Abstract
Background
Information regarding clinical signs, assessment, treatment, and outcome in cats with hiatal hernia (HH) is limited.
Objectives
To characterize the clinical presentation of HH and medical and surgical outcomes in a cohort of affected cats.
Animals
Thirty‐one client‐owned cats with HH.
Methods
Medical records of cats with HH were retrospectively reviewed for signalment, history, results of diagnostic tests, details of surgical and medical treatments, complications, and outcome. Long‐term follow‐up data were obtained by telephone communication. Relationships between clinical variables and outcome were evaluated by regression analysis.
Results
Type I HH was present in 85.7% (24/28) of cats, and 64.5% (20/31) were >3 years of age at diagnosis. Twenty‐one of 31 (67.7%) cats underwent surgical repair including phrenoplasty, esophagopexy, and left‐sided gastropexy, and 10 of 31 cats were treated medically without surgery. Concurrent illness was common, and 77.4% cats had comorbidities. All cats survived to discharge, and median time to death or follow‐up was 959 days (range, 3‐4015 days). Cats treated medically survived longer than cats treated surgically, with median time to death or follow‐up of 2559 and 771 days, respectively.
Conclusions and Clinical Importance
Type I HH is the most common type of HH in cats. A congenital etiology is possible, but many cats with HH were >3 years of age at diagnosis and suffered from comorbidities, including upper airway obstruction. Case selection and the presence of comorbidities likely influenced the outcome. Cats with HH may not be diagnosed until disease is advanced or concurrent illness draws attention to clinical signs.
Keywords: esophagopexy, gastroesophageal junction, gastropexy, phrenoplasty, reflux esophagitis
Abbreviations
- CT
computed tomography
- GEJ
gastroesophageal junction
- GER
gastroesophageal reflux
- H2RA
histamine‐2 receptor antagonist
- HH
hiatal hernia
- PPI
proton pump inhibitor
1. INTRODUCTION
Hiatal hernia (HH) in dogs and cats is defined as protrusion of abdominal contents, most often the cardia and fundus of the stomach, through the esophageal hiatus into the caudal mediastinum.1, 2, 3, 4, 5, 6, 7, 8, 9 In people, 4 types of HH are described in a classification scheme that also is applied to dogs and cats.1, 2, 3, 4, 5, 6, 7, 8, 9 Although limited data are available for cats, type I sliding HH is the most common type of HH in dogs, and simple and complicated type II paraesophageal hernias also are described relatively commonly.1, 2, 3, 4, 5, 7, 8 Hiatal hernia reportedly is often congenital in small animals, and affected dogs commonly display clinical signs before 1 year of age.1, 3, 5, 7, 9 Concurrent respiratory conditions may predispose to clinical signs, and brachycephalic syndrome, other causes of upper airway obstruction, and lower airway diseases have been reported in dogs with HH.4, 7, 9 Inspiratory dyspnea causes more subatmospheric intrapleural and intraesophageal pressures that may facilitate stretching of the phrenoesophageal ligament and cranial displacement of the stomach through the esophageal hiatus.1, 4, 7, 9, 10, 11
Clinical signs in dogs with HH include regurgitation, vomiting, hypersalivation, dysphagia, anorexia, respiratory distress, and weight loss.1, 5, 9, 12, 13, 14 Recommendations for treatment are debated, and the choice between medical and surgical management often is dictated by clinician preference and the severity of clinical signs.13, 15 Because information regarding HH in cats is limited to that derived from single case reports or small case series, little is known regarding clinical signs, superiority of any assessment tool or treatment option, and outcome in cats with HH.2, 8, 10, 12, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26
Our objective was to characterize the clinical presentation of HH in cats, including historical and physical examination findings and results of diagnostic imaging, and medical and surgical outcomes.
2. MATERIALS AND METHODS
2.1. Case selection criteria
Medical records of cats treated for HH at 6 tertiary care veterinary teaching hospitals between January 1, 1995, and December 31, 2016, were retrospectively reviewed. Cats were included in the study if gastrointestinal signs were present and HH was diagnosed by survey radiography, contrast esophagography, computed tomography (CT), esophagoscopy, or videofluoroscopic swallowing studies. Cats diagnosed with HH after known trauma were excluded.
2.2. Medical records review
History; signalment; body weight at presentation; results of physical examination and diagnostic imaging; surgical technique, operative findings, and complications; medical treatments; histopathologic diagnoses; survival to discharge and overall survival; necropsy results; and outcome were recorded. Respiratory rate <40 breaths per minute was considered normal and >40 bpm was considered abnormal. Respiratory effort was coded as normal or as abnormal characterized by short, shallow breathing; increased abdominal effort on inspiration; paradoxical abdominal motion; or open‐mouth breathing. Outcome was characterized by the occurrence and type of complications after treatment, resolution of clinical signs, and median number of days to death or follow‐up. Follow‐up was obtained by telephone communication with the client using a standardized questionnaire (see Supporting Information). In the questionnaire, owners were asked to rate the severity of their cats' clinical signs on a scale of 1 to 5, with a score of 1 being mild and 5 being severe. Surgical treatment was defined as an abdominal approach to the esophageal hiatus, reduction of hernia contents, and ≥1 of the following procedures: phrenoplasty (esophageal hiatus plication), esophagopexy, gastropexy, or fundoplication as previously described.1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 26 Medical management was defined as treatment with histamine‐2 receptor antagonists (H2RAs), proton pump inhibitors (PPIs), gastrointestinal prokinetic drugs (cisapride or metoclopramide), cytoprotective medications (sucralfate), or dietary modification including frequent, small‐volume feeding of a fat‐restricted diet, feeding from an elevated position, or administering food of a more liquid consistency to enhance gastric emptying. Swallowing studies evaluated all phases of swallowing from oropharyngeal to esophageal to gastroesophageal. The following were considered abnormal findings: gastric rugae cranial to the diaphragm indicative of HH, esophageal stricture, gastroesophageal reflux (GER), focal or diffuse esophageal dysmotility represented by the absence of peristaltic waves or inappropriate timing of peristaltic waves relative to bolus presentation, barium aspiration, irregular esophageal or gastric mucosa, or esophageal perforation.
2.3. Statistical analysis
Descriptive statistics, related plots, and preliminary statistical analyses were obtained using menu‐driven Systat 13.1 (Systat Inc, San Jose, California). Mean, median, standard deviation (SD), and n were calculated for all variables for all cats, and P < .05 was considered significant. These statistical variables also were calculated for subsets having survival times: medical treatment with abdominal surgery, medical treatment without abdominal surgery, and both groups combined for age, weight, and days to death or follow‐up. Contingency tables were used to compare the groups managed by medical treatment with and without abdominal surgery for differences related to pathophysiology, HH type, presence or absence of complications or upper airway obstruction, and resolution of clinical signs. Variables with >10 missing values were deleted. Differences between the abdominal surgery subsets were determined using repeated‐measures analysis (SAS 9.4 PROC Mixed) and confirmed graphically. Survival to discharge was considered uncensored. Kaplan‐Meier survival analysis was carried out using PROC LIFETEST (SAS).
3. RESULTS
Thirty‐one cats met the inclusion criteria. Seventeen (54.8%) cats were male (15 castrated and 2 sexually intact) and 16 (51.6%) were female (2 spayed and 14 sexually intact). Median age at presentation was 5.7 years (mean, 5.7 years; range, 0.2‐18.8 years). Eight (25.8%) cats were <1 year of age, and 20 (64.5%) cats were >3 years of age at the time of diagnosis. Median age of cats treated medically without surgery was significantly higher than that of cats treated surgically (7.9 years [range, 0.83‐18.7 years] versus 3 years [range, 0.8‐13.1 years], respectively [P = .02]). Median weight of all cats was 3.2 kg (range, 0.5‐6.9 kg). Median weight of cats treated medically without surgery was significantly higher than that of cats treated surgically (4.7 kg [range, 2.8‐6.8 kg] versus 2.4 kg [range, 0.5‐6.9 kg], respectively [P = .005]).
All 31 (100%) cats survived to discharge a median of 2.8 days (range, 0‐15 days) after presentation. Postdischarge survival data were available for 20 cats, of which 4 of 20 (20%) cats were treated medically without surgery and 16 of 20 (80%) were treated surgically. Table 1 summarizes median days to death or follow‐up in cats treated medically with or without surgery. Median days to death or follow‐up for cats treated medically without surgery were 2559 days (range, 1095‐4015 days), and median days to death or follow‐up for cats treated surgically were 771 days (range, 3‐3599 days). Median days to death or follow‐up for all cats were 959 days (range, 3‐4015 days).
Table 1.
Survival data for cats with hiatal hernia treated medically with or without abdominal surgery
| Quartile estimates | |||
|---|---|---|---|
| Point | 95% Confidence interval | ||
| Percent | Estimate | Lower | Upper |
| All cats = 31 | |||
| 75 | 1858.5 | 1095 | 3963 |
| 50 | 959 | 31 | 1825 |
| 25 | 169 | 3 | 813 |
| Cats treated medically without surgery = 4 | |||
| 75 | 3989 | 1095 | 4015 |
| 50 | 2559 | 1095 | 4015 |
| 25 | 1125 | 1095 | 3963 |
| Cats treated surgically = 16 | |||
| 75 | 1539.5 | 730 | 3188 |
| 50 | 771.5 | 29 | 1254 |
| 25 | 30 | 3 | 730 |
Confidence limits for medians are nonparametric estimates and do not assume normality of data.
Median duration of clinical signs was 170 days (range, 7‐3650 days). Gastrointestinal signs were present in 30 of 31 (96.8%) cats, and vomiting, weight loss, and anorexia were most common, reported in 13 (43.3%), 9 (30%), and 7 (23.3%) of 30 cats, respectively. Regurgitation was reported in 3 of 30 (10%) cats, and tenesmus was reported in 2 of 30 (6.7%) cats. Hiding and gagging were reported rarely. Type and duration of clinical signs did not differ significantly between cats treated medically with or without surgery.
On physical examination, 9 (29%) cats had evidence of upper or lower airway disease or obstruction. Of these, 3 (33.3%) cats were brachycephalic breeds including Persian (n = 2) and Himalayan (n = 1) breeds. Three (33.3%) cats had chronic upper respiratory infection with marked nasal congestion, 1 cat had a nasopharyngeal polyp, and 2 (22.2%) cats had an unknown respiratory disease causing increased abdominal effort; short, shallow breathing; wheezing; stertor; or occasional open‐mouth breathing. Respiratory rate and effort were characterized in 30 of 31 (96.8%) cats, and 16 of 30 (53.3%) cats were tachypneic (>40 bpm). Five (16.7%) cats had increased abdominal effort of breathing. The number of days to death or follow‐up between cats breathing normally or with abnormal rate (P = .74) or effort (P = .98) was not significantly different. Eleven (35.5%) cats had abnormalities detected on abdominal palpation including pain, gas‐distended bowel loops, and a firm caudoventral abdominal mass.
All 31 cats underwent thoracic radiography. A diagnosis of HH was made on survey radiography in 12 of 31 (38.7%) cats (Figure 1). Hiatal hernia subsequently was confirmed with additional imaging in all 12 cats. Type of HH was characterized in 28 of 31 (90.3%) cats: type I in 24 (85.7%) cats, type II in 1 (3.6%) cat, type III in 1 (3.6%) cat, and type IV in 2 (7.2%) cats. Cats with type II and type III HH underwent emergency surgery including phrenoplasty, esophagopexy, and left‐sided incisional gastropexy after appropriate resuscitation and stabilization. Owners of 2 cats with type IV HH did not elect surgery because of the presence of clinically relevant comorbidities. One cat was diagnosed with aspiration pneumonia preoperatively on thoracic radiography, and this cat also had pneumonia postoperatively. Three (9.7%) additional cats had radiographic evidence of aspiration pneumonia postoperatively. Mild esophageal dilatation with gas or fluid consistent with reflux and esophagitis was noted in 6 (19.4%) cats, predominantly in the distal esophagus in 4 cats and throughout the esophagus in 2 cats. Nineteen (61.3%) cats underwent contrast esophagography and upper gastrointestinal radiographic studies using barium or iohexol, and 17 of 19 cats (89.5%) cats were diagnosed with HH. Caudal pectus excavatum, pleural effusion, cardiomegaly, and a single pulmonary nodule also were seen in 1 cat each. Thirteen (41.9%) cats underwent videofluoroscopic swallowing studies using barium paste with food or liquid barium. Findings included rugal folds or the gastroesophageal junction (GEJ) positioned cranial to the diaphragm in 13 of 13 (100%) cats, intermittent herniation during retching in 1 of 13 (7.7%), esophageal dilatation in 4 of 13 (30.8%), GER, hypomotility in 3 of 13 (23.1%), and dysmotility in 2 of 13 (15.4%) cats. Six (18.2%) cats underwent upper gastrointestinal endoscopy. Abnormalities included edema and erythema of the esophageal mucosa consistent with esophagitis and reflux of gastric fluid through the GEJ in 4 of 6 (66.7%) cats. Fifteen (48.4%) cats underwent abdominal ultrasonography. Abnormalities included type I HH in 8 of 15 (53.3%) cats, and small, irregular, or mineralized kidneys (n = 6), renal or cystic calculi or pyelectasia (n = 3), thickened small intestines (n = 2), abdominal lymphadenopathy (n = 2), bile duct distension (n = 2), small intestinal mass (n = 1), or cholelithiasis (n = 1). Thoracic CT identified HH in 3 of 3 (100%) cats and a dilated esophagus, small cardiac silhouette consistent with presumed hypovolemia, and chronic bronchial disease in 1 cat each.
Figure 1.
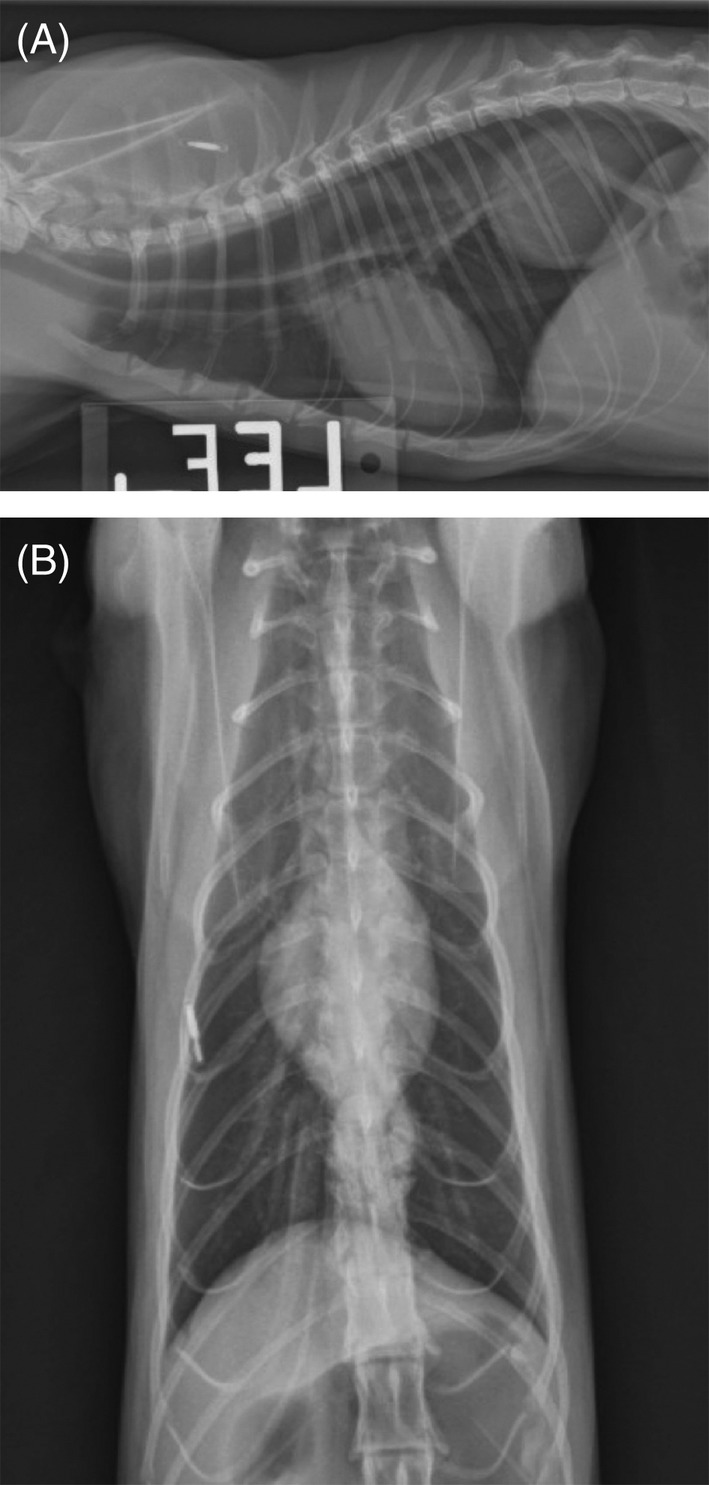
A, Left lateral thoracic radiograph of a cat with Type I hiatal hernia. Note the soft tissue opacity in the caudodorsal thorax. B, Ventrodorsal thoracic radiograph of a cat with Type I hiatal hernia. Note the soft tissue opacity in the caudodorsal thorax on the lateral view (A) and the inability to discern the hernia on the VD view (B), consistent with intermittent herniation that changes with patient positioning
Before referral for tertiary care, 9 of 31 (29%) cats received no medical treatment, 11 (35.5%) cats received >1 medication, and 11 (35.5%) cats received ≥2 medications including H2RAs (n = 9), prednisolone (n = 5), sucralfate (n = 5), metoclopramide (n = 4), antibiotics (n = 4), cisapride (n = 3), PPIs (n = 2), cyproheptadine (n = 2), and lactulose and polyethylene glycol (n = 1). On presentation to 1 of 6 veterinary teaching hospitals contributing to this multi‐institutional study, all 31 cats received ≥1 medications and 29 (95.5%) cats received ≥2 medications including H2RAs (n = 28), PPIs (n = 16), metoclopramide (n = 12), sucralfate (n = 5), cisapride (n = 4), and antibiotics (n = 4).
After diagnosis of HH, 10 of 31(23.3%) cats were treated medically without surgery and 21 of 31 (67.7%) cats were treated surgically. Eight of 10 (80%) cats treated medically without surgery and 16 of 21 (76.2%) cats treated surgically had concurrent abnormalities with etiology unrelated to HH. Abnormalities related to the upper airway included suppurative rhinitis (n = 4); stenotic nares (n = 3); pharyngitis related to gingivitis, stomatitis, and odontoclastic resorptive lesions (n = 2); and nasopharyngeal polyp (n = 1). Other clinically relevant comorbidities included hyperthyroidism (n = 2); thyroid adenoma (n = 1); thyroid carcinoma (n = 1); pulmonary adenocarcinoma (n = 1); cholestasis, cholelithiasis, cholangitis, or some combination of these (n = 2); gastric and small intestinal lymphoplasmacytic enteritis (n = 3); small intestinal or colonic adenocarcinoma (n = 3); chronic renal disease or pyelectasia (n = 4); renal or cystic calculi (n = 2); diabetes mellitus (n = 1); pancreatitis (n = 1); exocrine pancreatic insufficiency (n = 1); pancreatic carcinoma (n = 1); aortic thromboembolism (n = 1); and metastatic hemangiosarcoma (n = 1).
The most common surgery involved a combination of 3 procedures in 11 of 21 (52.4%) cats: phrenoplasty, esophagopexy, and left‐sided incisional gastropexy. Other procedures included left‐sided tube or belt‐loop gastropexy, right‐sided gastropexy, and modified Nissen fundoplication. There was no difference in survival to discharge, days to death or follow‐up, or incidence of complications attributable to the type of surgical procedure. Eleven of 21 (52.4%) cats treated surgically also had additional abdominal procedures performed at the time of hernia repair, including small intestinal biopsy (n = 4); gastric, mesenteric, or colonic lymph node excision (n = 3); liver biopsy (n = 2); small intestinal resection and anastomosis (n = 1); and colonic resection and anastomosis (n = 1). All 21 cats undergoing abdominal surgery to correct HH survived surgery and 5 of 21 (23.8%) cats experienced 7 complications. Acute complications included accidental entry into the mediastinum during surgery (n = 1), postoperative aspiration pneumonia (n = 3), postoperative hypoglycemia (n = 1), and leakage of the tube gastropexy site with septic peritonitis (n = 1). Chronic complications included postoperative esophageal stricture 19 days after presentation (n = 1). No complications were reported for cats treated medically without surgery.
Owners of 20 of 31 (64.5%) cats were available for questioning by telephone a median of 3162 days after presentation (range, 813‐7367 days). Twelve of 20 (60%) of the cats died a median of 372 days (range, 3‐4015 days) after presentation: 3 treated medically without surgery and 9 treated surgically. Owners of 1 of 3 (33.3%) cats treated medically and 5 of 9 (55.6%) cats treated surgically attributed death to persistent clinical signs or treatment of HH. Eight of 20 (42%) cats were alive at the time of follow‐up. One cat alive at the time of follow‐up had been treated medically without surgery for HH and was alive 3963 days after presentation. Seven of 8 (87.5%) cats had been treated surgically for HH and were alive a median of 1573 days (range, 813‐3599 days) after presentation. One cat treated surgically had mild persistent gastrointestinal signs and had been diagnosed with concurrent lymphoplasmacytic enteritis based on small intestinal biopsy specimens obtained at the time of HH repair. This cat vomited a mean of 0.5 times a week and required daily medical treatment for management of gastrointestinal signs 3599 days after presentation. Using a 5‐point grading scale ranging from mild to severe, the owner of this cat reported the cat to have very mild clinical signs. Owners of 1 cat treated medically and 6 of 7 cats treated surgically reported complete resolution and no recurrence of signs a median of 1254 days after presentation.
4. DISCUSSION
For 31 cats undergoing medical treatment for HH with or without surgery, survival to discharge was excellent and the median survival time or time to follow‐up for all cats was 959 days. Of 20 cats for which follow‐up information was available, median days to death or follow‐up was longer for 4 cats treated medically than for 16 cats treated surgically. Complications related to treatment were uncommon. Cats in our study were older than those reported in previous studies, and clinically relevant concurrent abnormalities were common and may have contributed to outcome.
Median age and weight were significantly lower for cats treated surgically than for cats treated medically. Although some cats treated surgically were young and not fully grown, older cats treated surgically also had low body weights, and the low median weight of all cats was likely reflective of the predominance of gastrointestinal clinical signs associated with HH.
The most common type of HH diagnosed in this cohort was type I sliding HH; a predominance of type I HH also is observed in people and dogs.1, 3, 5, 7, 9 Hiatal hernia often is congenital in origin, and over 75% of reported dogs and 67% of previously reported cats with HH were <1 year of age at diagnosis, although fewer than 30 cats with HH were reported in the veterinary literature before our study.1, 2, 5, 6, 8, 9, 12, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Only 8 of 31 (25.8%) cats in our study were <1 year of age, and the majority of cats in our study (20/31 [64.5%]) were >3 years of age at diagnosis. The etiology of HH in cats therefore may have a degenerative component because the amount of elastic tissue in the phrenoesophageal membrane can decrease with age, increasing its laxity.2, 6, 7, 8, 28 Alternatively, cats with congenital HH may not be diagnosed until the disease is advanced or until concurrent abnormalities exacerbate the HH with subsequent development of clinical signs.
Brachycephalic dogs with HH exhibit frequent vomiting, regurgitation, and hypersalivation with esophagitis and diffuse gastric and duodenal inflammation.3, 32, 33 Brachycephalic syndrome has been implicated as a cause for type I HH in dogs because increased inspiratory effort causes more subatomspheric intraesophageal and intrapleural pressures, stretching of the phrenoesophageal ligament, paradoxical cranial movement of the stomach, and HH.1, 9, 17, 32, 33 Our findings suggest that diseases causing airway obstruction also may be important in the development of HH in cats. Airway obstruction was diagnosed concurrently with HH in 9 of 31 (29%) cats in our study, including brachycephalic cats and cats with chronic rhinitis. Hiatal hernia has been described previously in a cat associated with chronic rhinitis causing nasopharyngeal stenosis and upper airway obstruction.9, 17 Further prospective studies are needed to determine the prevalence of HH and GER in cats with airway obstruction. Hiatal hernia should be considered as a cause of gastrointestinal signs in any cat with airway disease or obstruction, including brachycephalic cats.
Nearly 80% of cats in our study had clinically relevant comorbidities. Concurrent diseases of cats treated medically without surgery included hyperthyroidism, diabetes mellitus, stomatitis, and mild cholestasis and pancreatitis. Concurrent diseases of cats treated surgically often were severe and included chronic destructive rhinitis with severe congestion, megaesophagus, aspiration pneumonia, pulmonary adenocarcinoma, chronic renal disease, lymphoplasmacytic enteritis, and small intestinal adenocarcinoma. The prevalence of concurrent diseases with etiology distinct from HH is clinically important because prognosis may be affected by severity of comorbidities. Because of incomplete follow‐up, we were unable to determine if cats with comorbidities had a worse prognosis. However, all cats that died or were euthanized within 6 months of treatment for HH had major concurrent diseases.
Survey thoracic radiography was performed in all cats but only resulted in diagnosis of HH in 38.7% cats. False‐negative results are possible for cats with intermittent herniation, and survey thoracic radiography is most useful for diagnosis of aspiration pneumonia, megaesophagus, or fluid in the distal esophagus.1, 2, 3, 6, 7, 12 In the veterinary literature, survey and contrast esophagography failed to identify HH in 10%‐20% of cases, but diagnosis was achieved in nearly all cases by contrast‐enhanced videofluoroscopy.1, 12, 13, 14 Videofluoroscopic esophagography was diagnostic for HH in 100% of the cats in our study in which it was performed, as was thoracic CT.2, 7, 9, 14, 31 Cats with HH may elude diagnosis by any imaging modality, however, because type I herniation is intermittent and varies in severity.
The most common presenting clinical sign was vomiting that occurred in 13 of 31 (41.9%) cats, similar to what is observed in dogs.1, 5, 9, 12, 13, 30 Gastrointestinal signs are attributable to GER and reflux esophagitis. Given the poorly understood pathophysiology of HH, GER, and reflux esophagitis in cats, careful patient consideration is required when recommending medical or surgical treatment for HH. All cats in our study received ≥1 medications for gastrointestinal clinical signs, and 29 of 31 (95.5%) cats received ≥2 medications. Goals of medical treatment are to resolve reflux esophagitis and minimize development of aspiration pneumonia.2, 5, 6, 13 Complications such as aspiration pneumonia and esophageal stricture were rare in our study indicating that medical treatment plays an important role in decreasing morbidity associated with HH, GER, and reflux esophagitis, or that chronic intermittent reflux is rarely associated with stricture formation in cats, independent of medical treatment. Medical treatment for 30 days has been advocated before considering surgery in dogs with HH.7, 12, 13 Delay of definitive correction, however, may lead to worsened reflux esophagitis, esophageal stricture, aspiration pneumonia, mucosal hemorrhage, or strangulation of incarcerated viscera.2, 4, 5, 8, 12, 13, 14, 29, 30 Medical treatments recommended for HH include a combination of acid suppressants, prokinetics, and cytoprotective agents. Acid‐suppressant medications such as H2RAs and PPIs decrease acidity of refluxed material and limit damage to esophageal mucosa.6 A recent study showed that omeprazole q12h provided superior acid suppression in cats compared to famotidine q12h or placebo.34 Prokinetic agents such as metoclopramide and cisapride increase the rate of gastric emptying, and cisapride is superior to metoclopramide for enhancing GEJ tone in the dog.35 Cisapride and metoclopramide also stimulate esophageal peristalsis in the distal third of the feline esophagus where smooth muscle predominates.35, 36 Cisapride is more potent than metoclopramide in treating delayed gastric emptying in small animals and may be preferred for decreasing GER in cats.36 Cytoprotective agents such as sucralfate increase the resistance of the gastric and esophageal mucosa to acid injury.2, 5, 6, 13 Because of low case numbers, we were unable to determine the superiority of any medication in cats with HH. However, we support the use of PPIs, cisapride, and sucralfate for medical treatment of HH and associated reflux esophagitis in cats.6, 34, 36
Surgical management of HH in dogs and cats includes a combination of procedures, specifically phrenoplasty, esophagopexy, and gastropexy, resulting in reported success rates of 67%‐100%.3, 9 Combination procedures increase pressure at the GEJ and reinforce the anti‐reflux barrier, and gastropexy has been recommended in conjunction with reduction of HH to enhance barrier pressure.2, 7, 11, 37 Although only a short‐term effect on barrier pressure has been demonstrated with gastropexy, even transient increases in barrier pressure may provide long‐term benefit by decreasing GER‐ and esophagitis‐associated inflammation, and 4 of 4 (100%) dogs in 1 study showed no signs of HH recurrence 18‐24 months after phrenoplasty, esophagopexy, and left‐sided gastropexy.2, 37 All cats treated surgically in our study had some type of gastropexy performed, either as a solitary procedure or in combination with phrenoplasty or esophagopexy. Small sample size precluded determination of differences in overall survival or incidence of complications attributable to type of surgical procedure.
Our study had some limitations. The multi‐institutional nature of the study contributed to variations in evaluation and treatment, and techniques were not standardized among cases. Data generated by this retrospective study reflected the quality of information available in medical records, and not all records were complete. Direct comparison among cases from different institutions was difficult because of variation in reference intervals, imaging protocols, surgical technique, and treatment plans. Cats with lower body weights and more severe signs may have been more likely to be selected for surgery or have clinically relevant comorbidities. The presence of major comorbidities in many cats may have created a bias toward selection of surgical treatment, because many of these cats had concurrent airway and other diseases and had additional abdominal procedures performed. Because of the low number of cases overall and especially of cases treated medically without surgery, findings must be interpreted with caution because sample sizes were too small to use sample medians as estimates of treatment population medians. Post‐discharge survival data were available only for 20 of 31 (64.5%) cats, and the majority (16/20) of these cats were treated surgically. Follow‐up also was limited, because owners could not always be contacted or could not recall specific details.
Surgery has been recommended in dogs and cats with type I HH when severe esophageal ulceration or strictures are present and when the hernia is large or fixed in position.2, 3, 15, 30 However, given the predominance of clinically relevant comorbidities and lower weights in cats treated surgically, our findings suggest that the choice of medical or surgical treatment should be dictated by severity of herniation, response to medical treatment, and whether or not any clinically relevant concurrent diseases are present. Although HH is infrequent in cats, prognosis for survival to discharge was excellent and overall survival was good for cats treated medically or surgically. The presence of comorbidities may affect overall survival, and cats with HH may not show clinical signs until the disease is advanced or comorbidities precipitate clinical signs. Gastrointestinal signs in any cat with airway disease or obstruction, including brachycephalic cats, should prompt consideration of HH. Persistence of gastrointestinal signs is not uncommon and may be a consequence of ineffective medical or surgical management or concurrent gastrointestinal or other disease necessitating ongoing medical management. Complete resolution of signs is possible in a subset of cats, and further studies are needed to identify prognostic factors.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supporting Information
Figure S1 Supporting Information
ACKNOWLEDGMENTS
Abstract presented at the American College of Veterinary Surgeons Surgical Summit, Indianapolis, Oct 12‐15, 2017. The authors thank Dr. Jessica Hanlon, DVM, for assistance with data acquisition.
Phillips H, Corrie J, Engel DM, et al. Clinical findings, diagnostic test results, and treatment outcome in cats with hiatal hernia: 31 cases (1995‐2018). J Vet Intern Med. 2019;33:1970–1976. 10.1111/jvim.15583
Present address Daniel J. Duffy, Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, 1052 William Moore Drive, Raleigh, NC 27607. Allison R. Kendall, Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, 1052 William Moore Drive, Raleigh, NC 27607. Ilyssa L. Meren, The Animal Neurology and Imaging Center, Animal Medical and Surgical Center, 17477 North 82nd Street, Scottsdale, AZ 85255.
REFERENCES
- 1. Ellison G, Lewis D, Phillips L. Esophageal hiatal hernia in small animals: literature review and a modified surgical technique. J Am Anim Hosp Assoc. 1987;23:391‐399. [Google Scholar]
- 2. Prymak C, Saunders HM, Washabau RJ. Hiatal hernia repair by restoration and stabilization of normal anatomy—an evaluation in four dogs and one cat. Vet Surg. 1989;18:386‐391. [DOI] [PubMed] [Google Scholar]
- 3. Callan MB, Washabau RJ, Saunders HM, Kerr L, Prymak C, Holt D. Congenital esophageal hiatal hernia in the Chinese shar‐pei dog. J Vet Intern Med. 1993;7:210‐215. [DOI] [PubMed] [Google Scholar]
- 4. Sivacolundhu RK, Read RA, Marchevsky AM. Hiatal hernia controversies—a review of pathophysiology and treatment options. Aust Vet J. 2002;80:48‐53. [DOI] [PubMed] [Google Scholar]
- 5. Guiot LP, Lansdowne JL, Rouppert P, Stanley BJ. Hiatal hernia in the dog: a clinical report of four Chinese shar‐peis. J Am Anim Hosp Assoc. 2008;44:335‐341. [DOI] [PubMed] [Google Scholar]
- 6. Kahrilas PJ, Hyon CK, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keeley B, Puggioni A, Pratschke K. Congenital oesophageal hiatal hernia in a pug. Ir Vet J. 2008;61:389‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tong K, Guillou R. Congenital paraesophageal hernia in a cat. J Am Anim Hosp Assoc. 2015;51:252‐255. [DOI] [PubMed] [Google Scholar]
- 9. Mayhew PD, Marks SL, Pollard R, Culp WTN, Kass PH. Prospective evaluation of surgical management of sliding hiatal hernia and gastroesophageal reflux in dogs. Vet Surg. 2017;46:1098‐1109. [DOI] [PubMed] [Google Scholar]
- 10. DeSandre‐Robinson DM, Madden SN, Walker JT. Nasopharyngeal stenosis with concurrent hiatal hernia and megaesophagus in an 8‐year‐old cat. J Feline Med Surg. 2011;13:454‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratschke KM, Fitzpatrick E, Campion D, McAllister H, Bellenger CR. Topography of the gastro‐oesophageal junction in the dog revisited: possible clinical implications. Res Vet Sci. 2004;76:171‐177. [DOI] [PubMed] [Google Scholar]
- 12. Bright RM, Sackman JE, DeNovo C, Toal C. Hiatal hernia in the dog and cat: a retrospective study of 16 cases. J Small Anim Pract. 1990;31:244‐250. [Google Scholar]
- 13. Lorinson D, Bright RM. Long‐term outcome of medical and surgical treatment of hiatal hernias in dogs and cats: 27 cases (1978‐1996). J Am Vet Med Assoc. 1998;213:381‐384. [PubMed] [Google Scholar]
- 14. Levine JS, Pollard RE, Marks SL. Contrast videofluoroscopic assessment of dysphagic cats. Vet Radiol Ultrasound. 2014;55:465‐471. [DOI] [PubMed] [Google Scholar]
- 15. Holt D, Callan MB, Washabau RJ, et al. Medical treatment versus surgery for hiatal hernias. J Am Vet Med Assoc. 1998;213:800. [PubMed] [Google Scholar]
- 16. Waldron DR, Moon M, Leib MS, Barber D, Mays KA. Oesophageal hiatal hernia in two cats. J Small Anim Pract. 1990;31:259‐263. [Google Scholar]
- 17. Hardie EM, Ramirez O, Clary EM, et al. Abnormalities of the thoracic bellows: stress fractures of the ribs and hiatal hernia. J Vet Intern Med. 1998;12:279‐287. [DOI] [PubMed] [Google Scholar]
- 18. Frye FL. Hiatal diaphragmatic hernia and tricholithiasis in a Golden cat (a case history). Vet Med Small Anim Clin. 1972;67:391‐392. [PubMed] [Google Scholar]
- 19. Robotham GR. Congenital hiatal hernia in a cat. Feline Pract. 1979;9:37‐39. [Google Scholar]
- 20. Peterson SL. Esophageal hiatal hernia in a cat. J Am Vet Med Assoc. 1983;183:325‐326. [PubMed] [Google Scholar]
- 21. Papazoglou LG, Patsikas M, Rallis T, et al. Hiatal hernia with esophageal stricture in a cat. Feline Pract. 2000;28:10‐11. [Google Scholar]
- 22. Brinkley CH. Hiatus hernia in a cat. Vet Rec. 1990;127:46‐47. [PubMed] [Google Scholar]
- 23. Mitsuoka K, Tanaka R, Nagashima Y, et al. Omental herniation through the esophageal hiatus in a cat. J Vet Med Sci. 2002;64:1157‐1159. [DOI] [PubMed] [Google Scholar]
- 24. Owen MC, Morris PJ, Bateman RS. Concurrent gastro‐esophageal intussusception, trichobezoar, and hiatal hernia in a cat. N Z Vet J. 2005;53:371‐374. [DOI] [PubMed] [Google Scholar]
- 25. Gualtieri M, Olivero D. Reflux esophagitis in three cats associated with metaplastic columnar esophageal epithelium. J Am Anim Hosp Assoc. 2006;42:65‐70. [DOI] [PubMed] [Google Scholar]
- 26. Mohajeri SF, Molazem M, Dehghan MM, et al. Diagnosis, surgery, and follow‐up of sliding hiatal hernia in two cats. Iran J Vet Surg. 2013;8:61‐65. [Google Scholar]
- 27. Cornell K. Hiatal hernia In: Tobias KM, Johnston SA, eds. Veterinary Surgery: Small Animal. Vol 2 1st ed. St. Louis, MO: Elsevier Saunders; 2012:1500‐1503. [Google Scholar]
- 28. Pettersson GB, Bombeck CT, Nyhus LM. Influence of hiatal hernia on lower esophageal sphincter function. Ann Surg. 1981;193:214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han E. Diagnosis and management of reflux esophagitis. Clin Tech Small Anim Pract. 2003;18:231‐238. [DOI] [PubMed] [Google Scholar]
- 30. Williams JM. Hiatal hernia in a shar‐pei. J Small Anim Pract. 1990;31:251‐254. [Google Scholar]
- 31. Stickle R, Sparschu G, Love N, Walshaw R. Radiographic evaluation of esophageal function in Chinese shar‐pei pups. J Am Vet Med Assoc. 1992;201:81‐84. [PubMed] [Google Scholar]
- 32. Poncet CM, Dupre GP, Freiche VG, Estrada MM, Poubanne YA, Bouvy BM. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract. 2005;46:273‐279. [DOI] [PubMed] [Google Scholar]
- 33. Meola SD. Brachycephalic airway syndrome. Top Companion Anim Med. 2013;28:91‐96. [DOI] [PubMed] [Google Scholar]
- 34. Parkinson S, Tolbert K, Messenger K, et al. Evaluation of the effect of orally administered acid suppressants on intragastric pH in cats. J Vet Intern Med. 2015;29:104‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kempf J, Lewis F, Reusch CE, Kook PH. High‐resolution manometric evaluation of the effects of cisapride and metoclopramide hydrochloride administered orally on lower esophageal sphincter pressure in awake dogs. Am J Vet Res. 2014;75:361‐366. [DOI] [PubMed] [Google Scholar]
- 36. Whitehead K, Cortez Y, Eirmann L. Gastrointestinal dysmotility disorders in critically ill dogs and cats. J Vet Emerg Crit Care. 2016;26:234‐253. [DOI] [PubMed] [Google Scholar]
- 37. Pratschke KM, Bellenger CR, McAllister H, Campion D. Barrier pressure at the gastroesophageal junction in anesthetized dogs. Am J Vet Res. 2001;62:1068‐1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Figure S1 Supporting Information


