Abstract
Background
Hospital‐acquired acute kidney injury (AKI) in humans and dogs increases morbidity and nonsurvival. Azotemia at presentation has been associated with a poor outcome in horses; however, prevalence and consequences of hospital‐acquired AKI are unreported.
Hypothesis/Objectives
To evaluate the prevalence of AKI in hospitalized horses, risk factors associated with AKI, and the effect of AKI on short‐term survival. We hypothesized that the prevalence of AKI in horses is similar to that reported in other domestic mammalian species and would be associated with nonsurvival.
Animals
Adult horses hospitalized for >2 days from which a minimum of 2 measurements of serum creatinine concentration were available.
Methods
Retrospective cohort study. Clinical records were reviewed and horses grouped according to their baseline serum creatinine concentration and change in serum creatinine concentration from baseline. The associations between signalment, diagnosis, and treatment variables, and the presence of azotemia or AKI were assessed using multinomial logistic regression. The relationship between these conditions and survival to discharge was evaluated.
Results
Three hundred twenty‐five horses were included; 4.3% (14/325) had azotemia at baseline and 14.8% (48/325) developed AKI. There were no significant associations between investigated risk factors and development of AKI. The presence of azotemia and AKI did not significantly affect survival to discharge (P = .08 and .81, respectively).
Conclusions and Clinical Importance
The prevalence of AKI in this population of hospitalized horses is similar to that reported in dogs and humans; however, in this study population, there was less impact on morbidity and short‐term survival.
Keywords: AKI, azotemia, creatinine, equine, renal
Abbreviations
- AKI
acute kidney injury
- CI
confidence interval
- GFR
glomerular filtration rate
- KDIGO
kidney disease improving global outcomes
- NSAID
nonsteroidal anti‐inflammatory
- OR
odds ratio
- sCr
serum creatinine concentration
- SDMA
symmetric dimethylarginine
- SIRS
systemic inflammatory response syndrome
- VAKI
veterinary acute kidney injury
1. INTRODUCTION
Acute kidney injury (AKI) is a common and clinically important complication in hospitalized human patients. In humans, the prevalence of AKI varies depending on the definition used and the population studied, but can be as high as 67% in intensive care settings.1 Acute kidney injury increases morbidity and nonsurvival, duration of hospitalization, and cost of treatment.2, 3 An increase in serum creatinine concentration (sCr) of 0.3 mg/dL (26.5 μmol/L) increases the risk of death by 70% in hospitalized humans.2 Patients with sepsis or cardiac disease are particularly susceptible.4, 5 These results are mirrored in dogs, where the prevalence of AKI was 14.9% and the diagnosis corresponded with an increase in nonsurvival from 15.7% to 54.2%.6 In horses, severe (>10 mg/dL) or persistent (duration over 72 hours) azotemia has been associated with a poor outcome; however, smaller changes in sCr that could identify development of hospital‐acquired AKI have not been assessed.7, 8
Historically, variations in the definition of AKI have made consistent identification and classification of patients difficult. Recently, however, the Kidney Disease Improving Global Outcomes (KDIGO) group defined AKI in humans as any of the following: (1) increase in sCr by 0.3 mg/dL (26.5 μmol/L) or more within 48 hours, (2) increase in sCr 1.5 times baseline or more within 7 days, or (3) oliguria (<0.5 mL/kg/h) for 6 hours.9 Importantly, this definition recognizes that kidney injury is a dynamic process and that sCr can remain within the reference range despite AKI. These criteria have been adapted for use in veterinary medicine to create the Veterinary Acute Kidney Injury (VAKI) scoring system, which has been used in dogs.6
Kidney injury in horses has been identified as a complication of hypovolemia, administration of nephrotoxic drugs, diarrhea or colitis, and sepsis.8, 10, 11, 12, 13 The objectives of the current study were (1) to apply the VAKI scoring system to a population of hospitalized horses to identify the prevalence of AKI, (2) to identify risk factors associated with the development of AKI, and (3) to assess the association of AKI with short‐term survival.
We hypothesized that the prevalence of AKI in hospitalized horses would be 15%‐25% (extrapolated from studies performed in adult humans and dogs), and that the presence of AKI would be associated with a decreased short‐term survival.6, 14 We hypothesized that the administration of drugs with known nephrotoxic effects would be associated with increased AKI.
2. MATERIALS AND METHODS
This was a single‐center, retrospective cohort study. Medical records of all horses admitted to Rossdales Equine Hospital between August 2015 and January 2019 were reviewed. Power calculation using variance and effect size data from canine and human AKI characterization studies identified that a sample size of 119 horses would be required to reliably detect a 15% prevalence of AKI with 80% power and 95% confidence. Horses were included for further analysis if they had an sCr measurement within 24 hours of admission (baseline sCr) and at least 1 more sCr measurement in the first 7 days of hospitalization. Cases were excluded if they were admitted with a history suggestive of primary renal disease, were less than 3 months of age, or were hospitalized for less than 2 days.
Variables identified from case records included signalment (sex, breed, age, and weight). Clinical variables (heart rate, respiratory rate, and temperature) and laboratory data (PCV, total solids, peripheral lactate concentration, and leukocyte count) on admission were used to assess systemic inflammatory response and the presence of hypovolemia. Hypovolemia was defined as horses showing 2 of the following 3 variables: tachycardia (>60 beats/min), PCV >45%, and peripheral lactate >31.5 mg/dL (3.5 mmol/L).15 Systemic inflammatory response syndrome (SIRS) was defined as the presence of 2 or more abnormalities, including hyperthermia (>38.5°C) or hypothermia (<37.0°C), tachycardia (>45 beats/min), tachypnea (>20 breaths/min), leukopenia (leukocyte count <6000/μL), and leukocytosis (leukocyte count >12 000/μL).16
Emergency admissions were differentiated from those admitted for elective procedures. Diagnoses were recorded and categorized as small intestinal lesion, large intestinal lesion (excluding colon torsion), colon torsion, peritonitis, other abdomen, respiratory, orthopedic, reproductive, and “other.” Horses with >1 condition were included in all appropriate diagnostic categories and the total number of diagnoses was recorded.
Treatment variables included the number of general anesthesia episodes, administration of potentially nephrotoxic drugs (nonsteroidal anti‐inflammatory drugs [NSAIDs], gentamicin, tetracyclines, polymyxin B, and synthetic colloids), and whether crystalloid IV fluid therapy was administered. Duration of hospitalization and survival to discharge were recorded.
Creatinine concentrations were measured in serum samples in a single laboratory using the Jaffe reaction with alkaline picrate (IL ILab IL650 Chemistry Analyzer; Instrumentation Laboratory, Lexington, Massachusetts). Azotemia was defined as any horse with a baseline sCr >1.9 mg/dL (167 μmol/L).17 Azotemia was considered secondary to a primary disease when there was no history suggesting preexisting renal injury, and other clinical abnormalities were likely to have led to AKI.
Horses were categorized into groups for statistical analysis according to their kidney status as follows:
Azotemia on baseline; sCr measurement >1.9 mg/dL (167 μmol/L). These were analyzed separately to those that developed AKI in hospital.
- For horses that did not have azotemia on baseline sCr measurement, AKI staging was performed using the VAKI staging system.6
- Stage 0 (S0): increase in sCr <150% from baseline
- Stage 1 (S1): increase in sCr of 150%‐199% or an absolute increase in sCr 0.3 mg/dL (≥26.5 μmol/L) from baseline
- Stage 2 (S2): increase in sCr from 200‐299% from baseline
- Stage 3 (S3): increase in sCr of ≥300% from baseline or an absolute sCr 4.0 mg/dL (>354 μmol/L).
For horses with >2 sCr measurements during the hospitalization period, the sCr value with the greatest deviation from baseline was used to calculate the change in sCr. If sCr only decreased during hospitalization, the maximum change in sCr was recorded as a negative value.
2.1. Statistical analysis
Analysis was performed using the statistical software package R (Version 3.4.4; R Inc., Boston, Massachusetts). The single outcome variable was kidney status (S0, S1, S2, S3, or azotemia). Due to the small number of horses in groups S2 and S3, for the purposes of statistical analysis, S1, S2, and S3 horses were grouped together (S1‐S3). Independent variables assessed included signalment, diagnostic and treatment categories as described above. For continuous variables (age, duration of hospitalization), the median and interquartile range were reported and a Kruskal‐Wallis test was applied for statistical analysis of associations with kidney status. All other variables contained categorical data, which was analyzed using either a chi‐square test or Fisher's exact test depending on the number of animals in each subgroup. A Bonferroni correction for multiple testing was applied to the results. Multinomial logistic regression was used to assess associations between kidney status and all of the signalment, diagnostic and treatment categories independently: variables where associations with the dependent variable had a P‐value of .2 or less were used in the initial multivariable model. Variables were subsequently removed in a stepwise fashion where P‐value for an effect was less than .2. In order to avoid overfitting, the final model included main effects only without interactions. The effect of kidney status on survival to discharge was analyzed using a Kaplan‐Meier survival curve and a log‐rank test. P‐value <.05 was considered statistically significant.
3. RESULTS
3.1. Study population
A total of 325 horses were included. Age was known for 316 horses (median 9 years; range 3 months‐24 years); 66 horses were ≥15 years, 195 were 3‐14 years, and 55 were 3 months to 2 years of age. There were 160 females, 156 males (125 geldings and 31 stallions), and 9 unrecorded. Breeds included Thoroughbreds (141), Warmbloods (30), Ponies (28), Cob (31), Sports‐horse (45), other (37), and 13 unrecorded. Three horses were excluded due to primary renal disease, which had been identified before admission and was the reason for referral.
Horses were admitted as an emergency on 300 occasions (92.3%); 25 horses were admitted for elective procedures. Intra‐abdominal disease was diagnosed in 272 instances: 86 small intestinal, 111 large intestinal, 27 colon torsion, 23 peritonitis, and 25 other abdominal; 34 had a respiratory diagnosis; 42 orthopedic; 25 reproductive; and 55 had “other” diagnoses. Multiple diagnoses were present in 102 horses, 82 of which had 2 diagnoses, 17 had 3 diagnoses, and 3 had 4 diagnoses.
One hundred seventy‐nine horses underwent general anesthesia, of which 19 horses were anesthetized twice or more. Drugs with reported potential for nephrotoxicity were administered to 294 horses; NSAIDs (n = 294), gentamicin (n = 219), tetracycline antibiotic (n = 43), polymyxin B (n = 28), and synthetic colloids (n = 38). Intravenous isotonic fluid therapy was administered to 234 horses. The median duration of hospitalization was 8 days (range 2‐48 days; Table 1).
Table 1.
Characteristics, diagnoses, and treatments of 325 hospitalized horses according to their kidney status
| Variable | Stage 0 n (%) |
Stages 1‐3 | Azotemia at baseline | ||
|---|---|---|---|---|---|
| n (%) | P | n (%) | P | ||
| Signalment | |||||
| Sex | n = 254 | n = 48 | .68 | n = 13 | .99 |
| Mare | 102 (40.2) | 17 (35.4) | 6 (46.2) | ||
| Gelding | 128 (50.4) | 25 (52.1) | 6 (46.2) | ||
| Stallion | 24 (9.4) | 6 (12.5) | 1 (7.7) | ||
| Breed | n = 252 | n = 47 | .12 | n = 13 | .24 |
| Thoroughbred | 118 (46.8) | 19 (40.4) | 4 (30.8) | ||
| Warmblood | 20 (7.9) | 8 (17.0) | 2 (15.4) | ||
| Pony | 24 (9.5) | 4 (8.5) | 0 | ||
| Cob | 29 (11.5) | 1 (2.1) | 1 (7.7) | ||
| Sports Horse | 34 (13.5) | 9 (19.1) | 2 (15.4) | ||
| Other | 27 (10.7) | 6 (12.7) | 4 (30.1) | ||
| Age (years) a | 9 (0‐24) | 9 (0‐20) | .99 | 13 (0‐19) | .97 |
| Laboratory parameters | |||||
| SIRS | n = 160 | n = 35 | .05 | n = 9 | .32 |
| 67 (41.8) | 8 (22.9) | 5 (55.6) | |||
| Hypovolemia | n = 166 | n = 38 | .36 | n = 10 | .12 |
| 34 (20.5) | 5 (13.2) | 4 (40) | |||
| Diagnosis | n = 263 | n = 48 | n = 14 | ||
| Small intestinal | 64 (24.3) | 17 (35.4) | .11 | 5 (35.7) | .53 |
| Large intestinal | 88 (33.4) | 16 (33.3) | .99 | 7 (50) | .25 |
| Colon torsion | 23 (8.7) | 4 (8.3) | .99 | 0 | .62 |
| Peritonitis | 19 (7.2) | 4 (8.3) | .77 | 0 | .61 |
| Other abdominal | 20 (7.6) | 2 (4.2) | .55 | 3 (21.4) | .08 |
| Respiratory | 30 (11.4) | 4 (8.3) | .80 | 0 | .38 |
| Orthopedic | 36 (13.7) | 4 (8.3) | .36 | 2 (14.2) | .70 |
| Reproductive | 19 (7.2) | 4 (8.3) | .77 | 2 (14.2) | .29 |
| Number of diagnoses | .47 | .01* a | |||
| 1 | 183 (69.5) | 35 (72.9) | 5 (35.7) | ||
| 2 | 66 (25.1) | 9 (18.8) | 6 (42.3) | ||
| ≥3 | 14 (5.3) | 4 (8.3) | 3 (21.4) | ||
| Treatment | n = 263 | n = 48 | n = 14 | ||
| Number of general anesthetic episodes | .21 | .12 | |||
| 0 | 120 (45.6) | 18 (37.5) | 8 (57.1) | ||
| 1 | 131 (49.8) | 25 (52.1) | 4 (28.6) | ||
| ≥2 | 12 (4.6) | 5 (10.4) | 2 (14.3) | ||
| NSAIDs | 236 (89.7) | 45 (93.8) | .59 | 13 (92.9) | .99 |
| Gentamicin | 174 (66.2) | 37 (77.1) | .18 | 8 (57.1) | .40 |
| Tetracycline | 41 (15.6) | 1 (2.1) | .01* | 2 (14.3) | .70 |
| Polymyxin B | 23 (8.7) | 4 (8.3) | .99 | 1 (7.1) | .99 |
| Synthetic colloids | 30 (11.4) | 3 (6.3) | .44 | 5 (35.7) | .02* |
| Duration of hospitalization (days) a | 8 (2‐48) | 11 (2‐29) | .62 | 7 (3‐21) | .44 |
P‐values identify univariable associations between S0 and S1‐S3 or azotemia, respectively.
Abbreviations: n, number in subset; NSAIDs, nonsteroidal anti‐inflammatory drugs; SIRS, Systemic inflammatory response syndrome.
P‐values <.05 indicate a statistically significant difference when compared to S0 group.
Kruskal‐Wallis applied for continuous variables; median and range reported.
Of the 325 horses, hypovolemia was identified in 43 on admission, 171 did not have evidence of hypovolemia, and for 112 horses, data were insufficient. Of the 43 hypovolemic horses, 4 had azotemia on admission and 5 developed AKI during hospitalization.
Evidence of SIRS was found in 80 of 325 horses. Of the 325 horses, 124 did not have evidence of SIRS and for 121 data were insufficient to assess. Of the 80 horses with SIRS, 5 had azotemia and 8 developed AKI. For horses where SIRS status was known, P for a statistical association between the presence of SIRS and development of AKI was .05 (odds ratio [OR], 0.41; 95% confidence intervals [CI], 0.15‐1.01) and .32 for an association between the presence of SIRS and azotemia (OR, 1.99; 95% CI, 0.41‐10.4). For horses with SIRS, azotemia and the development of AKI were not associated with survival to discharge (P = .99 and P = .68, respectively).
3.2. Kidney status
Of the 325 horses, 4.3% (14 horses; 95% CI 2.1‐6.5) had azotemia on baseline sCr (mean 2.4 mg/dL, range 1.9‐3.5 mg/dL). Serum creatinine normalized during hospitalization for all horses with azotemia. As defined by the VAKI staging system, 80.9% (263/325; 95% CI, 76.7‐85.2) were S0, 13.5% (44/325; 95% CI, 9.8‐17.3) were S1, and 1.2% (4/325; 95% CI, 0.03‐2.4) were S2. No horses in this study were S3. The overall prevalence of AKI in this population was 14.8% (95% CI, 10.9‐18.6).
3.3. Risk factors
In an exploratory analysis using chi‐square tests, azotemia was significantly associated with the number of diagnoses and treatment with synthetic colloids (P = .01 and P = .02, respectively). S1‐S3 was significantly associated with treatment with tetracycline antibiotics (P = .01; Table 1). However, applying a Bonferroni correction for multiple testing removed significance for these variables.
Following univariable analysis, multinomial logistic regression was used to assess factors associated with the risk of AKI (3 levels of kidney status: S0, S1‐S3, or azotemia). Factors included in the multinomial model (P < .2) included breed, SIRS, hypovolemia, small intestine and other abdominal diagnoses, number of diagnoses, treatment with gentamicin or tetracycline antibiotics, and the number of general anesthetics. After stepwise removal of variables where P > .2, the final multivariable model included SIRS (P = .06), hypovolemia (P = .08), number of diagnoses (P = .17), and treatment with tetracycline antibiotics (P = .16; Table 2).
Table 2.
Multinomial logistic regression model identifying associations between risk factors and either the development of acute kidney injury (stages 1‐3) or the presence of azotemia on baseline with reference to horses with no kidney dysfunction (stage 0)
| Stage 0 vs stages 1‐3 | Stage 0 vs azotemia | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| (Intercept) | 0.073 | 0.02‐0.2 | <.001 | 0 | NA | <.001 |
| SIRS | 0.15 | 0.02‐1.07 | .06 | NS | NS | NS |
| Tetracycline | 0.015 | 0‐1.62 | .08 | NS | NS | NS |
| Hypovolemia | NS | NS | NS | 8.6 | 1.8‐41.1 | .17 |
| Number of diagnoses | NS | NS | NS | 4.1 | 1.5‐11.2 | .16 |
Abbreviations: CI, confidence interval; NS, Not significant in univariable therefore not included in multinomial model; OR, odds ratio; SIRS, systemic inflammatory response syndrome.
3.4. Survival
Overall survival to discharge was 87.7% (285/325; 95% CI, 83.7‐90.1). For S0 horses, survival was 88.6% (233/263; 95% CI, 84.2‐91.9). For S1‐S3 horses, survival was 87.5% (42/48; 95% CI, 75.3‐94.1). For azotemia horses, survival was 71.4% (10/14; 95% CI, 45.4‐88.3). Survival to discharge was not significantly different between S0 and horses with azotemia (P = .08) and between S0 and S1‐S3 horses (P = .81; Figure 1).
Figure 1.
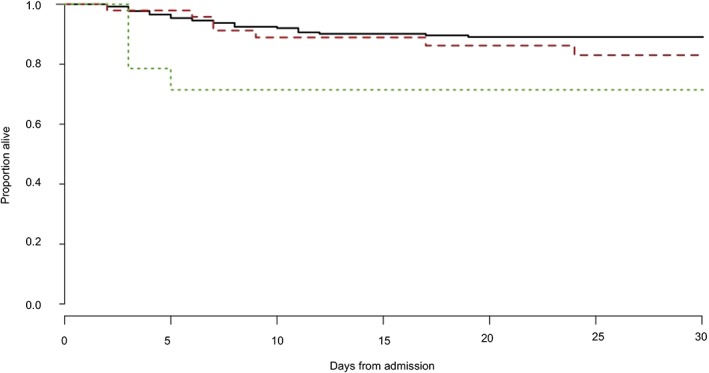
Comparison of survival to discharge curves for horses with no kidney disease (S0) (black solid line), those that developed an acute kidney injury while hospitalized (S1‐S3) (red dashed line), and those that had azotemia on arrival (green dotted line) by the Kaplan‐Meier method (log‐rank test, P < .05)
4. DISCUSSION
4.1. Prevalence
Using the VAKI criteria, the prevalence of AKI in this population of hospitalized horses was 14.7% (95% CI, 10.9‐18.6). This is lower than the reported prevalence of AKI in humans; a worldwide meta‐analysis of hospital‐acquired AKI using the KDIGO definition found a pooled incidence of 21.6% (95% CI, 19.3‐24.1).14 Other studies focusing on predominantly intensive care unit populations report higher prevalence, up to 67%.1 In humans, kidney injury can be accompanied by other organ system failure, especially in critically ill patients, and development of AKI is related to disease severity.1, 2, 14 Overall, despite the majority of horses in this study being admitted as an emergency (92.3%) and predominantly for gastrointestinal disorders, they appeared less systemically compromised than the human hospital populations that have been studied previously. Indeed, our study also included a number of horses hospitalized for treatment of orthopedic disorders in which generalized systemic disease would not be expected. In both univariable and multinomial regression analysis, the association between SIRS and the development of AKI did not reach significance. This is contrary to existing evidence from other species of association between level of systemic illness and development of AKI. Due to the retrospective nature of this study, missing data meant SIRS status was not established in 121 horses; this could have predisposed to type 2 errors. Larger numbers in this subset analysis might have added significance to this observation.
4.2. Risk factors
Baseline blood samples were taken within 24 hours of admission. Although the majority of these were likely to have been taken during admission, this could not be confirmed retrospectively. In addition, treatments administered by owners and referring veterinarians before referral were not assessed. Therefore, some potentially nephrotoxic treatments will have been administered before the baseline sCr being established, confounding risk factor analysis.
There was no association between age, breed, or sex on the presence of azotemia or development of AKI. In humans, advanced age and female sex are associated with increased susceptibility to AKI, and age over 65 years is associated with reduced recovery rates following kidney injury.9 This is due to age‐associated structural and functional renal deterioration, decreased renal reserve, and a high level of comorbidities in the geriatric population.9, 18 The median age of horses in this population was 9 years, with only 64 of 325 (19.7%) horses ≥15 years of age, whereas the average age of adult humans with hospital‐acquired AKI is 61 years.14 Our equine study population is relatively younger group of mammals presenting with acute disorders than studied hospitalized aged human populations, who are likely to have chronic disease and multiple comorbidities.
Multinomial logistic regression did not identify any clinical or laboratory diagnoses significantly associated with azotemia or development of AKI (Table 2). This is unexpected as although little studied in horses, renal hypoperfusion secondary to hypovolemia and kidney injury secondary to systemic inflammation are considered common mechanisms for development of kidney injury in other species.19 Indeed, renal disease secondary to gastrointestinal disease in horses has been appreciated for some time, and an association has been shown between kidney injury and gastrointestinal lesions that involve sequestration and loss of fluid and electrolytes, such as diarrhea or gastric reflux.8, 20 In the current study, risk factor analysis of the subset of 80 horses positively identified with SIRS was not possible due to small group sizes.
No association was identified in this study between treatments and the development of AKI, although care must be taken in interpretation as subdivision of horses has reduced the power of this analysis. In a previous study of horses with colic and colitis, no association was identified between differences in treatments, including NSAIDs, aminoglycoside antibiotics or administration of IV fluids, and resolution of azotemia, suggesting that treatments did not influence resolution of kidney injury.8 This could reflect clinician choice; horses with azotemia or considered at risk of AKI might have been less likely to receive nephrotoxic drugs. A larger study would be useful to be quantify the use of drugs with nephrotoxic effects and possible association with AKI.
4.3. Survival
In this study, no significant difference was identified between horses with azotemia or AKI and survival to discharge, unlike the direct correlation between the severity of AKI and the risk of non‐survival in humans.2, 3, 21, 22
Previous studies have documented an association between the presence of azotemia on admission and decreased survival to discharge.7, 8, 23, 24 These studies included different populations to that described in the current study; septic pleuropneumonia, colitis, and equine neorickettsiosis are diseases that carry a significant risk of hypovolemia as part of their pathophysiology, and, therefore, renal hypoperfusion is likely to be a more prominent feature in these populations than in the current study where only 43 horses were identified as hypovolemic on admission. In addition, an association between increased severity (>10 mg/dL) and persistence of azotemia over 72 hours has been associated with decreased survival.7, 8 The highest admission sCr in this study population was 3.5 mg/dL and sCr normalized during hospitalization for all horses in the azotemia group; therefore, the severity of azotemia was less than previously studied populations. A numerical 17% difference in survival to discharge between azotemia (71.4%, 95% CI, 45.4‐88.3) and S0 (88.6%, 95% CI, 84.2‐91.9) horses was observed (P = .08; Figure 1); increased sample size would be required to investigate this further. Based on the data collected, a sample size estimate that would identify a 10% difference in survival between the groups, as indicated in this study, with 80% power and 95% confidence would require 699 horses.
The majority of horses in this study that developed AKI were classified as S1 (13.5%), with only 2 S2 animals (1.2%) and no S3 horses. The severity of AKI in this population is therefore less than that in a generalized hospitalized human population where approximately 11.5% are S1, 4.8% S2, and 4.0% S3 using the KDIGO criteria. The death rates for these groups were 15.9%, 28.5%, and 47.8%, respectively, identifying a statistically significant increase in non‐survival with increasing AKI stage in humans.14 These results are mirrored in dogs.22 This might be attributable to the difference in the level of intensive care that can be provided to the different species.25
4.4. Limitations
The investigation of AKI is currently limited by the lack of a sensitive diagnostic test; sCr is a commonly used clinical indicator of glomerular filtration rate (GFR). However, it is an insensitive marker of renal function, as it increases only after an approximately 75% loss of GFR. In addition, sCr can vary with age, breed, sex, and muscle mass.26 Although currently gold standard, the Jaffe reaction for measurement of sCr is prone to interference both by a number of organic compounds (pseudochromogens), including bilirubin and glucose, and various antibiotics.27, 28 Other, more sensitive biomarkers of renal function could become available. Symmetric dimethylarginine (SDMA) is formed from the methylation of l‐arginine in cells and is excreted solely by the kidneys and more accurately reflects GFR than sCr in humans, dogs, and cats; concentrations increase in response to a 40% loss of GFR, and it is less affected by extrinsic factors such as low muscle mass, sex, and age.26, 29, 30, 31 Reference ranges for SDMA in adult horses have been established (Data on file at IDEXX Laboratories, Inc, Westbrook, Maine), and preliminary studies have identified that variability between healthy draft horses was low, with no effect of age or sex.32 In addition, biomarkers of renal injury have diagnostic potential. Increased urinary matrix metalloproteinase‐9 has been identified in horses with colic and might be related to early renal tubular damage.33 Other direct biomarkers of renal injury, including neutrophil gelatinase‐associated lipocalin, clusterin, cystatin B, and inosine have yet to be investigated.
This study was limited to identifying the association between changes in sCr on short‐term survival (to discharge). In humans, it is reported that a 25% increase in sCr in the first week postoperatively is associated with decreased long‐term (>8 years) survival.34 Therefore, long‐term follow‐up would be valuable in future research to investigate delayed or long‐term effects of kidney injury. Additionally, only horses older than 3 months were included in this study in order to eliminate the confounding effects of spurious hypercreatininemia and immature renal metabolism.35 Because of these factors, identifying renal disease in foals is a unique diagnostic challenge and specific biomarkers of renal function in this population would be valuable.
5. CONCLUSIONS
The prevalence of hospital‐acquired AKI was 14.8% in this population of horses, and AKI severity was lower than has been observed in other species. In human and canine patients, systemic illness predisposes to AKI, and severity of AKI is associated with non‐survival. However, the presence of azotemia within 24 hours of admission or the development of an AKI while hospitalized did not affect survival to discharge in this population of horses. Further investigation is required to identify more sensitive markers of equine AKI and the impact of AKI in critically ill equine patients.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
The antibiotic doxycycline was used off‐label due to the lack of a licensed alternative.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This research was presented at the 2018 ACVIM Forum, Seattle, WA.
Savage VL, Marr CM, Bailey M, Smith S. Prevalence of acute kidney injury in a population of hospitalized horses. J Vet Intern Med. 2019;33:2294–2301. 10.1111/jvim.15569
REFERENCES
- 1. Hoste EAJ, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2004;8(4):P162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365‐3370. [DOI] [PubMed] [Google Scholar]
- 3. Lee J, Baek SH, Ahn SY, et al. Pre‐stage acute kidney injury can predict mortality and medical costs in hospitalized patients. PLoS One. 2016;11(12):e0167038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597‐1605. [DOI] [PubMed] [Google Scholar]
- 5. James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the prevalence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096‐2103. [DOI] [PubMed] [Google Scholar]
- 6. Thoen ME, Kerl ME. Characterization of acute kidney injury in hospitalized dogs and evaluation of a veterinary acute kidney injury staging system. J Vet Emerg Crit Care. 2011;21(6):648‐657. [DOI] [PubMed] [Google Scholar]
- 7. Schott HC, Woodie JB. Chapter 64. Diagnostic techniques and principles of urinary tract surgery In: Auer JA, Stick JA, Kummerle JM, Prange T, eds. Equine Surgery. 5th ed. St. Louis, MO: Elsevier; 2019:1095‐1111. [Google Scholar]
- 8. Groover ES, Woolums AR, Cole DJ, LeRoy BE. Risk factors associated with renal insufficiency in horses with primary gastrointestinal disease: 26 cases (2000–2003). J Am Vet Med Assoc 2006; 6;228(4):572–577. [DOI] [PubMed] [Google Scholar]
- 9. Dewalt E. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1‐138. [Google Scholar]
- 10. El‐Ashker M, Risha E, Abdelhamid F, et al. Gentamicin‐induced acute kidney injury in equines is associated with marked acute phase response: an experimental study on donkey (Equus asinus). J Vet Sci Med Diagn. 2015;04(02):4. [Google Scholar]
- 11. Mozaffari AA, Derakhshanfar A, Alinejad A, Morovati M. A comparative study on the adverse effects of flunixin, ketoprofen and phenylbutazone in miniature donkeys: Haematological, biochemical and pathological findings. N Z Vet J. 2011;58(5):224‐228. [DOI] [PubMed] [Google Scholar]
- 12. Vivrette S, Cowgill LD, Pascoe J, Suter C, Becker T. Hemodialysis for treatment of oxytetracycline‐induced acute renal failure in a neonatal foal. J Am Vet Med Assoc. 1993;203(1):105‐107. [PubMed] [Google Scholar]
- 13. Divers TJ, Whitlock RH, Byars TD, Leitch M, Crowell WA. Acute renal failure in six horses resulting from haemodynamic causes. Equine Vet J. 1987;19(3):178‐184. [DOI] [PubMed] [Google Scholar]
- 14. Susantitaphong P, Cruz DN, Cerda J, et al. World prevalence of AKI: a meta‐analysis. Clin J Am Soc Nephrol. 2013;8(9):1482‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hollis AR, Boston RC, Corley KTT. Plasma aldosterone, vasopressin and atrial natriuretic peptide in hypovolaemia: a preliminary comparative study of neonatal and mature horses. Equine Vet J. 2010;40(1):64‐69. [DOI] [PubMed] [Google Scholar]
- 16. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The AACP/SCCM consensus conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644‐1655. [DOI] [PubMed] [Google Scholar]
- 17. McConachie E, Giguère S, Barton MH. Scoring system for multiple organ dysfunction in adult horses with acute surgical gastrointestinal disease. J Vet Intern Med. 2016;30(4):1276‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chao CT, Tsai HB, Lin YF, et al. Acute kidney injury in the elderly: only the tip of the iceberg. J Clin Gerontol and Geriatr. 2013;5:7‐12. [Google Scholar]
- 19. Schott HCII. Chapter 14. Disorders of the urinary system In: Reed SM, Bayly WM, Sellon DC, eds. Equine Internal Medicine. 4th ed. Missouri: Elsevier; 2018:888‐990. [Google Scholar]
- 20. Seanor JW, Byars TD, Boutcher JK. Renal disease associated with colic in horses. Mod Vet Prac. 1984;65:A26‐A29. [PubMed] [Google Scholar]
- 21. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee YJ, Chang CC, Chan JPW, Hsu WL, Lin KW, Wong ML. Prognosis of acute kidney injury in dogs using RIFLE (risk, injury, failure, loss and end‐stage renal failure)‐like criteria. Vet Rec. 2011;168(10):264‐264. [DOI] [PubMed] [Google Scholar]
- 23. Arroyo MG, Slovis NM, Moore GE, Taylor SD. Factors associated with survival in 97 horses with septic pleuropneumonia. J Vet Intern Med. 2017;31(3):894‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bertin FR, Reising A, Slovis NM, Constable PD, Taylor SD. Clinical and clinicopathological factors associated with survival in 44 horses with equine neorickettsiosis (Potomac horse fever). J Vet Intern Med. 2013;27(6):1528‐1534. [DOI] [PubMed] [Google Scholar]
- 25. Roy MF, Kwong GPS, Lambert J, Massie S, Lockhart S. Prognostic value and development of a scoring system in horses with systemic inflammatory response syndrome. J Vet Intern Med. 2017;31(2):582‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29(3):808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srisawasdi P, Chaichanajarernkul U, Teerakanjana N, Vanavanan S, Kroll MH. Exogenous interferences with Jaffe creatinine assays: addition of sodium dodecyl sulfate to reagent eliminates bilirubin and total protein interference with Jaffe methods. J Clin Lab Anal. 2010;24(3):123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Syal K, Srinivasan A, Banerjee D. Streptomycin interference in Jaffe reaction—possible false positive creatinine estimation in excessive dose exposure. Clin Biochem. 2013;46(1–2):177‐179. [DOI] [PubMed] [Google Scholar]
- 29. Nabity MB, Lees GE, Boggess MM, et al. Symmetric Dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29(4):1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kielstein JT, Salpeter SR, Bode‐Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta‐analysis. Nephrol Dial Transplant. 2006;21(9):2446‐2451. [DOI] [PubMed] [Google Scholar]
- 31. Hall JA, Obare E, Yerramilli M, Almes K, Jewell DE. Serum concentrations of symmetric Dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Intern Med. 2016;30(3):794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schott HC, Gallant L, Coyne M, et al. Symmetric dimethylarginine and creatinine concentrations in draft horse breeds. J. Vet. Int. Med. 2018;32:2128‐2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arosalo BM, Raekallio M, Rajamäki M, et al. Detecting early kidney damage in horses with colic by measuring matrix metalloproteinase −9 and −2, other enzymes, urinary glucose and total proteins. Acta Vet Scand. 2007;49(1):287‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loef BG. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in‐hospital mortality and long‐term survival. J Am Soc Nephrol. 2004;16(1):195‐200. [DOI] [PubMed] [Google Scholar]
- 35. Chaney KP, Holcombe SJ, Schott HC II, Barr BS. Spurious hypercreatininemia: 28 neonatal foals (2000‐2008). J Vet Emerg Crit Care. 2010;20(2):244‐249. [DOI] [PubMed] [Google Scholar]


