological and histologic scores with treatment status and outcome was also performed (for correlation analysis with cobalamin values, concentrations reported as <150 ng/mL were set to 149 ng/mL). A Kaplan‐Maier diagram was used for survival analysis comparing group P and S (Log rank test). All statistical tests were conducted and graphs created using commercially available statistical software (IBM SPSS Statistics, version 22, IBM Corporation, New York, New York; GraphPad Prism, version 8.0.2, GraphPad Software Inc, San Diego, California); and P < .05 was considered significant.
3. RESULTS
Retrospective database search yielded 298 “hits” for the used search terms. After removal of duplicates, 244 cases remained. Cases initially excluded were: n = 13 revisits, n = 5 serum albumin concentration not recorded, n = 1 diagnosed with Immerslund‐Gräsbeck syndrome. Of the remaining 225 dogs, 63 were hypoalbuminemic. Of these, n = 11 were excluded as they had a final diagnosis not consistent with inflammatory PLE (lymphoma n = 3, intestinal intussusception n = 3, intestinal adenocarcinoma n = 2, granulomatous colitis n = 2, and low albumin because of vegan diet n = 1). A further 9 were excluded as no final diagnosis was reached. Five hypoalbuminemic dogs were excluded because of concurrent diseases interfering with the diagnosis of PLE (n = 1 of each of the following: diabetes mellitus, protein‐losing nephropathy, multiple endocrinopathies and IMHA, exocrine pancreatic insufficiency, bilateral adrenal masses with vascular invasion, and an aortic thrombus). Four dogs were excluded because of suspected or proven intestinal blood loss or iron deficiency (based on serum iron panel) and a single dog was excluded as the low serum albumin concentration was a clerical error (the dog was eventually diagnosed with food‐responsive enteropathy). This left a final number of 33 dogs with PLE because of CIE. Signalment, history, diagnostic procedures performed, and main findings of these 33 dogs can be found in Table S1.
Thirty‐one out of 33 dogs followed the hospital's diagnostic and treatment protocol: 17 dogs (55%) in group P and 14 dogs (45%) in group S. The remaining 2 dogs were mildly hypoalbuminemic and treated with diet and tylosin only and therefore not included in further analyses. Thirteen of the 14 dogs in group S received cyclosporine as SLI and the remaining dog received chlorambucil. The mean serum albumin concentration of all dogs at time of first presentation was 19.4 g/L (SD, 4.5), the mean of the lowest serum albumin concentration recorded was 16.8 g/L (SD, 4.4). Additional biochemical abnormalities included: hypoglobulinemia (15/31), hypocholesterolemia (21/31), total hypocalcemia (31/31), ionized hypocalcemia (4/25), and total hypomagnesemia (17/31). Hypocobalaminemia was present in 19/31 dogs (61%) with 8 of those 19 dogs showing cobalamin values lower than the measuring threshold of the laboratory (GraphPad Software Inc; <150 μmoL/L). Folate was below the reference range in 12/29 dogs (41%), above it in 4/29 (14%), and within the reference range in the remaining dogs in which it was measured.
Thirty dogs had a full abdominal ultrasound performed. The remaining dog had a point‐of‐care ultrasound performed that revealed marked ascites, followed by an abdominal CT. Lesions found on ultrasound included abnormalities of the small intestine (n = 27/30), large intestine (n = 13/30), abdominal lymph nodes (n = 12/30, described as reactive changes, local, or diffuse mild to marked lymphadenomegaly), excessive peritoneal fluid (n = 23/31), and dysmotility/intestinal ileus (n = 7/30). Specific abnormalities of the small intestines reported were generalized mucosal lesions (n = 14/30, described as hyperechoic striations and speckles, hyperechoic mucosa, or enteritis), generalized lesions beyond the mucosa (n = 8/30, described as diffusely thickened or thickening of the muscularis layer). Jejunal ulcers were found in 2 cases. Colitis was described in 3 dogs and nonobstructive foreign bodies were found in 2 dogs.
Gastroduodenoscopy and colonoscopy with mucosal pinch biopsies was performed in all dogs. Ileoscopy was performed in 21/31 dogs. Endoscopic scores were available for the stomach, duodenum, and colon from 27/31 dogs and for the ileum from 19/21 dogs (Table S2). Overall, median endoscopic severity was 1 (range, 0‐9) for the stomach, 7 (range, 0‐20) for the duodenum, 0 (range, 0‐10) for the colon, and 6 (range, 0‐19) for the ileum, respectively.
Histological diagnoses are summarized in Table S3: most frequent findings were lymphoplasmacytic gastritis (n = 17/31) and colitis (n = 17/31), whereas in the duodenum and ileum, lymphoplasmacytic plus eosinophilic inflammation was most common (n = 10 for both locations, respectively), followed by “chronic‐active” lymphoplasmacytic plus neutrophilic inflammation (n = 9 duodenum, n = 7 ileum). Crypt abscessation or dilation was found in 17/31, villous blunting in 14/31, and lacteal dilation in 10/31 cases, respectively. Median WSAVA histology scores (Table S2) for the stomach, duodenum, ileum, and colon were 2 (range, 0‐12), 6 (range, 1‐13), 7 (range, 0‐15) and 2 (range, 0‐10), respectively, resulting in a median total score (omitting ileal scores, as they were not available for all dogs) of 2 (range, 0‐10).
Multivariate analysis of variance between group P and S highlighted significant or borderline significant differences for initial serum albumin (P = .05), total protein (P = .007), cholesterol (P = .04), white blood cell count (WBC, P = .05), folate (P < .0001), cobalamin (P = .01), endoscopy score of the duodenum (P = .007), and WSAVA histology score of the ileum (P = .03) and colon (P = .04) (Table 1 and Figures 1 and 2). However, for pairwise/univariate comparisons (Table 1), only the WSAVA histology score of the duodenum was significantly higher (P = .05) in group P (mean, 7.12; SD, 3.19) than in group S (mean, 4.79; SD, 3.09).
Table 1.
Comparison of selected parameters between dogs with protein‐losing enteropathy (PLE) treated with (group S, n = 14) and without (group P, n = 17) a second‐line immunosuppressive drug in addition to glucocorticoids
| Variable tested | Group P median (range) or mean (SD) | Group S median (range) or mean (SD) | Significance level (P‐value) |
|---|---|---|---|
| Age (years)a | 7.7 (1.7‐11) | 6.3 (2.5‐12.6) | .04 |
| Serum albumin at presentation (g/L)b | 19.1 (4.6) | 19.7 (4.6) | .69 |
| Lowest recorded serum albumin (g/L)b | 17.1 (4.1)b | 16.5 (5.0) | .70 |
| Serum globulin (g/L)b | 19.7 (5.6)b | 19.3 (6.5) | .86 |
| Total protein (g/L)b | 38.8 (8.5)b | 39.1 (10.3) | .94 |
| Serum cholesterol (mmol/L)a | 2.8 (1.6‐8.3) | 2.5 (1.7‐4.7) | .25 |
| Total calcium (mmol/L)a | 2.0 (1.1‐2.3) | 2.0 (0.7‐2.3) | .82 |
| Ionized calcium (mmol/L)a | 1.2 (0.8‐1.4) | 1.3 (0.6‐1.4) | .69 |
| Total magnesium (mmol/L)b | 0.7 (0.2) | 0.6 (0.2) | .29 |
| BUN/urea (mmol/L)b | 5.3 (2.7) | 5.2 (2.1) | .89 |
| WBC (×10e9/L)b | 11.5 (6.5) | 13.4 (8.2) | .90 |
| Lymphocytes (×10e9/L)b | 1.3 (1.0) | 1.1 (0.6) | .49 |
| PCV (L/L)b | 0.4 (0.1) | 0.4 (0.1) | .63 |
| Platelets (10e9/L)b | 433 (199) | 399 (152) | .60 |
| Reticulocytes (10e9/L)b | 52.8 (33.9) | 100.0 (75.3) | .11 |
| Serum folate (μg/L)a | 8.0 (2.1‐18.7) | 10.0 (4.2‐22.5) | .11 |
| Serum cobalamin (ng/L)a | 204 (<150‐676) | 183 (<150‐1489) | .72 |
| Cortisol (nmol/L)a | 88 (36.1‐315) | 74 (37‐241) | .68 |
| Intestinal mucosal lesions on ultrasoundb | 1.5 (0.5) | 1.5 (0.5) | .72 |
| Intestinal full thickness lesions on ultrasoundb | 1.7 (0.5) | 1.7 (0.5) | .94 |
| Lymphadenopathy on ultrasoundb | 1.6 (0.5) | 1.5 (0.5) | .33 |
| Free fluid on ultrasoundb | 1.3 (0.5) | 1.2 (0.4) | .62 |
| Abnormal stomach on ultrasoundb | 1.7 (0.5) | 1.8 (0.4) | .71 |
| Abnormal small intestine on ultrasoundb | 1.1 (0.2) | 1.2 (0.4) | .44 |
| Abnormal large intestine on ultrasoundb | 1.7 (0.5) | 1.4 (0.5) | .09 |
| Dysmotility/ ileus on ultrasoundb | 1.6 (0.5) | 1.9 (0.3) | .06 |
| Gastric endoscopy scorea | 1.5 (0‐9) | 1.0 (0‐7) | .76 |
| Duodenal endoscopy scoreb | 7.1 (5.6) | 7.1 (4.8) | .97 |
| Ileal endoscopy scoreb | 6.0 (5.3) | 4.5 (4.1) | .28 |
| Colonic endoscopy scorea | 1.0 (0–10) | 0 (0‐6) | .71 |
| WSAVA histology score stomacha | 2.0 (0‐12) | 2.0 (0‐4) | .74 |
| WSAVA histology score duodenumb | 7.1 (3.2) | 4.8 (3.1) | .05 |
| WSAVA histology score ileumb | 6.8 (3.9) | 7.3 (3.4) | .72 |
| WSAVA histology score colona | 2.0 (0‐6) | 2.5 (0‐10) | .52 |
| WSAVA histology score totalb | 12.2 (4.1) | 9.6 (4.1) | .09 |
| Time to reach serum albumin >20 g/L (d)a | 7 (0–33) | 14 (0‐77) | .71 |
| Survival time after diagnosis (d)a | 85 (13‐463) | 166 (8‐390) | .39 |
Abbreviations: BUN, blood urea nitrogen; WBC, white blood cell; WSAVA, World Small Animal Veterinary Association.
Data not normally distributed.
Data of normal distribution.
Figure 1.
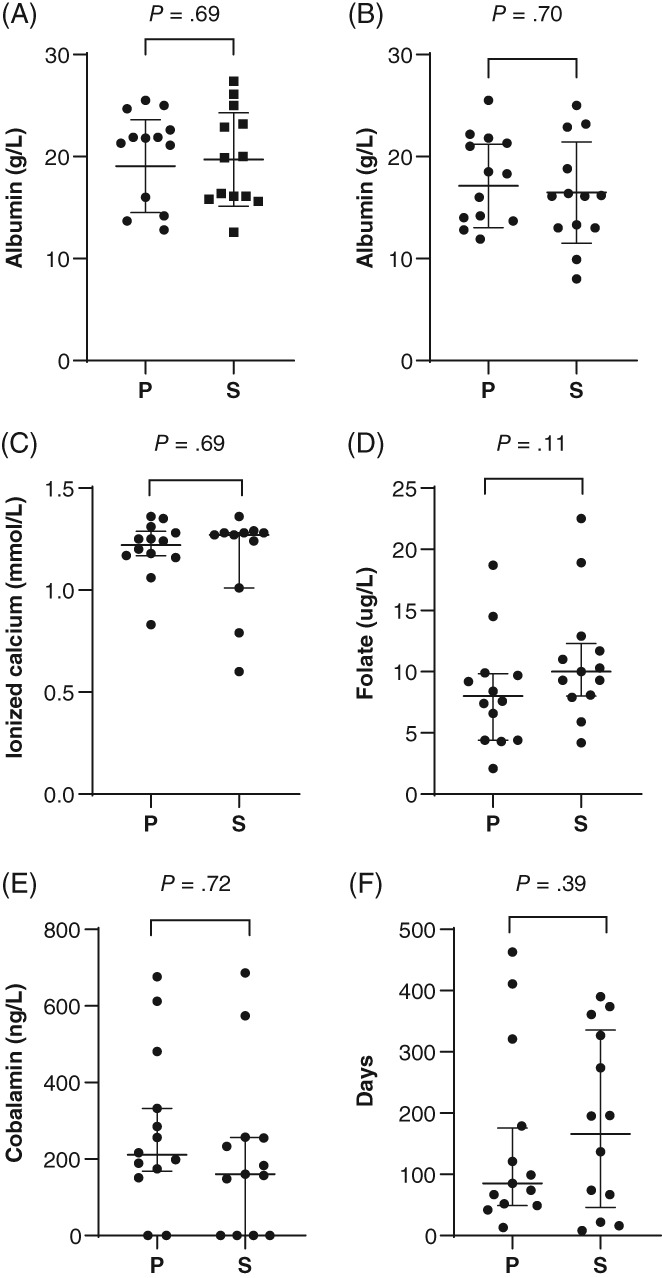
Selected clinicopathological data from 31 dogs with protein‐losing enteropathy (PLE) treated with prednisolone alone (group P) or with prednisolone and a second‐line immunosuppressant (group S): A, Serum albumin concentration at presentation; B, lowest recorded serum albumin concentration; C, ionized calcium concentration; D, serum folate concentration; E, serum cobalamin concentration; and F survival time. For albumin, data are presented as mean (horizontal bar) and SD (whiskers). All other data are presented as median and interquartile range (whiskers)
Figure 2.
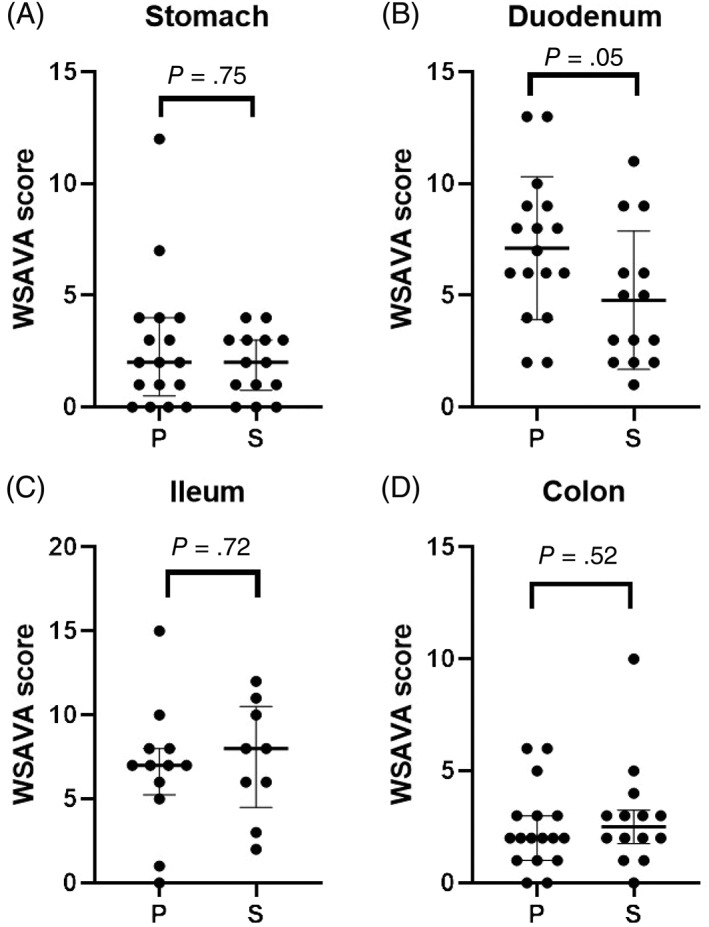
World Small Animal Veterinary Association (WSAVA) histology scores14 (A, stomach; B, duodenum; C, ileum; and D, colon) from 31 dogs with protein‐losing enteropathy (PLE) treated with prednisolone alone (group P) or with prednisolone and a second‐line immunosuppressant (group S). A and D present data as median (horizontal bar) and interquartile range (whiskers). B and C present data as mean (horizontal bar) and SD (whiskers)
There were no differences in MANOVA or univariate comparisons of those same data between dogs that survived and those that died (Figure 3). When dogs were grouped by cobalamin status, analysis of variance revealed significant differences for total protein (P = .01), WBC (P = .05), folate (P < .0001), endoscopy score of the duodenum (P = .01), and WSAVA score of the duodenum (P = .05), and ileum (P = .04), with only folate remaining significantly different on post hoc analysis (Figure 4). Folate was significantly lower in normocobalaminemic dogs compared to mildly (P = .04) or severely hypocobalaminemic dogs (P = .01), but not between mildly and severely hypocobalaminemic dogs. Also, no differences in time to normalization of serum albumin (Figure 5) or outcome could be detected across different cobalamin status. Detailed P‐values for comparisons of all data from different data groupings are summarized in Table S4. The relationship between serum folate and cobalamin values was confirmed by an inverse correlation (Figure 6). Strong positive pairwise correlations were also detected among serum albumin concentration, serum globulin concentration, serum total protein concentration, serum cholesterol concentration, total calcium concentration, and ionized calcium concentration, respectively. In addition, total serum magnesium concentration correlated positively with serum cholesterol concentration (P = .02; r = 0.427), total calcium concentration (P = .009; r = 0.460), and ionized calcium concentration (P < .0001; r = 0.909). Duodenal endoscopic score correlated with serum albumin concentration (P = .022; r = − 0.438), serum globulin concentration (P = .002; r = −0.567), serum total protein concentration (P = .002; r = −0.569), serum cholesterol concentration (P = .04; r = −0.403), total calcium concentration (P = .01; r = −0.475), and ionized calcium concentration (P = .04; r = −0.442), whereas ileal endoscopic score only correlated with lowest (not initial) serum albumin concentration (P = .02; r = −0.513), serum globulin concentration (P = .01; r = −0.558), and serum total protein concentration (P = .01; r = −0.567). When assessing WSAVA histology scores, correlation was observed between duodenal histology scores and duodenal endoscopy scores (P = .02; r = 0.431). There was no correlation of any of the examined values with time to normalization of serum albumin concentration or survival time.
Figure 3.
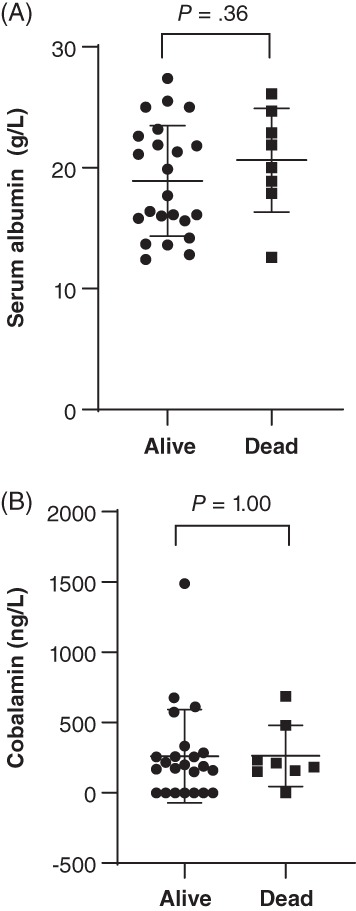
A, Serum albumin concentration and B, serum cobalamin concentration of 31 dogs with protein‐losing enteropathy (PLE) separated by outcome. Data presented as mean (horizontal line) and SD (whiskers)
Figure 4.
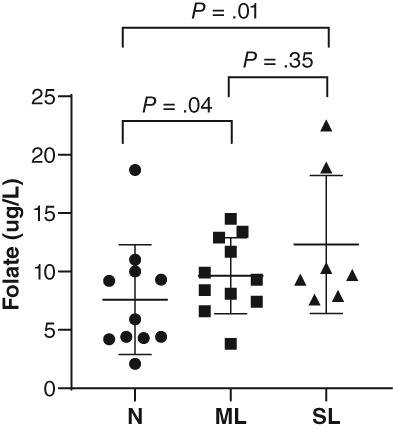
Serum folate concentration in dogs with protein‐losing enteropathy (PLE) grouped by their cobalamin status. N, normal cobalamin >250 ng/L; ML, mildly low cobalamin between 150 and 250 ng/L; SL, severely low cobalamin <150 ng/L. Data presented as mean (horizontal bar) and SD (whiskers)
Figure 5.
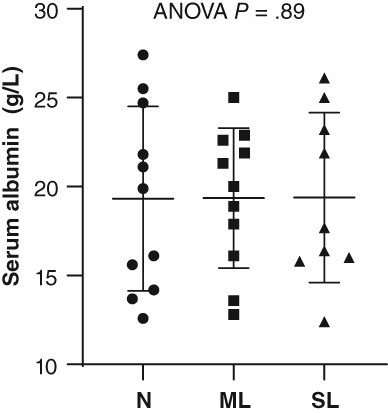
Serum albumin concentration of dogs with protein‐losing enteropathy (PLE) by their serum cobalamin status. N, normal serum cobalamin concentration >250 ng/L; ML, mildly low serum cobalamin concentration between 150 and 250 ng/L; SL, severely low serum cobalamin concentration <150 ng/L. Data presented as mean (horizontal bar) and SD (whiskers)
Figure 6.
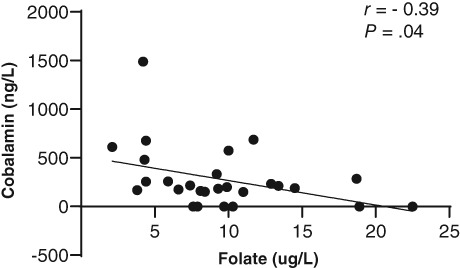
Pearson's correlation between serum folate concentration and serum cobalamin concentration in 31 dogs with protein‐losing enteropathy (PLE)
Overall median time until the serum albumin concentration reached >20 g/L was 13 days (range, 0‐77 days), which was not significantly different between groups P and S (P = .71; Table 1). Median overall follow‐up was 99 days (range, 4‐463 days). Eight dogs were deceased by the end of the study (26%), 3 of which were in treatment group P and 5 in group S (P = .28). Median survival was 85 days in group P and 166 days in group S (Figure 7), which was not significantly different (P = .35; Table 1).
Figure 7.
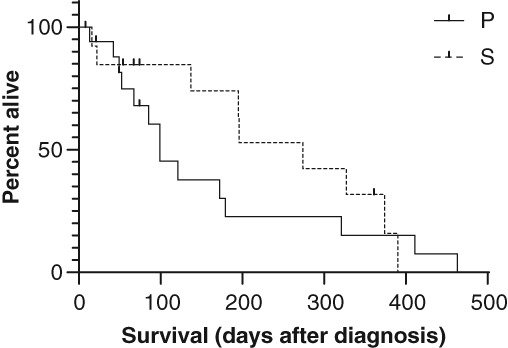
Kaplan‐Meier survival plot of dogs with protein‐losing enteropathy (PLE) treated with prednisolone alone (group P) and treated with prednisolone and a second‐line immunosuppressant (group S). Vertical lines indicate censored cases
4. DISCUSSION
Results of this study challenge the practice of immunosuppressive treatment in addition to administration of glucocorticoids in dogs with PLE,8 even though the judgment of steroid responsiveness was not consistently or objectively defined because of the retrospective nature of this study. It also challenges the use of commonly determined clinicopathological values to predict outcome in these dogs. Especially severity of hypoalbuminemia (neither at initial presentation to the referral center or the lowest recorded value) and the presence of hypocobalaminemia did not correlate with time to reach an “acceptable” serum albumin (which was chosen to be >20 g/L for the purpose of this study) or overall outcome, even though these values are reported to be negative prognostic indicators.1, 2, 3, 8, 9, 10, 12, 13 Based on the current findings, it might be justified to attempt treatment with prednisolone first and to assess response to treatment before adding a SLI, instead of the commonly used and advocated “step‐down approach” of immunosuppressive treatment (usually glucocorticoids combined with cyclosporine, azathioprine, chlorambucil, mycophelonate mofetil, or others) with the aim to slowly withdraw treatment once an acceptable response or “remission” is achieved.
Ultrasonographic findings did not correlate with the severity of hypoproteinemia or severity of disease, and hence were not associated with treatment requirements or outcome in this study. Mucosal abnormalities previously associated with PLE were present in 47% of cases, even though hyperechoic speckles and striations are reported to have a sensitivity of 75% and specificity of 96% for PLE, repectively.17 However, reevaluation of ultrasound findings with a standardized scoring system as used in other studies was not performed. One study has questioned the diagnostic usefulness of abdominal ultrasound in dogs with chronic diarrhea overall.22 However, this study was no specific to PLE and omits the usefulness of being able to exclude certain (often sinister) differential diagnoses in dogs with chronic diarrhea (eg, lymphadenopathy, solitary intestinal masses, chronic obstructions, and so forth).
The inverse correlation of cobalamin and folate has not been reported in dogs with CE or PLE before although serum folate levels rise in 20%‐30% of people with cobalamin deficiency.23, 24 Hypofolatemia is a result of chronic malabsorption, but not specific for CIE or PLE. Equally, normofolatemia does not exclude a diagnosis of CIE or PLE.11 Medical consequences of folate deficiency are difficult to predict, as neither serum folate concentrations nor serum cobalamin concentrations were associated with treatment or outcome in the population of dogs examined here. It is equally difficult to extrapolate the clinical relevance of serum folate concentrations above the reference range (which was only present in 4 out of the 31 dogs). For example, among people with cobalamin deficiency, patients with neurological defects have higher mean concentrations of serum folate,24 but neuropathies are not frequently reported in dogs with acquired cobalamin deficiency. In addition, there are limited to no similarities between hematological abnormalities often observed with cobalamin deficiency in people and dogs.25 Hyperfolatemia or “falsely normal” serum folate values in dogs have traditionally been attributed to small intestinal dysbiosis with increased folate production by some members of the intestinal microbiota.11, 26, 27 However, there are no investigations into this specifically in dogs with PLE.
To the authors' knowledge, the correlation between WSAVA histology scores of duodenal biopsies and endoscopic scores of the duodenum (but not between other histology and endoscopy scores) has not been reported previously for dogs with PLE. This might help clinicians to more confidently estimate severity of disease during endoscopy. Severe endoscopic duodenal scores were associated with negative outcome in a previous study,8 and with CIBDAI scores in another. The fact that this was not true for the ileum might be because of the small number of dogs in which ileal biopsies were performed. Also, standardized scoring of both endoscopy and histology findings specifically for the ileum is not included in the WSAVA guidelines,20 even though the scoring system used for duodenal biopsies has been previously validated for ileal lesions.28, 29 This study highlights the need for a more comprehensive histological assessment of the ileum.29
This study has the typical limitations of any retrospective study, in that additional treatments and diets were not standardized, which could haven skewed outcome measures. Clinical signs were not objectively assessed using, for example, the CIBDAI or CCECAI scoring systems7, 8; there was no exact definition of what constituted an acceptable partial or complete response to treatment, and revisits and follow‐up times were not standardized. It is possible that the decision of adding an SLI in individual dogs was also taken because of the presence of unacceptable glucocorticoid adverse effects despite clinical or biochemical improvement. It was up to the respective clinicians which SLI was chosen, even though in the all but 1 case this was cyclosporine. We can also not fully exclude that dogs with undetected neoplasia (especially small cell lymphoma) were erroneously included in this study, as immunohistochemistry was only performed in cases in which initial histology assessment could not clearly differentiate inflammation from neoplasia.
This study did not investigate the effect of diet (alone or in combination with other treatments) on the progression or outcome of PLE in dogs, and all dogs were fed a hypoallergenic/hydrolyzed diet rather than a low fat diet. This could have influenced overall outcome, as good response to low‐fat diets alone has been reported.30, 31
It is worth considering if cyclosporine is an adequate SLI in dogs with PLE and whether it is an appropriate first choice once steroid treatment alone (or diet) has been deemed insufficient. There are no studies comparing the use of cyclosporine with another SLI in dogs with PLE. Chlorambucil produced a better response to treatment and outcome compared with azathioprine, but has not been directly compared to cyclosporine.1 Depending on the SLI chosen, the decision to omit SLI treatment can be an economic advantage for the client. In addition, most drugs used as SLI can cause adverse effects, the majority of which associated with the GI tract. Not only could this lead to decreased compliance with drug administration or the inability to administer oral drugs, it could also interfere with monitoring clinical improvement and cause additional concern to dog owners.
In conclusion, this study should encourage veterinarians to consider treating PLE with glucocorticoids alone, especially if dietary management failed and financial concerns are present.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplementary figure S1: Algorithm used in the authors' institution for diagnostic workup and sequential treatment of dogs with protein‐losing enteropathy.
Supplementary table S1: Summary of main findings from signalement, biochemistry, ultrasound and outcome in 31 dogs treated for PLE. HyperS = hyperechoic stripes and speckles, FF = free fluid, LN = lymphadenomegaly, SI = small intestine, hypomot = subjective hypomotility or ileus, thickn = thickened/ thickening, FB = non‐obstructive foreign body. “Enteritis” used to describe fluid filled, corrugated small intestinal segments, “colitis” used to describe fluid‐filled corrugated colon. N/A = non‐applicable (dog's serum albumin never reached >20 g/L), D = deceased, A = alive at last follow‐up.
Supplementary table S2: Endoscopic (Endo)13 and histology14 scores based on WSAVA guidelines for 31 dogs with PLE.
Supplementary table S3: Summary of histopathological abnormalities of 31 dogs with PLE. LP = lymphoplasmacytic, EO = eosinophilic, N = neutrophilic
Supplementary table S4: P‐values for comparison of selected data from dogs with PLE, grouped either by treatment (prednisolone alone vs prednisolone and a second‐line immunosuppressant) or by survival status. a = significant difference observed. $ = based on Mann‐Whitney U test, † = based on student t‐test (equal variances not assumed), * = based on 1‐way ANOVA with post‐hoc LSD. n/a = not applicable. Serum cobalamin values were defined as normal when >250 ng/L, mildly decreased when between 150‐250 ng/mL and severely decreased when <150 ng/mL.
ACKNOWLEDGMENTS
The authors thank all clinicians and nurses working at the Hospital for Small Animals in Edinburgh, Susan Campbell, Nicole Pitulia, and Mazdak Salavati, who assisted with statistics. Parts of this paper were presented as an oral abstract at the ECVIM congress 2018 in Rotterdam, the Netherlands.
Salavati Schmitz S, Gow A, Bommer N, Morrison L, Mellanby R. Diagnostic features, treatment, and outcome of dogs with inflammatory protein‐losing enteropathy. J Vet Intern Med. 2019;33:2005–2013. 10.1111/jvim.15571
REFERENCES
- 1. Dandrieux JRS, Noble PM, Scase TJ, Cripps PJ, German AJ. Comparison of a chlorambucil‐prednisolone combination with an azathioprine‐prednisolone combination for treatment of chronic enteropathy with concurrent protein‐losing enteropathy in dogs: 27 cases (2007–2010). J Am Vet Med Assoc. 2013;242(12):1705‐1714. [DOI] [PubMed] [Google Scholar]
- 2. Peterson PB, Willard MD. Protein‐losing enteropathies. Vet Clin North Am Small Anim Pract. 2003;33(5):1061‐1082. [DOI] [PubMed] [Google Scholar]
- 3. Dossin O, Lavoué R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract. 2011;41(2):399‐418. [DOI] [PubMed] [Google Scholar]
- 4. Flesjå K, Yri T. Protein‐losing enteropathy in the Lundehund. J Small Anim Pract. 1977;18(1):11‐23. [DOI] [PubMed] [Google Scholar]
- 5. Melville‐Walker SW, Smith KC, Elwood CM. Protein‐losing enteropathy in a soft‐coated wheaten terrier in the United Kingdom. Vet Rec. 2004;154(14):440‐441. [DOI] [PubMed] [Google Scholar]
- 6. Littman MP, Dambach DM, Vaden SL, Giger U. Familial protein‐losing enteropathy and protein‐losing nephropathy in soft coated wheaten terriers: 222 cases (1983‐1997). J Vet Intern Med. 2000;14(1):68‐80. [DOI] [PubMed] [Google Scholar]
- 7. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17(3):291‐297. [DOI] [PubMed] [Google Scholar]
- 8. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21(4):700‐708. [DOI] [PubMed] [Google Scholar]
- 9. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995‐2002). J Small Anim Pract. 2004;45(7):336‐342. [DOI] [PubMed] [Google Scholar]
- 10. Simmerson SM, Armstrong PJ, Wünschmann A, Jessen CR, Crews LJ, Washabau RJ. Clinical features, intestinal histopathology, and outcome in protein‐losing enteropathy in Yorkshire Terrier dogs. J Vet Intern Med. 2014;28(2):331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Equilino M, Théodoloz V, Gorgas D, et al. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein‐losing enteropathy. J Am Vet Med Assoc. 2015;246(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 12. Gianella P, Lotti U, Bellino C, et al. Clinicopathologic and prognostic factors in short‐ and long‐term surviving dogs with protein‐losing enteropathy. Schweiz Arch Tierheilkd. 2017;159(3):163‐169. [DOI] [PubMed] [Google Scholar]
- 13. Nakashima K, Hiyoshi S, Ohno K, et al. Prognostic factors in dogs with protein‐losing enteropathy. Vet J. 2015;205(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 14. Allenspach K, Rizzo J, Jergens AE, Chang YM. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet Res. 2017;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craven MD, Washabau RJ. Comparative pathophysiology and management of protein‐losing enteropathy. J Vet Intern Med. 2019;33(2):383‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutherland‐Smith J, Penninck DG, Keating JH, Webster CRL. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilation. Vet Radiol Ultrasound. 2007;48(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 17. Gaschen L, Kircher P, Stüssi A, et al. Comparison of ultrasonographic findings with clinical activity index (CIBDAI) and diagnosis in dogs with chronic enteropathies. Vet Radiol Ultrasound. 2008;49(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 18. Yuki M, Sugimoto N, Takahashi K, et al. A case of protein‐losing enteropathy treated with methotrexate in a dog. J Vet Med Sci. 2006;68(4):397‐399. [DOI] [PubMed] [Google Scholar]
- 19. Slovak JE, Wang C, Sun Y, et al. Development and validation of an endoscopic activity score for canine inflammatory bowel disease. Vet J. 2015;203(3):290‐295. [DOI] [PubMed] [Google Scholar]
- 20. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24(1):10‐26. [DOI] [PubMed] [Google Scholar]
- 21. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008. Feb;138(Suppl 1):S1‐S43. [DOI] [PubMed] [Google Scholar]
- 22. Leib MS, Larson MM, Grant DC, et al. Diagnostic utility of abdominal ultrasonography in dogs with chronic diarrhea. J Vet Intern Med. 2012;26(6):1288‐1294. [DOI] [PubMed] [Google Scholar]
- 23. Shane B. Folate and vitamin B12 metabolism: overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food Nutr Bull. 2008;29(2 Suppl):S5‐S16. discussion S17‐9. [DOI] [PubMed] [Google Scholar]
- 24. Carmel R, Melnyk S, James SJ. Cobalamin deficiency with and without neurologic abnormalities: differences in homocysteine and methionine metabolism: presented in preliminary form at the 44th Annual Meeting of the American Society of Hematology, Philadelphia, PA, December 7, 2002. Blood. 2003;101(8):3302‐3308. [DOI] [PubMed] [Google Scholar]
- 25. Stanley E, Appleman E, Schlag A, Siegel A. Relationship between cobalamin and folate deficiencies and anemia in dogs. J Vet Intern Med. 2019;33(1):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruaux CG, Steiner JM, Williams DA. Protein‐losing enteropathy in dogs is associated with decreased fecal proteolytic activity. Vet Clin Pathol. 2004;33(1):20‐22. [DOI] [PubMed] [Google Scholar]
- 27. Ruaux CG. Cobalamin in companion animals: diagnostic marker, deficiency states and therapeutic implications. Vet J. 2013;196(2):145‐152. [DOI] [PubMed] [Google Scholar]
- 28. Allenspach KA, Mochel JP, Du Y, et al. Correlating gastrointestinal histopathologic changes to clinical disease activity in dogs with idiopathic inflammatory bowel disease. Vet Pathol. 2019;56(3):435‐443. [DOI] [PubMed] [Google Scholar]
- 29. Procoli F, Mõtsküla PF, Keyte SV, Priestnall S, Allenspach K. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med. 2013. Mar;27(2):268‐274. [DOI] [PubMed] [Google Scholar]
- 30. Okanishi H, Yoshioka R, Kagawa Y, Watari T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J Vet Intern Med. 2014;28(3):809‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rudinsky AJ, Howard JP, Bishop MA, Sherding RG, Parker VJ, Gilor C. Dietary management of presumptive protein‐losing enteropathy in Yorkshire Terriers. J Small Anim Pract. 2017;58(2):103‐108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure S1: Algorithm used in the authors' institution for diagnostic workup and sequential treatment of dogs with protein‐losing enteropathy.
Supplementary table S1: Summary of main findings from signalement, biochemistry, ultrasound and outcome in 31 dogs treated for PLE. HyperS = hyperechoic stripes and speckles, FF = free fluid, LN = lymphadenomegaly, SI = small intestine, hypomot = subjective hypomotility or ileus, thickn = thickened/ thickening, FB = non‐obstructive foreign body. “Enteritis” used to describe fluid filled, corrugated small intestinal segments, “colitis” used to describe fluid‐filled corrugated colon. N/A = non‐applicable (dog's serum albumin never reached >20 g/L), D = deceased, A = alive at last follow‐up.
Supplementary table S2: Endoscopic (Endo)13 and histology14 scores based on WSAVA guidelines for 31 dogs with PLE.
Supplementary table S3: Summary of histopathological abnormalities of 31 dogs with PLE. LP = lymphoplasmacytic, EO = eosinophilic, N = neutrophilic
Supplementary table S4: P‐values for comparison of selected data from dogs with PLE, grouped either by treatment (prednisolone alone vs prednisolone and a second‐line immunosuppressant) or by survival status. a = significant difference observed. $ = based on Mann‐Whitney U test, † = based on student t‐test (equal variances not assumed), * = based on 1‐way ANOVA with post‐hoc LSD. n/a = not applicable. Serum cobalamin values were defined as normal when >250 ng/L, mildly decreased when between 150‐250 ng/mL and severely decreased when <150 ng/mL.


