Abstract
Background
Urine specific gravity (USG) is an integral part of the urinalysis and a key component of many clinical decisions, and fluctuations in USG have the potential to impact case management.
Objectives
To determine the intraindividual variability of first morning USG results in healthy dogs.
Animals
One hundred three healthy client‐owned dogs.
Methods
Dogs were deemed healthy based on clinical history and physical examination findings. Repeated USG measurements were performed over the course of 2 weeks. Three urine samples were collected each week for a total of 6 samples per dog. Sample collection was distributed evenly throughout the week. Urine samples were acquired immediately upon waking and before any ingestion of liquids, food, or exertion of physical activity in the dogs. All measurements were made using the same Misco digital refractometer.
Results
Intraindividual USG was variable over the course of the study. The mean difference between the minimum and maximum USG for each dog was 0.015 (SD, 0.007). The within‐week difference between the minimum and maximum USG was less than over the complete 2‐week study (0.009 [SD 0.006] for week 1 and 0.010 [SD 0.007] for week 2). The mean coefficient of variance across all 6 time points was 15.4% (SD 8.97%).
Conclusions and Clinical Importance
Clinically important variation occurs in USG in healthy animals and might impact clinical decision‐making when diagnostic cutoff points are utilized. Clinicians should be aware of inherent variability in this clinical variable when analyzing results.
Keywords: concentration, urinalysis, variance
Abbreviations
- ANOVA
analysis of variance
- SD
standard deviation
- UA
urinalysis
- Uosm
urine osmolality
- USG
urine specific gravity
1. INTRODUCTION
Routine urinalysis (UA) is an integral diagnostic test in the clinical evaluation of diseased animals as well as in healthy animals as part of a comprehensive preventative medicine program. Urinalysis aids in screening asymptomatic animals, providing supportive information during diagnostic evaluations, establishing a definitive diagnosis, and monitoring animals.1 Urine specific gravity (USG) provides an easy estimation of urine concentration that is important in evaluation of renal function and determination of the response to dehydration.1, 2
Both urine osmolality (Uosm) and USG have been used to assess the ability of renal tubules to concentrate urine in dogs.3, 4, 5 Measurement of osmolality is considered the gold standard for determination of concentrating ability, as changes in plasma osmolality are sensed by hypothalamic osmoreceptors. The amount of antidiuretic hormone subsequently released into circulation and its effect after binding to collecting tubule receptors largely determines the degree of urinary concentration.6, 7 However, given the time and expense involved in measuring osmolality, it is not routinely used in clinical practice. Urine specific gravity provides an inexpensive and quick alternative for measurement of urinary concentrating ability.
Urine specific gravity can vary depending on environment and activity level among individual dogs.8, 9 Increased activity is often associated with increased food and water intake. Urine specific gravity can change within the same dog depending on the timing of urine collection in relation to eating and the volume of water consumed.10 Interpretation of variable USG values for an individual dog could present a clinical challenge, as different values might be indicative of disease or might just be due to individual variation. Current recommendations for determination of urinary concentrating ability include collection of a first morning urine sample, as this presumably represents the most concentrated USG for that animal throughout the day.11 One study of 89 clinically healthy dogs found that Uosm and USG varied widely among healthy dogs. This study also found a wide variation in Uosm during the day among individual dogs.11 However, this study only looked at 2 time points for comparison of variability in the morning and did not specify when the sample was acquired other than during the first morning walk.
The purpose of this study was to report the variability of USG measured in the first morning urine for individual dogs within a week and between weeks. Determination of normal variability might better allow clinicians to determine when a change in USG could be indicative of clinically relevant disease. We hypothesize that standardizing the timing of first morning urine collection will decrease day‐to‐day variability and ultimately allow for more accurate clinical interpretations of variable individual animal USG values.
2. MATERIALS AND METHODS
Dogs that were owned by faculty, staff, and students at The Ohio State University College of Veterinary Medicine were screened for inclusion in the study based on a standardized history and physical examination. Signalment information collected included age (years), weight (categorized as <20 kg or ≥20 kg), sex, spay/neuter status, and breed (classified as purebred or mixed). Dogs were included in the study if they had no history of polyuria, polydipsia, or clinical signs consistent with systemic disease known to alter USG (eg, kidney disease, hyperadrenocorticism, diabetes, etc), and were otherwise considered healthy by the owners and based on history and physical examination. Dogs were also excluded if they had been sick or had been treated with any medications (eg, corticosteroids), which might interfere with urine‐concentrating ability during the 6‐month period before the start of the study. Dogs less than 5 months of age were also excluded to avoid potential interference from immature renal function.
Each owner collected the dog's first morning urine by free catch immediately upon waking and before the dogs ate or drank. Samples were collected on 3 consecutive days for 2 consecutive weeks, for a total of 6 urine samples per dog. Urine was aspirated into 12 mL syringes, and the sample was immediately refrigerated until it was processed within 12 hours. Before measurement, the urine was allowed to warm to room temperature. The information collected on each urine sample included a urine dipstick (Chemstrip Test Strips, Roche Diagnostics, Indianapolis, Indiana) and USG measured by a digital refractometer (Palm Abbe Digital Refractometer #PA203, Misco, Cleveland, Ohio).4 Patients were excluded if they had a urine sample positive for glucose, blood, or ketones or if they had a urine sample with a protein concentration of greater than 100 mg/dL (+2 on the Chemstrip).
2.1. Statistical analysis
Means and standard deviations (SDs) were calculated for responses that consisted of continuous data, and proportions were calculated for responses that consisted of categorical data. Mean USG values for each dog were calculated by week and for all samples. The difference between the lowest and highest USG for each dog by week and for all samples was calculated. Because >1.030 USG is traditionally considered to be the cutoff for adequate tubular concentrating capacity, the number of dogs that had at least 1 sample both above and below 1.030 was calculated.12, 13 Specific comparisons to look at the differences in mean USG among sex, spay/neuter status, purebred classification, and weight were performed using the Wilcoxon rank sum test. Daily and weekly intraindividual differences in USG measurement were compared with a repeated‐measures analysis of variance (ANOVA).
Multilevel mixed effects linear regression modeling was used to model the effect of the independent variables age, weight, purebred classification, spay/neuter status, sex, and day on the dependent variable USG. Week nested within dog were modeled as random effects. Urine specific gravity and all standard transformations were tested for normality using the Shapiro‐Wilk test and a visual standardized normal probability plot. As no transformation was normal using the Shapiro‐Wilk test but the distributional diagnostic plot was approximately normal, USG was modeled without transformation. Data were assessed for linearity and normal residual distributions graphically. Levene's test was used to assess homogeneity of variance. Assumptions for the statistical tests used in this study were met.
For all analyses, P‐values of <.05 were considered significant. Standard statistical software was used (GraphPad Prism and Stata V11.0).
3. RESULTS
There were 103 dogs enrolled in the study. The mean age was 3.8 years (SD 2.90) with a range of 0.5 to 14 years. Fifty‐five (53.4%) were male and 48 (46.4%) were female with 97 (94.2%) being spayed/neutered. Slightly over half of the dogs were purebred (53, 51.5%) representing 29 breeds, and the remainder were classified as mixed breed (50, 48.5%). Sixty‐three (61.2%) of the dogs weighed 20 kg or more and 40 (38.8%) weighed less than 20 kg. There were no differences in mean USG among sex, spay/neuter status, purebred classification, or weight.
The overall mean USG was 1.040 (SD 0.011) with a range of 1.011 to 1.060. A detailed description of the mean USG is given in Table 1. The mean difference between the minimum and maximum USG for each dog was 0.015 (SD 0.007). The range of USG measurements acquired over the course of the study is shown in Figure 1. The mean difference was much smaller, however, when examined within week. The mean difference between the minimum and maximum USG for each dog was 0.009 (SD 0.006) for week 1 and 0.010 (SD 0.007) for week 2. The frequency that individual dogs had USG that was above and below the standard USG cutoffs during the 2‐week study period is reported in Table 2. The mean coefficient of variance across all 6 time points was 15.4% (SD 8.97%). The repeated‐measures 1‐way ANOVA did not identify a difference in USG between time points.
Table 1.
Summary of mean urine specific gravity (USG) measurements for 103 dogs from first morning voided samples
| Mean USG range | Number of dogs | Percentage of study population |
|---|---|---|
| 1.010‐1.020 | 4 | 3.4 |
| 1.021‐1.030 | 14 | 13.6 |
| 1.031‐1.040 | 22 | 21.4 |
| 1.041‐1.050 | 42 | 40.8 |
| 1.051‐1.060 | 21 | 20.4 |
| Total | 103 | 100 |
Figure 1.
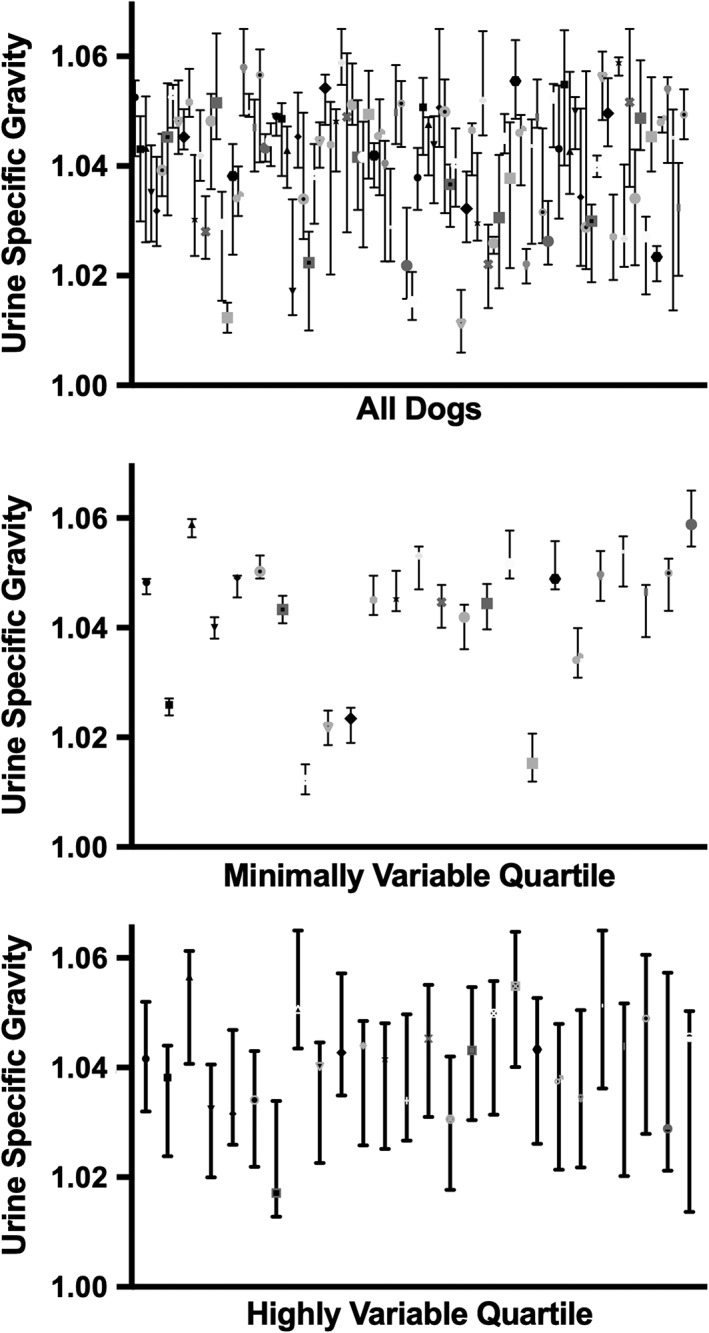
Data from all dogs were analyzed and separated into quartiles based on the range of urine specific gravities measured over the course of the study. Median and range of first morning urine specific gravities are graphed for individual dogs. The graphs represent data from all dogs in the study, the data from dogs in the least variable quartile, and data from the dogs in the most variable quartile
Table 2.
Frequency of urine specific gravity results above and below multiple theoretical diagnostic cutoff points reported over the entire course of the study and within week
| Cutoff point | Number of dogs above and below cutoff during the total study, n (%) | Number of dogs above and below cutoff during week 1, n (%) | Number of dogs above and below cutoff during week 2, n (%) |
|---|---|---|---|
| 1.010 | 2 (1.9) | 2 (1.9) | 1 (0.9) |
| 1.020 | 14 (13.6) | 8 (7.8) | 8 (7.8) |
| 1.030 | 34 (33) | 19 (18.5) | 22 (21.4) |
| 1.040 | 49 (47.6) | 32 (31.1) | 32 (31.1) |
| 1.050 | 44 (42.7) | 31 (30.1) | 34 (33.0) |
When accounting for the effect of day, age, weight, purebred classification, spay/neuter status, and sex, the only variable that had a significant impact on mean USG was age, with a 0.001 (95% CI, 0.0003‐0.0016) decline in USG for each 1 year increase in age. The within‐dog variation was very small with an SD for the within‐dog effect of 0.0063 (95% CI, 0.0060‐0.0067). The within‐week variation (nested within dog) was small as well with an SD of 0.0027 (95% CI, 0.0019‐0.0039).
4. DISCUSSION
The results of this study demonstrated clinically important variability among first morning USG measurements in healthy dogs. In many clinical settings, cutoff points and set classification ranges are used to assist with USG interpretation.14 The average difference noted within the dogs in this study (0.015) among the 6 time points is large enough to change diagnostic interpretation from day to day and week to week. The variability between time points was decreased when week 1 was analyzed separately from week 2. The reason for daily and weekly fluctuations in urine concentration is not evident in the study. A repeated‐measures analysis was performed to investigate at‐large trends in the data but did not reveal significant differences between time points. Environmental and physiologic factors affecting the need for water conservation change daily and fluctuate over time. It is possible that the changing needs in the individual animal might introduce more variability as the time between sampling periods is increased.
Previous intraindividual and interindividual variation in urine concentration was reported in the article by van Vonderen et al in healthy pet dogs.11 They reported a similar range in USG values obtained from morning urine samples in healthy dogs. Coefficient of variation was not reported on USG measurements in that study. However, Uosm was reported to have a coefficient of variation of approximately 34.2%. In our study, less variation (15.4%) was noted on average in USG measurements in dogs over all 6 time points, which might indicate the relative variability in the samples in this study was decreased. However, as the variability of USG was not reported in the previous study, and Uosm measurements were not performed in the study described here, the ability for a direct comparison is limited. The major difference in the study described here is the rigor of the sampling time point in the morning. If there is a true difference, it might be related to the fact that samples were taken immediately upon waking and before morning walks, meals, or drinking, so that sampling would be tightly controlled for the effect of daily routine variables.
Multiple variables were examined for a correlation with USG measurement and did not reveal any significant associations. The only variable that did correlate with decreasing USG values was age. This finding was identified in the previous study published on the variability of USG in healthy dogs and has also been documented in other species.11, 15 In rats, there is decreased expression of aquaporins, urea transporters, and vasopressin V‐2 receptors that are important in urine concentration, which occurs during aging.15 If a similar phenomenon occurs in dogs, this could be responsible for decreased concentrating ability and water conservation in aged dogs. Alternatively, it is possible that the older dogs enrolled in this study had occult disease that was below the limit of detection in the screening protocol.
Variability exists when using refractometers to measure urine containing other analytes, such as marked protein or glucosuria.2 For example, USG increases approximately 0.004 for each g/dL of glucose and 0.003 for each g/dL of protein.16, 17, 18 This study was performed in healthy dogs that were required to be nonproteinuric and nonglycosuric for study inclusion. Future studies should examine urine affected by these variables to further examine the impact they might have on the range of fluctuations in first morning urine concentration. Clinicians should be aware of the fluctuations in the first morning USG measurements either day to day or over the course of longer intervals. These USG measurements may still reflect a true change in the concentrating ability; however, consideration should also be given to normal fluctuations. Further studies should be conducted in dogs affected by polyuria and polydipsia to determine the amount of variability noted in different disease states as well as using USG as a monitoring tool for disease progression or response to treatment. This evidence would provide clinicians useful information to make the most medically sound conclusions about urine concentration.
CONFLICT OF INTEREST DECLARATION
Stephen DiBartola serves as co‐Editor‐in‐Chief for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the animal care and use committee as well as the committee for research conduct at The Ohio State University.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Rudinsky A, Cortright C, Purcell S, et al. Variability of first morning urine specific gravity in 103 healthy dogs. J Vet Intern Med. 2019;33:2133–2137. 10.1111/jvim.15592
REFERENCES
- 1. Fry MM. Urinalysis In: Bartges JP, Polzin DJ, eds. Nephrology and Urology of Small Animals. Ames, IA: Blackwell Publishing Ltd; 2011:46‐57. [Google Scholar]
- 2. George JW. The usefulness and limitations of hand‐held refractometers in veterinary laboratory medicine: an historical and technical review. Vet Clin Pathol. 2001;30:201‐210. [DOI] [PubMed] [Google Scholar]
- 3. Hendriks HJ, de Bruijne JJ, van den Brom WE. The clinical refractometer: a useful tool for the determination of specific gravity and osmolality in canine urine. Tijdschr Diergeneeskd. 1978;103:1065‐1068. [PubMed] [Google Scholar]
- 4. Rudinsky AJ, Wellman ML, Tracy GM, Stoltenberg L, DiBartola SP, Chew DJ. Variability among four refractometers for measurement of urine specific gravity and comparison to urine osmolality in dogs. Vet Clin Pathol. (in Press). [DOI] [PubMed] [Google Scholar]
- 5. Dossin O, Germain C, Braun JP. Comparison of the techniques of evaluation of urine dilution/concentration in the dog. J Vet Med A Physiol Pathol Clin Med. 2003;50:322‐325. [DOI] [PubMed] [Google Scholar]
- 6. Penney MD, Walters G. Are osmolality measurements clinically useful? Ann Clin Biochem. 1987;24:566‐571. [DOI] [PubMed] [Google Scholar]
- 7. Bovee KC. Urine osmolarity as a definitive indicator of renal concentrating capacity. J Am Vet Med Assoc. 1969;155:30‐35. [PubMed] [Google Scholar]
- 8. O'Connor WJ. Drinking by dogs during and after running. J Physiol. 1975;250:247‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Connor WJ, Potts DJ. The external water exchanges of normal laboratory dogs. Q J Exp Physiol Cogn Med Sci. 1969;54:244‐265. [DOI] [PubMed] [Google Scholar]
- 10. Burger IH, Anderson RS, Holme DW. Nutritional factors affecting water balance in the dog and cat In: Anderson RS, ed. Nutrition of the Dog and Cat. Oxford, UK: Pergamon Press; 1980. [Google Scholar]
- 11.van Vonderen IK, Kooistra HS, Rijnberk A. Intra‐ and interindividual variation in urine osmolality and urine specific gravity in healthy pet dogs of various ages. J Vet Intern Med 1997;11:30–35. [DOI] [PubMed] [Google Scholar]
- 12. Reine NJ, Langston CE. Urinalysis interpretation: how to squeeze out the maximum information from a small sample. Clin Tech Small Anim Pract. 2005;20:2‐10. [DOI] [PubMed] [Google Scholar]
- 13. Watson ADJ, Lefebvre HP, Elliot J. Urine specific gravity. International Renal Interest Society. http://www.iris-kidney.com/education/urine_specific_gravity.html. Accessed June 5, 2019.
- 14. Lefebvre H. Renal function testing In: Bartges J, Polzin D, eds. Nephrology and Urology of Small Animals. 1st ed. West Sussex, UK: Wiley‐Blackwell; 2011. [Google Scholar]
- 15. Sands JM. Urine concentrating and diluting ability during aging. J Gerontol A Biol Sci Med Sci. 2012;67:1352‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strasinger SK, Di Lorenzo MS. Physical examination of urine Urinalysis and Body Fluids. Philadelphia, PA: F.A. Davis Company; 2014:647‐661. [Google Scholar]
- 17. Osborne CA, Stevens JB. Urine specific gravity, refractive index, or osmolality: which one would you chose? In: Stevens JB, Osborne CA, eds. Urinalysis: A Clinical Guide to Compassionate Patient Care. Bayer: Shawnee Mission, KS; 1999:73‐85. [Google Scholar]
- 18. Duncan JR, Prasse KW. Clinical examination of the urine. Vet Clin North Am. 1976;6:647‐661. [DOI] [PubMed] [Google Scholar]


