Abstract
Background
Focused cardiac ultrasound (FCU) helps detect occult heart disease in human patients.
Hypothesis
Focused cardiac ultrasound by a nonspecialist practitioner (NSP) will increase the detection of occult heart disease in asymptomatic cats compared with physical examination and ECG.
Animals
Three hundred forty‐three client‐owned cats: 54 excluded and 289 analyzed.
Methods
Multicenter prospective cohort study. Twenty‐two NSPs were trained to perform FCU. Cats without clinical signs of heart disease were recruited, and NSPs performed the following in sequential order: physical examination, ECG, FCU, and point‐of‐care N‐terminal pro‐B‐type natriuretic peptide assay (POC‐BNP). After each step, NSPs indicated yes, no, or equivocal as to whether they believed heart disease was present. The level of agreement between the NSP diagnosis and a blinded cardiologist's diagnosis after echocardiogram was evaluated using Cohen's kappa test.
Results
Cardiologist diagnoses included 148 normal cats, 102 with heart disease, and 39 equivocal ones. Agreement between NSP and cardiologist was slight after physical examination (kappa 0.253 [95% CI, 0.172‐0.340]), did not increase after ECG (0.256 [0.161‐0.345]; P = .96), increased after FCU (0.468 [0.376‐0.558]; P = .002), and the level of agreement was similar after POC‐BNP (0.498 [0.419‐0.580]; P = .67). In cats with mild, moderate, and marked occult heart disease, the proportion of cats having a NSP diagnosis of heart disease after FCU was 45.6%, 93.1%, and 100%, respectively.
Conclusions and Clinical Importance
Focused cardiac ultrasound performed by NSPs increased the detection of occult heart disease, especially in cats with moderate to marked disease. Focused cardiac ultrasound appears to be a feasible and useful tool to assist NSPs in the detection of heart disease in cats.
Keywords: cardiomyopathy, echocardiography, ECG, feline, myocardial disease
Abbreviations
- CI
confidence interval
- FCU
focused cardiac ultrasound
- IVSd
interventricular septum thickness at end‐diastole
- LA:Ao
left atrium to aortic root dimension ratio
- NSP
nonspecialist practitioner
- POC‐BNP
point‐of‐care N‐terminal pro‐B‐type natriuretic peptide assay
1. INTRODUCTION
Heart disease is a common cause of morbidity and mortality in cats.1, 2, 3 Some affected cats are perpetually asymptomatic, whereas others experience life‐threatening clinical manifestations, including cardiac arrhythmias, arterial thromboembolism, syncope, congestive heart failure, or sudden death.4, 5, 6 Identifying cats with occult heart disease is difficult in general practice because the diagnostic tools available to the nonspecialist practitioners (NSPs), including physical examination,1, 2, 5, 7 thoracic radiographs,5, 7, 8, 9 ECG,8, 9 genetic testing,10, 11 and biomarker measurement,12, 13, 14, 15, 16, 17, 18, 19 have limitations. For example, the point‐of‐care N‐terminal pro‐B‐type natriuretic peptide assay (POC‐BNP) quickly and reliably detects moderate to severe occult cardiomyopathy in cats.15 However, false‐negative results are possible in cats with mild heart disease, and false‐positive results are possible in cats with systemic illness such as chronic kidney disease20 or noncardiac respiratory disease.21 Echocardiograms performed by board‐certified veterinary cardiology specialists are considered the clinical reference standard for diagnosing cardiomyopathy in cats5, 22, 23, 24; however, most cats do not ever receive an echocardiographic evaluation.23 Reasons for this include the following: (1) echocardiography requires specialized equipment and advanced technical skills, (2) echocardiography is typically expensive compared with the other available cardiac tests, and (3) there exist a limited number of veterinary cardiologists relative to the number of household cats. It is therefore not uncommon for cats to present to veterinary hospitals with serious cardiac complications without having any documented history of cardiac disease.25, 26, 27
Focused cardiac ultrasound (FCU) is defined as “a focused examination of the cardiovascular system performed by a [veterinarian] by using ultrasound as an adjunct to the physical examination to recognize specific ultrasonic signs that represent a narrow list of potential diagnoses in specific clinical settings.”28 In humans, FCU accurately detects left ventricular hypertrophy, left atrial enlargement, systolic dysfunction, and cavitary effusion, among other findings,28, 29 and when used in conjunction with other diagnostics such as physical examination and ECG, it improves clinical decision‐making.28 In both human patients and in veterinary species in the emergency room setting, focused assessment with sonography for trauma, triage, and tracking protocols help diagnose certain conditions, including cavitary effusions, pleural space disease, and pulmonary disease.28, 29, 30, 31, 32, 33, 34, 35 Focused cardiac ultrasound is useful for differentiating congestive heart failure from non‐cardiac causes of dyspnea in cats.21 The FCU in the present study is distinct from previously described sonographic protocols due to its use in nonemergent clinical scenarios and its concentration on specific heart structures visualized from a limited number of acquired images.28, 29
The use of FCU to screen asymptomatic cats for occult cardiac disease has not been previously described. The goal of this study is to evaluate the use of FCU by NSPs to detect important sonographic cardiac features characteristic of occult heart disease in cats. The hypothesis of this study is that the agreement between NSPs and cardiologists regarding the presence or absence of occult heart disease in cats will improve when FCU is performed after physical examination and ECG.
2. MATERIALS AND METHODS
Enrollment in this multicenter diagnostic test evaluation via prospective cohort spanned from April 2016 through December 2017. All study procedures were approved by the Clinical Studies Review Committee at the Cummings School of Veterinary Medicine at Tufts University and the Institutional Animal Care and Use Committee at the University of Pennsylvania School of Veterinary Medicine. Informed owner consent was obtained for each participating cat.
2.1. Animals
Inclusion criteria were cats older than 1 year of age without signs of heart disease, such as exercise intolerance, difficulty breathing, or collapsing episodes. Exclusion criteria were cats with known prior congestive heart failure or arterial thromboembolism, cats treated with cardiac medications, cats with known systemic hypertension, cats with known uncontrolled hyperthyroidism, cats with IRIS stage 3 or greater kidney disease, cats receiving fluids, cats with active systemic illness, and cats that had an echocardiogram by someone with advanced echocardiographic skills in the last 2 years.
2.2. NSP enrollment
General practice veterinarians, conveniently located near the study centers and with no prior formal cardiac ultrasound training, were recruited to participate in this study via email or telephone to form a cohort of NSPs. Training of the NSPs was modeled after a previously described FCU training course.36 The protocol for teaching NSPs how to perform FCU included the following: (1) participation in an on‐site training session that included a short didactic lesson and a live FCU demonstration by the cardiologist; (2) viewing an online instructional video (https://www.youtube.com/watch?v=I4U8AoxYmok&feature=youtube); and (3) practicing FCU on a cohort of cats with or without cardiologist supervision. The goal of training was to enable NSPs first to obtain adequate right parasternal short axis views of the left atrium and left ventricle and second to subjectively evaluate key cardiac features such as the left atrial size, left ventricular wall thickness, left ventricular contractile function, as well as the presence or absence of cavitary effusions. Nonspecialist practitioners were instructed to decide whether cardiac abnormalities were present using the following guidelines: (1) when looking at the short axis view of the left atrium, if the area of the left atrium is more than 2.5 times the area of the aorta, the diameter of the left atrium is more than 1.5 times the diameter of the aorta, or both, then the left atrium is likely enlarged; (2) when looking at the short axis view of the left ventricle, if the wall thickness is more than 40% of the left ventricle cavity diameter, if there are subjectively prominent papillary muscles, if the left ventricle cavity appears small in systole, or all the three, then the cardiac musculature is likely thickened; and (3) if the ventricle appears to contract less than half the way down with each contraction, if the vigor of contraction is subjectively less than normal, or both, then left ventricle contractile function could be compromised. The NSPs were shown case examples of these abnormalities via the online instructional video, presentation by the cardiologist, or both. Case examples included hypertrophic cardiomyopathy, dilated cardiomyopathy, pericardial effusion, and pleural effusion. Nonspecialist practitioners were encouraged to practice the FCU before enrolling cats in the study until they felt comfortable obtaining the views and identifying abnormalities. The recommended number of FCUs for practice was 25 to 30 cats before enrolling cats in the study. It was anticipated that knowledge regarding the variations of normal atrial size, ventricular function, and wall thickness would be further developed based on these 25 to 30 practice examinations.
2.3. Study sessions
After training was completed by a NSP, apparently healthy cats meeting the inclusion criteria were recruited from clients and clinic employees for the incentive of echocardiographic evaluation and advancing veterinary science. Cats with a history of a heart murmur, gallop, or arrhythmia were not excluded from participation. The cardiologist and the NSP both assessed all cats at the clinic where the NSP routinely practiced, and the NSP performed all FCUs using the ultrasound unit and probe already available at their clinic. The cardiologist and the NSP evaluated cats in separate rooms and were blinded to each other's findings. Technicians or other veterinarians were present to help with restraint, phlebotomy, and obtaining the ECG recordings.
2.4. NSP cardiac evaluation
Data collection was achieved using a standardized form, and the order in which the diagnostic tests were performed was strictly controlled. After each of the diagnostic tests, the NSPs were asked whether they thought the cat had heart disease (yes, no, or equivocal). First, the NSP performed a physical examination with cardiac auscultation. Heart rate and respiratory rate as well as the presence of murmurs, gallops, and arrhythmias were recorded. Second, an ECG was obtained via a handheld smartphone device (Alivecor, Mountain View, California) or single or 6‐lead ECG machine. The NSPs were instructed to consider the ECG rhythm and the heart rate. Third, FCU was performed by the NSP. The NSPs were asked to subjectively assess whether left atrial enlargement, left ventricular hypertrophy, cavitary effusions, or decreased contractility were present. Fourth and finally, venous blood was collected for a commercial POC‐BNP (Idexx Laboratories, Westbrook, Maine), and the assay result was recorded as negative or positive. After each of the 4 diagnostic steps, and before performing the next diagnostic test, NSPs indicated yes, no, or equivocal as to whether they thought the cat had heart disease based on all the information and diagnostics that had been accumulated up until that point.
2.5. Board‐certified cardiologist evaluation
A board‐certified cardiology specialist made a clinical reference diagnosis based on (1) the review of the NSP's ECG and POC‐BNP result, (2) their own physical examination, and (3) M‐mode, 2D, and Doppler color flow echocardiogram. In addition to echocardiographic evaluation, cardiologists specifically noted the degree of left atrial enlargement and left ventricular hypertrophy as none, mild, moderate, or marked. Based on their findings, the cardiology specialist diagnosed each cat as normal, equivocal, or having heart disease. For those cats with heart disease, the cardiologist reported a subjective interpretation of overall disease severity as mild, moderate, or marked.
2.6. Statistical analysis
Descriptive statistics of cat demographics and diagnostic findings were generated. Normality of data was tested using Shapiro‐Wilk tests and visual inspection of frequency histograms. Comparisons between cat groups were performed either using Kruskal‐Wallis tests followed by Wilcoxon rank‐sum tests for pairwise comparisons or using one‐way analysis of variance followed by pairwise t‐tests or Sidek multiple comparisons tests. The level of agreement between NSPs and cardiologists was determined by calculation of Cohen's kappa. With respect to evaluation of the presence or absence of heart disease after each diagnostic step, NSPs could select 1 of 3 different responses (yes, no, or equivocal). An equivocal response meant that the observer's certainty about the presence of heart disease was between a yes and a no response. To account for the presence of this uncertainty, the prespecified analysis plan called for the use of weighted kappa analyses,37 whereby concordant diagnoses of yes, no, or equivocal were assigned a weight of 1, discordant diagnoses that involved an equivocal diagnosis by either the NSP or cardiologist were assigned a weight of 0.5, and discordant diagnoses not involving an equivocal diagnosis were assigned a weight of 0. The 3 × 3 table of kappa weights is shown in Figure 1. For secondary end points involving the level of agreement between dichotomous responses, unweighted kappa analyses of the 2 × 2 table were performed. Levels of agreement based on kappa values were prespecified as follows: poor, 0.00‐0.20; slight, 0.21‐0.40; moderate, 0.41‐0.60; good, 0.61‐0.80; very good, 0.81‐1.00.38 To determine the effect of each individual diagnostic test on the number and proportion of cats that received a correct (ie, concordant) or incorrect (ie, discordant) diagnosis, cases were tabulated and assigned to 1 of 4 categories after each diagnostic step as follows: category C‐C, cases with a correct diagnosis both before and after the diagnostic test; category I‐C, cases with an incorrect diagnosis before and correct diagnosis after the test; category C‐I, cases with a correct diagnosis before and incorrect diagnosis after the test; category I‐I, cases with an incorrect diagnosis both before and after the test. Cases were further tabulated by disease severity. To explore how different diagnostic findings influenced the likelihood that the NSP would diagnose a case as having heart disease after FCU, univariate and multivariate logistic regression was performed with the NSP diagnosis after FCU as the dependent variable. Cases with an equivocal diagnosis by the NSP were excluded. Variables with P values <.2 on univariable regression were entered into a multivariable model, and backward selection was performed until all remaining variables had a P value <.05. Statistical analysis was performed using commercial software (Stata 14.2, Stata Corporation, College Station, Texas; Prism 7.0d, GraphPad Software, Inc, La Jolla, California). P < .05 was considered significant. Continuous data are displayed as median (IQR) unless otherwise specified.
Figure 1.
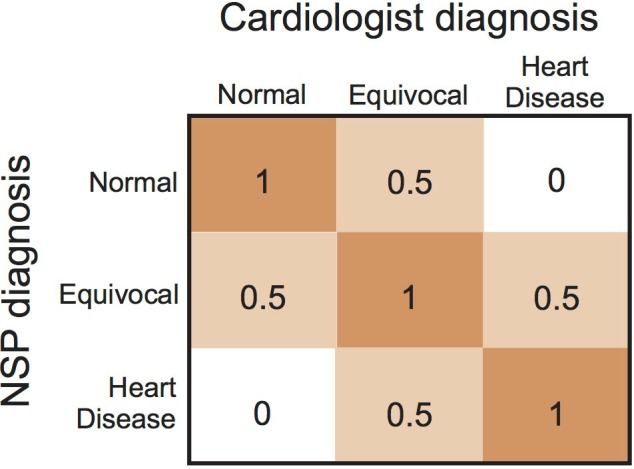
Kappa weights for analyses of the level of agreement between assessment of the nonspecialist practitioner (NSP) and cardiologist for the presence or absence of heart disease in cats
3. RESULTS
Three hundred forty‐three cats were enrolled. No cats were sedated for the purpose of FCU. Twenty‐eight cats were too fractious to be restrained for the study procedures and were excluded. An additional 26 cats were excluded due to inability of the NSPs to obtain FCU images they felt were suitable for interpretation. The remaining 289 of 343 cats (84.3%) formed the analysis set. Within this set, cardiologists assigned the following diagnoses: normal, 148 of 289 (51.2%); heart disease, 102 of 289 (35.3%); and equivocal, 39 of 289 (13.5%). Three cardiology specialists from 2 veterinary medical universities participated. The Cummings School of Veterinary Medicine at Tufts University served as the primary coordinating site for 209 of 289 cats (72.3%) and the University of Pennsylvania School of Veterinary Medicine for 80 of 289 cats (27.7%). Twenty‐two different NSPs from 16 different veterinary practices participated in the study. The median number of cats examined by each NSP was 11 (range, 1‐44).
Differences in signalment and physical examination variables among cats diagnosed as normal, with heart disease, and equivocal are shown in Table 1. The median age of the entire study population was 9 (5‐12) years, with normal cats being significantly younger than cats diagnosed with heart disease or being equivocal. The median body weight was 5.2 (4.3‐6.0) kg, with cats having heart disease being significantly heavier than normal cats. One hundred fourteen (39.4%) cats were female, and 175 of 289 cats (60.6%) were male. Male cats made up a significantly higher proportion of cats with heart disease compared to cats that were normal (P = .02). The median heart rate was 200 (180‐220) bpm, and the median respiratory rate was 40 (30‐52) breaths per minute with no significant differences between cat groups for either variable. Most cats in the study were domestic short hair (187/289, 64.7%), followed by domestic long hair (52/289, 18.0%) and Maine Coon (11/289, 3.8%).
Table 1.
Signalment, body weight, heart rate, and respiratory rate of the study cohort
| Normal n = 148 | Heart disease n = 102 | Equivocal n = 39 | P | |
|---|---|---|---|---|
| Agea | 7 (4‐10) | 10 (7‐12)b | 10 (7‐14)c | <.001 |
| n | 147 | 101 | bversus normal, <.001 | |
| cversus normal, <.001 | ||||
| Body weight | 4.9 (4.0‐6.0) | 5.5 (4.7‐6.0)b | 5.3 (3.8‐6.0) | .008 |
| bversus normal, .002 | ||||
| Sex (F/M) | 68/80 | 29/73b | 17/22 | .02 |
| bversus normal, .02 | ||||
| Heart rate | 200 (180‐200) | 200 (180‐220) | 190 (180‐210) | .23 |
| Respiratory rate | 40 (30‐60) | 40 (32‐50) | 40 (30‐50) | .84 |
| n | 146 | 100 | 38 |
The age of 2 cats that were adopted from a shelter could not be accurately determined.
The specific diagnoses and subjective disease severities in the 102 cats with a reference diagnosis of heart disease are shown in Table 2. Hypertrophic cardiomyopathy was the most common diagnosis (42/102, 41.2%), followed by hypertrophic obstructive cardiomyopathy (37/102, 36.3%) and restrictive or unclassified cardiomyopathy (10/102, 9.8%). Sixty‐seven of 102 (65.7%) cats were judged to have mild disease; 29 of 102 (28.4%) cats to have moderate disease, and 6 of 102 (5.9%) cats to have marked disease.
Table 2.
Primary diagnosis and disease severity of 102 cats with a reference diagnosis of heart disease by a cardiologist
| Severity | ||||
|---|---|---|---|---|
| Diagnosis | Mild | Moderate | Marked | Total |
| Hypertrophic cardiomyopathy | 37 | 3 | 2 | 42 |
| Hypertrophic obstructive cardiomyopathy | 19 | 17 | 1 | 37 |
| Restrictive/unclassified cardiomyopathy | 6 | 3 | 1 | 10 |
| Valve disease | 2 | 1 | 1 | 4 |
| Dilated cardiomyopathy | 0 | 3 | 1 | 4 |
| Othera | 3 | 2 | 0 | 5 |
| Total | 67 | 29 | 6 | 102 |
Other includes 1 cat with each of the following diagnoses: pericardial effusion, patent ductus arteriosus, ventricular septal defect, complete atrioventricular nodal block, and arrhythmogenic right ventricular cardiomyopathy.
The level of agreement between the NSP and the cardiologist's reference diagnosis after each diagnostic step is shown in Table 3. After physical examination, agreement between the NSP and cardiologist diagnosis was slight, with kappa of 0.253 (95% CI, 0.172‐0.340). After physical examination and ECG, agreement remained slight, with kappa of 0.256 (95% CI, 0.161‐0.345) and no significant difference in the level of agreement versus the previous assessment (P = .96). After physical examination, ECG, and FCU, agreement between the NSP and cardiologist diagnosis increased to moderate, with kappa of 0.468 (95% CI, 0.376‐0.558). The level of agreement after FCU was significantly increased versus the evaluation based on physical examination and ECG alone (P = .002). After performing POC‐BNP in addition to the physical examination, ECG, and FCU, agreement remained moderate, with kappa of 0.498 (95% CI, 0.419‐0.580). The level of agreement after POC‐BNP was not significantly different versus the previous step (P = .67).
Table 3.
Level of agreement (kappa) and 95% confidence intervals between the NSP and cardiologist diagnosis after sequential performance of physical examination, ECG, FCU, and POC‐BNP
| Level of agreement with cardiologist | ||
|---|---|---|
| Diagnosis by NSP | Kappa | Pairwise difference |
| 1. After physical examination | 0.253 | |
| (0.172‐0.340) | ||
| P < .001 | ||
| n = 288 | ||
| 2. After physical examination and ECG | 0.256 | versus 1: P = .96 |
| (0.161‐0.345) | ||
| P < .001 | ||
| n = 278 | ||
| 3. After physical examination, ECG, and FCU | 0.468 | versus 2: P = .002 |
| (0.376‐0.558) | ||
| P < .001 | ||
| n = 289 | ||
| 4. After physical examination, ECG, FCU, and POC‐BNP | 0.498 | versus 3: P = .67 |
| (0.419‐0.580) | ||
| P < .001 | ||
| n = 287 | ||
Pairwise P values indicate whether or not kappa was significantly different between each sequential diagnostic step.
Abbreviations: FCU, focused cardiac ultrasound; NSP, nonspecialist practitioner; POC‐BNP, point‐of‐care N‐terminal pro‐B‐type natriuretic peptide assay.
Agreement between the NSP and cardiologist after each diagnostic step is shown in Figure 2. Focused cardiac ultrasound examiners correctly diagnosed 121 of 289 (41.9%) cats after physical examination and 124 of 278 (44.6%) cats after ECG. This number increased to 180 of 289 (62.3%) cats after FCU and remained similar at 180 of 287 (62.7%) cats after POC‐BNP. The number of cats in each of the 4 reclassification categories based on the correctness of the before‐versus‐after diagnostic test results is shown in Figure 3. The largest gain in the number of cats in Category I‐C occurred after FCU. Specifically, 79 cats with an incorrect diagnosis before FCU were correctly diagnosed after FCU, including 35 normal cats, 39 with heart disease, and 5 equivocal. Twenty‐seven cats in Category C‐I were misclassified after FCU, including 13 normal cats, 3 with heart disease, and 11 equivocal.
Figure 2.
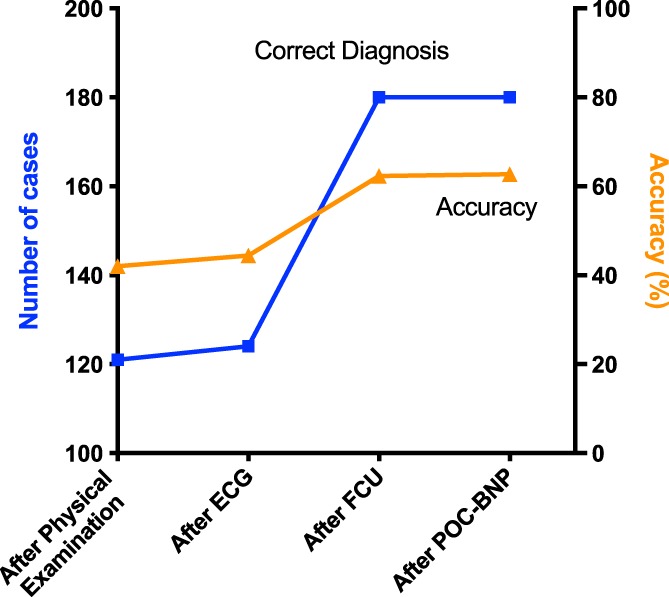
Number (blue line and squares, left‐hand axis) and percentage (orange line and triangles, right‐hand axis) of cats correctly diagnosed by the nonspecialist practitioner after each step of the diagnostic plan, namely after physical examination, after ECG, after focused cardiac ultrasound (FCU) and after point‐of‐care N‐terminal pro‐B‐type natriuretic peptide assay (POC‐BNP). The greatest increase in the number and percentage of cases correctly diagnosed occurred after FCU
Figure 3.
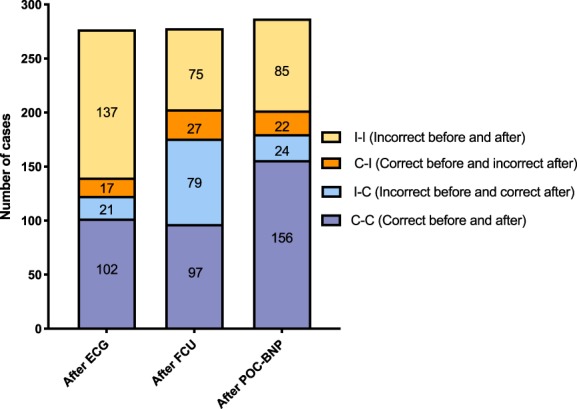
Number of cats in each of 4 reclassification categories based on whether the nonspecialist practitioner's (NSP's) diagnosis of heart disease was correct before or after ECG, focused cardiac ultrasound (FCU), or point‐of‐care N‐terminal pro‐B‐type natriuretic peptide assay (POC‐BNP). The value of performing a diagnostic test lies with either confirming a diagnosis that is already correct (C‐C) or changing an incorrect diagnosis to a correct one (I‐C). The negative value of a diagnostic test occurs when an incorrect diagnosis is not corrected (I‐I) or a correct diagnosis is changed as a result of the test to an incorrect one (C‐I). After performing ECG, the NSP changed the diagnosis from an incorrect one to correct one in 21 cats and from a correct to incorrect diagnosis in 17 cats; 102 cats had a correct diagnosis and 137 cats had an incorrect diagnosis both before and after ECG diagnosis. The NSP then performed FCU, after which the diagnosis was changed from an incorrect one to a correct one in 79 cats and from a correct to incorrect one in 27 cats; 97 cats had a correct diagnosis and 75 cats had an incorrect diagnosis both before and after FCU diagnosis. After performing POC‐BNP, the NSP diagnosis was changed from an incorrect one to a correct one in 24 cats and from a correct to incorrect diagnosis in 22 cats; 156 cats had a correct diagnosis and 85 cats had an incorrect diagnosis both before and after FCU diagnosis
After performance of FCU, 88 of 289 (30.4%) cats were judged by the NSP to have heart disease, including 6 of 6 (100%) cats assessed as having marked heart disease by the cardiologist, 27 of 29 (93.1%) cats with moderate heart disease, and 31 of 67 (46.3%) cats with mild heart disease.
The presence or absence of select physical examination and qualitative FCU findings along with the associated level of agreement between the NSP and the cardiologist in detecting these findings is shown in Table 4. Heart murmurs were commonly heard in cats with heart disease as well as in normal cats by both types of examiners. The detection of arrhythmias on physical examination or ECG was uncommon. There was moderate agreement between the NSP and cardiologist regarding the presence of a heart murmur, slight agreement regarding the presence of a gallop or arrhythmia, and slight agreement regarding qualitative assessment of left atrial enlargement or left ventricular hypertrophy found via FCU or echocardiogram.
Table 4.
Proportion of cats assessed as having selected physical examination or ultrasound findings based on whether the examiner was the NSP or the cardiologist and the level of agreement (kappa) and 95% confidence intervals between the 2 examiner types across the study cohort
| Proportion of cats in reference diagnosis groups (normal; heart disease; equivocal) | Level of agreement between NSP and cardiologist | |||
|---|---|---|---|---|
| Parameter | NSP | Cardiologist | Kappa (95% CI) | P |
| Heart murmur (Y/N) | 61/87; 75/27; 23/16 | 68/80; 86/16; 22/17 | 0.583 | <.001 |
| (0.485‐0.676) | ||||
| Gallop rhythm (Y/N) | 11/137; 8/93; 5/34 | 5/143; 11/91; 3/36 | 0.272 | <.001 |
| (0.077‐0.449) | ||||
| Arrhythmia‐auscultation (Y/N) | 8/140; 11/90; 2/36 | 3/145; 25/77; 0/38 | 0.309 | <.001 |
| (0.124‐0.486) | ||||
| Left atrial enlargement‐FCU (Y/N) | 6/142; 23/79; 3/36 | 1/147; 53/49; 7/32 | 0.333 | <.001 |
| (0.205‐0.460) | ||||
| Left ventricular‐hypertrophy FCU (Y/N) | 33/115; 68/32; 15/24 | 4/144; 82/20; 12/27 | 0.375 | <.001 |
| (0.269‐0.472) | ||||
Abbreviations: CI, confidence interval; FCU, focused cardiac ultrasound; NSP, nonspecialist practitioner.
The factors associated with the likelihood of a NSP achieving a diagnosis of heart disease after FCU was explored by univariable and multivariable logistic regression. The NSPs diagnosed heart disease in 26 of 26 (100%) cats they assessed to have atrial enlargement, which precluded entry of this variable into the exploratory analysis. To explore the influence of the left heart size and structure on the NSP diagnosis, cardiologist‐derived quantitative echocardiographic data were included in the analysis, with the exploratory theory that the magnitude of structural change would impact the NSP assessment. Table 5 displays univariate regressions of 13 different signalment, physical examination, and echocardiographic variables. Based on these results, 7 variables were carried into the multivariable model. Following backward selection, 3 variables, including the presence of a murmur, increased interventricular septum thickness at end‐diastole (IVSd), and increased left atrium to aortic root dimension ratio (LA:Ao), were found to independently predict a diagnosis of heart disease by the NSP after FCU. The presence of a heart murmur increased the likelihood of the NSP diagnosing heart disease after FCU 11.8 (95% CI, 5.15‐27.0; P < .001) times. For every 0.1 cm increase in the IVSd, the likelihood increased by 3.66 (2.31‐5.78; P < .001) times, and for every 0.1 increase in the LA:Ao, the likelihood of increased by 1.42 (1.19‐1.70; P < .001) times.
Table 5.
Univariable logistic regression analysis exploring the effect of various cat characteristics and diagnostic findings on whether or not the NSP diagnosed heart disease in asymptomatic cats after performing focused cardiac ultrasound examination
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Signalment | |||
| Age | 1.08 | 1.01‐1.15 | .02 |
| Weight | 0.93 | 0.76‐1.15 | .52 |
| Sex (M) | 1.31 | 0.76‐2.26 | .33 |
| NSP evaluation | |||
| Heart murmur (Y) | 10.9 | 5.59‐21.2 | <.001 |
| Gallop rhythm (Y) | 1.32 | 0.47‐3.67 | .60 |
| Arrhythmia (Y) | 2.20 | 0.83‐5.79 | .11 |
| Heart rate (10 bpm) | 1.09 | 0.99‐1.19 | .07 |
| Respiratory rate (10 bpm) | 0.91 | 0.78‐1.08 | .28 |
| Cardiologist echocardiogram | |||
| LVIDd (0.1 cm) | 0.93 | 0.81‐1.06 | .27 |
| LVIDs (0.1 cm) | 0.95 | 0.83‐1.08 | .42 |
| IVSd (0.1 cm) | 3.64 | 2.48‐5.33 | <.001 |
| LVPWd (0.1 cm) | 2.27 | 1.66‐3.09 | <.001 |
| LA:Ao (0.1) | 1.39 | 1.21‐1.61 | <.001 |
Abbreviations: CI, confidence interval; IVSd, interventricular septum thickness at end‐diastole; LA:Ao, left atrium to aortic root dimension ratio; LVIDd, left ventricular internal diameter at end‐diastole; LVIDs, left ventricular internal diameter at end‐systole; LVPWd, left ventricular posterior wall thickness at end‐diastole; NSP, nonspecialist practitioner.
4. DISCUSSION
This study reports the utility of FCU performed by NSPs for the detection of occult heart disease in cats. Focused cardiac ultrasound performed by trained NSPs resulted in a significant improvement in agreement with a cardiologist's diagnosis as compared to NSP assessment based on physical examination and ECG alone, especially in cats with moderate or marked occult heart disease. Early diagnosis of cardiac disease in cats via FCU could lead to better medical management and improved veterinary care because certain medications might be helpful in reducing the risk of congestive heart failure or arterial thromboembolism and because veterinarians might be better able to assess risk in cats being treated for other medical conditions.23, 39 For example, knowledge of cardiac status could alter decisions about surgical procedures, anesthetic protocols, fluid therapy plans, and medication administration.
The results of the present study are in general agreement with studies investigating the use of FCU as a diagnostic modality in human patients. Focused cardiac ultrasound is used to screen populations of young athletes for predisposing factors of sudden death40, 41; screen resource‐limited populations for evidence of rheumatic heart disease, hypertension, or other cardiac abnormalities42, 43; and identify cardiac‐related anesthetic risk factors in patients preoperatively.44, 45 The World Interactive Network Focused on Critical UltraSound stated that FCU used in asymptomatic human populations shows, “promise in detecting unexpected relevant cardiac abnormalities” and made a strong recommendation that FCU “is useful in screening human patients at risk for cardiovascular disease.”29
An important aspect of our study was its ability to assess the sequential and additive value of various diagnostic tests to the NSP's evaluation. The study design enabled quantification of the added benefit of each test as opposed to evaluation of each test in isolation. This approach more closely mimics how a NSP would evaluate cats in general practice, first by performing physical examination, possibly followed by an ECG, and then by performing FCU to determine whether more intensive examination by a cardiologist would be warranted. Our results are consistent with previous reports that found physical examination1, 2 and ECG7, 8, 9 to be unreliable in diagnosing heart disease in cats. In our study, physical examination alone as well as physical examination plus ECG performed poorly in detecting occult disease.
The greatest incremental benefit with regard to diagnostic accuracy came from performing FCU. The ability of FCU to improve the detection of heart disease in cats compared with physical examination and ECG is consistent with what has been shown in human medicine.28, 29, 46 For example, FCU significantly improves the diagnostic accuracy of medical students and junior doctors in identification of various abnormalities, including the left and right ventricular systolic dysfunction, valvular changes, pericardial effusions, and aortic root dilatation when it is performed after history taking, physical examination, and ECG.46 Agreement between the medical student or junior doctor and the cardiologist significantly increases from moderate (kappa of 0.49 ± 0.22) after history, physical examination, and ECG to good (kappa of 0.75 ± 0.28) after FCU is performed.46
In the current study, NSP diagnostic accuracy after POC‐BNP was not significantly increased compared with diagnostic accuracy after FCU. A previous study reported that POC‐BNP detected occult cardiomyopathy in cats with relatively high sensitivity and specificity.15 Future studies might be designed to directly compare the utility of FCU versus POC‐BNP or to investigate the cost‐effectiveness of the available diagnostics, including ECG, POC‐BNP, FCU, and traditional echocardiography. Results could be helpful to identify instances in which one test might be beneficial over another and to determine the appropriate cost to the client for performance of FCU by a NSP. A cost analysis study in human medicine revealed that FCU was the most cost‐effective strategy for identifying occult left ventricular dysfunction in human patients with abnormal ECGs or natriuretic peptide levels.47
Diagnostic tests are 2‐edged swords: benefit lies in an incorrect diagnosis before a test being corrected after the test (ie, reclassification category I‐C), while potential harm lies in a correct diagnosis being erroneously changed to an incorrect diagnosis after the test (ie, reclassification category C‐I). Therefore, despite the ability of FCU to increase the number of cats that NSPs correctly identify with heart disease and correctly identify as not having heart disease, it is important to evaluate the effect of erroneously changing a correct diagnosis and the potential effects on medical care. In the present study, 3 cats previously diagnosed correctly were misclassified as not having heart disease by the NSP after FCU. All 3 cats were assessed as having mild heart disease by the cardiologist. Thus, it seems unlikely that misclassification of these 3 cats would lead to a significant change in case management. Thirteen cats were misclassified as having heart disease by the NSP after FCU. In these cats, suspicion of heart disease when heart disease is not present could result in unnecessary referral costs and delayed or canceled procedures or treatments. These cases highlight the fact that FCU is not a substitute for further evaluation by a specialist. A key premise of FCU is that it will facilitate “subsequent referral for an echocardiographic study [that] will delineate and measure all findings … which may go unrecognized by FCU.”28
In the present study, agreement between the NSP and the cardiologist regarding the diagnosis of heart disease after FCU significantly improved despite a level of agreement for individual echocardiographic variables, such as the presence of left atrial enlargement or left ventricular hypertrophy, which was less than in studies of human patients.48, 49, 50, 51, 52, 53, 54, 55, 56 These results suggest that the NSPs in our study considered a variety of different variables, both FCU and non‐FCU based, in making their diagnosis. Indeed, our exploratory analysis indicated that the 3 most influential variables leading NSPs to diagnose heart disease included the presence or absence of a heart murmur as well as the left atrial size and thickness of the interventricular septum. In our study, NSPs were relatively good at identifying cats with severe wall thickening but were not consistently able to identify cats with mild wall thickening. Therefore, some cats with mild heart disease will be missed by FCU. Although a full echocardiogram is recommended for cats with findings that are questionable or suggestive of heart disease, not all cats will receive follow‐up with a cardiologist due to logistical or financial limitations. The usefulness of serial FCUs to monitor cats for progression into the moderate or marked stage of disease could be considered an area for future study.
In many respects, the current study evaluated the effectiveness (as opposed to the efficacy) of FCU‐based detection of occult heart disease in cats. In general, efficacy refers to the utility of an intervention under ideal circumstances, whereas effectiveness refers to the utility under less than ideal (ie, “real‐world”) circumstances.57 Important “real‐world” aspects of the current study involved training of personnel that had little or no experience with cardiac ultrasonography. Practitioners in general practice were particularly recruited, as this is the population of veterinarians most likely to perform FCU‐based screening for occult heart disease. NSPs performed FCU using ultrasound machines that existed in their practices, the vast majority of which were small portable general‐purpose machines. Cats can be difficult to restrain, and the study found that FCU could not be performed on 15.7% of the enrolled cases due to either fractious temperament or inability of the NSP to obtain images suitable for interpretation. Despite these less‐than‐ideal conditions, FCU improved the detection of occult disease. Studies in human medicine suggest that FCU improves the accuracy of clinical diagnosis regardless of whether or not the person performing the examination has extensive experience with ultrasound imaging.29, 46, 52, 54 Further study might investigate whether alternate training methods or more experience can lower the rate of inadequate FCU examinations or improve the diagnostic accuracy of FCU in cats.
Our study had several limitations. Diagnosing mild forms of heart disease in cats is difficult due to substantial overlap between pathology and normal physiology, especially in geriatric cats.5, 22, 24, 58 Echocardiography is the clinical norm for antemortem diagnosis and is commonly used as the gold standard in clinical studies.39 Future studies could consider using a panel of cardiologists to achieve a consensus diagnosis. In the current study, physical examination by the attending cardiologist was considered an important piece of the eventual diagnosis, and examination of each cat by multiple cardiologists was not feasible. The clinical utility of FCU demonstrated in the present study is only applicable to cats with a previously performed physical examination and ECG. As previously mentioned, our study cannot determine the performance of the diagnostic tests done in a different order. It is unknown whether FCU would improve detection if POC‐BNP had been done before and not after FCU. Certain diagnostic tests, including thoracic radiographs, blood pressure, genetic testing, and thyroid measurement, were not evaluated by this study, and how the performance of these tests before FCU would affect NSP agreement with cardiologist diagnosis requires further study. Various inherent aspects of the FCU itself were not evaluated. Further study might investigate differences in ultrasound devices, quality of FCU attributable to cat weight, body condition, restraint, or other physical factors, alternate FCU training protocols, NSP experience, and cost‐effectiveness of FCU compared to standard practice. Finally, the effect of FCU detection of occult disease on future morbidity and mortality in individual cats is an important aspect that warrants further study.
In conclusion, FCU performed by NSPs significantly increased the differentiation of cats with occult heart disease that had previously received physical examination and ECG. A high proportion of cats diagnosed with moderate to marked occult disease were detected by FCU. Focused cardiac ultrasound performed by NSPs is a practicable and helpful diagnostic tool, and further study of FCU is warranted.
CONFLICT OF INTEREST DECLARATION
Within the past 3 years, John E. Rush has received speaker honoraria, travel, and accommodation reimbursement and grant funding from IDEXX. Mark A. Oyama has received grant funding from IDEXX.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Penn (#805655); Clinical Studies Review Committee: Tufts (#033‐14).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank the practitioners that participated in the study as well as technicians Kristen Antoon, Sarah Cass, and Lila Sierra and doctors Lisa Freeman, Emily Karlin, Amelie Beaumier‐Primeau, Megan Poad, and Alex Crooks for their support. Special thanks to Kevin Connelly for assistance in developing the training video used by the primary care veterinarians.
Loughran KA, Rush JE, Rozanski EA, Oyama MA, Larouche‐Lebel É, Kraus MS. The use of focused cardiac ultrasound to screen for occult heart disease in asymptomatic cats. J Vet Intern Med. 2019;33:1892–1901. 10.1111/jvim.15549
Funding information Barkley Fund; Morris Animal Foundation, Grant/Award Number: D14FE‐502
REFERENCES
- 1. Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc. 2009;234:1398‐1403. [DOI] [PubMed] [Google Scholar]
- 2. Wagner T, Fuentes VL, Payne JR, McDermott N, Brodbelt D. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol. 2010;12:171‐182. [DOI] [PubMed] [Google Scholar]
- 3. Payne JR, Brodbelt DC, Luis FV. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol. 2015;17(Suppl 1):S244‐S257. [DOI] [PubMed] [Google Scholar]
- 4. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985‐1989). J Am Vet Med Assoc. 1992;201:613‐618. [PubMed] [Google Scholar]
- 5. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990‐1999). J Am Vet Med Assoc. 2002;220:202‐207. [DOI] [PubMed] [Google Scholar]
- 6. Payne JR, Borgeat K, Brodbelt DC, et al. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015;17(Suppl 1):S318‐S328. [DOI] [PubMed] [Google Scholar]
- 7. Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994‐2001). J Feline Med Surg. 2003;5:151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schober KE, Maerz I, Ludewig E, Stern JA. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2‐dimensional echocardiography. J Vet Intern Med. 2007;21:709‐718. [DOI] [PubMed] [Google Scholar]
- 9. Moise NS, Dietze AE, Mezza LE, et al. Echocardiography, electrocardiography, and radiography of cats with dilatation cardiomyopathy, hypertrophic cardiomyopathy, and hyperthyroidism. Am J Vet Res. 1986;47:1476‐1486. [PubMed] [Google Scholar]
- 10. Wess G, Schinner C, Weber K, Küchenhoff H, Hartmann K. Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine coon and other breed cats. J Vet Intern Med. 2010;24:527‐532. [DOI] [PubMed] [Google Scholar]
- 11. Granstrom S, Godiksen MT, Christiansen M, et al. Genotype‐phenotype correlation between the cardiac myosin binding protein C mutation A31P and hypertrophic cardiomyopathy in a cohort of Maine coon cats: a longitudinal study. J Vet Cardiol. 2015;17(Suppl 1):S268‐S281. [DOI] [PubMed] [Google Scholar]
- 12. Hsu A, Kittleson MD, Paling A. Investigation into the use of plasma NT‐proBNP concentration to screen for feline hypertrophic cardiomyopathy. J Vet Cardiol. 2009;11(Suppl 1):S63‐S70. [DOI] [PubMed] [Google Scholar]
- 13. Connolly DJ, Magalhaes RJ, Syme HM, et al. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med. 2008;22:96‐105. [DOI] [PubMed] [Google Scholar]
- 14. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med. 2011;25:1010‐1016. [DOI] [PubMed] [Google Scholar]
- 15. Machen MC, Oyama MA, Gordon SG, et al. Multi‐centered investigation of a point‐of‐care NT‐proBNP ELISA assay to detect moderate to severe occult (pre‐clinical) feline heart disease in cats referred for cardiac evaluation. J Vet Cardiol. 2014;16:245‐255. [DOI] [PubMed] [Google Scholar]
- 16. Langhorn R, Tarnow I, Willesen JL, Kjelgaard‐Hansen M, Skovgaard IM, Koch J. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28:1485‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borgeat K, Sherwood K, Payne JR, Luis Fuentes V, Connolly DJ. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28:1731‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tominaga Y, Miyagawa Y, Toda N, et al. The diagnostic significance of the plasma N‐terminal pro‐B‐type natriuretic peptide concentration in asymptomatic cats with cardiac enlargement. J Vet Med Sci. 2011;73:971‐975. [DOI] [PubMed] [Google Scholar]
- 19. Wess G, Daisenberger P, Mahling M, Hirschberger J, Hartmann K. Utility of measuring plasma N‐terminal pro‐brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol. 2011;40:237‐244. [DOI] [PubMed] [Google Scholar]
- 20. Lalor SM, Connolly DJ, Elliott J, Syme HM. Plasma concentrations of natriuretic peptides in normal cats and normotensive and hypertensive cats with chronic kidney disease. J Vet Cardiol. 2009;11(Suppl 1):S71‐S79. [DOI] [PubMed] [Google Scholar]
- 21. Ward JL, Lisciandro GR, Ware WA, et al. Evaluation of point‐of‐care thoracic ultrasound and NT‐proBNP for the diagnosis of congestive heart failure in cats with respiratory distress. J Vet Intern Med. 2018;32:1530‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92:2645‐2651. [DOI] [PubMed] [Google Scholar]
- 23. Fox PR, Schober KA. Management of asymptomatic (occult) feline cardiomyopathy: challenges and realities. J Vet Cardiol. 2015;17(Suppl 1):S150‐S158. [DOI] [PubMed] [Google Scholar]
- 24. Haggstrom J, Luis Fuentes V, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. 2015;17(Suppl 1):S134‐S149. [DOI] [PubMed] [Google Scholar]
- 25. Smith SA, Tobias AH, Jacob KA, Fine DM, Grumbles PL. Arterial thromboembolism in cats: acute crisis in 127 cases (1992‐2001) and long‐term management with low‐dose aspirin in 24 cases. J Vet Intern Med. 2003;17:73‐83. [DOI] [PubMed] [Google Scholar]
- 26. Schoeman JP. Feline distal aortic thromboembolism: a review of 44 cases (1990‐1998). J Feline Med Surg. 1999;1:221‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilkie LJ, Smith K, Luis FV. Cardiac pathology findings in 252 cats presented for necropsy; a comparison of cats with unexpected death versus other deaths. J Vet Cardiol. 2015;17(Suppl 1):S329‐S340. [DOI] [PubMed] [Google Scholar]
- 28. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567‐581. [DOI] [PubMed] [Google Scholar]
- 29. Via G, Hussain A, Wells M, et al. International evidence‐based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27:683 e681‐683 e633. [DOI] [PubMed] [Google Scholar]
- 30. Boysen SR, Lisciandro GR. The use of ultrasound for dogs and cats in the emergency room: AFAST and TFAST. Vet Clin North Am Small Anim Pract. 2013;43:773‐797. [DOI] [PubMed] [Google Scholar]
- 31. Lisciandro GR. The use of the diaphragmatico‐hepatic (DH) views of the abdominal and thoracic focused assessment with sonography for triage (AFAST/TFAST) examinations for the detection of pericardial effusion in 24 dogs (2011‐2012). J Vet Emerg Crit Care (San Antonio). 2016;26:125‐131. [DOI] [PubMed] [Google Scholar]
- 32. Lisciandro GR. Abdominal and thoracic focused assessment with sonography for trauma, triage, and monitoring in small animals. J Vet Emerg Crit Care (San Antonio). 2011;21:104‐122. [DOI] [PubMed] [Google Scholar]
- 33. Lisciandro GR, Fosgate GT, Fulton RM. Frequency and number of ultrasound lung rockets (B‐lines) using a regionally based lung ultrasound examination named vet BLUE (veterinary bedside lung ultrasound exam) in dogs with radiographically normal lung findings. Vet Radiol Ultrasound. 2014;55:315‐322. [DOI] [PubMed] [Google Scholar]
- 34. Lisciandro GR, Fulton RM, Fosgate GT, Mann KA. Frequency and number of B‐lines using a regionally based lung ultrasound examination in cats with radiographically normal lungs compared to cats with left‐sided congestive heart failure. J Vet Emerg Crit Care (San Antonio). 2017;27:499‐505. [DOI] [PubMed] [Google Scholar]
- 35. Ward JL, Lisciandro GR, Keene BW, Tou SP, DeFrancesco T. Accuracy of point‐of‐care lung ultrasonography for the diagnosis of cardiogenic pulmonary edema in dogs and cats with acute dyspnea. J Am Vet Med Assoc. 2017;250:666‐675. [DOI] [PubMed] [Google Scholar]
- 36. Tse YC, Rush JE, Cunningham SM, et al. Evaluation of a training course in focused echocardiography for noncardiology house officers. J Vet Emerg Crit Care (San Antonio). 2013;23:268‐273. [DOI] [PubMed] [Google Scholar]
- 37. Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213‐220. [DOI] [PubMed] [Google Scholar]
- 38. Altman DG. Practical Statistics for Medical Research. 1st ed. London; New York: Chapman and Hall; 1991:xii 611 pages. [Google Scholar]
- 39. Abbott JA. Feline hypertrophic cardiomyopathy: an update. Vet Clin North Am Small Anim Pract. 2010;40:685‐700. [DOI] [PubMed] [Google Scholar]
- 40. Kerkhof DL, Gleason CN, Basilico FC, Corrado GD. Is there a role for limited echocardiography during the Preparticipation physical examination? Pm R. 2016;8:S36‐S44. [DOI] [PubMed] [Google Scholar]
- 41. Yim ES. Aortic root disease in athletes: aortic root dilation, anomalous coronary artery, bicuspid aortic valve, and Marfan's syndrome. Sports Med. 2013;43:721‐732. [DOI] [PubMed] [Google Scholar]
- 42. Engelman D, Kado JH, Remenyi B, et al. Focused cardiac ultrasound screening for rheumatic heart disease by briefly trained health workers: a study of diagnostic accuracy. Lancet Glob Health. 2016;4:e386‐e394. [DOI] [PubMed] [Google Scholar]
- 43. Galasko GI, Lahiri A, Senior R. Portable echocardiography: an innovative tool in screening for cardiac abnormalities in the community. Eur J Echocardiogr. 2003;4:119‐127. [DOI] [PubMed] [Google Scholar]
- 44. Canty DJ, Heiberg J, Yang Y, et al. Pilot multi‐Centre randomised trial of the impact of pre‐operative focused cardiac ultrasound on mortality and morbidity in patients having surgery for femoral neck fractures (ECHONOF‐2 pilot). Anaesthesia. 2018;73:428‐437. [DOI] [PubMed] [Google Scholar]
- 45. Heiberg J, El‐Ansary D, Canty DJ, et al. Focused echocardiography: a systematic review of diagnostic and clinical decision‐making in anaesthesia and critical care. Anaesthesia. 2016;71:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 46. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket‐size hand‐held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323‐330. [DOI] [PubMed] [Google Scholar]
- 47. Galasko GI, Barnes SC, Collinson P, et al. What is the most cost‐effective strategy to screen for left ventricular systolic dysfunction: natriuretic peptides, the electrocardiogram, hand‐held echocardiography, traditional echocardiography, or their combination? Eur Heart J. 2006;27:193‐200. [DOI] [PubMed] [Google Scholar]
- 48. Biais M, Carrie C, Delaunay F, et al. Evaluation of a new pocket echoscopic device for focused cardiac ultrasonography in an emergency setting. Crit Care. 2012;16:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gianstefani S, Catibog N, Whittaker AR, et al. Pocket‐size imaging device: effectiveness for ward‐based transthoracic studies. Eur Heart J Cardiovasc Imaging. 2013;14:1132‐1139. [DOI] [PubMed] [Google Scholar]
- 50. Giusca S, Jurcut R, Ticulescu R, et al. Accuracy of handheld echocardiography for bedside diagnostic evaluation in a tertiary cardiology center: comparison with standard echocardiography. Echocardiography. 2011;28:136‐141. [DOI] [PubMed] [Google Scholar]
- 51. Prinz C, Voigt JU. Diagnostic accuracy of a hand‐held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111‐116. [DOI] [PubMed] [Google Scholar]
- 52. Prinz C, Dohrmann J, van Buuren F, et al. The importance of training in echocardiography: a validation study using pocket echocardiography. J Cardiovasc Med (Hagerstown). 2012;13:700‐707. [DOI] [PubMed] [Google Scholar]
- 53. Coletta C, De Marchis E, Lenoli M, et al. Reliability of cardiac dimensions and valvular regurgitation assessment by sonographers using hand‐carried ultrasound devices. Eur J Echocardiogr. 2006;7:275‐283. [DOI] [PubMed] [Google Scholar]
- 54. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96:1002‐1006. [DOI] [PubMed] [Google Scholar]
- 55. Andruszkiewicz P, Sobczyk D, Gorkiewicz‐Kot I, et al. Reliability of focused cardiac ultrasound by novice sonographer in preoperative anaesthetic assessment: an observational study. Cardiovasc Ultrasound. 2015;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruddox V, Norum IB, Stokke TM, Edvardsen T, Otterstad JE. Focused cardiac ultrasound by unselected residents‐the challenges. BMC Med Imaging. 2017;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haynes B. Can it work? Does it work? Is it worth it? BMJ. 1999;319:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fox PR. Hypertrophic cardiomyopathy. Clinical and pathologic correlates. J Vet Cardiol. 2003;5:39‐45. [DOI] [PubMed] [Google Scholar]


