Abstract
Background
Eosinophilic lung disease is a poorly understood inflammatory airway disease that results in substantial morbidity.
Objective
To describe clinical findings in dogs with eosinophilic lung disease defined on the basis of radiographic, bronchoscopic, and bronchoalveolar lavage fluid (BAL) analysis. Categories included eosinophilic bronchitis (EB), eosinophilic granuloma (EG), and eosinophilic bronchopneumopathy (EBP).
Animals
Seventy‐five client owned dogs.
Methods
Medical records were retrospectively reviewed for dogs with idiopathic BAL fluid eosinophilia. Information abstracted included duration and nature of clinical signs, bronchoscopic findings, and laboratory data. Thoracic radiographs were evaluated for the pattern of infiltrate, bronchiectasis, and lymphadenomegaly.
Results
Thoracic radiographs were normal or demonstrated a bronchial pattern in 31 dogs assigned a diagnosis of EB. Nine dogs had intraluminal mass lesions and were bronchoscopically diagnosed with EG. The remaining 35 dogs were categorized as having EBP based on radiographic changes, yellow green mucus in the airways, mucosal changes, and airway collapse. Age and duration of cough did not differ among groups. Dogs with EB were less likely to have bronchiectasis or peripheral eosinophilia, had lower total nucleated cell count in BAL fluid, and lower percentage of eosinophils in BAL fluid compared to dogs in the other 2 groups. In contrast to previous reports, prolonged survival (>55 months) was documented in dogs with EG.
Conclusions and Clinical Importance
Dogs with eosinophilic lung disease can be categorized based on imaging, bronchoscopic and BAL fluid cytologic findings. Further studies are needed to establish response to treatment in these groups.
Keywords: bronchitis, bronchomalacia, bronchopneumopathy, granuloma, infection
Abbreviations
- BAL
bronchoalveolar lavage
- CT
computed tomography
- EB
eosinophilic bronchitis
- EBP
eosinophilic bronchopneumopathy
- EG
eosinophilic granuloma
- TNCC
total nucleated cell count
1. INTRODUCTION
Pulmonary eosinophilia can develop in response to parasitic, fungal, or neoplastic diseases or as an immunologic response to an unknown trigger. Dogs with idiopathic pulmonary eosinophilia have a spectrum of clinical presentations and bronchoscopic appearances,1 but little is known about the clinical features of these various manifestations of disease. Mild changes in the airways of some dogs were interpreted to represent a form of bronchitis.1 Some dogs have severe radiographic abnormalities and bronchoscopic changes including a polypoid appearance to the airways, visible bronchiectasis, yellow green mucus coating multiple airways, and airway collapse. This pattern was described as eosinophilic bronchopneumopathy (EBP), a systemic and parenchymal disease process causing cough and nasal discharge in young‐ to middle‐aged dogs.2
Several reports have defined a variant of eosinophilic disease characterized by granulomatous masses.3, 4, 5, 6 Diagnostic imaging findings in these cases often resemble a neoplastic process.5, 7 Once, this disorder was considered the most common form of eosinophilic lung disease1 and diagnosis was associated with a guarded prognosis for recovery. Clinical experience has demonstrated that intraluminal airway lesions in eosinophilic granuloma (EG) often appear similar to the fungal granulomas visualized in sinonasal aspergillosis. Aspergillus organisms were isolated from culture of bronchoalveolar lavage fluid (BAL) in 2 of 22 dogs with EBP,2 but no studies to date have identified a fungal origin for this disease or for EG.
Eosinophilic lung disease in dogs previously has been described as a heterogenous group of disorders similar to the description used in humans, with disease primarily in the airways or parenchyma, or in both segments of the lung,8, 9 but large‐scale descriptive studies are lacking. The aim of our retrospective study was to define clinical findings in dogs that could be categorized as eosinophilic bronchitis (EB), EBP, and EG. We hypothesized that cases would be similar in demographics but that dogs with EB would have shorter duration of clinical signs, less severe peripheral eosinophilia and basophilia, and less marked evidence of inflammation in BAL fluid. We also hypothesized that dogs with EG would more often display radiographic evidence of bronchiectasis, because of severe inflammation or as a predisposing factor for mucus inspissation and plugging.
2. MATERIALS AND METHODS
Medical records at the William R. Pritchard Veterinary Medical Teaching Hospital at the University of California‐Davis were searched for all dogs having bronchoscopy performed between January 1, 2006, and January 1, 2016. Bronchoscopy records were evaluated for cases with BAL fluid eosinophilia. At least 1 lobar lavage cytology had to contain >14% eosinophils, which represented the 90th percentile for this cell type determined in a BAL study of dogs with histologically normal lungs.10 In addition, cases with a clinical diagnosis of eosinophilic lung disease (recorded as EBP, EB, pulmonary infiltrates with eosinophilia, or EG) were identified from the clinical diagnosis field or pathology results in the medical record. Inclusion criteria were documentation of respiratory signs (eg, cough, nasal discharge, difficulty breathing, or tachypnea) in conjunction with eosinophilic lower respiratory tract cytology or histopathology. Cases were excluded if pulmonary eosinophilia could be ascribed to cardiac or pulmonary parasitism, foreign body disease, or a paraneoplastic process, based on clinical data obtained.
Dogs were placed under general anesthesia using a protocol deemed most appropriate by the anesthesia service and were positioned in sternal recumbency. Computed tomography (CT), when performed, was completed immediately before bronchoscopy, and results were abstracted from the imaging report. When an endotracheal tube size >#7 could be placed, bronchoscopy was performed through a bronchoscopic adaptor attached to the endotracheal tube. A 4.9‐mm outer diameter flexible videoendoscope (Olympus P180 or GIF‐XP180N, Center Valley, Pennsylvania) was utilized, and isoflurane was used to provide general anesthesia. Smaller dogs were maintained at a stable plane of anesthesia with a constant rate IV infusion of propofol at 6 μg/kg/min; oxygenation was maintained by the use of jet ventilation via a 14‐gauge angiocatheter at a rate of 180 breaths per minute, and a 3.8‐mm outer diameter flexible videoendoscope was used (Olympus BIF 3C160). Bronchoscopy was performed by 1 of the authors (L.R.J., S.E.H., J.D.D.) or a house officer trained by the authors. Pulse oximetry was monitored throughout the procedure, and the procedure was interrupted if hemoglobin saturation with oxygen decreased below 85%.
The endoscopist was aware of prior diagnostic imaging results at the time of the procedure. All dogs were evaluated in standardized fashion, with interrogation of all accessible lobar bronchi in the following order: left cranial (cranial and caudal segments), left caudal, right cranial, right middle, accessory, and right caudal lung lobes. Bronchoscopy findings of moderate to severe airway hyperemia, mucus exudation, airway collapse, and presence of intraluminal material were recorded using a combination of drop‐down menus and free field text boxes within the endoscopy report.
After complete visual inspection of the airways, the bronchoscope was withdrawn from the airways to irrigate the channel with sterile saline and wipe debris from the outer surface, then reinserted to complete BAL at specific site or sites chosen by the endoscopist based on abnormalities found on radiographs or bronchoscopy. When no specific abnormalities were identified, the right middle lobe and the caudal portion of the left cranial lung lobe were sampled most commonly. Lavage was performed at ≥1 sites by wedging of the endoscope in the smallest possible airway followed by instillation and aspiration of warm, sterile saline through the biopsy channel of the endoscope. Lavage volume was based on the size of the dog, the outer diameter of the bronchoscope used, and the ability to wedge the bronchoscope in the airway in a manner that provided adequate return. An approximate guideline of 1 mL/kg/lavage site was utilized, and fluid retrieval was considered acceptable if visible foam was present in the syringe suggesting the presence of surfactant or if lavage fluid was obviously flocculent or contained mucoid material. One to 4 lavages were performed as clinically indicated, and the volume recovered was recorded for each separate lavage. When indicated, endobronchial biopsy samples or samples from intraluminal mass lesions were collected and placed in 10% neutral buffered formalin for histopathologic evaluation.
Bronchoalveolar lavage fluid was processed within 1 hour of collection. Lavages from distinct lobar sites were submitted separately for cytologic analysis by board‐certified clinical pathologists. Total nucleated cell counts (TNCC) were performed on unfiltered BAL fluid using an automated cell counter (Advia 120; Siemens, Deerfield, Illinois) and reported as TNCC/μL. Slides were prepared for cytologic analysis by cytocentrifugation (Cytospin3; ThermoShandon, Pittsburgh Pennsylvania) followed by Wright‐Giemsa staining using an automated cell stainer (Model 7151 Wescor Aerospray Hematology Pro; ELITech Bio‐Medical Systems, Logan, Utah). A differential cell count was based on an assessment of 200 cells. Normal reference values used for canine BAL fluid differential cell counts in our laboratory are approximately 80% macrophages, 7% lymphocytes, 5% neutrophils, 6% eosinophils, 1% mast cells, and 1% epithelial cells in dogs.10 The number of lavages per dog with BAL eosinophilia, highest TNCC/μL, and the highest eosinophil percentage for each dog were recorded. Septic suppurative inflammation was documented by identification of bacterial phagocytosis by neutrophils and was used to identify infectious lower respiratory disease.
A pooled sample of BAL fluid was plated onto 5% sheep blood agar and MacConkey's agar for isolation of aerobic organisms and pleuropneumonia‐like organism base with thallium acetate (antifungal) and penicillin G (antibacterial) additions for isolation of Mycoplasma spp. At the discretion of the attending clinician, samples were plated onto pre‐reduced anaerobic Brucella plates (Anaerobe Systems, Morgan Hill, California) for anaerobic culture or inhibitory mold agar (Hardy Diagnostics, Santa Maria, California) for fungal culture. Bacterial growth was assessed in a semi‐quantitative fashion and was reported as 1+, 2+, 3+, or 4+ based on the number of quadrants containing bacterial growth. Standard biochemical methods were used to identify cultured bacteria before September 2013 and afterward by matrix‐assisted laser desorption/ionization time‐of‐flight (Bruker Daltonics Microflex LT, Billerica, Massachusetts). Dogs with >2+ growth of potential pathogens on aerobic, anaerobic, Mycoplasma, or fungal culture with or without septic cytology were diagnosed with lower respiratory tract infection.
Underlying or coincident disease processes associated with airway eosinophilia were investigated by ancillary tests, including airway BAL fluid cultures, fecal parasite evaluations, serologic heartworm testing, or histopathology, at the discretion of the attending clinician.
History and physical examination findings were retrieved from medical records included in the electronic database of the hospital and were reviewed by 1 of the authors (L.R.J.). Data abstracted from the medical record included age, breed, sex, type, and duration of clinical signs, medication administered within the previous week, and results of a CBC. Complete blood counts were evaluated for total white blood cell count, count above or below the reference range for our laboratory (6000‐13 000/μL) and for total eosinophil and basophil counts, as well as those above the normal range (eosinophils >1500/μL and basophils >50/μL). Radiographs typically were performed the day before bronchoscopy and ≤1 week before the procedure. All radiographs were retrospectively reviewed by a board‐certified radiologist (E.G.J.) who was aware of pulmonary eosinophilia. Radiographs were interpreted as either normal or as having interstitial, nodular, bronchial, or alveolar patterns, with many dogs having >1 type of radiographic pattern. The presence of bronchiectasis, bronchial plugging (characterized by elongated tubular or cylindrical soft tissue infiltrates filling bronchi), and lymphadenomegaly was recorded.
Dogs were divided into 3 groups for statistical comparison. Dogs with normal thoracic radiographs or primarily a bronchial pattern in conjunction with bronchoscopic findings of minimal mucosal irregularities, hyperemia alone, mild or focal mucus, and lack of airway collapse were categorized as having EB (Figure 1). Dogs with bronchoscopic evidence of intraluminal eosinophilic mass lesions plugging ≥1 airways were described as having EG (Figure 2). Given the historically guarded prognosis of dogs with EG, records of affected dogs also were evaluated for information describing treatment response and survival. Dogs with bronchoscopic findings of thick mucoid exudate, mucus plugging, polypoid proliferations of the mucosa of the airways, bronchiectasis, airway collapse, lack of granuloma formation, and diagnostic imaging abnormalities including bronchial, interstitial, nodular, or alveolar patterns were diagnosed with EBP (Figure 3).
Figure 1.
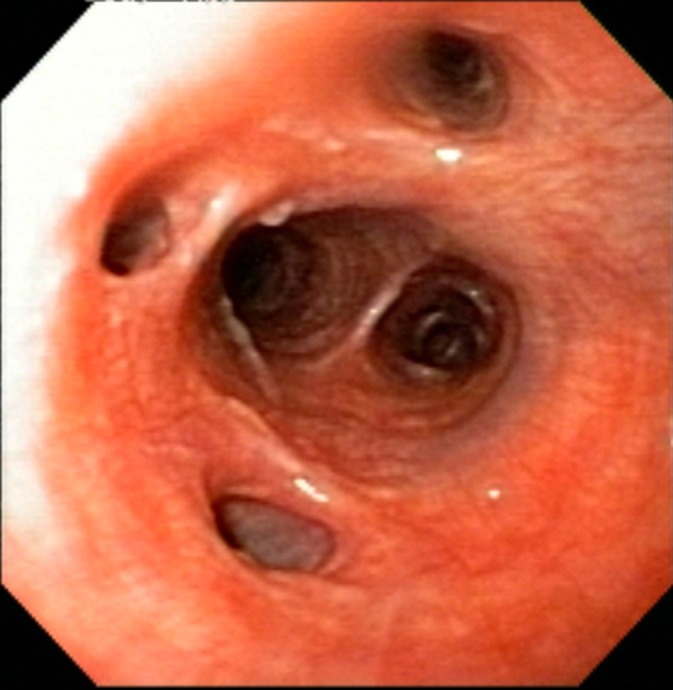
Bronchoscopic image from a dog with eosinophilic bronchitis reveals airway hyperemia. Airway openings are normal in shape and size, the epithelial lining is smooth, minimal mucus is present, and no airway collapse is noted
Figure 2.
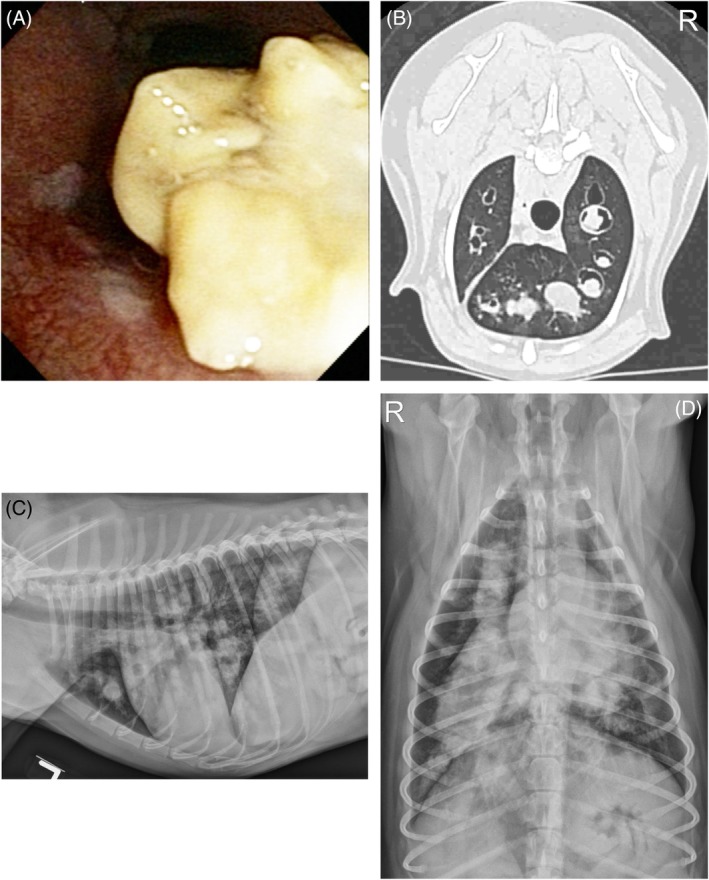
Bronchoscopic (A) and CT image (B) from a dog with eosinophilic granuloma. In (A), a large yellow mass lesion is partially obstructing a bronchus. The CT in (B) demonstrates generalized and severe saccular dilation of bronchi with thickening of airway walls and a large amount of soft tissue attenuating material obstructing the bronchial lumen at multiple locations. Left lateral (C) and dorsoventral radiographs (D) show a severe bronchial pattern with bronchiectasis and, in particular, gross enlargement of the right cranial and right caudal lobar bronchi that are filled with soft tissue opacity and are tubular in shape
Figure 3.
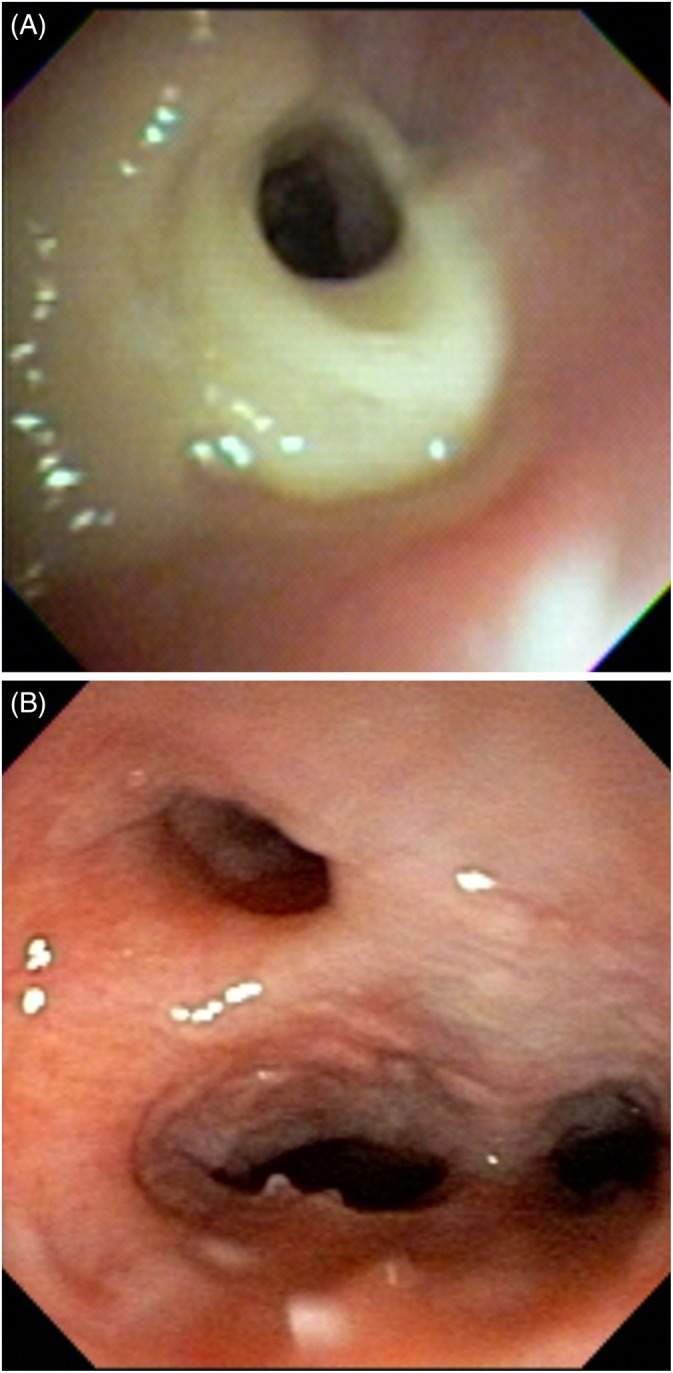
Bronchoscopic images from two different dogs with eosinophilic bronchopneumopathy reveal yellow green mucus obscuring an airway (A) and airway collapse with nodular epithelial changes (B)
2.1. Statistical analysis
Data were assessed for normality using the D'Agostino and Pearson omnibus normality test (GraphPad Prism version 5.0f, San Diego, California). Normally distributed data are presented as mean ± standard deviation, and data with a non‐Gaussian distribution are presented as median with range. Results for nonparametric data were compared among groups using the Kruskal‐Wallis test with post hoc Dunn's multiple comparison test. Chi‐square analysis or Fisher's exact test was used to compare the number of dogs among groups with leukocytosis, peripheral eosinophilia, bronchiectasis, and other radiographic infiltrates.
3. RESULTS
Between January 1, 2006, and January 1, 2016, 569 dogs had bronchoscopy performed. Bronchoalveolar lavage eosinophilia >14% in at least 1 lobar lavage was documented in 85 dogs. Ten dogs were excluded from further analysis, including 6 dogs with foreign body migration or broncho‐pneumonia, 2 dogs with pulmonary carcinoma, and 2 dogs with disease related to Dirofilaria immitis or Oslerus osleri infections. In the remaining 75 dogs, heartworm antigen test (Dirocheck Heartworm Antigen test kit; Zoetis, Parsippany, New Jersey) was negative, and fecal examinations, including flotation, sedimentation, and Baermann examination were negative in 24 of 24 dogs. Coccidioidomycosis serology was negative in 13 of 13 dogs tested.
Overall, the group of 75 affected dogs ranged in age from 0.6‐15 years at the time of diagnosis (median, 6 years) and was comprised of 4 Labrador Retrievers and 4 Standard Poodles, 3 each of Cocker Spaniel, German Shepherd, and Pomeranian, and 2 each of Rottweiler, Australian Shepherd, Jack Russell Terrier, and Shih Tzu. The remaining dogs were breeds represented once or were mixed breed dogs. There were 3 intact female, 35 female spayed, 3 intact male, and 34 male neutered dogs. The most common clinical complaint was cough (69/75; 92%), with a median duration before presentation of 6 months (range, 1.5 days to 96 months). Nasal discharge was recorded in 21 of 75 dogs (28%) with duration ranging from 0.2 to 36 months (median, 5 months). Three dogs (4%) were presented for tachypnea and 3 dogs (4%) were presented for abnormal respiratory sounds described by owners as gurgling, snorting, or wheezing. Medications administered in the week before diagnostic testing included corticosteroids in 5 dogs and antimicrobials in 21 dogs.
A diagnosis of EB was made in 31 of 75 dogs (41%, Table 1). Radiographs were available for review in 28 of 31 EB dogs and were reported as normal in 17 of 28 (61%) dogs, and a bronchial pattern was described in 11 of 28 (39%) dogs. Bronchoscopy was reported as normal in 6 of 31 dogs, airway hyperemia was present in 25 of 31 dogs, and mild deposits of mucus were noted in 7 of 31 dogs. Bronchoalveolar lavage eosinophilia was present in only 1 of multiple lung lavages in 19 of 31 (61%) dogs. In 10 dogs, BAL fluid eosinophilia was noted in 2 of 2 lavages. One dog each had BAL fluid eosinophilia in 2 of 3 or 3 of 3 lavage sites. One dog with concurrent laryngeal dysfunction had bacterial sepsis and growth of E. coli and Streptococcus on culture. A second dog had a single colony of Aspergillus detected on the aerobic bacterial culture, which was considered a contaminant. Dogs with EB included 4 Labrador Retrievers and 2 each of Jack Russell Terrier and Australian Shepherd. The group comprised 15 female (1 intact) and 16 male (1 intact) dogs. Median weight was 23.6 kg with 7 dogs <10 kg, 3 dogs 10‐20 kg, and 20 dogs >20 kg.
Table 1.
Radiographic findings in 72 of 75 dogs with BAL fluid eosinophilia that had radiographs available for review
| EB (n = 28) | EG (n = 9) | EBP (n = 35) | Total (%) | P value | |
|---|---|---|---|---|---|
| Normal radiographs | 17 | 0 | 0 | 17/73 (23) | NA |
| Bronchial pattern | 11 | 9 | 32 | 52/73 (71) | NA |
| Interstitial infiltrates | 0 | 6 | 0 | 6/73 (8) | ND |
| Alveolar infiltrates | 0 | 4 | 7 | 11/73 (15) | .20 |
| Bronchial plugging | 0 | 5 | 12 | 17/73 (23) | .27 |
| Nodular pattern | 0 | 5a | 3 | 8/73 (11) | .005 |
| Bronchiectasis | 6a | 7 | 21 | 35/73 (48) | .007 |
| Lymphadenopathy | 0 | 1 | 3 | 4/73 (5) | ND |
Individual dogs had more than one radiographic pattern.
Abbreviations: EB, eosinophilic bronchitis; EBP, eosinophilic bronchopneumopathy; EG, eosinophilic granuloma; NA, test not applicable due to inclusion criteria; ND, test not performed due to low numbers.
Significantly different than other entries within the row.
Bronchoscopy identified intraluminal EG in 9 of 75 dogs (12%). Airway hyperemia was present in 5 of 9 dogs and mucus exudation and inspissation in 9 of 9 dogs. Thoracic radiographs were abnormal in all dogs, with a bronchial pattern (9/9), bronchiectasis (6/9), bronchial plugging with intraluminal soft tissue opacities (5/9), nodular or mass lesions (5/9), alveolar or interstitial infiltrates (4/9), and hilar lymphadenomegaly (1/9). In 1 dog, BAL fluid eosinophilia was found in only 1 of 3 lavages, and in a second dog, BAL fluid cytology identified neutrophilic inflammation, but EG was reported on histopathologic examination of intraluminal mass lesions. In the remaining 7 dogs, BAL fluid eosinophilia was found in 1/1 lavage, 2/2 lavages, 2/3 lavages, 3/3 lavages (3 dogs) and 4/4 lavages. One dog had septic suppurative inflammation with isolation of Pasteurella canis considered a secondary infection. Fungal culture was negative in 3 of 3 dogs tested, and histopathology of the intraluminal granulomas failed to identify infectious organisms in 2 of 2 dogs evaluated. In dogs with EG, mixed breed dogs were most common (4/9) and no dog breed was represented more than once. There were 4 female spayed, 2 male, and 3 male neutered dogs. Median weight was 21.4 kg with 3 dogs <10 kg and the remainder above 19 kg.
The remaining 35 dogs (47%) were considered to have EBP based on multiple radiographic and bronchoscopic findings. A bronchial pattern was the most common radiographic abnormality (29/35; 83%) followed by bronchiectasis (21/35; 60%). Hilar lymphadenomegaly was found in 3 of 35 (9%) dogs. Bronchoalveolar lavage fluid analysis identified septic suppurative inflammation in 1 dog with isolation of Pasteurella spp., and BAL fluid fungal culture was negative in 4 of 4 dogs tested. Eosinophilia in BAL fluid cytology was found in 1/1 lavages in 3 of 35 dogs, 2/2 lavages in 23 of 35 dogs, 2/3 lavages in 2 of 35 dogs, and 3/3 lavages in 7 of 35 dogs. In this group, 3 were Standard Poodles, and there were 2 each of Cocker Spaniels, Pomeranians, and Shih Tzus. There were 2 intact female dogs, 16 female spayed, and 17 male neutered dogs. Median weight was 21.2 kg with 13 dogs <10 kg, 4 dogs 10‐20 kg, and 18 dogs >20 kg.
Overall, in 72 dogs with BAL fluid eosinophilia that had radiographs available for review, a bronchial pattern was the most common radiographic finding followed by bronchiectasis (Table 1). Dogs with EB had radiographic evidence of bronchiectasis detected significantly less often than dogs did with EG or EBP (P = .002), and a nodular infiltrate was significantly more common in EG than in other diseases (P = .005). Computed tomography was performed in 3 of 31 dogs with EB, 5 of 9 dogs with EG, and 4 of 35 dogs with EBP. Findings are not specifically addressed here given the small number of cases that had CT performed, and the availability of previous descriptions of CT findings in dogs with pulmonary eosinophilia.5, 7
Median duration of cough did not differ among groups (P = .27, Table 2). Dogs with EBP were significantly more likely to have peripheral leukocytosis than were dogs with EB or EG (P = .03), and peripheral eosinophilia was present in significantly fewer dogs with EB than in dogs with EBP or EG (P < .0001, Figure 4). There were no differences in other white blood cells including basophils among groups. Total nucleated cell counts in BAL fluid of dogs with EB were significantly lower (median, 980; range, 300‐4240) than those in dogs with EBP (median, 3360; range, 550‐33 800) or EG (median, 2630, range, 1920‐16 940, P < .0001), and percentages of BAL eosinophils were significantly lower in dogs with EB than in dogs with EBP and EG (P < .0001, Figure 5).
Table 2.
Demographic and clinicopathologic findings in dogs with BAL fluid eosinophilia
| EB (n = 31) | EG (n = 9) | EBP (n = 35) | P‐value | |
|---|---|---|---|---|
| Age (y) | 8.5 (0.6‐15) | 6 (4‐10) | 4.0 (1‐13) | .08 |
| Weight (kg) | 23.6 (5.4‐41.8) | 21.4 (5.9‐44.6) | 20.5 (2.1‐40) | .28 |
| Nasal discharge | 11/31 | 3/9 | 7/35 | .27 |
| Cough | 27/33 | 9/9 | 33/35 | .41 |
| Duration of cough (mo) | 7 (0.5‐60) | 2 (0.5‐60) | 6 (0.07‐96) | .31 |
| WBC/μL | 11 920 (7760‐21 287) | 12 900 (10 190‐29 150) | 14 190 (6860‐32 390) | .21 |
| Number of dogs with leukocytosis (>13 000) | 8/28 | 3/9 | 21/35a | .03 |
| Peripheral eosinophils/μL | 676 (25‐2064)a | 2202 (621‐15 450) | 1651 (185‐17 003) | <.0001 |
| Number of dogs with peripheral eosinophilia >1500 | 2/28a | 6/9 | 18/35 | <.0001 |
| Peripheral basophils/μL | 30 (0‐246) | 22 (0‐1458) | 26 (0‐2520) | .93 |
| Number of dogs with peripheral basophilia >50 | 6/28 | 3/9 | 14/35 | .25 |
| BAL TNCC/μL | 980 (300‐4240)a | 2630 (1920‐16 940) | 3360 (550‐33 800) | <.0001 |
| %BAL eosinophils | 21 (15‐64)a | 55 (24‐91) | 61 (23‐95) | <.0001 |
Values represent medians (range).
Abbreviations: EB, eosinophilic bronchitis; EBP, eosinophilic bronchopneumopathy; EG, eosinophilic granuloma; TNCC, total nucleated cell count; WBC, white blood cell count.
Significantly different than other entries within the row.
Figure 4.
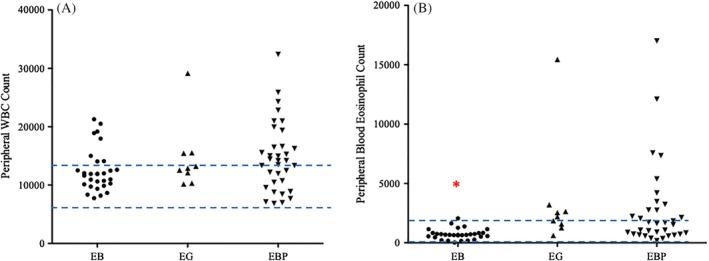
Total WBC did not differ among groups (A) although the percentage of dogs with EBP that had peripheral leukocytosis was significantly higher than in the other 2 groups. Peripheral blood eosinophils (B) were significantly lower in dogs with EB in comparison to dogs with EG and EBP. Dashed lines represent reference intervals
Figure 5.
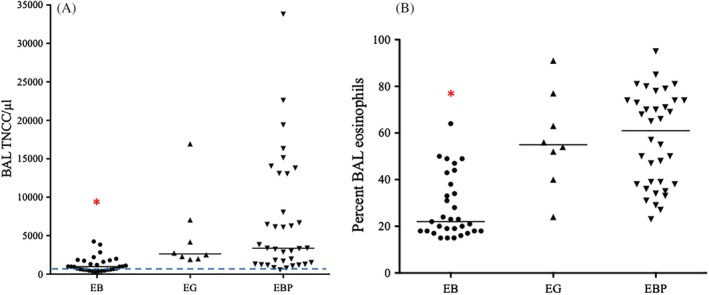
Total nucleated cell counts in BAL fluid were significantly lower in dogs with EB compared to those with EG and EBP (A; dashed line represents the normal value), and percentage BAL fluid eosinophilia was also lower in that group (B). Only dogs with BAL fluid eosinophil percentage exceeding 14% were evaluated here
Follow‐up information was obtained from the medical records of 9 dogs with EG. Although treatment was not standardized prospectively, all dogs were started on prednisone at approximately 1 mg/kg PO q12h. Nebulization with saline was initiated in 6 of 9 dogs and N‐acetylcysteine was administered PO to 5 of 9 dogs. One dog underwent lobectomy of the left caudal lobe and then the left cranial lung lobe and has been maintained on inhaled corticosteroids and cyclosporine PO for >55 months at the time of writing. One dog was known to have died 4 months after the diagnosis, and 1 dog was tapered off corticosteroids 39 months after diagnosis when bacterial of developed. The remaining 6 dogs were reportedly doing well on corticosteroids 2 weeks to 15 months (median, 8 months) after the diagnosis of EG before being lost to follow‐up.
4. DISCUSSION
To our knowledge, this study is the first to report features highlighting the diversity of eosinophilic lung disease in a large group of dogs and to describe the prevalence of intraluminal EG as part of this syndrome. Dogs with minimal to no radiographic and minimal bronchoscopic changes were categorized as having EB. These dogs less commonly had peripheral eosinophilia, had lower TNCC in BAL fluid, and had lower percentages of eosinophils in BAL fluid. The most common category comprised dogs diagnosed with EBP based on radiographic infiltrates and bronchoscopic abnormalities, whereas the least common category consisted of dogs with eosinophilic material filling the airways (ie, EG).
Our study identified multiple clear differences among the 3 disease syndromes, with the most easily identified being the presence of large intra‐airway mass lesions in EG. It is unclear whether the syndrome characterized here is the same as that described as eosinophilic pulmonary granulomatosis in the veterinary literature. Two reports of that condition describe parenchymal lesions infiltrated by epithelioid cells, macrophages, and eosinophils surrounded by fibroplasia that destroy the local pulmonary architecture.6, 11 In those reports, the alveolar architecture surrounding the granulomas remained intact and was histologically characterized by type II pneumocyte proliferation with eosinophilic infiltration. Some cases11 were characterized by eosinophilic infiltration of other organs, resembling hypereosinophilic syndrome.12 In contrast, the disease in dogs with EG described here generally was confined to intra‐airway accumulation of mass‐like material, more similar to what was described in a CT study of dogs with eosinophilic granulomatosis.5 Given that some previous reports did not employ bronchoscopy,6, 11 it is possible that only pulmonary parenchymal involvement was reported for those cases because the possibility of intra‐airway disease was not investigated.
A second clear difference among the 3 types of eosinophilic lung disease categorized in our study was the difference in peripheral leukocyte variables and pulmonary eosinophilic responses. Leukocytosis was present in 44% of all dogs with BAL fluid eosinophilia reported here, with a significantly higher number of dogs with EBP demonstrating leukocytosis (60%) in comparison to dogs with EB or EG, although neutrophils, lymphocytes, monocytes, and basophils did not differ among groups. Dogs with EB had lower numbers of peripheral eosinophils and more typically had a peripheral eosinophil number within the reference range compared to dogs with EG or EBP, suggesting more severe pulmonary involvement in the latter 2 conditions.
In addition, dogs with EB had lower percentages of eosinophils in BAL fluid compared to the other 2 disease syndromes. These findings provide an interesting contrast to an earlier description of EBP.2 In that study, the severity of eosinophilic lung disease was categorized based on BAL eosinophil percentages of 0%‐20% (mild), 20%‐50% (moderate), and >50% (severe). Had that methodology been employed here, slightly <50% of dogs with EB would fit in the mild category of EBP, but all remaining dogs in that group and most dogs in the other 2 groups would fit into the moderate to severe category of inflammation. Therefore, the method of severity scoring based on BAL eosinophil percentages appears to be less discriminating than our proposed categorization, which takes into account multiple clinical features. A stricter definition of pulmonary eosinophilia might be obtained by requiring increased eosinophil percentages in >1 lavage fluid, particularly given the knowledge that cytologic inflammatory responses can differ among lobes in 37% of dogs with pulmonary disease.13 Nonetheless, in our study, dogs with EG and EBP had higher TNCC in BAL fluid as well as higher percentages of BAL fluid eosinophils, which indicates more marked airway inflammation.
A final indicator of increased severity of disease in EG and EBP was the increased radiographic detection of bronchiectasis in these conditions in comparison to EB. Bronchiectasis has been a consistent finding in dogs with eosinophilic lung disease and EG.5, 7, 14 This observation is likely not a consequence of longer duration of disease, because there was no significant difference in duration of cough among the 3 disease groups and there was no difference in the age of affected dogs. Instead, bronchiectasis is likely a consequence of the tissue damaging effects of eosinophils on the support structures of the airways, given that dogs with EG and EBP had both higher BAL fluid TNCC and percentage eosinophils.
In humans, eosinophilic lung disease has many different forms that often are divided into disorders that affect the airways, those that involve the alveolar space, and those that are part of a systemic response.9 Variants of airway disease include asthma and allergic bronchopulmonary aspergillosis (ABPA), a form of asthma characterized by hypersensitivity to Aspergillus. Eosinophilic granulomatosis with vasculitis (previously referred to as Churg‐Strauss syndrome) is a very rare disorder characterized by nodules surrounding blood vessels that can be found in association with severe asthma,15, 16 and it does not resemble the EG described here. The only syndrome in humans with a presentation somewhat similar to that of EG in dogs described here is aspergilloma, a noninvasive intra‐airway fungal infection treated with itraconazole or surgical excision.17, 18 It is often found in association with bronchiectasis and also can be found with ABPA.19, 20 However, in our study, all dogs with EG tested for fungal infection were negative on culture or histopathology, indicating a lack of similarity to this condition in humans.
Although our study was not designed to assess treatment response in EG, given the time frame in which the dogs were examined, some follow‐up information was available for all 9 dogs. Dogs were all treated similarly with high doses of glucocorticoids PO, and most were treated with medication (eg, N‐acetylcysteine PO) or airway treatment such as saline nebulization designed to improve mobilization of secretions. Despite the reported poor prognosis for this disease, virtually all dogs showed improvement or resolution of clinical signs, with documented survival >39 and 55 months in 2 dogs. Further prospective studies of eosinophilic lung disease are needed to determine optimal treatment.
The original report of EBP identified a predilection for the disease in Siberian Huskies and Alaskan Malamutes,2 but only 1 Malamute and 1 Husky were included in our study. Interestingly, sled dogs trained in cold weather have been reported to develop airway eosinophilia,21 which could reflect a breed predilection or shared environmental trigger leading to this type of response to injury. Multiple breeds were affected by the 3 forms of eosinophilic disease described in our study, and the disease was more common in large breed dogs, as >50% of dogs examined here weighed >20 kg. Only 5 dogs weighed <5 kg, all of which were diagnosed with EBP rather than EB or EG.
Eosinophilic lung disease has been associated with increased tissue destruction and remodeling, collagenolytic activity and collagen turnover, and damage to epithelial attachment to the basement membrane, as indicated by increased procollagen type III aminoterminal peptide, matrix metalloproteinases, and laminin‐5 gamma 2‐chain in BAL fluid.22, 23, 24, 25 However, some of these findings also have been reported in BAL fluid of healthy dogs or dogs with other lung diseases. The type of eosinophilic lung disease examined in those studies is not clear, and it appears that dogs we would characterize as EB were combined with those we would define as EBP.22, 23, 24 Future studies with more strict definitions of the subsets of eosinophilic lung disease could provide better understanding of the molecular mechanisms and underlying immunopathogenesis of disease.
The retrospective nature of our study limited standardized collection of results, particularly information on recall of primary presenting complaints, duration of signs, and previous administration of medications. Multiple bronchoscopists performed the procedures over this 10‐year time frame, and videotapes were unavailable for review that could have been used to clarify the severity of lesions and to identify bronchiectasis. However, 3 of the authors (L.R.J., S.E.H., J.D.D.) performed or attended over 67% of the procedures, and all endoscopists were similarly trained in identification of lesions and completion of reports, lending some reliability to the methodology. Interestingly, bronchiectasis could be identified radiographically in a number of dogs, including those with EB. We did not include a severity score for bronchiectasis or for interpretation of other radiographic lesions and believe that CT data would be better utilized for that assessment in a prospective study. Finally, pulmonary parasitism is very uncommon in our area of the country (western USA), which resulted in few dogs having fecal evaluations performed. Therefore, it is possible that some of the cases that we classified as having idiopathic BAL eosinophilia actually had parasitic lung disease, but we believe this to be unlikely.
In conclusion, dogs with eosinophilic lung disease have different manifestations of disease and can be assigned to 1 of 3 categories based on hematologic, imaging, and bronchoscopic findings in conjunction with BAL fluid cytologic assessment. It is unclear whether these various forms of pulmonary eosinophilia share the same etiopathogenesis or represent distinct disease processes. If they are variants of a single disease, then they might represent a continuum of the disease spectrum. Alternately, variations could result from different host or environmental influences in the dogs affected. Determining the specific type of eosinophilic lung disease and its immunopathogenesis could provide improved treatment modalities and more predictable response to treatment. Eosinophilic granuloma often has been given a guarded prognosis, although disease remission has been reported,4 and acceptable response to treatment was noted in several dogs in our study. Response to PO glucocorticoid treatment has been associated with normalization of parameters of immune function in dogs with EBP,2 but prolonged treatment is often required because relapses are common. The use of inhalation treatment in dogs with EBP has been suggested to result in only partial response,26 but it is possible that the less severe form of EB might prove highly responsive to topical treatment.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Presented in part at the European College of Veterinary Internal Medicine—Companion Animal, Rotterdam, The Netherlands on September 2018.
Johnson LR, Johnson EG, Hulsebosch SE, Dear JD, Vernau W. Eosinophilic bronchitis, eosinophilic granuloma, and eosinophilic bronchopneumopathy in 75 dogs (2006‐2016). J Vet Intern Med. 2019;33:2217–2226. 10.1111/jvim.15605
REFERENCES
- 1. Corcoran BM, Thoday KL, Henfrey JI, Simpson JW, Burnie AG, Mooney CT. Pulmonary infiltration with eosinophils in 14 dogs. J Small Anim Pract. 1991;32(10):494‐502. [Google Scholar]
- 2. Clercx C, Peeters D, Snaps F, et al. Eosinophilic bronchopneumopathy in dogs. J Vet Intern Med. 2000;14(3):282‐291. [DOI] [PubMed] [Google Scholar]
- 3. Calvert CA, Mahaffey MB, Lappin MR, et al. Pulmonary and disseminated eosinophilic granulomatosis in dogs. J Am Anim Hosp Assoc. 1988;24:311‐320. [Google Scholar]
- 4. Katajavuori P, Melamies M, Rajamäki MM. Eosinophilic pulmonary granulomatosis in a young dog with prolonged remission after treatment. J Small Anim Pract. 2013;54(1):40‐43. [DOI] [PubMed] [Google Scholar]
- 5. Fina C, Vignoli M, Terragni R, Rossi F, Wisner E, Saunders JH. Computed tomographic characteristics of eosinophilic pulmonary granulomatosis in five dogs. Vet Radiol Ultrasound. 2014;55(1):16‐22. [DOI] [PubMed] [Google Scholar]
- 6. Dehghanpir SD, Leissinger MK, Jambhekar A, Kawabata A, Ryan KA, Wilson LD, Gaunt SD Pathology in practice J Am Vet Med Assoc 2019;254(4):479–482, Pathology in Practice. [DOI] [PubMed] [Google Scholar]
- 7. Mesquita L, Lam R, Lamb CR, McConnell JF. Computed tomographic findings in 15 dogs with eosinophilic bronchopneumopathy. Vet Radiol Ultrasound. 2015;56(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 8. Clercx C, Peeters D. Canine eosinophilic bronchopneumopathy. Vet Clin North Am Small Anim Pract. 2007;37(5):917‐935. [DOI] [PubMed] [Google Scholar]
- 9. Weissler JC. Eosinophilic lung disease. Am J Med Sci. 2017;354(4):339‐349. [DOI] [PubMed] [Google Scholar]
- 10. Hawkins EC, DeNicola DB, Kuehn NF. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat. State of the Art. J Vet Intern Med. 1990;4:267‐274. [DOI] [PubMed] [Google Scholar]
- 11. Confer AW, Qualls CW Jr, MacWilliams PS, Root CR. Four cases of pulmonary nodular eosinophilic granulomatosis in dogs. Cornell Vet. 1983;73(1):41‐51. [PubMed] [Google Scholar]
- 12. Sykes JE, Weiss DJ, Buoen LC, Blauvelt MM, Hayden DW. Idiopathic hypereosinophilic syndrome in 3 Rottweilers. J Vet Intern Med. 2001;15(2):162‐166. [DOI] [PubMed] [Google Scholar]
- 13. Hawkins EC, DeNicola DB, Plier ML. Cytological analysis of bronchoalveolar lavage fluid in the diagnosis of spontaneous respiratory tract disease in dogs: a retrospective study. J Vet Intern Med. 1995;9:386‐392. [DOI] [PubMed] [Google Scholar]
- 14. Johnson LR, Johnson EG, Vernau W, Kass PH, Byrne BA. Bronchoscopy, imaging, and concurrent diseases in dogs with bronchiectasis: (2003‐2014). J Vet Intern Med. 2016;30(1):247‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gioffredi A, Maritati F, Oliva E, Buzio C. Eosinophilic granulomatosis with polyangiitis: an overview. Front Immunol. 2014;3(5):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen Y, Guillevin L. Eosinophilic granulomatosis with polyangiitis (Churg‐Strauss). Semin Respir Crit Care Med. 2018;39(4):471‐481. [DOI] [PubMed] [Google Scholar]
- 17. Ofori A, Steinmetz AR, Akaasi J, et al. Pulmonary aspergilloma: an evasive disease. Int J Mycobacteriol. 2016;5(2):235‐239. [DOI] [PubMed] [Google Scholar]
- 18. Horiuchi K, Asakura T, Hasegawa N, Saito F. Recurrence of allergic bronchopulmonary aspergillosis after adjunctive surgery for aspergilloma: a case report with long‐term follow‐up. BMC Pulm Med. 2018;18(1):185‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Soyza A, Aliberti S. Bronchiectasis and Aspergillus: how are they linked? Med Mycol. 2017;55(1):69‐81. [DOI] [PubMed] [Google Scholar]
- 20. Yii AC, Koh MS, Lapperre TS, Tan GL, Chotirmall SH. The emergence of Aspergillus species in chronic respiratory disease. Front Biosci (Schol Ed). 2017;9:127‐138. [DOI] [PubMed] [Google Scholar]
- 21. Davis MS, McKiernan B, McCullough S, et al. Racing Alaskan sled dogs as a model of "ski asthma". Am J Respir Crit Care Med. 2002;166(6):878‐882. [DOI] [PubMed] [Google Scholar]
- 22. Rajamäki MM, Järvinen AK, Sorsa T, Maisi P. Clinical findings, bronchoalveolar lavage fluid cytology and matrix metalloproteinase‐2 and ‐9 in canine pulmonary eosinophilia. Vet J. 2002;163(2):168‐181. [DOI] [PubMed] [Google Scholar]
- 23. Rajamaki MM, Jarvinen AK, Sorsa T, Maisi P. Collagenolytic activity in bronchoalveolar lavage fluid in canine pulmonary eosinophilia. J Vet Intern Med. 2002;16(6):658‐664. [DOI] [PubMed] [Google Scholar]
- 24. Rajamaki MM, Jarvinen AK, Sorsa TA, et al. Elevated levels of fragmented laminin‐5 gamma2‐chain in bronchoalveolar lavage fluid from dogs with pulmonary eosinophilia. Vet J. 2006;171(3):562‐565. [DOI] [PubMed] [Google Scholar]
- 25. Heikkilä HP, Krafft E, Jespers P, McEntee K, Rajamäki MM, Clercx C. Procollagen type III amino terminal propeptide concentrations in dogs with idiopathic pulmonary fibrosis compared with chronic bronchitis and eosinophilic bronchopneumopathy. Vet J. 2013;196(1):52‐56. [DOI] [PubMed] [Google Scholar]
- 26. Canonne AM, Bolen G, Peeters D, Billen F, Clercx C. Long‐term follow‐up in dogs with idiopathic eosinophilic bronchopneumopathy treated with inhaled steroid therapy. J Small Anim Pract. 2016;57(10):537‐542. [DOI] [PubMed] [Google Scholar]


