Abstract
Background
Measurement of serum ionized calcium is not always available in practice. Total calcium (tCa) might not be reliable for determination of calcium status in cats.
Objectives
To predict serum ionized calcium concentration from signalment, biochemistry profile and T4, and compare predicted ionized calcium (piCa) to tCa.
Animals
A total of 1701 cats from two hospitals.
Methods
Cross‐sectional study. Cats with serum ionized calcium, biochemistry profile and T4 available were screened over 6 years and included in the training set (569 cats) to create a multivariate adaptive regression splines model to calculate piCa. Diagnostic performances of tCa and piCa and its prediction interval (PI) were compared in 652 cats from the same institution (test set) and 480 cats from a different hospital (external set).
Results
The final model included tCa, chloride, albumin, cholesterol, creatinine, BUN, body condition score, GGT, age, and potassium. For hypercalcemia, piCa was highly specific (test set: 99.8%; confidence interval [CI]: 99.5‐100; external set: 97%; CI: 95.3‐98.7) but poorly sensitive (test set: 30.4%; CI: 18.3‐42.4; external set: 42.5%; CI: 31.7‐53.3). For hypocalcemia, piCa was also highly specific (test set: 81.6%; CI: 78‐85; external set: 99.6%; CI: 99‐100) but poorly sensitive (test set: 57.6%; CI: 50.6‐64.6; external set: 0%). These diagnostic performances were comparable to those of tCa. The upper and lower limits of piCa PI had high sensitivity for detecting ionized hypercalcemia and hypocalcemia, respectively.
Conclusions and clinical importance
Predicted ionized calcium is useful to confirm suspected hypercalcemia in cats and screen for hypercalcemia and hypocalcemia.
Keywords: feline, hypercalcemia, hypocalcemia, prediction
Abbreviations
- ALP
alkaline phosphatase
- BCS
body condition Score
- BUN
blood urea nitrogen
- CI
confidence interval
- GCV
generalized cross‐validation
- GGT
gamma‐glutamyltransferase
- MARS
multivariate adaptive regression splines
- miCa
measured ionized calcium
- NDLR
negative diagnostic likelihood ratio
- NPV
negative predictive value
- PDLR
positive diagnostic likelihood ratio
- PI
prediction interval
- piCa
predicted ionized calcium
- PPV
positive predictive value
- RI
reference interval
- RMSE
root mean squared error
- tCa
total calcium
1. INTRODUCTION
There are three fractions of serum total calcium (tCa): ionized, protein‐bound, and complexed calcium. Ionized calcium is the free, biologically active form of calcium within the blood that is tightly regulated.1 Ionized calcium concentration, measured by analyzers with ion‐specific electrodes, is considered the gold standard for evaluation of calcium homeostasis in both humans and animals.2 Although the availability of portable, cost‐effective point‐of‐care analyzers has facilitated measurement of ionized calcium concentration, many veterinary hospitals do not have immediate access to measured ionized calcium (miCa) and rely on tCa concentration. Measurement of tCa might not be a reflection of miCa, especially in cats, and therefore, might not reflect the correct calcium status of the cat.3 Fluctuations in the fractions of tCa might be responsible for the discordance between tCa and miCa.1 Correction equations, which adjust the tCa for either albumin or total protein concentration, have attempted to improve the correlation between tCa and miCa. Unfortunately, these corrected tCa formulas are highly unreliable in cats to predict the ionized calcium status.3 In dogs, a recent study developed a model for predicting miCa based on numerous values available on a biochemical profile.4 This model was developed using data from 1200 dogs and used nonparametric statistics (ie, a multivariate adaptive regression splines [MARS] model) to determine the relationships between biochemical results and variation of miCa, and to provide a prediction of ionized calcium concentration. Although this model was only evaluated with variables measured from a single laboratory, it showed overall higher performance than tCa and corrected tCa to classify dogs according to their miCa status.
The objectives of this study were (a) to employ the same statistical technique as in dogs to create a multivariate predictive model of ionized calcium concentration in cats, using only readily available data such as signalment, routine biochemistry measurements, and T4 values, and (b) to evaluate the diagnostic performances of predicted ionized calcium (piCa) using data from two laboratories.
2. MATERIALS AND METHODS
2.1. Study population and data collection
A. Predictive model development: Medical records of cats were retrospectively reviewed between 2008 and 2016 at the University of Illinois Veterinary Teaching Hospital. Cats were included if they had a measurement of ionized calcium concentration, a serum biochemistry panel (creatinine, blood urea nitrogen [BUN], total protein, albumin, globulin, tCa, phosphorus, sodium, potassium, chloride, glucose, alkaline phosphatase [ALP], alanine transferase, gamma‐glutamyltransferase [GGT], total bilirubin, cholesterol, triglycerides, and bicarbonate), and a T4 level, all performed within 24 hours of each other. Cats were excluded if any laboratory values were missing. Ionized calcium concentration was measured by sampling 0.4 mL of whole blood that was placed directly into a lithium heparin‐coated plastic tube containing <15 USP units of heparin/mL of blood. To ensure proper mixing of blood with anticoagulant, the tube was inverted 8‐10 times and was analyzed within 15 minutes of collection, according to the manufacturer's recommendations. The blood was withdrawn from the tube using a syringe and analyzed with a stat blood gas analyzer that uses ion‐selective electrode technology (NovaStat CCX, Waltham, Massachusetts). The ionized calcium concentration reported was unadjusted. The reference interval (RI) for miCa was determined using 31 healthy cats and was 1.14‐1.33 mmol/L. The biochemistry panel was obtained from two chemistry analyzers (Hitachi 917 Automatic Analyzer, Roche Ltd., Basel, Switzerland from February 2008 to August 2010 and Olympus AU680, Beckman Coulter, Inc, Brea, California from August 2010 to November 2016 [Table S1]).
The miCa and all values obtained from the biochemistry panel, including T4, were recorded for each patient. Additional information such as age, breed, sex, body weight, and body condition score (BCS)5 were also recorded.
B. Assessment of predictive model performance: Both internal validation (ie, evaluation of the reproducibility of the model on cats from the same population and same laboratory as those used for model development) and external validation (ie, evaluation of the transportability or the generalizability of the model by cats from different population and laboratory) were assessed. For internal validation, two situations were considered: if T4 level was eventually retained in the predictive model, no additional cats would be available for model testing and internal validation would be performed via 10‐fold cross validation using the same cats as those included for development of the model. If T4 level was not part of the final predictive model, all cats that fulfilled inclusion criteria except for T4 level availability would be included to form a test set, on which the performance of the newly created model would be assessed. From the authors' point of view, a test set would be preferable over a cross validation technique, in order to limit overfitting.6
For external validation, a different institution, from a different geographical area, was contacted for eligibility of their cases (Queen Mother Hospital for Animals, Royal Veterinary College, London, UK). Eligibility criteria were the use of a different biochemistry analyzer than the ones used for model development, and availability of miCa and of the predictors required for piCa calculation. If only one predictor was not available, eligibility would still be considered if the missing predictor had low contribution to the prediction (defined by a generalized cross‐validation statistics [GCV] < 10 in Table 3 and a small range of miCa variation on the corresponding graph on Figure 1). As the institution fulfilled eligibility criteria, cats were retrospectively selected from the Royal Veterinary College database from 2008 to 2018. Ionized calcium concentration was measured on nonheparinized whole blood immediately after venipuncture by a point‐of‐care analyzer (i‐STAT 1, Abbott, Princeton, New Jersey). The unadjusted ionized calcium concentration was reported. The RI for miCa was determined using 52 healthy cats >9 years and was 1.19‐1.37 mmol/L.7 For the biochemistry panel, blood was obtained by jugular venipuncture, collected in a heparinized tube, and stored on ice for a maximum of 6 hours until centrifugation and separation. Heparinized plasma was sent to an external laboratory (Idexx laboratories, Wetherby, UK) for analysis with a Beckman coulter AU50800 analyzer. Time between miCa and tCa measurement was approximately 24 hours. GGT was not included in the biochemistry panel and was arbitrarily set to 0 U/L for all cats, based on the median GGT value of the cats from the University of Illinois Veterinary Teaching Hospital, which was 0. To guarantee independence of observations, an individual could not be included more than once; if the cat was evaluated several times, only the first evaluation was included. Differences regarding the demographic data between the training set, the test set and the external set were assessed by the Mann‐Whitney test with Bonferroni correction and the Chi‐square test for continuous and dichotomous variables, respectively, as continuous data significantly differed from a Gaussian distribution (assessed via Shapiro‐Wilk test, P < .05).
Figure 1.
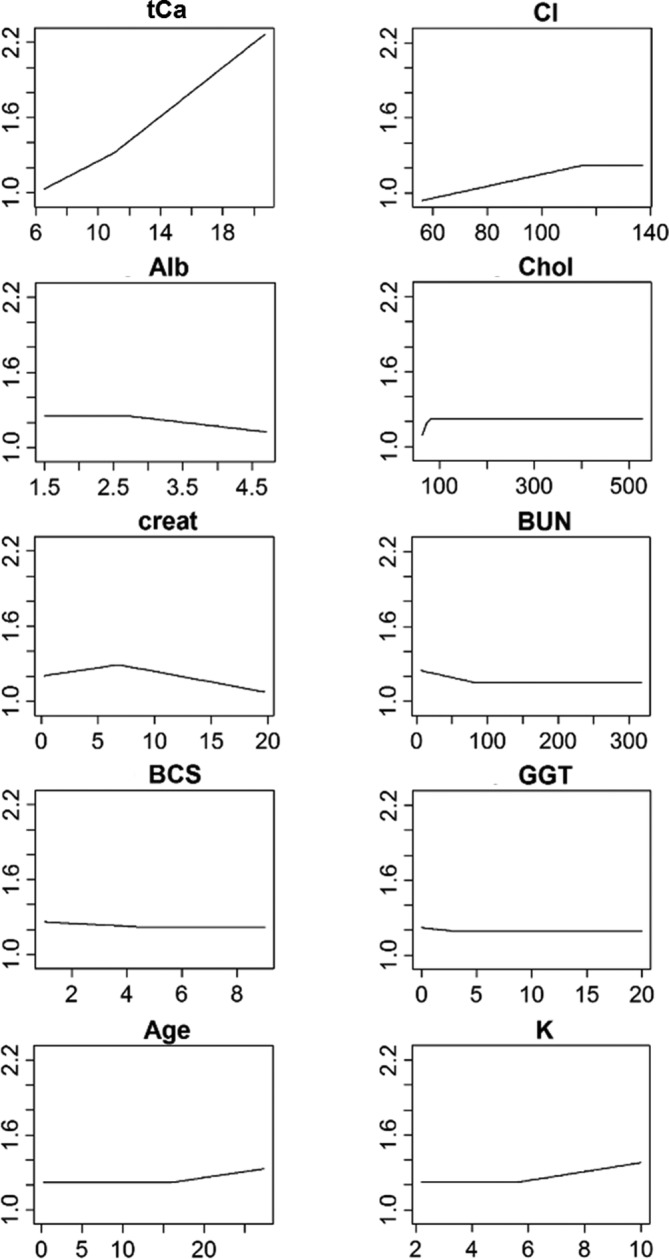
Individual relationships between measured ionized calcium and the predictors that have been retained in the final predictive model. The measured ionized calcium value is represented on the y‐axis, against the value of the predictor variables on the x‐axis
2.2. Multivariate adaptive regression splines predictive model creation
MARS model statistics were performed by the software of the public domain R version 3.4.2 (R project, Vienna, Austria). A MARS model was used to create the predictive algorithm in the same way as it was done in dogs.4, 8 Briefly, a MARS model is a nonparametric regression technique that approximates complex non‐linear relationships by a series of hinge functions of the predictors expressed by the formula:
where a 0 is the intercept of the model, p is the total number of hinge functions, a k is the coefficient of the k th hinge function h k (x), and x is a predictor. The hinge functions are pairs of two‐sided truncated functions applied to all the predictors and described by the following equations:
where t is the joining point of the polynomial called knot. Therefore, the hinge functions break each predictor variable into two groups centered on a knot value and determine a linear relationship between the predictor and miCa in each group. The final relationship between the predictors and miCa is therefore nonlinear. The MARS model selection followed a two‐step process: the initial forward pass selected the full model, which included all of the biochemical variables that were useful a priori to predict miCa. Then, multiple backward elimination passes were used for final model selection and predictive performance optimization, by removing hinge functions 1 by 1 from the model by all possible combinations. Each time a variable was removed, the accuracy and complexity of the new model were assessed by GCV statistics. The smaller the GCV, the more accurate and less complex the model. The model with the lowest GCV value was selected as the final model. Finally, a prediction interval (PI) was defined as follows: piCa ±2x regression SE.9
2.3. Description of the relationship between biochemistry predictors and ionized calcium concentration
Importance of the independent variables in miCa prediction was assessed by the GCV criterion, which corresponds to the increase in GCV when a variable is removed from the model. For ease of interpretation, the increases in GCV were scaled so the largest increase was 100. Variables that caused larger increase in GCV were considered more important. The predicted relationship between miCa and the predictors was depicted graphically for each predictor.
2.4. Assessment of predictive model performance
For both test and external sets, accuracy of the prediction was measured by calculating the root mean squared error (RMSE), which can be interpreted as the average distance between the observed values and the model prediction. The accuracy of the prediction interval (PI) was evaluated by measuring how often the miCa values were included inside the PI.
Samples were classified as hypercalcemic, hypocalcemic, and normocalcemic if miCa was greater than the upper limit of the RI, lower than the lower limit of the RI, or in between the two limits of the RI, respectively. For each set, sensitivity, specificity, and negative (NPV) and positive predictive values (PPV) were calculated for piCa and its PI and tCa by logistic regression and predictive margins using STATA version 14.2 software (StataCorp LLC, College Station, Texas).10 Specification errors and goodness‐of‐fit of the logistic regression were assessed by the linktest and lfit functions of the software. Negative (NDLR) and positive diagnostic likelihood ratios (PDLR) were directly calculated from the contingency tables. For all these indices, 95% confidence intervals (CI) were calculated.
2.5. Comparison between total calcium and predicted ionized calcium diagnosic performance
The same logistic regression method was applied to determine the diagnostic performances of tCa, and of piCa PI lower and upper limits. Performances of piCa and tCa were compared by evaluating the 95% CI of Sen, Spe, PPV, NPV, PDLR, and NDLR. Significant differences between piCa and tCa were considered if their 95% CI did not overlap for a same index. Furthermore, diagnostic discordance was evaluated for piCa and tCa. Samples for which the calcium status differed when determined by miCa and piCa (or tCa) were coded with a score of 1 (as opposed to 0 if there was agreement between miCa and piCa [or tCa]). Diagnostic discordance and its 95% CI was estimated for piCa and tCa by a logistic model in which the dependent variable was presence/absence of diagnostic discordance, and the independent variable was centers (ie, University of Illinois Veterinary Teaching Hospital or Royal Veterinary College Queen Mother Hospital for Animals).
3. RESULTS
3.1. Study population
In total, 1538 cats met inclusion criteria at the University of Illinois Veterinary Teaching Hospital, with 317 cats excluded because of missing values. Among the remaining 1221 cats, 569 had a miCa, a full biochemistry panel, and a T4 level measured within 24 hours over the inclusion period, and, therefore, constituted the training set to create the predictive model. As T4 was not retained in the model (see Table 2), a test set of 652 cats (ie, all the cats with a miCa and a biochemistry panel but no T4 measurement over the inclusion period) was created for internal validation. Comparison between the training and test sets are shown in Table 1. There were significant differences in age, miCa, tCa, albumin, and chloride levels between the training and test sets. Prevalence of ionized hypercalcemia was not significantly different between the two sets (training set: 7.7%, test set: 8.6%, P = .59), but prevalence of ionized hypocalcemia was (training set: 19.3%, test set: 29.3%, P < .001).
Table 1.
Demographic data and selected biochemical variables for the cats of the training set, test set, and external set
| Variable | Training set (n = 569) | Test set (n = 652) | P1 | External set (n = 480) | P2 | P3 |
|---|---|---|---|---|---|---|
| Age (years) | 10.2 (0.2‐27) | 8.6 (0.2‐23.8) | <.001 | 14.7 (0.9‐26) | <.001 | <.001 |
| BCS (9‐point scale) | 5 (1‐9) | 5 (1‐9) | .07 | 4 (1‐9) | <.001 | <.001 |
| Sex | .11 | .06 | <.001 | |||
| Neutered males | 328 (57.6%) | 390 (59.8%) | 241 (50.2%) | |||
| Intact males | 7 (1.2%) | 15 (2.3%) | 5 (1%) | |||
| Spayed females | 228 (40.1%) | 233 (35.7%) | 231 (48.1%) | |||
| Intact females | 6 (1.1%) | 14 (2.2%) | 3 (0.7%) | |||
| Ionized calcium (mmol/L) | 1.22 (0.8‐2.3) | 1.19 (0.6‐1.8) | <.001 | 1.3 (0.9‐1.8) | <.001 | <.001 |
| Ionized hypercalcemia | 44 (7.7%) | 56 (8.6%) | .59 | 80 (16.7%) | <.001 | <.001 |
| Ionized hypocalcemia | 110 (19.3%) | 191 (29.3%) | <.001 | 18 (3.7%) | <.001 | <.001 |
| Ionized normocalcemia | 415 (73%) | 405 (62.1%) | <.001 | 382 (79.6%) | .01 | <.001 |
| Total calcium (mg/dL) | 9.6 (6.5‐20.7) | 9.0 (4.2‐13.9) | <.001 | 10.0 (7.6‐13.7) | <.001 | <.001 |
| Total hypercalcemia | 107 (18.8%) | 77 (11.8%) | <.001 | 12 (2.5%) | <.001 | <.001 |
| Total hypocalcemia | 94 (16.5%) | 255 (39.1%) | <.001 | 2 (.4%) | <.001 | <.001 |
| Total normocalcemia | 368 (64.7%) | 320 (49.1%) | <.001 | 466 (97.1%) | <.001 | <.001 |
| Albumin (g/dL) | 3.2 (1.5‐4.7) | 2.9 (0.4‐4.5) | <.001 | 3.1 (1.9‐4.0) | <.001 | <.001 |
| Hyperalbuminemia | 7 (1.2%) | 5 (0.8%) | .56 | 0 (0%) | .02 | .08 |
| Hypoalbuminemia | 127 (22.3%) | 313 (48%) | <.001 | 11 (2.3%) | <.001 | <.001 |
| Normoalbuminemia | 435 (76.5%) | 334 (51.2%) | <.001 | 469 (97.7%) | <.001 | <.001 |
| Creatinine (mg/dL) | 1.4 (0.2‐19.7) | 1.3 (0.2‐31.5) | .06 | 2.0 (0.6‐10.9) | <.001 | <.001 |
| Normal creatinine | 428 (75.2%) | 500 (76.7%) | .55 | 230 (48%) | <.001 | <.001 |
| Creatinine >2.0 mg/dL | 141 (24.8%) | 152 (23.3%) | 250 (52%) | |||
| Chloride (mmol/L) | 116 (56‐137) | 115 (67‐143) | <.001 | 118 (101‐129) | <.001 | <.001 |
| Hyperchloridemia | 12 (2.1%) | 13 (2%) | .89 | 15 (3.1%) | .30 | .23 |
| Hypochloridemia | 56 (9.9%) | 157 (24.1%) | <.001 | 0 (0%) | <.001 | <.001 |
| Normochloridemia | 501 (88%) | 482 (73.9%) | <.001 | 465 (96.9%) | <.001 | <.001 |
Note: Table entries represent median values (minimum–maximum) for continuous variables and number of cats (percent of cats) for categorical variables. Significant differences between two sets were defined by a P < .017 and are bolded. BCS, body condition score; P1: P value for the comparison between the training and test sets; P2: P value for the comparison between the training and external sets; P3: P value for the comparison between the test and external sets.
Four hundred eighty cats from the Royal Veterinary College Queen Mother Hospital for Animals were included for external validation. Comparisons between the external set and the training and test sets are shown in Table 1. There were significant differences in almost all of the variables evaluated, including prevalence of ionized hypercalcemia (external set: 16.7%, training set: 7.7%, P < .001, test set: 8.6%, P < .001), and ionized hypocalcemia (external set: 3.7%, training set: 19.3%, P < .001, test set: 29.3%, P < .001).
3.2. Final model
The final model included creatinine, BUN, albumin, tCa, potassium, chloride, GGT, cholesterol, age, and BCS, and is presented in Table 2. Appendix 1 shows an example of how to calculate piCa by this model in cats.
Table 2.
Final multivariate adaptive regression splines model for prediction of ionized calcium from routine biochemical and cat variables determined from the cats of the training set
| Hinge function of the predictors | Coefficient |
|---|---|
| (Intercept) | 1.32146983 |
| h(6.8‐creatinine) | −0.01343127 |
| h(creatinine‐6.8) | −0.01683551 |
| h(82‐BUN) | 0.00126050 |
| h(Albumin‐2.7) | −0.06398259 |
| h(11‐total calcium) | −0.06373329 |
| h(total calcium‐11) | 0.09763819 |
| h(potassium‐5.6) | 0.03565030 |
| h(115‐Chloride) | −0.00476348 |
| h(3‐GGT) | 0.00999586 |
| h(76‐Cholesterol) | −0.00990447 |
| h(Age‐16) | 0.00946669 |
| h(4.5‐Body Condition Score) | 0.01128579 |
Abbreviations: BUN, blood urea nitrogen; GGT, gamma glutamyltransferase; h(), hinge function.
3.3. Description of the relationship between biochemistry predictors and ionized calcium concentration
As shown in Table 3, the most important variables for prediction of miCa, in descending order, were tCa, chloride, albumin, cholesterol, creatinine, BUN, BCS, GGT, age, and potassium.
Table 3.
Evaluation of the importance of the predictor variables that form the model for predicting measured ionized calcium changes
| Variables in order of importance (from top to bottom) | GCV |
|---|---|
| Total calcium | 100 |
| Chloride | 47.2 |
| Albumin | 26.4 |
| Cholesterol | 13.9 |
| Creatinine | 11.9 |
| BUN | 9.5 |
| Body condition score | 8.8 |
| GGT | 6.8 |
| Age | 4.6 |
| Potassium | 3.1 |
Abbreviations: BUN, blood urea nitrogen; GGT, gamma glutamyltransferase.
The individual relationships between miCa and the predictor variables are presented in Figure 1. The miCa increased almost linearly as tCa increased. In addition, miCa increased as chloride and cholesterol concentrations increased, but only to a certain point (chloride value of 115 mmol/L and cholesterol value of 76 mg/dL), where the relationships ceased. Conversely, miCa had no change associated with age and potassium values until a certain value (16 years old and 5.6 mmol/L, respectively), where miCa increased as age and potassium concentration increased further.
On the other hand, miCa decreased as BCS, BUN and GGT concentrations increased, but only to a certain point (BCS of 4.5, BUN value of 82 mg/dL, and GGT value of 3 U/L), where the relationships ceased. Conversely, miCa had no change associated with albumin values until a certain value (2.7 mg/dL), where miCa decreased as albumin concentration increased further.
Finally, the most complex association was found between miCa and creatinine, as miCa increased with increasing creatinine values, up to a certain point (6.8 mg/dL), but then decreased when creatinine concentration increased further.
3.4. Assessment of predictive model performance and comparison with total calcium
The observed‐versus‐predicted plots, displaying miCa on the y‐axis and piCa on the x‐axis, are presented in Figure 2A,B for the test set and the external set, respectively. If miCa and piCa were exactly the same, all of the points would lay on a single line, which would be the first bisector (miCa = 0 + 1 × piCa). Points tended to dispersed away from the first bisector, especially at lower miCa values, indicating a greater difference between miCa and piCa in ionized hypocalcemic cats. The average difference between piCa and miCa, as estimated by RMSE, was 0.1 mmol/L and 0.08 mmol/L in the test set and external set, respectively. The PI included miCa 78% and 85% of the time in the test set and external set, respectively.
Figure 2.
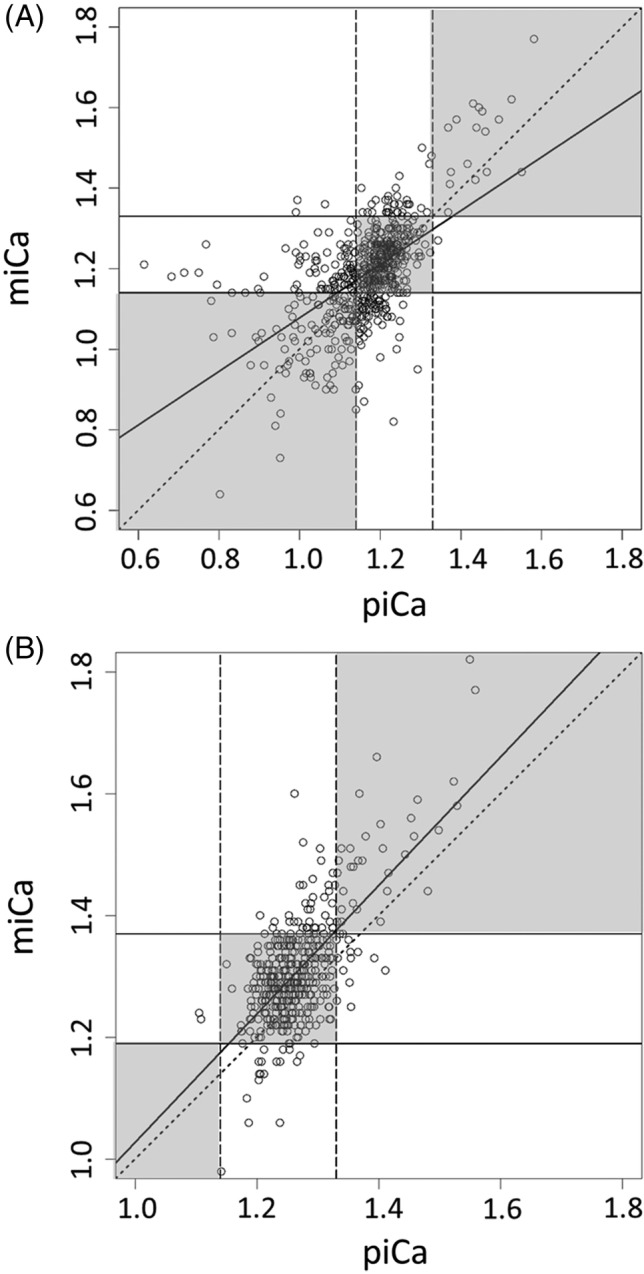
Observed‐versus‐predicted plot showing the relationship between measured and predicted ionized calcium in the (A) test set and (B) external set. The upper and lower limits of normocalcemia are represented by the vertical dashed lines for the predicted ionized calcium (1.14‐1.33 mmol/L), and the horizontal solid line for the measured ionized calcium (A: 1.14‐1.33 mmol/L; B: 1.19‐1.37 mmol/L). The diagonal dotted line represents the first bisector, on which predicted ionized calcium values that perfectly match measured ionized calcium fall in. The thick solid line represents the regression line. Points that fall within the three gray boxes along the first bisector were properly classified by predicted ionized calcium, and those points outside these gray boxes were misclassified by predicted ionized calcium. miCa, measured ionized calcium; piCa, predicted ionized calcium
Sensitivity, specificity, NPV, PPV, NDLR, and PDLR for piCa and its PI and tCa for diagnosis of ionized hypercalcemia or hypocalcemia are presented in Table 4A,B, respectively. Unlike tCa, sensitivity of piCa to detect ionized hypercalcemia was not significantly different between the test (30.4%; 95% CI: 18.3‐42.2) and external sets (42.5%, 95% CI: 31.7‐53.3). Owing to the difference in sensitivity of tCa between the 2 sets (test set: 58.9%, 95% CI: 46‐71.8; external set: 15%, 95% CI: 7.2‐22.8), piCa was significantly less sensitive than tCa in the test set, but more sensitive than tCa in the external set, to detect ionized hypercalcemia.
Table 4.
Sensitivity, specificity, negative and positive predictive values, and negative and positive diagnostic likelihood ratios of predicted ionized calcium and its prediction interval and total calcium for diagnosis of (A) hypercalcemia in cats of the test and external sets (prevalence of hypercalcemia = 8.6% and 16.7%, respectively), and (B) hypocalcemia in cats of the test and external sets (prevalence of hypocalcemia = 29.3% and 3.7%, respectively). Statistically significant differences for the same index between the 2 sets (ie, within the same row) are indicated by a. Statistically significant differences for the same index between piCa and/or the limits of its PI and tCa inside a same set (ie, within the same column) are indicated by b
| Test set (n = 652) | External set (n = 480) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | NPV | PPV | PDLR | NDLR | Sensitivity | Specificity | NPV | PPV | PDLR | NDLR | |
| (A) Ionized hypercalcemia | ||||||||||||
| piCa >1.33 mmol/L | 30.4%b (18.3‐42.4) | 99.8%a,b (99.5‐100) | 93.8% (92‐95.7) | 94.4%b (83.9‐100) | 181b (24‐1334) | .7 (.6‐.8) | 42.5%b (31.7‐53.3) | 97%a (95.3‐98.7) | 89.4% (86.5‐92.3) | 73.9% (61.2‐86.6) | 14.2 (7.7‐26) | .6b (.5‐.7) |
| Upper end of PI >1.33 mmol/L | 69.6%a,b (57.6‐81.7) | 79.2%b (75.9‐82.5) | 96.5% (94.9‐98.2) | 23.9%b (17.4‐30.5) | 3.3a,b (2.6‐4.2) | .4 (.3‐.6) | 97.5%a,b (94.1‐100) | 23.5% (19.4‐27.7) | 97.9%b (95.1‐100) | 20.3% (16.3‐24.3) | 1.3a (1.2‐1.4) | .1b (.05‐.4) |
| Lower end of PI >1.33 mmol/L | 16.1%b (6.5‐25.7) | 100% (NE) | 92.7%a (90.7‐94.7) | 100% (NE) | ∞ (NE) | .8b (.7‐.9) | 12.5% (5.3‐19.7) | 100% (NE) | 85.1%a (81.9‐88.3) | 100% (NE) | ∞ (NE) | .9 (.8–.9) |
| tCa > 10.2 mg/dL (test); 11.8 mg/dL (external) | 58.9%a,b (46‐71.8) | 92.6%b (90.5‐94.7) | 96%a (94.4‐97.6) | 42.8%b (31.8‐53.9) | 8b (5.6‐11.4) | .4a,b (.3‐.6) | 15%a,b (7.2‐22.8) | 100% (NE) | 85.5%a,b (82.3‐88.7) | 100% (NE) | ∞ (NE) | .8a,b (.8‐.9) |
| (B) Ionized hypocalcemia | ||||||||||||
| piCa <1.14 mmol/L | 57.6% (50.6‐64.6) | 81.6%a,b (78‐85) | 82.3%a (78.8‐85.8) | 56.4% (49.5‐63.4) | 3.1 (2.5‐3.9) | .5a (.4–.6) | 0% (NE) | 99.6%a (99‐100) | 96.2%a (94.5‐97.9) | 0% (NE) | 0 (NE) | 1a (1) |
| Lower end of PI <1.14 mmol/L | 96.3%b (93.7‐99) | 16.1%a,b (12.7‐19.4) | 91.4% (85.3‐97.5) | 32.2%a,b (28.4‐36.1) | 1.1a,b (1.1‐1.2) | .2 (.1‐.5) | 83.3%b (66.1‐100) | 61.5%a (57‐65.9) | 98.9% (97.8‐100) | 7.8%a (4‐11.5) | 2.2a (1.7‐2.7) | .3 (.1–.8) |
| Upper end of PI <1.14 mmol/L | 22.5%b (16.6‐28.4) | 95.7%b (93.8‐97.5) | 74.9%a,b (71.4‐78.4) | 68.3% (56.8‐79.7) | 5.2 (3.1‐8.6) | .8b (.7‐.9) | 0% (NE) | 100% (NE) | 96.3%a (94.6‐98) | 0% (NE) | NE | 1 (NE) |
| tCa < 8.8 mg/dL (test); 8.2 mg/dL (external) | 70.2%a,b (63.7‐76.6) | 73.7%b (69.7‐77.8) | 85.6%a (82.2‐89.1) | 52.5%b (46.4‐58.7) | 2.7b (2.2‐3.2) | .4a,b (.3‐.5) | 11.1%a,b (0‐25.6) | 100% (NE) | 96.7%a (95.1‐98.3) | 100% (NE) | ∞ (NE) | .9a (.7‐1) |
If the upper limit of piCa PI was used to screen for ionized hypercalcemia (ie, if the upper limit of piCa PI was >1.33 mmol/L, piCa may actually be >1.33 mmol/L), sensitivity increased to 69.6% (95% CI: 57.6‐81.7) and 97.5% (95% CI: 94.1‐100) in the test set and external set, respectively.
Specificity of piCa and tCa to diagnose ionized hypercalcemia could only be compared in the test set, as a 95% CI could not be calculated for tCa specificity in the external set because of the absence of false positive results. In the test set, piCa was significantly more specific than tCa to diagnose hypercalcemia.
If the lower limit of piCa PI was used to diagnose ionized hypercalcemia (ie, if the lower limit of piCa PI was >1.33 mmol/L, piCa must be >1.33 mmol/L), specificity was 100% in both the test and external sets.
In the test set, the use of piCa and its PI was significantly better than the use of tCa to diagnose ionized hypercalcemia, as demonstrated by significantly higher PPV (piCa: 94.4%, 95% CI: 83.9‐100; lower limit of PI: 100%; tCa: 42.8%, 95% CI: 31.8‐53.9) and PDLR (piCa: 181, 95% CI: 24‐1334; lower limit of PI: ∞; tCa: 8, 95% CI: 5.6‐11.4). Such a comparison was not possible in the external set because of the lack of false positive hypercalcemia by tCa. However, the use of piCa and its PI was significantly better than the use of tCa to exclude ionized hypercalcemia, as demonstrated by significantly higher NPV (upper limit of PI: 97.9%, 95% CI: 95.1‐100; tCa: 85.5%, 95% CI: 82.3‐88.7) and significantly lower NDLR (piCa: .6, 95% CI: .5‐.7; upper limit of PI: .1, 95% CI: .05‐.4; tCa: .8, 95% CI:.8‐.9) in the external set.
Finally, diagnostic discordance for ionized hypercalcemia between piCa and miCa varied between the test and the external sets and was 6.1% (95% CI: 4.5‐8.2) and 12.1% (95% CI: 9.5‐15.3), respectively. It was slightly lower than the diagnostic discordance between tCa and miCa, which also varied between the test and the external sets (10.3%; 95% CI: 8.2‐12.9 and 14.2%; 95% CI: 11.3‐17.6, respectively).
Sensitivity of piCa to screen for ionized hypocalcemia could not be compared between the test and external sets because of the lack of true positive results in the external set. However, sensitivity of the lower end of the PI was not significantly different between the two sets. The lower end of piCa PI was significantly more sensitive than tCa to detect ionized hypocalcemia in both sets (test set: lower end of PI: 96.3%, 95% CI: 93.7‐99, tCa: 70.2%, 95% CI: 63.7‐76.6%; external set: lower end of PI: 83.3%, 95% CI: 66.1‐100, tCa: 11.1%, 95% CI: 0‐25.6).
Specificity of piCa to diagnose ionized hypocalcemia was significantly lower in the test set compared to the external set. However, it was significantly more specific than tCa in the test set (piCa: 81.6%, 95% CI: 78‐85; tCa: 73.7%, 95% CI: 69.7‐77.8), and as specific as tCa in the external set (piCa: 99.6%, 95% CI: 99‐100; tCa: 100%). Predictive values and diagnostic likelihood ratios of piCa and its PI and tCa were either similar or not comparable owing to the lack of true or false positive results.
Finally, diagnostic discordance for ionized hypocalcemia between piCa and miCa varied between the test and the external sets and was 25.5% (95% CI: 22.3‐28.9) and 4.2% (95% CI: 2.7‐6.4), respectively. It was not statistically different from the diagnostic discordance between tCa and miCa, which also varied between the test and the external sets (27.3%; 95% CI: 24.0‐30.9 and 3.3%; 95% CI: 2.1‐5.4, respectively).
4. DISCUSSION
In this study, a MARS model was created to predict serum ionized calcium in cats and was tested in two different datasets, from two different countries. The purpose of the study was to calculate piCa by measurements universally available; the variables included in the study were all easily obtained and readily available. Similar to the analogous dog model, the variables that had a major impact on miCa variations in cats included tCa, albumin, and chloride; however, there was also variation associated with age, creatinine, and potassium levels. Unlike in dogs, cholesterol, BUN, BCS, and GGT were also associated with miCa changes.
For tCa, chloride, and albumin, the relationship with miCa was relatively similar to what was described in dogs.4 A previous study found a weaker relationship between tCa and albumin in cats compared to dogs,11 which might explain why miCa was affected only with increased albumin levels. The lack of correlation between hypoalbuminemia and miCa in this study might be a reasonable explanation to the inadequacy of albumin‐corrected formulas for calcium in cats. On the other hand, the relationships between miCa and creatinine, age, and potassium measurements were different in dogs and cats. In dogs, miCa decreased as creatinine concentration increased to 5 mg/dL, where it no longer had an effect on miCa.4 In cats, miCa increased as creatinine concentration increased until 6.8 mg/dL. At higher creatinine concentrations, miCa subsequently decreased. This species difference has been reported in the literature, as cats with chronic kidney disease might have either ionized hypocalcemia or hypercalcemia whereas dogs with chronic kidney disease tend to only demonstrate ionized hypocalcemia.12, 13 Kidney disease might be a risk factor for the development of ionized hypercalcemia because of decreased glomerular filtration, increased tubular reabsorption, and decreased calcium bone storage.14 As azotemia progresses in cats, hypocalcemia becomes more prevalent.12 As creatinine continues to increase, decreased renal calcitriol synthesis and phosphorus retention could cause the observed hypocalcemia.13, 15 Ionized hypocalcemia becomes especially prevalent in uremic cats, as opposed to cats with compensated kidney disease.12 This inverse relationship between increasing BUN and decreasing miCa was also reflected by our model. Potassium had no effect on miCa in both cats and dogs until a certain point, where potassium and miCa decreased linearly in dogs but increased in cats. This positive correlation between miCa and potassium at high potassium levels is surprising, as the opposite is typically observed in cats with urethral obstruction.16 Further investigations on the relationship between miCa and potassium levels in cats are warranted to understand the association found by the MARS model. Age was inversely correlated with miCa in dogs younger than 2.27 years old, likely related to skeletal maturation.4 Conversely, age was not associated with miCa in cats until >16 years old. The increased calcium in older cats could be the result of systemic illnesses causing hypercalcemia.17, 18 BCS might decrease as a result of illnesses associated with hypercalcemia, explaining the relationship found by the MARS model. The slight decrease of miCa while GGT increases could be attributed to pancreatitis, as GGT can be increased with pancreatitis, and as such can be associated with fat saponification and calcium sequestration.19 An increase in calcium might contribute to an increase in cholesterol by decreasing an enzyme, 7α‐hydroxylase, involved in cholesterol catabolism.20 These numerous complex relationships between miCa and biochemical measurements illustrate how a simple equation cannot predict miCa levels adequately.
Although eventually not retained in the predictive model, T4 was considered as a potential predictor in this study. Thyroid disorders affect calcium homeostasis in both humans and cats. In humans with hyperthyroidism there is a shift toward hypercalcemia with many suspected explanations, including increased bone resorption, increased renal reabsorption, and altered fractional excretion of calcium.21, 22 Conversely, in cats, hyperthyroidism is associated with hypocalcemia.23, 24 The mechanism for low calcium levels in hyperthyroid cats is unknown but has been postulated to be secondary to hyperphosphatemia or because of renal secondary hyperparathyroidism for those with underlying chronic kidney disease.23 However, the MARS model did not retain T4 in our study, which might indicate that the relationship between hyperthyroidism in cats and miCa might have actually been confounded by a comorbidity in previous studies (eg, chronic kidney disease, which is common in hyperthyroid cats).
In this study, a MARS model was utilized for better accuracy. A MARS model is nonparametric and nonlinear, which supports better predictive values than classic parametric linear regression.25 Both internal and external validations were assessed to evaluate the reproducibility and transportability of the model. Internal validation assesses the validity of the predictive model by the original population, and external validation assesses the validity by a different but related population. In our study, a population of cats from a different institution and a new geographical area was used for external validation. Although there were statistically significant differences in the diagnostic performances of piCa and its PI between the test and the external sets, the results can be considered close enough from a clinical point of view to demonstrate generalizability of the predictive model. The statistical differences were likely because of dissimilarities in prevalence of ionized hypocalcemia, ionized hypercalcemia, and azotemia between the two sets. Overall, piCa and its PI behaved similarly in the two sets. For both ionized hypercalcemia and hypocalcemia, piCa presented high specificity, PPV and PDLR, which made it useful to diagnose calcium disorders but not necessarily to detect them. However, the PI showed high NPV and low NDLR, which indicated good ability to screen for ionized hypercalcemia (for the PI upper limit) and hypocalcemia (for the PI lower limit). Finally, the PI also showed high specificity, PPV and PDLR, which permitted diagnosis of ionized hypercalcemia (for the PI lower limit) and hypocalcemia (for the PI upper limit) with greater confidence. Notably, the diagnosis of ionized hypercalcemia can be considered certain if the lower limit of the PI is above 1.33 mmol/L, without the need for miCa. However, for ionized hypocalcemia, the suspicion can be only reinforced if the upper limit of the PI is below 1.14 mmol/L, as piCa PI does not seem to be good enough to diagnose ionized hypocalcemia with certainty in cats and results should be verified by miCa.
As demonstrated in previous studies, our results confirm that relying on tCa to classify cats as hypercalcemic is problematic mainly because of a lack of sensitivity.3, 26 In other terms, high tCa values in cats support ionized hypercalcemia, but many cats with ionized hypercalcemia do not have high tCa values. Despite statistically significant differences between piCa and tCa, both showed comparable diagnostic performances regarding ionized hypercalcemia from a clinical point of view. For hypocalcemia, tCa was moderately sensitive and specific in the test set, and highly specific but poorly sensitive in the external set. This difference between the two sets is likely related to the higher prevalence of renal azotemia in the external set. These results are in accordance with a previous study.3 Again, despite statistically significant differences between piCa and tCa, both showed similar diagnostic performances regarding ionized hypocalcemia from a clinical point of view, as reflected by similar diagnostic discordance between piCa and miCa and tCa and miCa. Hence, the main advantage of piCa over tCa resides in its PI, which allowed high sensitivity, high NPV and low NDLR for both ionized hypercalcemia (for the PI upper end) and hypocalcemia (for the PI lower end) and, therefore, represents a much better screening tool than tCa.
There were several limitations to this study, mainly because of its retrospective nature. Records with absent or missing data were not included in the analysis, reducing the number of cats available for model development and testing. Cats' prior conditions or medications might have been overlooked while obtaining a history and thus not recorded, which could have affected their calcium homeostasis or other biochemical variables. Although clinician motives for measuring ionized calcium cannot be determined for absolutely all cats, miCa was part of the routine bloodwork requested on most cats at both the University of Illinois Veterinary Teaching Hospital (where most of the included cats were cats that entered through the Emergency Room) and the Royal Veterinary College Queen Mother Hospital for Animals (where most of the cats were azotemic cats), and was not motivated by a disturbance of tCa or a suspicion of calcium disorders. Another limitation is that two different biochemical analyzers were utilized during the study time frame. However, it is unlikely that these had a substantial impact on the predictive ability of the model, as predictive performance was relatively stable over the three sets of cats. An additional limitation, because of the retrospective nature of the study, was that proper handling technique of the blood samples cannot be ensured. However, the standard of care at both hospitals strictly enforces proper handling of blood samples. If proper handling was not performed, then air exposure might result in a decrease in miCa for example.27 The blood collection methodology was different between the test set, in which miCa was measured on heparinized blood, and the external set, in which miCa was measured on non‐heparinized blood. Ionized calcium values would be different in a same cat if measured from heparinized whole blood and from non‐heparinized whole blood.28 However, both values would hopefully classify the cat similarly regarding its calcium status respectively to the RIs, which would also be different for miCa measured from heparinized blood and for miCa measured from whole blood. This was the case in our study, as the RIs of miCa when measured from heparinized blood was 1.14‐1.33 mmol/L, whereas it was 1.19‐1.37 mmol/L when measured from whole blood. Hence, although the methodology was different between the external set and the test set, it should not impact the calcium status determination in both sets. Furthermore, prevalence of ionized hypocalcemia was different between the test and the external sets: only 18 cats had ionized hypocalcemia in the external set (prevalence: 3.7%) versus 191 cats in the test set (prevalence: 29.3%). The small number of hypocalcemic cats in the external set likely impacted the diagnostic performance assessment, as reflected by the large difference in terms of diagnostic discordance between the test set (diagnostic discordance between piCa or tCa and miCa around 25‐27%) and the external set (diagnostic discordance around 3‐4%).
In our study, the training set and the test set were not randomly extracted from the same population of cats. The cats of both sets are different in several ways, as those included in the training set all had T4 measurement performed and were therefore older than the cats of the test set. Testing a predictive model from a dataset that comes from a population that is different from the data used to build the model is called extrapolation, as opposed to interpolation. However, the cats of the test set should reflect the data to which the model will be applied in practice. As the cats of the test set corresponded to a broad and general population of cats, they likely represent an appropriate sample for model testing.
The MARS model identified the BCS as a predictor of ionized calcium. As BCS is a subjective measurement, it may add variability to the prediction. However, BCS has previously been showed to be repeatable both within scorers and between scorers in cats.5 Hence, if the BCS is different between two clinicians for the same cat, the difference should be limited. A one‐point difference in BCS would produce either no change (if both BCS are >4.5) or a minimal change (±0.01 mmol/L) in piCa value. It would be unlikely for this 0.01 mmol/L difference to be clinically relevant in practice. Similarly, the lack of GGT data available for the cats of the external set likely had a limited impact on piCa calculation, owing to the low contribution of GGT to the prediction. Indeed, GGT can only produce a maximal change of ±0.03 mmol/L in piCa value. It would be unlikely that this change in piCa value resulted in relevant inaccuracies in the calcium status classification of the cats of the external set. However, in order to get a prediction as accurate as possible in practice, clinicians are strongly encouraged to include all the predictors, including GGT value, in the model.
Lastly, other variables such as ionized magnesium could have influenced calcium homeostasis.29, 30, 31, 32 Magnesium was not included in the study, as it is not readily available to many veterinary practitioners. Inclusion of this variable may have improved the model performance.
In conclusion, a novel equation was developed that calculates piCa from readily available signalment and biochemical data points. This formula may be useful to those who do not have rapid access to miCa concentration. This piCa may help confirm suspected hypercalcemia in cats, owing to its high specificity. Its PI may be used to screen hypercalcemia and hypocalcemia, owing to its high sensitivity, which represents a compelling advantage over tCa.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supporting informtion
ACKNOWLEDGMENT
Preliminary results were presented as a poster at the European College of Veterinary Internal Medicine Congress, Rotterdam, Holland, September 2018.
Hodgson N, McMichael MA, Jepson RE, Le Boedec K. Development and validation of a multivariate predictive model to estimate serum ionized calcium concentration from serum biochemical profile results in cats. J Vet Intern Med. 2019;33:1943–1953. 10.1111/jvim.15622
REFERENCES
- 1. de Brito Galvão JF, Parker V, Schenck PA, Chew DJ. Update on feline ionized hypercalcemia. Vet Clin North Am Small Anim Pract. 2017;47:273‐292. [DOI] [PubMed] [Google Scholar]
- 2. Schenck PA, Chew DJ, Nagode LA, Rosol TJ. Disorders of calcium: hypercalcemia and hypocalcemia In: DiBartola SP, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. 4th ed. St. Louis (MO): Elsevier Science & Saunders; 2012:120‐194. [Google Scholar]
- 3. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res. 2010;74:209‐213. [PMC free article] [PubMed] [Google Scholar]
- 4. Danner J, Ridgway MD, Rubin SI, Le Boedec K. Development of a multivariate predictive model to estimate ionized calcium concentration from serum biochemical profile results in dogs. J Vet Intern Med. 2017;31:1392‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Practice. 1997;25:13‐17. [Google Scholar]
- 6. Waljee AK, Higgins PDR, Singal AG. A primer on predictive models. Clin Transl Gastroenterol. 2014;5:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geddes RF. Calcium‐Phosphate Homeostasis in Feline Chronic Kidney Disease [PhD thesis]. London: Royal Veterinary College, University of London; 2014.
- 8. Friedman JH. Multivariate adaptive regression splines. Ann Stat. 1991;19:1‐67. [DOI] [PubMed] [Google Scholar]
- 9. Pardoe I. Simple linear regression In: Pardoe I, ed. Applied Regression Modeling. 2nd ed. Hoboken (NJ): John Wiley & Sons; 2012:35‐82. [Google Scholar]
- 10. Coughlin SS, Trock B, Criqui MH, Pickle LW, Browner D, Tefft MC. The logistic modeling of sensitivity, specificity, and predictive value of a diagnostic test. J Clin Epidemiol. 1992;45:1‐7. [DOI] [PubMed] [Google Scholar]
- 11. Flanders JA, Scarlett JM, Blue JT, Neth S. Adjustment of total serum calcium concentration for binding to albumin and protein in cats: 291 cases (1986‐1987). J Am Vet Med Assoc. 1989;194:1609‐1611. [PubMed] [Google Scholar]
- 12. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:108‐116. [DOI] [PubMed] [Google Scholar]
- 13. Cortadellas O, Fernández del Palacio MJ, Talavera J, Bayón A. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. J Vet Intern Med. 2010;24:73‐79. [DOI] [PubMed] [Google Scholar]
- 14. Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 1):S23‐S30. [DOI] [PubMed] [Google Scholar]
- 15. Kogika MM, Lustoza MD, Notomi MK, Wirthl VABF, Mirandola RMS, Hagiwara MK. Serum ionized calcium in dogs with chronic renal failure and metabolic acidosis. Vet Clin Pathol. 2006;35:441‐445. [DOI] [PubMed] [Google Scholar]
- 16. Drobatz KJ, Hughes D. Concentration of ionized calcium in plasma from cats with urethral obstruction. J Am Vet Med Assoc. 1997;211:1392‐1395. [PubMed] [Google Scholar]
- 17. Sayyid M, Gilor C, Parker V, et al. Ionized hypercalcemia in cats: etiologies and associated clinical signs. In: 2016 ACVIM Forum Research Abstract Program. Denver, CO; 2016.
- 18. Hardy BT, de Brito Galvao JF, Green TA, et al. Treatment of ionized hypercalcemia in 12 cats (2006‐2008) using PO‐administered alendronate. J Vet Intern Med. 2015;29:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kimmel SE, Washabau RJ, Drobatz KJ. Incidence and prognostic value of low plasma ionized calcium concentration in cats with acute pancreatitis: 46 cases (1996‐1998). J Am Vet Med Assoc. 2001;219:1105‐1109. [DOI] [PubMed] [Google Scholar]
- 20. Gallo L, Faniello MC, Canino G, et al. Serum calcium increase correlates with worsening of lipid profile: an observational study on a large cohort from South Italy. Medicine (Baltimore). 2016;95:e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burman KD, Monchik JM, Earll JM, Wartofsky L. Ionized and total serum calcium and parathyroid hormone in hyperthyroidism. Ann Intern Med. 1976;84:668‐671. [DOI] [PubMed] [Google Scholar]
- 22. Schenck PA. Calcium homeostasis in thyroid disease in dogs and cats. Vet Clin North Am Small Anim Pract. 2007;37:693‐708. [DOI] [PubMed] [Google Scholar]
- 23. Williams TL, Elliott J, Berry J, Syme HM. Investigation of the pathophysiological mechanism for altered calcium homeostasis in hyperthyroid cats. J Small Anim Pract. 2013;54:367‐373. [DOI] [PubMed] [Google Scholar]
- 24. Barber PJ, Elliott J. Study of calcium homeostasis in feline hyperthyroidism. J Small Anim Pract. 1996;37:575‐582. [DOI] [PubMed] [Google Scholar]
- 25. Kuhn M, Johnson K. Nonlinear regression models In: Kuhn M, Johnson K, eds. Applied Predictive Modeling. New York: Springer; 2013:141‐171. [Google Scholar]
- 26. van den Broek DHN, Chang Y‐M, Elliott J, Jepson RE. Chronic kidney disease in cats and the risk of Total Hypercalcemia. J Vet Intern Med. 2017;31:465‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unterer S, Gerber B, Glaus TM, Hässig M, Reusch CE. Evaluation of an electrolyte analyser for measurement of concentrations of ionized calcium and magnesium in cats. Vet Res Commun. 2005;29:647‐659. [DOI] [PubMed] [Google Scholar]
- 28. Bachmann K, Kutter A, Jud Schefer RS, Sigrist N. Determination of reference intervals and comparison of venous blood gas parameters using a standard and nonstandard collection method in 51 dogs. Schweiz Arch Tierheilkd. 2018;160:163‐170. [DOI] [PubMed] [Google Scholar]
- 29. Holowaychuk MK. Hypocalcemia of critical illness in dogs and cats. Vet Clin North Am Small Anim Pract. 2013;43:1299‐1317. [DOI] [PubMed] [Google Scholar]
- 30. Gitelman HJ, Kukolj S, Welt LG. Inhibition of parathyroid gland activity by hypermagnesemia. Am J Physiol. 1968;215:483‐485. [DOI] [PubMed] [Google Scholar]
- 31. Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med. 1984;310:1221‐1225. [DOI] [PubMed] [Google Scholar]
- 32. Shils ME. Magnesium, calcium, and parathyroid hormone interactions. Ann NY Acad Sci. 1980;355:165‐180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting informtion


