Abstract
Background
Broadly applicable reference intervals (RIs) for measurements of left atrial (LA) and left ventricular (LV) size and function generated prospectively using statistically appropriate methods are limited.
Objectives
To generate body size‐independent RIs for linear, area, and volume measurements of LA size and LV size and function.
Animals
Healthy adult dogs (n = 122) of variable size and somatotype.
Methods
Prospective study. All dogs underwent an echocardiogram performed by the same examiner. Effects of body weight, sex, age, and heart rate were evaluated by regression and correlation analyses. Scaling exponents and prediction intervals were generated for linear measurements using the allometric equation. After normalization to body weight, 95% RIs were determined using nonparametric methods with 2.5 and 97.5 percentiles serving as the lower and upper limits (each with 90% confidence intervals), respectively.
Results
Linear LA and LV measurements were strongly correlated (R 2 ≥ 0.79) with body weight. Scaling exponents were close to the expected 1/3 (0.299‐0.392). Prediction intervals for linear measurements of LV chamber size were considerably narrower than previously reported. Weak correlations (r = −0.42 to −0.50) among LV fractional shortening, fractional area change, and ejection fraction and body weight were identified. No other meaningful relationships were identified between the measurements and sex, age, and heart rate.
Conclusions and Clinical Importance
Body size‐independent RIs for several linear, area, and volume measurements of LA and LV size and function were generated prospectively from a large and diverse reference population and are available for clinical use.
Keywords: ejection fraction, fractional shortening, left atrium, left ventricle, reference range, reproducibility
Abbreviations
- 2DE
2‐dimensional echocardiography
- AoD
aortic valve diameter
- BSA
body surface area
- CLSI
Clinical and Laboratory Standards Institute
- CV
coefficient of variation
- EF
ejection fraction
- FAC
fractional area change
- FS
fractional shortening
- ICC
intraclass correlation coefficient
- IQR
interquartile range
- LA
left atrium/atrial
- LAD
left atrial dimension
- LAV
left atrial volume
- LV
left ventricle/ventricular
- LVAd
left ventricular area at end‐diastole
- LVAs
left ventricular area at end‐systole
- LVIDd
left ventricular internal dimension at end‐diastole
- LVIDs
left ventricular internal dimension at end‐systole
- LVVd
left ventricular volume at end‐diastole
- LVVs
left ventricular volume at end‐systole
- Lx
long‐axis
- MM
M‐mode echocardiography
- RI
reference interval(s)
- Sx
short‐axis
1. INTRODUCTION
Quantitation of cardiac chamber size is an important aspect of cardiac imaging. In diseases such as dilated cardiomyopathy and myxomatous mitral valve disease in dogs, identification of cardiac chamber enlargement greatly aids in clinical decision making with regard to diagnosis and clinical staging,1, 2 risk assessment,3, 4, 5 treatment decisions in the preclinical stage,6, 7, 8 and prognosis.7, 8, 9, 10, 11, 12, 13, 14 Routine echocardiographic assessment of cardiac chamber size usually consists of linear measurements of the diameter or minor axis dimension (ie, the shortest diameter of an ellipse) using M‐mode (MM) or 2‐dimensional echocardiography (2DE).15 Area measurements or estimates of chamber volume using 3‐dimensional echocardiography or area‐length methods derived from 2DE also may be used in some situations. Linear and area measurements serve as surrogates for chamber volume.
Reference intervals (RIs) are useful to help identify cardiac chamber enlargement and can help stratify dogs into categories of mild, moderate, or severe chamber enlargement to aid clinical decision making. Data collected from a reference population should be performed in a standardized prescribed manner that includes rigorous screening to determine if the reference individual is normal or healthy.16, 17 The Clinical and Standards Laboratory Institute guidelines recommend having a reference population of at least 120 subjects. Reference intervals conventionally encompass the central 95% of the reference values.17 This sample size also permits an estimation of the precision around the lower 2.5% and upper 97.5% reference limits.18
Few measurements of cardiac chamber size and function in dogs have RIs that were determined using the aforementioned recommendations. Studies commonly cited to serve as RIs for popular linear measurements of left atrial (LA) and left ventricular (LV) chamber size and function in dogs have used small sample sizes,19, 20 were derived from a single breed,19 or have not been collected in the prescribed manner for the purpose of establishing RIs.21
Broadly applicable RIs are challenging in dogs given the marked range of body shapes and sizes encountered in clinical practice. Common approaches to overcome this challenge include using breed‐specific RIs, normalizing chamber measurements to an internal control such as the aorta,19, 20, 22, 23, 24, 25 or normalizing chamber measurements to body weight using nonlinear regression or allometric scaling.21, 26 Although breed‐specific RIs might offer advantages compared to normalizing by body size alone,27 breed‐specific RIs are impractical for all breeds and not applicable to mixed breed dogs.
With the exception of LA volume estimated from left apical imaging planes28, 29 and estimates of LV volume evaluated in single breeds,30, 31, 32, 33 methods used to estimate LA and LV chamber volume from 2DE have not been well studied in large and diverse populations of dogs for the purpose of generating RIs. Estimates of LA and LV chamber volume from 2DE (eg, Simpson's method of discs) might be more sensitive to changes in chamber size. These measurements have been shown to be superior to linear measurements to detect early changes in Dobermans with dilated cardiomyopathy32 and in dogs with myxomatous mitral valve disease.34
Our primary objective was to generate body size‐independent RIs for several linear, area, and volume measurements of LA size and LV size and function acquired prospectively in a standardized manner using 2DE of a large and diverse sample of healthy dogs. We also sought to report measurement agreement and day‐to‐day repeatability of these measurements.
2. MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Davis (protocol #: 19867). All dog owners gave written consent before enrollment.
2.1. Animals
Dogs at least 1 year of age consisting of various somatotypes and body weights were prospectively recruited for the study. Dogs were owned by members of the University of California, Davis, School of Veterinary Medicine community. Each dog was determined to be healthy and free of cardiac disease based on history, physical examination, and a complete echocardiographic examination (details below). Echocardiographic images were evaluated subjectively by a cardiologist (L.C.V.) before study enrollment and data collection. Exclusion criteria were: (1) pathologic heart murmur, gallop sound, or nonsinus arrhythmia; (2) current or recent evidence of any systemic illness based on history and physical examination; (3) medications known to affect the cardiovascular system; (4) uncooperative temperament for echocardiography; and (5) cardiac abnormalities identified on the 2DE, MM, and Doppler echocardiographic examinations. Trivial atrioventricular or semilunar valve regurgitation was not an exclusion criterion provided it was not heard on auscultation and no structural valve abnormalities were identified. Sample size was based on Clinical and Laboratory Standards Institute (CLSI) guidelines for establishing RIs where at least 120 reference subjects are recommended to determine reference limits by nonparametric methods with 90% confidence intervals around the limits.17 All dogs were weighed on the same digital floor scale (TI‐500E, Transcell Technology, Inc, Buffalo Grove, Illinois) at the time of echocardiographic examination. Dogs were recruited so that there were approximately equal numbers of dogs in the following weight categories: <10 kg, ≥10 to <20 kg, ≥20 to <30 kg, and ≥30 kg.
2.2. Echocardiographic examinations
2.2.1. Image acquisition
Echocardiographic examinations were performed by a single operator (L.C.V.) using an ultrasound unit (Philips EPIQ 7C, Philips Healthcare, Andover, Massachusetts) equipped with several phased‐array transducers (5‐12 MHz) that were matched to the size of the dog. Simultaneous ECG was recorded. All dogs had 2DE, MM, and Doppler echocardiographic examinations using recommended right parasternal, subcostal, and left parasternal imaging planes.35, 36 The same standardized imaging protocol was used for each examination. From the right parasternal 4‐chamber long‐axis (Lx) imaging plane, care was taken to avoid foreshortening of the LA and LV. When the LV apex appeared cutoff in the standard right parasternal 4‐chamber Lx imaging plane, separate cine loops focused on the elongated LV were acquired. At least 6 cardiac cycles from each imaging plane were acquired. Sweep speeds of at least 100 mm/s (the echocardiography system's default setting) were utilized for MM and spectral Doppler imaging. Dogs were restrained manually in right and left lateral recumbency. Sedation was not utilized. Raw imaging data from each study were captured digitally for off‐line analysis, which was performed later using dedicated software (Syngo Dynamic Workplace, Siemens Medical Solutions, Inc, Malvem, Pennsylvania) at an off‐cart workstation.
2.2.2. Echocardiographic measurements and calculations
A single trained investigator (M.M.C.) performed all echocardiographic measurements. The value recorded for each measurement consisted of the average of 3, usually consecutive cardiac cycles. For 2DE images in which the mitral valve was visible, LV end‐diastole was defined as the frame that coincided with mitral valve closure. For images in which the mitral valve was not visible, end‐diastole represented the maximum chamber dimension at or near the onset of the QRS complex. End‐systole of the LV was defined as 1‐to‐2 frames before mitral valve opening. For images in which the mitral valve was not visible, the minimum chamber dimension was used to define end‐systole. For all cardiac chamber measurements, the blood‐tissue interface (ie, inner edge‐to‐inner edge measurement technique) was utilized. For all LV chamber measurements, the papillary muscles (if visualized) were ignored (ie, the papillary muscles were not traced and their respective volume and area were included along with the LV volume and area chamber quantitation). The heart rate value recorded represented the mean of the 3 instantaneous heart rates determined from the LV MM measurements.
All cardiac measurements used were performed from right parasternal imaging planes and, unless specified, used 2DE. The right parasternal Lx 4‐chamber view was used for linear and volume measurements of the LA and LV. Maximum LA dimension (LAD) was measured mid‐chamber (bisecting Lx atrial area) at end‐systole (of the ventricle) using a line drawn approximately parallel to the mitral annulus.25 This measurement extends from the inner edge of the region of the fossa ovale to the internal reflection of the bright (hyperechoic) pericardium in the far field approximately parallel to the mitral annulus. Maximum LA volume (LAV) was determined by manually tracing the internal border of the LA at ventricular end‐systole and applying the monoplane Simpson's method of discs to estimate volume from 2DE (Figure 1A). Volume was estimated from a series of stacked discs and was calculated by the echocardiography software package. A straight line drawn from hinge point to hinge point across the mitral valve annulus defined the boundary of the LA and LV. The confluence of the pulmonary vein was excluded. The height of the stacked discs was always selected to be perpendicular to the midpoint of the mitral valve annulus, bisecting the atrial area in the Lx. The LV internal dimension was measured using 2DE at end‐diastole (LVIDd) and end‐systole (LVIDs) at the level of the chordae tendineae approximately perpendicular to the Lx of the ventricular septum and LV free wall.25 Left ventricular volume was determined at end‐diastole (LVVd) and end‐systole (LVVs) by tracing the internal border of the LV and applying the same monoplane Simpson's method as for LAV (Figure 1B,C). A straight line was drawn from hinge point to hinge point (ventricular side) across the mitral valve annulus to define the boundary between the LV and LA for these measurements.
Figure 1.
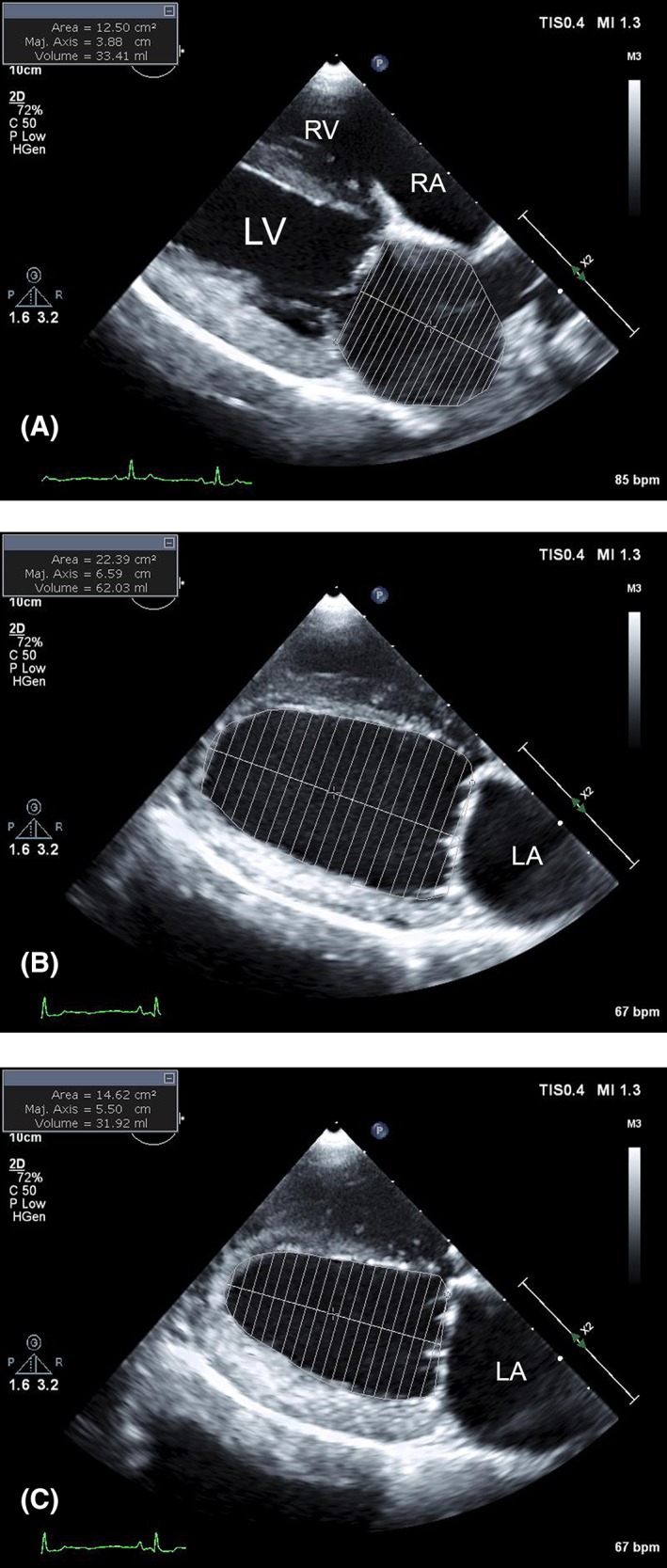
Representative measurements of maximum left atrial (LA) volume (LAV) (A), left ventricular (LV) volume at end‐diastole (LVVd) (B), and end‐systole (LVVs) (C) from the right parasternal long‐axis tomographic planes used in this study. For all measurements, the internal border (blood‐tissue interface) was manually traced. The echocardiography software calculated estimates of chamber volume from a series of stacked discs using a monoplane Simpson's method. The height of the stacked discs was always selected to be perpendicular to the midpoint of the mitral valve annulus, bisecting the chamber area in the long (major)‐axis. For LAV (A), a straight line was drawn from hinge point to hinge point across the mitral valve annulus and defined the boundary of the LA and LV. The confluence of the pulmonary vein was excluded. For LVVd and LVVs, a straight line was drawn from hinge point to hinge point (ventricular side) across the mitral valve annulus to define the boundary between the LV and LA. If encountered, the papillary muscles were excluded. RA, right atrium; RV, right ventricle
The aortic valve diameter (AoD; ie, aortic annulus) was measured from the right parasternal Lx outflow view. Measurements were made during early to mid‐systole between the hinge points of the maximally opened aortic valve.
The right parasternal short‐axis (Sx) view at the high papillary muscle level was used for linear and area measurements of the LV using 2DE. Measurements of LVIDd and LVIDs were made by drawing a line starting from the midpoint of the septal arc to the LV free wall between and equidistant from the 2 papillary muscles. Left ventricular area at end‐diastole (LVAd) and end‐systole (LVAs) were determined using planimetry by manually tracing the internal border of the LV (excluding the papillary muscles). M‐mode recordings acquired from the same right parasternal Sx view also were used to measure LVIDd and LVIDs. Thus, from the right parasternal Sx view, both 2DE and MM were acquired and linear measurements of LVIDd and LVIDs were obtained using each modality.
The LA‐to‐aortic root ratio (LA/Ao) was measured from the right parasternal Sx view at the level of the aortic root.19, 20 Both linear measurements were made in early diastole, which was defined by the earliest frame in which the closed aortic valve cusps could be visualized. The aortic root measurement was made starting from the midpoint of the convex curvature of the internal wall of the right aortic sinus of Valsalva and continuing along the commissure of the left and noncoronary cusps to the junction of the aortic wall, left coronary cusp, and noncoronary cusp. The LA measurement was made from the internal border of the LA extending from and parallel to the commissure between left and noncoronary cusp (ie, continued along the trajectory of the aortic root measurement) to the internal border of the distant LA wall in the far field, often near a pulmonary venous ostium. If a pulmonary vein entered the LA at the desired measurement point, the line was placed on what was considered to be an extrapolation of the atrial border.19 Care was taken to avoid including a pulmonary vein.
Fractional shortening (FS) was calculated as ([LVIDd − LVIDs]/LVIDd) × 100. Fractional area change (FAC), also called shortening area, was calculated as ([LVAd − LVAs]/LVAd) × 100. Ejection fraction (EF) was calculated as ([LVVd − LVVs]/LVVd) × 100.
2.3. Echocardiographic measurement agreement and day‐to‐day repeatability
Intraobserver measurement agreement (variability) was determined by having the same investigator (M.M.C.) perform all measurements on the echocardiographic examinations from the same 12 randomly selected dogs (3 randomly selected from each weight class: <10 kg, ≥10 to <20 kg, ≥20 to <30 kg, and ≥30 kg) on 3 separate occasions at least 1 week apart. Interobserver measurement agreement was determined by having 3 investigators (M.M.C., A.N.S., and L.C.V.) perform all measurements on the echocardiographic examinations from the same 12 randomly selected dogs. Intraoperator, day‐to‐day repeatability (echocardiographic recording reproducibility) was performed by randomly selecting 10 dogs (5 dogs <20 kg and 5 dogs ≥20 kg) to undergo a second echocardiographic examination performed by the same sonographer (L.C.V.) 48 hours after the first. The same investigator (M.M.C.) performed all measurements in these 10 dogs approximately 3 months after the initial measurements. For all echocardiographic measurement agreement and repeatability studies, investigators were blinded to each other's measurements or their previous measurements. Images or frames to measure were selected at the discretion of the investigator.
2.4. Statistical analysis
Statistical analyses were performed using commercially available computer software (Prism 7, GraphPad Software, Inc, La Jolla, California, and MedCalc Statistical Software, MedCalc Software bvba, Ostend, Belgium). All linear echocardiographic measurements were normalized to body weight (kg) using the constants generated from the allometric scaling or power equation, Y = ax b, where a is the proportionality constant, b is the scaling exponent, Y is the linear echocardiographic measurement, and x represents body weight. Accordingly, simple linear regression was performed on log10 body weight (explanatory variable) versus each log10 linear echocardiographic measurement (response variable), which yields the log10 form of the allometric scaling equation, log (Y) = log(a) + b × log(x).21 This approach provides the necessary allometric scaling constants b and a, which represent the slope and antilog Y intercept, respectively. Prediction intervals were determined from the constant (a c) according to the formula: , where a is the proportionality constant derived from the aforementioned linear regression equation, t is the desired Student's t statistic for n − 2 degrees of freedom and the desired degree of confidence, and S x,y is the SE of the Y estimate also derived from linear regression.21 For each regression, residual plots were visually inspected for model adequacy and tested for normality using the Shapiro‐Wilk test.
For the purpose of generating body size‐independent RIs, all linear echocardiographic measurements were normalized (indexed) to body size either by dividing by AoD or by dividing by body weight (kg)b, where b is the scaling exponent respective to each index and was determined from the linear regression equation as described above. All area echocardiographic measurements were normalized to body size by dividing by body surface area (BSA). All volume echocardiographic measurements were normalized to body size by dividing by body weight. All echocardiographic indices subsequently were tabulated, visually inspected using a dot plot, tested for normality using a Shapiro‐Wilk test, and tested for outliers using Tukey's method. Statistical outliers were examined and only considered for removal if an obvious measurement error was thought to have occurred. The 95% RIs were determined using the nonparametric percentile method as recommended by the CLSI when the reference sample exceeds 120 subjects.17 The 2.5th and 97.5th percentiles were defined as the lower and upper reference limits, respectively. As recommended, 90% confidence intervals around these limits also were determined using CLSI guidelines and the statistical software (MedCalc Statistical Software, MedCalc Software bvba) to provide an estimate of the precision of these limits.17, 18
Multiple linear regression analyses were used to explore the relationship between selected normalized measurements of chamber size (LVIDd2DE, Sx, LVIDd2DE, Lx/AoD, LVVd, LVIDs2DE, Sx, LVIDs2DE, Lx/AoD, LVVs, LAD2DE, Lx, LAD/AoD, LA/Ao, and LAV) and ejection phase indices (FS2DE, Sx, FAC, and EF) with body weight (kg), age (years), and heart rate (bpm). Spearman's rank correlation coefficients were used to explore the relationship between sex and the same echocardiographic indices. Only semipartial and Spearman's correlation coefficients with r > 0.4 were considered to be potentially clinically relevant and therefore reported.
Intraclass correlation coefficient (ICC) was used to quantify echocardiographic intraobserver and interobserver measurement agreement. For ICC calculations, a 2‐way single measures mixed effect model (where all the subjects are measured by the same observers) for absolute agreement was selected.37 The agreement of the investigators performing the measurements was considered poor if the value was 0‐0.2, fair if 0.21‐0.40, moderate if 0.41‐0.6, substantial if 0.61‐0.8, and almost perfect if 0.81‐1.38, 39 The within‐subject coefficient of variation (CV) and 95% repeatability (reproducibility) coefficient (RC95%) were used to quantify intraoperator day‐to‐day repeatability. A 1‐way ANOVA of the repeated echocardiographic studies in which the grouping variable was the subject provided the within‐subject variance (residual mean square), and the within‐subject SD (S w) was calculated as the square root of the within‐subject variance. Within‐subject CV was calculated as: (S w/overall mean) × 100, and the RC95% was calculated as 1.96 × √2 × S w.40 Statistical significance was set at P < .05.
3. RESULTS
One hundred twenty‐eight dogs were recruited and underwent echocardiographic examination. Six dogs were excluded; 3 had mild myxomatous mitral valve disease and 3 had uncooperative temperament. Therefore, 122 dogs were enrolled and comprised the reference population. Sixty were male and 62 were female with a median age of 4.3 years (minimum, 1.1 years; maximum, 16.5 years; interquartile range [IQR], 3.0‐7.0 years). Median body weight was 22.8 kg (minimum, 2.6 kg; maximum, 67.8 kg; IQR, 10.6‐29.8 kg). Twenty‐six dogs weighed <10 kg, 23 were ≥10 to <20 kg, 43 were ≥20 to <30 kg, and 30 were ≥30 kg. For the dogs ≥30 kg, median body weight was 34 kg (minimum, 30.0 kg; maximum, 67.8 kg; IQR, 31.5‐39.3 kg). Sixty‐one dogs were mixed breed; 11 were Labrador Retrievers; 6 were American Pit Bull Terriers; 4 dogs each were Australian Shepherds, Golden Retrievers, and Chihuahuas; 3 dogs each were Border Collies and Dachshunds; 2 dogs each were Doberman Pinschers, German Shepherds, and Australian Cattle Dogs; and the remaining were a Belgian Malinois, Bishon Frise, Boxer, German Shorthaired Pointer, Great Dane, Maltese, Miniature American Eskimo, Rottweiler, Yorkshire Terrier, Beagle, Shih Tzu, Siberian Husky, Standard Poodle, Tibetan Mastiff, Basenji, English Springer Spaniel, French Bulldog, English Cocker Spaniel, Rhodesian Ridgeback, and Brittany Spaniel.
The 5 dogs <20 kg that were randomly selected for the intraoperator day‐to‐day repeatability study consisted of 2 mixed breeds (1 male, 1 female), a German Shorthaired Pointer (female), a Chihuahua (male), and a Yorkshire Terrier (male). Their median (IQR) age and body weight were 5.0 (1.8‐7.1) years and 8.6 (3.9‐15.9) kg, respectively. The 5 dogs ≥20 kg that were randomly selected for the intra‐operator day‐to‐day repeatability study consisted of 2 Labrador Retrievers (1 male, 1 female), 2 mixed breeds (1 male, 1 female), and an American Pit Bull Terrier (male). Their median (IQR) age and body weight were 5.2 (3.5‐8.5) years and 22.8 (20.8‐29.5) kg, respectively. None of the dogs used for repeatability studies had chest conformation abnormalities and were considered to have adequate imaging windows.
All echocardiographic indices could be acquired and measured in each dog. No obvious measurement errors were identified on the measurements determined to be statistical outliers. Therefore, no measurements were excluded from the study. A summary of the linear regression data describing the relationship between log10 of selected linear chamber measurements and log10 body weight are presented in Table 1. All linear cardiac measurements demonstrated a significant (all P < .001) and strong (R 2 ≥ 0.79) correlation with body weight. All scaling exponents were close to the theoretical value of 1/3.
Table 1.
Results of the linear regression analyses describing how log10 of selected linear chamber measurements relate to log10 body weight in 122 healthy dogs
| Echocardiographic chamber measurement | Proportionality constant (a) | SE of Y estimate | Scaling exponent (b) | SE of b | R 2 |
|---|---|---|---|---|---|
| LVIDd2DE, Lx | 1.36 | 0.034 | 0.316 | 0.010 | 0.894 |
| LVIDd2DE, Sx | 1.37 | 0.036 | 0.316 | 0.011 | 0.881 |
| LVIDdMM, Sx | 1.40 | 0.034 | 0.299 | 0.010 | 0.884 |
| LVIDs2DE, Lx | 0.89 | 0.050 | 0.351 | 0.015 | 0.828 |
| LVIDs2DE, Sx | 0.73 | 0.062 | 0.392 | 0.018 | 0.795 |
| LVIDsMM, Sx | 0.73 | 0.062 | 0.387 | 0.018 | 0.790 |
| LAD2DE, Lx | 1.37 | 0.030 | 0.309 | 0.009 | 0.911 |
Notes: The constants derived from linear regression permit the calculation of prediction intervals and normalized echocardiographic linear measurements for any body weight (in kg) using the equation: measured linear dimension (in cm)/body weight (in kg)b, where b is the scaling exponent respective to each index.
Abbreviations: 2DE, two‐dimensional echocardiography; LAD, left atrial dimension; LVIDd, left ventricular internal dimension at end‐diastole; LVIDs, left ventricular internal dimension at end‐systole; Lx, long‐axis; MM, M‐mode echocardiography; Sx, short‐axis.
All correlations were statistically significant (P < .001).
Descriptive statistics and proposed RIs for the echocardiographic measurements of LA and LV chamber size normalized to body weight and LV ejection phase indices are summarized in Table 2. Prediction intervals of selected linear measurements for left heart chamber size normalized to body weight are presented in Table 3. Weak statistically significant correlations were identified between FS2DE, Sx (r = −0.42; P < .001), FAC (r = −0.43, P < .001), and EF (r = −0.50, P < .001) and body weight. Otherwise, no clinically significant correlations were identified between the other selected echocardiographic indices and body weight. No clinically relevant correlations were identified between the echocardiographic indices and heart rate, age, and sex.
Table 2.
Descriptive statistics and proposed reference intervals for echocardiographic measurements of left heart chamber size normalized to body weight and LV ejection indices
| Echocardiographic indicesa | Median | 95% CI of median | Min‐Max | 95% reference interval 2.5 centile (90% CI) to 97.5 centile (90% CI) |
|---|---|---|---|---|
| LV end‐diastole (normalized) | ||||
| LVIDd2DE, Lx cm/kg0.316 | 1.36 | 1.35‐1.38 | 1.08‐1.60 | 1.15 (1.08, 1.17) to 1.55 (1.55, 1.60) |
| LVIDd2DE, Sx cm/kg0.316 | 1.38 | 1.36‐1.40 | 1.11‐1.75 | 1.14 (1.11, 1.17) to 1.61 (1.54, 1.75) |
| LVIDdMM, Sx cm/kg0.299 | 1.42 | 1.39‐1.44 | 1.17‐1.67 | 1.19 (1.17, 1.24) to 1.63 (1.57, 1.67) |
| bLVIDd2DE, Lx/AoD | 2.29 | 2.25‐2.34 | 1.75‐2.59 | 1.84 (1.75, 1.96) to 2.56 (2.53, 2.59) |
| LVIDd2DE, Sx/AoD | 2.32 | 2.26‐2.36 | 1.80‐2.89 | 1.84 (1.80, 1.88) to 2.71 (2.62, 2.89) |
| LVAd2DE, Sx cm2/m2 | 14.4 | 13.9‐14.9 | 8.70‐19.5 | 9.72 (8.70, 10.10) to 18.69 (18.10, 19.50) |
| LVVd2DE, Lx mL/kg | 2.26 | 2.15‐2.35 | 1.34‐3.06 | 1.45 (1.34, 1.60) to 2.99 (2.92, 3.06) |
| LV end‐systole (normalized) | ||||
| LVIDs2DE, Lx cm/kg0.351 | 0.89 | 0.87‐0.92 | 0.65‐1.11 | 0.68 (0.65, 0.71) to 1.09 (1.05, 1.11) |
| LVIDs2DE, Sx cm/kg0.392 | 0.75 | 0.71‐0.77 | 0.42‐0.99 | 0.56 (0.42, 0.59) to 0.93 (0.89, 0.99) |
| LVIDsMM, Sx cm/kg0.387 | 0.74 | 0.72‐0.76 | 0.45‐0.96 | 0.50 (0.45, 0.55) to 0.92 (0.89, 0.96) |
| LVIDs2DE, Lx/AoD | 1.65 | 1.61‐1.69 | 1.13‐2.04 | 1.19 (1.13, 1.27) to 2.03 (1.95, 2.04) |
| LVAs2DE, Sx cm2/m2 | 6.26 | 5.68‐6.56 | 2.37‐11.13 | 3.41 (2.37, 3.67) to 10.07 (9.07, 11.13) |
| LVVs2DE, Lx mL/kg | 0.87 | 0.77‐0.94 | 0.29‐1.58 | 0.40 (0.29, 0.47) to 1.35 (1.27, 1.58) |
| LV ejection phase | ||||
| bFS2DE, Lx % | 26.6 | 25.5‐28.3 | 17.7‐43.9 | 19.1 (17.7, 19.6) to 41.7 (39.6, 43.9) |
| bFS2DE, Sx % | 32.2 | 31.0‐33.5 | 21.5‐55.0 | 21.9 (21.5, 24.2) to 49.3 (45.9, 55.0) |
| bFSMM, Sx % | 31.4 | 29.6‐34.5 | 20.2‐56.9 | 20.7 (20.2, 22.6) to 51.9 (47.7, 56.9) |
| FAC2DE, Sx % | 56.6 | 54.8‐59.9 | 39.8‐76.3 | 40.7 (39.8, 43.0) to 72.7 (69.6, 76.3) |
| bEF2DE, Lx % | 61.0 | 58.9‐63.2 | 46.0‐82.5 | 46.7 (46.0, 50.1) to 80.7 (75.9, 82.5) |
| LA size (normalized) | ||||
| LAD2DE, Lx cm/kg0.309 | 1.38 | 1.34‐1.39 | 1.14‐1.65 | 1.19 (1.14, 1.21) to 1.56 (1.49, 1.65) |
| LAD/AoD2DE, Lx | 2.24 | 2.21‐2.31 | 1.85‐2.57 | 1.88 (1.85, 1.95) to 2.54 (2.52, 2.57) |
| bLA/Ao2DE, Sx | 1.42 | 1.38‐1.44 | 0.92‐1.70 | 1.00 (0.92, 1.12) to 1.68 (1.65, 1.70) |
| LAV2DE, Lx mL/kg | 1.09 | 1.02‐1.44 | 0.65‐1.65 | 0.68 (0.65, 0.78) to 1.62 (1.47, 1.65) |
Abbreviations: Ao, aortic root; AoD, aortic valve diameter; CI, confidence interval; FAC, fractional area change; FS, fractional shortening; EF, ejection fraction; LA, left atrium; LAV, left atrial volume; LV, left ventricle; LVAd, left ventricular area at end‐diastole; LVAs, left ventricular area at end‐systole; LVVd, left ventricular volume at end‐diastole; LVVs, left ventricular volume at end‐systole. See Table 1 for the remainder of the abbreviations key.
All measurements were acquired from a right parasternal imaging window.
Not normally distributed.
Table 3.
Scaling exponents and prediction intervals of normalized linear left heart chamber measurements
| Echocardiographic chamber measurement | 97.5 percentile | 95 percentile | 75 percentile | 50 percentile | 25 percentile | 5 percentile | 2.5 percentile | Scaling exponent |
|---|---|---|---|---|---|---|---|---|
| LVIDd2DE, Lx | 1.59 | 1.55 | 1.43 | 1.36 | 1.29 | 1.19 | 1.16 | 0.316 |
| LVIDd2DE, Sx | 1.62 | 1.57 | 1.45 | 1.37 | 1.30 | 1.20 | 1.16 | 0.316 |
| LVIDdMM, Sx | 1.64 | 1.60 | 1.48 | 1.40 | 1.33 | 1.23 | 1.20 | 0.299 |
| LVIDs2DE, Lx | 1.11 | 1.07 | 0.96 | 0.89 | 0.82 | 0.73 | 0.71 | 0.351 |
| LVIDs2DE, Sx | 0.97 | 0.92 | 0.80 | 0.73 | 0.66 | 0.58 | 0.55 | 0.392 |
| LVIDsMM, Sx | 0.96 | 0.92 | 0.80 | 0.73 | 0.66 | 0.57 | 0.55 | 0.387 |
| LAD2DE, Lx | 1.57 | 1.53 | 1.43 | 1.37 | 1.30 | 1.22 | 1.19 | 0.309 |
Notes: Scaling exponents permit the prediction of normalized echocardiographic linear measurements (in cm) for any body weight (in kg) using the rearranged allometric equation: a = Y/x b and the desired prediction interval can be consulted to aid clinical decisions. For example, the LVIDd (MM, Sx) normalized for body weight for 95% of healthy dogs is predicted to be 1.20‐1.64. The equation used to normalize LVIDd (MM, Sx) for any body weight is the measured LVIDd (MM, Sx) / body weight0.299. See Table 1 for the abbreviations key.
Intraobserver and interobserver measurement agreement data are presented in Table 4. For intraobserver measurement agreement, measurements for all indices were considered to have almost perfect agreement with the exception of FS2DE, Lx, LAD/AoD2DE, Lx, and LA/Ao2DE, Sx, which exhibited substantial agreement. For interobserver measurement agreement, LA/Ao2DE, Sx exhibited fair agreement, FS2DE, Lx exhibited moderate agreement, LVIDd2DE, Lx/AoD, FSMM, Sx, EF2DE, Lx, and LAD/AoD2DE, Lx exhibited substantial agreement, whereas the remaining indices exhibited almost perfect interobserver measurement agreement.
Table 4.
Intraobserver and interobserver measurement agreement data from 12 randomly selected echocardiographic studies
| Echocardiographic measurements | Intraobserver measurement agreement ICC (95% CI) | Interobserver measurement agreement ICC (95% CI) |
|---|---|---|
| AoD | 0.96 (0.90, 0.99) | 0.94 (0.84, 0.98) |
| Ao | 0.96 (0.91, 0.99) | 0.90 (0.75, 0.97) |
| LVIDd2DE, Lx | 0.97 (0.92, 0.99) | 0.98 (0.94, 0.99) |
| LVIDd2DE, Sx | 0.99 (0.97, 1.0) | 0.97 (0.94, 0.99) |
| LVIDdMM, Sx | 0.96 (0.91, 0.99) | 0.97 (0.92, 0.99) |
| LVIDd2DE, Lx/AoD | 0.82 (0.60, 0.94) | 0.80 (0.58, 0.93) |
| LVAd2DE, Sx | 0.98 (0.89, 1.0) | 0.98 (0.94, 0.99) |
| LVVd2DE, Lx | 0.98 (0.96, 0.99) | 0.96 (0.66, 0.99) |
| LVIDs2DE, Lx | 0.98 (0.96, 0.99) | 0.94 (0.81, 0.98) |
| LVIDs2DE, Sx | 0.99 (0.98, 1.0) | 0.98 (0.91, 0.99) |
| LVIDsMM, Sx | 0.99 (0.98, 1.0) | 0.96 (0.90, 0.99) |
| LVAs2DE, Sx | 0.97 (0.77, 0.99) | 0.97 (0.91, 0.99) |
| LVVs2DE, Lx | 0.98 (0.96, 0.99) | 0.99 (0.97, 1.0) |
| FS2DE, Lx | 0.75 (0.48, 0.91) | 0.50 (0.14, 0.80) |
| FS2DE, Sx | 0.92 (0.81, 0.97) | 0.82 (0.56, 0.94) |
| FSMM, Sx | 0.88 (0.73, 0.96) | 0.78 (0.54, 0.92) |
| FAC2DE, Sx | 0.90 (0.58, 0.97) | 0.87 (0.70, 0.96) |
| EF2DE, Lx | 0.86 (0.68, 0.95) | 0.78 (0.14, 0.94) |
| LAD2DE, Lx | 0.95 (0.88, 0.98) | 0.96 (0.90, 0.99) |
| LAD/AoD2DE, Lx | 0.77 (0.53, 0.92) | 0.70 (0.41, 0.90) |
| LA2DE, Sx | 0.96 (0.91, 0.98) | 0.88 (0.73, 0.96) |
| LA/Ao2DE, Sx | 0.69 (0.39, 0.89) | 0.39 (0.07, 0.73) |
| LAV2DE, Lx | 0.98 (0.96, 0.99) | 0.95 (0.85, 0.98) |
Note: Bold values represent less than substantial agreement (ICC < 0.61).
Abbreviations: ICC, intraclass correlation coefficient. See Table 1 for the remainder of the abbreviations key.
Coefficients of variation and RCs for intraoperator day‐to‐day repeatability are presented in Table 5. All CVs were <13.4%, and with the exception of LVAs2DE, Sx, LVVs2DE, Lx, FS2DE, Lx, FS2DE, Sx, FSMM, Sx, FAC2DE, Sx, and LA/Ao2DE, Sx; the remainder were <10%.
Table 5.
Day‐to‐day intraoperator repeatability (echocardiographic recording reproducibility) from 10 randomly selected dogs that had 2 echocardiographic examinations 48 hours apart
| Echocardiographic indices (normalized) | CV (%) | RC95% a |
|---|---|---|
| LVIDd2DE, Lx (cm/kg0.316) | 1.8 | 0.07 |
| LVIDd2DE, Sx (cm/kg0.316) | 3.6 | 0.14 |
| LVIDdMM, Sx (cm/kg0.299) | 5.9 | 0.23 |
| LVIDd2DE, Lx/AoD | 4.0 | 0.26 |
| LVAd2DE, Sx (cm2/m2) | 6.4 | 2.56 |
| LVVd2DE, Lx (mL/kg) | 8.5 | 0.53 |
| LVIDs2DE, Lx (cm/kg0.351) | 4.7 | 0.11 |
| LVIDs2DE, Sx (cm/kg0.392) | 5.8 | 0.11 |
| LVIDsMM, Sx (cm/kg0.387) | 4.9 | 0.10 |
| LVAs2DE, Sx (cm2/m2) | 13.4 | 2.11 |
| LVVs2DE, Lx (mL/kg) | 10.5 | 0.22 |
| FS2DE, Lx (%) | 11.1 | 9.60 |
| FS2DE, Sx (%) | 10.9 | 11.5 |
| FSMM, Sx (%) | 10.2 | 9.60 |
| FAC2DE, Sx (%) | 10.4 | 17.6 |
| EF2DE, Lx (%) | 4.5 | 8.20 |
| LAD2DE, Lx (cm/kg0.309) | 2.8 | 0.10 |
| LAD/AoD2DE, Lx | 4.3 | 0.27 |
| LA/Ao2DE, Sx | 11.0 | 0.44 |
| LAV2DE, Lx (mL/kg) | 8.7 | 0.26 |
Note: Coefficients of variation values greater than 10% are in bold.
Abbreviations: CV, coefficient of variation; RC95%, 95% repeatability coefficient. See Table 1 for the remainder of the abbreviations key.
Note the RC is in the same unit as the echocardiographic index.
4. DISCUSSION
We evaluated numerous linear, area, and volume measurements of LA size and LV size and function that were acquired prospectively in a standardized manner using 2DE in a large (>120 dogs) and diverse sample of dogs for the purpose of generating RIs. Our study provides RIs for several indices that have not been previously reported, including volume estimates of LA and LV size acquired from the right parasternal Lx view, and reevaluates RIs and prediction intervals for some previously studied indices when collected in a standardized prescribed manner. Our data have expanded previous preliminary work evaluating LAV from the right parasternal Lx view in healthy dogs.41 Linear measurements identified the typical nonlinear relationship with body weight and allometric scaling, provided scaling exponents that were close to the theoretical valve of 1/3 and ranging from 0.299 to 0.392. Ejection phase indices (FS2DE, Sx, FAC, and EF) exhibited weak negative correlations with body weight. Intraobserver and interobserver measurement agreement and day‐to‐day repeatability were quantified to help delineate the precision and reliability of the studied indices.
Unlike in human medicine,42 recommended standards for quantitation of cardiac chamber size and function by echocardiography in dogs do not currently exist. Therefore, we evaluated several measurements of LA size and LV size and function acquired from MM and 2DE and in Sx or Lx imaging planes. In humans, the parasternal Lx views using 2DE are preferred for linear measurements of LV size.42 Left atrial and LV volumes measured using 2DE (using Simpson's method) or 3‐dimensional echocardiography from left apical imaging planes also are recommended.42 Without having consensus‐based recommendations for quantitation of cardiac chamber size in dogs, we elected to estimate LA and LV volumes from the right parasternal Lx imaging plane using a monoplane Simpson's method because monoplane assessment is more appealing for routine clinical use because of its convenience and efficiency compared to biplane assessment. Also, in our experience, foreshortening of the LV and LA are more likely to occur using the left apical imaging plane in dogs. This notion is strengthened by our results in that our normalized LA volume measurements (95th percentile of 1.50 mL/kg) were larger when compared with LA volumes acquired from the left apical imaging plane using similar methodology from other reference populations of healthy dogs (95th percentiles of 0.92‐1.07 mL/kg).28, 34 A study evaluating various methods of assessing LAV in healthy dogs also suggested that LA volume is slightly larger when measured from the right parasternal Lx view compared with left apical imaging plane.41 This difference potentially can be explained by less foreshortening of the LA from the right parasternal Lx view. We did not pursue 3‐dimensional echocardiography and strain imaging because they are less broadly applicable because of cost and availability limitations.
Currently, no accepted standard exists on how to determine RIs (ie, clinical cutoffs to help distinguish normal from abnormal) for echocardiography data sets in healthy dogs. Several strategies have been reported in the veterinary literature and have included reporting a range (minimum‐maximum),19 using predetermined percentiles (eg, upper 95th percentile or 97.5th percentile based on 1‐ or 2‐tailed analysis),20, 25, 28, 43 using the mean multiplied by 2 × the SD,31, 44 or reporting regression‐based 95% prediction intervals (based on body weight).21, 45 For our study, we elected to use the standards adopted by the CLSI and the American Society for Veterinary Clinical Pathology.16, 17 These standards provide guidelines on the statistical methods that are based on the size of the reference population and whether or not the data set is normally distributed. Therefore, as recommended and after normalization of the measurements for body weight, we proposed RIs based on nonparametric methods using the central 95% (lower 2.5 percentile and upper 97.5 percentile) of the data set with 90% confidence intervals around the upper and lower limits. For the purpose of direct comparison to a previous study,21 we also calculated 95% prediction intervals using the allometric scaling method based on body weight for the linear measurements of LA and LV size (Table 3).
There is no consensus regarding the ideal method to index (normalize) cardiac measurements to body size. However, it is clear that linear measurements of cardiac chamber size are not linearly related to body weight (or BSA).21, 24 Logic and physical principles dictate that linear measurements are linearly related to body length or body weight1/3, area measurements are linearly related to body weight2/3 or BSA, and volume measurements are linearly related to body weight. Indexing linear measurements to measurements of different orders (eg, length indexed to body weight, which equivalent to a volume) is flawed, and studies reporting strong linear correlations of a linear cardiac measurement to body weight are likely underpowered, have a small range of body size, or both. Our results agree with several large‐scale studies of healthy dogs21, 45 and humans46, 47 that linear cardiac measurements are not linearly related to body weight. Even adequately powered studies of cats,48, 49, 50 a species that exhibits a relatively small body weight range, have demonstrated the nonlinear relationship between linear cardiac measurements and body weight, including a study of cats all of the same breed (Bengals).50 Thus, common approaches for indexing linear measurement to body size have included breed‐specific reference values,27 nonlinear or allometric scaling (ie, power‐based regression models),21, 26 or indexing linear measurements to a linear internal control such as the aorta.19, 20, 22, 23, 24, 25 Pediatric cardiologists commonly utilize Z‐scores, which represents the number of standard deviations of a value from the mean value at a particular BSA.51 We elected to use the 2 most common approaches in the veterinary echocardiography literature for indexing linear measurements to body weight: allometric scaling and indexing to the aorta. This approach permits direct comparisons to previous studies. For example, our scaling exponent for LVIDdMM, Sx (0.299) was nearly identical to that of a previous study's MM LVIDd (0.294).21 Lastly, we also chose to follow geometric principles by matching orders for area and volume measurements (ie, area measurements were indexed to BSA and volume measurements were indexed to body weight as opposed to indexing volumes to BSA).
A study evaluating allometric scaling of MM cardiac measurements in normal adult dogs21 commonly cited to serve as RIs for linear measurements of the minor LV dimension acquired with MM reported considerably wider prediction interval cut‐offs for normalized LVIDd and LVIDs (2.5‐97.5 percentiles, 1.27‐1.85 and 0.71‐1.26, respectively) when compared to our study (2.5‐97.5 percentiles, 1.20‐1.64 and 0.55‐0.96, respectively; Table 3). This most likely can be explained by the considerable differences in study design. The aforementioned study21 utilized cardiac chamber measurements that were collected retrospectively, not collected in a standardized fashion (eg, 9 different sonographers with some using blinded MM), and had a skewed reference population with 29% consisting of a single giant breed (Irish Wolfhounds). The authors of this study rightfully acknowledged that their wide prediction intervals should not serve as RIs or cutoffs intended to distinguish normal dogs from dogs with cardiac disease, particularly those with mild cardiac disease.21 To this point, it is also noteworthy that 1 of the enrollment criteria of a recent large prospective clinical trial in dogs with preclinical myxomatous mitral valve disease, cardiomegaly, and considerable risk for developing heart failure was an LVIDd normalized to body weight ≥1.70.7 This value easily falls within the 95% prediction interval of 1.27 to 1.85 reported in the aforementioned study.21 This value would be consistent with LV enlargement based on the 95% prediction interval for LVIDdMM, Sx of 1.20 to 1.64 in our study. The RIs and prediction intervals generated from our study are more likely to identify LV enlargement using linear measurements of the minor LV dimension. This likely will permit earlier detection of cardiovascular disease, which might impact future clinical decision making.
Several other studies have evaluated indices of LA/Ao (LA/Ao2DE, Sx or LAD/AoD2DE, Lx) using 2DE in healthy dogs for the purpose of generating RIs. Compared to a recent study25 that also evaluated LAD/AoD2DE, Lx (median, 2.09; minimum‐maximum, 1.82‐2.48), the values in our study were similar but slightly larger (median, 2.24; minimum‐maximum, 1.85‐2.57). Compared to a previous study evaluating LA/Ao2DE, Sx in multiple breeds (median, 1.31; maximum, 1.59),20 our results (median, 1.42; maximum, 1.70) were again similar but slightly larger. This was despite a considerably smaller reference population (36 dogs) and a slightly different measurement technique of the aortic root used in that study.20 Our results for LA/Ao2DE, Sx were nearly identical to a study of 40 healthy Dachshunds (median, 1.41; minimum‐maximum, 1.19‐1.65).52 Furthermore, our LA/Ao2DE, Sx ratios were considerably larger than those reported in a study of 134 Cavalier King Charles Spaniels (median, 0.74; minimum‐maximum, 0.47‐0.94).43 This difference is because of the use of end‐diastolic measurements in that study43 compared to the use of early diastolic measurements in our study. Lastly, our maximum LA/Ao2DE, Sx (1.70) was considerably larger than the maximum LA/Ao2DE, Sx (1.27) reported in another study (median was not reported for comparison) of healthy Cavalier King Charles Spaniels.19 The reason for divergent results of that study19 compared to others20, 52 and ours is unclear but likely is a result of differences in measurement technique or the different reference populations. Specifically, the aforementioned study19 did not use an inner edge‐to‐inner edge technique for measurement of the LA. The LA measurement extended from the inner edge of the aortic root measurement to the blood‐tissue interface of caudal LA wall in the far field.19
Our study evaluated several ejection phase indices of LV systolic function, some of which (FAC and monoplane Simpson's EF) have not been evaluated in a large and diverse population of dogs for the purpose of generating RIs. Fractional shortening is a commonly used index of global LV systolic function and most examiners consider FSMM, Sx ≥ 25% to represent normal.15, 21 Indeed, 95% of the dogs in the aforementioned study of 494 normal dogs had a FS > 25%,21 whereas, our equivalent cutoff was slightly lower at a FSMM, Sx of 22%. Based on our clinical experience and that of others,15 many apparently healthy dogs can exhibit a FS closer to 20% for years without a known cardiac event. This might be particularly true in some large breed dogs, and is supported by our observation that some dogs had FS closer to 20% and the finding of weak negative correlation of FS2DE, Sx (r = −0.42; P < .001) to body weight. Interestingly, FAC and EF also exhibited weak negative correlations to body weight further suggesting larger dogs might exhibit relatively decreased systolic function compared to smaller dogs. These results are in agreement with a previous study53 of 60 Sighthounds with a large range in body weight (3.2 to 31.5 kg) but disagree with a large multibreed study,21 which failed to find a significant correlation between FS and body size. Higher resting vagal tone, behavioral differences (eg, calmer demeanor), increased athleticism, or some combination thereof are possible explanations for larger dogs to exhibit relatively decreased systolic function compared to smaller dogs. Further study is warranted to evaluate these possible explanations.
Given how close some of our RI cutoffs and scaling exponents are for normalized linear measurements of LV chamber size and function (LVIDd, LVIDs, and FS), one might be tempted to use RIs or scaling exponents of, for example, FS interchangeably. With the exception of LVIDd2DE, Sx and LVIDd2DE, Lx (given the identical scaling exponents and nearly identical RIs), we caution against this practice and encourage the use of the RIs and scaling exponents specific to each imaging plane (Lx versus Sx) and imaging modality (2DE versus MM). There are subtle but important differences in measurements performed with 2DE versus MM and Lx versus Sx, and they likely will behave differently, particularly in dogs with cardiac disease. One study found that LVIDd and LVIDs (acquired with MM) from Sx and Lx behaved similarly in healthy dogs but varied considerably in dogs with cardiac disease.54
A secondary objective of our study was to evaluate measurement agreement and the day‐to‐day repeatability of the echocardiographic indices. These analyses can be viewed as methods to evaluate the precision of the measurement of an echocardiographic index. The intraobserver measurement agreement was considered “almost perfect” for all indices except FS2DE, Lx, LAD/AoD2DE, Lx, and LA/Ao2DE, Sx, which were still considered “substantial” (as previously defined) in the context of echocardiography.39 This suggests that the measurements, when performed by the same individual, were consistent. When different individuals measured the same indices (all performed by the same sonographer), this high level of agreement was maintained for all but FS2DE, Lx and LA/Ao2DE, Sx. The LA/Ao2DE, Sx measurement in particular exhibited the worst interobserver agreement (ICC 0.39; “fair” agreement). We hypothesize that this result is a consequence of difficulties in consistently defining the far‐field LA border, maintaining a consistent path through the aortic root during image acquisition, and consistently defining aortic valve closure (timing of the measurement). The latter has been shown to affect this measurement.55
The day‐to‐day intraoperator repeatability data reported in our study (Table 5) helps to quantify the variability of an echocardiographic index caused by both image acquisition and measurement in addition to any physiologic variability that might occur within 48 hours. We showed the relative variability of the indices, conventionally defined by the CV. We also used the RC95% to help predict how a true change (beyond day‐to‐day variability) in the measured index can be defined. The latter provides clinically useful information by predicting where a future measurement is expected to be on 95% of occasions (ie, with 95% confidence), assuming there is no true change in the measurement caused by, for example, disease progression. For example, our results suggest that to document a true increase in LV size on serially obtained LVIDd2DE, Sx in the same dog (when acquired and measured by the same examiner), one should see an increase beyond 0.14 cm/kg0.316.
Our results should be considered within the context of the study's limitations. One examiner performed all of the echocardiographic examinations and 1 investigator performed all of the measurements in the study. Thus, our echocardiographic data might be biased by the methods, experience, and level of expertise of these individuals. Slightly different results likely will be observed across different echocardiography laboratories that have examiners with different levels of expertise and experience. However, to aid clinical decision making when using these RIs, we attempted to account for this possibility, to some degree, by reporting 90% confidence intervals around our proposed RI cutoffs and by evaluating the measurement variability and day‐to‐day repeatability of the echocardiographic indices. As with most RI studies in veterinary medicine, our study lacked longitudinal follow‐up of our dogs. Thus, we cannot be certain that some dogs were not affected by subclinical cardiac disease at the time of examination. Blood pressure and blood test data were not assessed before enrollment. Systemic hypertension or subclinical blood test result changes could have influenced cardiac measurements. Lastly, our reference population did not include all dog breeds and sizes. Half of our reference population was mixed breed dogs. We attempted to recruit a large variety of dogs but found it particularly difficult to recruit a variety of giant breed dogs. Therefore, our data might not be applicable to all dog breeds and caution is advised when applying these RIs to dogs outside the body weights of the dogs enrolled in our study (2.6‐67.8 kg).
In conclusion, body size‐independent RIs for a variety of linear, area, and volume measurements of LA size and LV size and function (mostly acquired using 2DE) are now available for clinical use. Because these RIs were acquired prospectively in a consistent manner with adequate statistical power, they likely represent reliable and broadly applicable cutoffs to help differentiate normal dogs from dogs with cardiac disease.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All procedures in this study were approved by the IACUC of the University of California, Davis (protocol #19867).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Brian Scansen for reviewing the manuscript, the contributions of Chrissy Kinkade, and the dog owners of the UC Davis School of Veterinary Medicine community for volunteering their dogs for this study.
Visser LC, Ciccozzi MM, Sintov DJ, Sharpe AN. Echocardiographic quantitation of left heart size and function in 122 healthy dogs: A prospective study proposing reference intervals and assessing repeatability. J Vet Intern Med. 2019;33:1909–1920. 10.1111/jvim.15562
REFERENCES
- 1. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142‐1150. [DOI] [PubMed] [Google Scholar]
- 2. Wess G, Domenech O, Dukes‐McEwan J, Häggström J, Gordon S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J Vet Cardiol. 2017;19:405‐415. [DOI] [PubMed] [Google Scholar]
- 3. Lord P, Hansson K, Kvart C, Häggström J. Rate of change of heart size before congestive heart failure in dogs with mitral regurgitation. J Small Anim Pract. 2010;51:210‐218. [DOI] [PubMed] [Google Scholar]
- 4. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: the PREDICT cohort study. J Vet Cardiol. 2012;14:193‐202. [DOI] [PubMed] [Google Scholar]
- 5. Hezzell MJ, Boswood A, Moonarmart W, Elliott J. Selected echocardiographic variables change more rapidly in dogs that die from myxomatous mitral valve disease. J Vet Cardiol. 2012;14:269‐279. [DOI] [PubMed] [Google Scholar]
- 6. Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc. 2007;231:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 7. Boswood A, Haggstrom J, Gordon SG, et al. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC study‐a randomized clinical trial. J Vet Intern Med. 2016;30:1765‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT study). J Vet Intern Med. 2012;26:1337‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borgarelli M, Crosara S, Lamb K, et al. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J Vet Intern Med. 2012;26:69‐75. [DOI] [PubMed] [Google Scholar]
- 10. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120‐128. [DOI] [PubMed] [Google Scholar]
- 11. Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22:1124‐1135. [DOI] [PubMed] [Google Scholar]
- 12. Moonarmart W, Boswood A, Luis Fuentes V, et al. N‐terminal pro B‐type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract. 2010;51:84‐96. [DOI] [PubMed] [Google Scholar]
- 13. Sargent J, Muzzi R, Mukherjee R, et al. Echocardiographic predictors of survival in dogs with myxomatous mitral valve disease. J Vet Cardiol. 2015;17:1‐12. [DOI] [PubMed] [Google Scholar]
- 14. Martin MW, Stafford Johnson MJ, Strehlau G, et al. Canine dilated cardiomyopathy: a retrospective study of prognostic findings in 367 clinical cases. J Small Anim Pract. 2010;51:428‐436. [DOI] [PubMed] [Google Scholar]
- 15. Bonagura JD, Luis Fuentes V. Echocardiography In: Mattoon J, Nyland T, eds. Small Animal Diagnostic Ultrasound. 3rd ed. Philadelphia, PA: Saunders; 2014:217‐331. [Google Scholar]
- 16. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol. 2012;41:441‐453. [DOI] [PubMed] [Google Scholar]
- 17. Clinical Laboratory Standards Institute . Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline CLSI document EP28‐A3c. 3rd ed. Wayne, PA; 2008. https://clsi.org/standards/products/method-evaluation/documents/ep28/. [Google Scholar]
- 18. Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334:5‐23. [DOI] [PubMed] [Google Scholar]
- 19. Hansson K, Haggstrom J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in Cavalier King Charles Spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 2002;43:568‐575. [DOI] [PubMed] [Google Scholar]
- 20. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med. 2000;14:429‐435. [DOI] [PubMed] [Google Scholar]
- 21. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18:311‐321. [DOI] [PubMed] [Google Scholar]
- 22. Brown DJ, Rush JE, MacGregor J, Ross JN Jr, Brewer B, Rand WM. M‐mode echocardiographic ratio indices in normal dogs, cats, and horses: a novel quantitative method. J Vet Intern Med. 2003;17:653‐662. [DOI] [PubMed] [Google Scholar]
- 23. Brown DJ, Rush JE, MacGregor J, Ross JN Jr, Brewer B, Rand WM. Quantitative echocardiographic [corrected] evaluation of mitral endocardiosis in dogs using ratio indices. J Vet Intern Med. 2005;19:542‐552. [DOI] [PubMed] [Google Scholar]
- 24. Hall DJ, Cornell CC, Crawford S, Brown DJ. Meta‐analysis of normal canine echocardiographic dimensional data using ratio indices. J Vet Cardiol. 2008;10:11‐23. [DOI] [PubMed] [Google Scholar]
- 25. Strohm LE, Visser LC, Chapel EH, Drost WT, Bonagura JD. Two‐dimensional, long‐axis echocardiographic ratios for assessment of left atrial and ventricular size in dogs. J Vet Cardiol. 2018;20:330‐342. [DOI] [PubMed] [Google Scholar]
- 26. Goncalves AC, Orton EC, Boon JA, Salman MD. Linear, logarithmic, and polynomial models of M‐mode echocardiographic measurements in dogs. Am J Vet Res. 2002;63:994‐999. [DOI] [PubMed] [Google Scholar]
- 27. Morrison SA, Moise NS, Scarlett J, Mohammed H, Yeager AE. Effect of breed and body weight on echocardiographic values in four breeds of dogs of differing somatotype. J Vet Intern Med. 1992;6:220‐224. [DOI] [PubMed] [Google Scholar]
- 28. Hollmer M, Willesen JL, Tolver A, et al. Left atrial volume and phasic function in clinically healthy dogs of 12 different breeds. Vet J. 2013;197:639‐645. [DOI] [PubMed] [Google Scholar]
- 29. Hollmer M, Willesen JL, Tolver A, et al. Comparison of four echocardiographic methods to determine left atrial size in dogs. J Vet Cardiol. 2016;18:137‐145. [DOI] [PubMed] [Google Scholar]
- 30. Seckerdieck M, Holler P, Smets P, Wess G. Simpson's method of discs in Salukis and Whippets: echocardiographic reference intervals for end‐diastolic and end‐systolic left ventricular volumes. J Vet Cardiol. 2015;17:271‐281. [DOI] [PubMed] [Google Scholar]
- 31. Smets P, Daminet S, Wess G. Simpson's method of discs for measurement of echocardiographic end‐diastolic and end‐systolic left ventricular volumes: breed‐specific reference ranges in Boxer dogs. J Vet Intern Med. 2014;28:116‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wess G, Maurer J, Simak J, et al. Use of Simpson's method of disc to detect early echocardiographic changes in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med. 2010;24:1069‐1076. [DOI] [PubMed] [Google Scholar]
- 33. Stephenson HM, Fonfara S, Lopez‐Alvarez J, et al. Screening for dilated cardiomyopathy in Great Danes in the United Kingdom. J Vet Intern Med. 2012;26:1140‐1147. [DOI] [PubMed] [Google Scholar]
- 34. Wesselowski S, Borgarelli M, Bello NM, Abbott J. Discrepancies in identification of left atrial enlargement using left atrial volume versus left atrial‐to‐aortic root ratio in dogs. J Vet Intern Med. 2014;28:1527‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 36. Abbott JA, MacLean HN. Comparison of Doppler‐derived peak aortic velocities obtained from subcostal and apical transducer sites in healthy dogs. Vet Rad Ultrasound. 2003;44:695‐698. [DOI] [PubMed] [Google Scholar]
- 37. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 39. Barnhart HX, Yow E, Crowley AL, et al. Choice of agreement indices for assessing and improving measurement reproducibility in a core laboratory setting. Stat Methods Med Res. 2016;25:2939‐2958. [DOI] [PubMed] [Google Scholar]
- 40. Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31:466‐475. [DOI] [PubMed] [Google Scholar]
- 41. LeBlanc N, Scollan K, Sisson D. Quantitative evaluation of left atrial volume and function by one‐dimensional, two‐dimensional, and three‐dimensional echocardiography in a population of normal dogs. J Vet Cardiol. 2016;18:336‐349. [DOI] [PubMed] [Google Scholar]
- 42. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1‐39 e14. [DOI] [PubMed] [Google Scholar]
- 43. Misbach C, Lefebvre HP, Concordet D, et al. Echocardiography and conventional Doppler examination in clinically healthy adult Cavalier King Charles Spaniels: effect of body weight, age, and gender, and establishment of reference intervals. J Vet Cardiol. 2014;16:91‐100. [DOI] [PubMed] [Google Scholar]
- 44. Vollmar AC. Echocardiographic measurements in the Irish wolfhound: reference values for the breed. J Am Anim Hosp Assoc. 1999;35:271‐277. [DOI] [PubMed] [Google Scholar]
- 45. Gentile‐Solomon JM, Abbott JA. Conventional echocardiographic assessment of the canine right heart: reference intervals and repeatability. J Vet Cardiol. 2016;18:234‐247. [DOI] [PubMed] [Google Scholar]
- 46. Neilan TG, Pradhan AD, King ME, Weyman AE. Derivation of a size‐independent variable for scaling of cardiac dimensions in a normal paediatric population. Eur J Echocardiogr. 2009;10:50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neilan TG, Pradhan AD, Weyman AE. Derivation of a size‐independent variable for scaling of cardiac dimensions in a normal adult population. J Am Soc Echocardiogr. 2008;21:779‐785. [DOI] [PubMed] [Google Scholar]
- 48. Haggstrom J, Andersson AO, Falk T, et al. Effect of body weight on echocardiographic measurements in 19,866 pure‐bred cats with or without heart disease. J Vet Intern Med. 2016;30:1601‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karsten S, Stephanie S, Vedat Y. Reference intervals and allometric scaling of two‐dimensional echocardiographic measurements in 150 healthy cats. J Vet Med Sci. 2017;79:1764‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scansen BA, Morgan KL. Reference intervals and allometric scaling of echocardiographic measurements in Bengal cats. J Vet Cardiol. 2015;17(Suppl 1):S282‐S295. [DOI] [PubMed] [Google Scholar]
- 51. Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465‐495. [DOI] [PubMed] [Google Scholar]
- 52. Lim CK, Fosgate GT, Green HW 3rd, et al. Two‐dimensional left atrium‐to‐aorta ratios and left ventricular M‐mode transthoracic echocardiographic measurements in clinically normal adult Dachshunds. Am J Vet Res. 2016;77:374‐382. [DOI] [PubMed] [Google Scholar]
- 53. della Torre PK, Kirby AC, Church DB, et al. Echocardiographic measurements in Greyhounds, Whippets and Italian Greyhounds–dogs with a similar conformation but different size. Aust Vet J. 2000;78:49‐55. [DOI] [PubMed] [Google Scholar]
- 54. Schober KE, Baade H. Comparability of left ventricular M‐mode echocardiography in dogs performed in long‐axis and short‐axis. Vet Radiol Ultrasound. 2000;41:543‐549. [DOI] [PubMed] [Google Scholar]
- 55. Dickson D, Caivano D, Patteson M, Rishniw M. The times they are a‐changin': two‐dimensional aortic valve measurements differ throughout diastole. J Vet Cardiol. 2016;18:15‐25. [DOI] [PubMed] [Google Scholar]


